Two pollen-specific aquaporins are localized in the vacuoles of the vegetative and sperm cells, respectively, and contribute to reproduction under adverse environmental conditions.
Abstract
The water and nutrient status of pollen is crucial to plant reproduction. Pollen grains of Arabidopsis (Arabidopsis thaliana) contain a large vegetative cell and two smaller sperm cells. Pollen grains express AtTIP1;3 and AtTIP5;1, two members of the Tonoplast Intrinsic Protein subfamily of aquaporins. To address the spatial and temporal expression pattern of the two homologs, C-terminal fusions of AtTIP1;3 and AtTIP5;1 with green fluorescent protein and mCherry, respectively, were expressed in transgenic Arabidopsis under the control of their native promoter. Confocal laser scanning microscopy revealed that AtTIP1;3 and AtTIP5;1 are specific for the vacuoles of the vegetative and sperm cells, respectively. The tonoplast localization of AtTIP5;1 was established by reference to fluorescent protein markers for the mitochondria and vacuoles of sperm and vegetative cells and is at variance with the claim that AtTIP5;1 is localized in vegetative cell mitochondria. AtTIP1;3-green fluorescent protein and AtTIP5;1-mCherry showed concomitant expression, from first pollen mitosis up to pollen tube penetration in the ovule, thereby revealing the dynamics of vacuole morphology in maturating and germinating pollen. Transfer DNA insertion mutants for either AtTIP1;3 or AtTIP5;1 showed no apparent growth phenotype and had no significant defect in male transmission of the mutated alleles. By contrast, a double knockout displayed an abnormal rate of barren siliques, this phenotype being more pronounced under limited water or nutrient supply. The overall data indicate that vacuoles of vegetative and sperm cells functionally interact and contribute to male fertility in adverse environmental conditions.
The male gametophyte or pollen of flowering plants is reduced to essential and indispensable constituents for male gamete formation and delivery to the egg cell and, accordingly, shows a very precise and compact cellular architecture (Mogensen, 1992). Pollen grains of angiosperms contain a large vegetative cell and one generative cell giving rise to one or two sperm cells (Brewbaker, 1967). Although the sperm shows an occasional absence of plastids, pollen grains harbor most of the typical organelles of plant cells (Van Aelst et al., 1993; Yamamoto et al., 2003; Russell and Strout, 2005; Matsushima et al., 2008; Tang et al., 2009). The maturation process of pollen grains associates multiple steps in the formation and differentiation of vacuoles (vacuolation; for review, see Pacini et al., 2011). Extensive anatomical studies have shown that the vacuolar apparatus of the vegetative cell undergoes two fluctuating phases of morphology during pollen maturation and germination (Owen and Makaroff, 1995; Yamamoto et al., 2003; Hicks et al., 2004; Dettmer et al., 2005, 2010). The highest level of vacuolation, as characterized by a large, space-filling central vacuole, is found in the polarized microspore and during pollen germination. This state is discontinued by fragmentation into dispatched vacuoles or de novo synthesis of small vacuoles at the other developmental stages. By contrast, little is known about the morphology and role of vacuoles in sperm cells. Their existence has been documented using electron microscopy in a few plant species (Weber, 1988; Yu et al., 1992; Russell and Strout, 2005), and expression of two soluble E subunits of vacuolar H+-ATPase has been reported in the tonoplast of Arabidopsis (Arabidopsis thaliana) sperm cells (Strompen et al., 2005).
Pollen represents a remarkable cellular model in plant biology, as the differentiation of this simplified organ ends with its progressive desiccation. Upon deposition on a receptive stigma, pollen undergoes a sudden imbibition, which triggers germination, that is, a dramatic elongation of the pollen tube (PT; Winship et al., 2010). As this sequence of events contributes to the efficiency of fertilization, the water status of both mature and germinating pollen is crucial to plant reproduction (Heslop-Harrison, 1979; Sarker et al., 1988; Firon et al., 2012). Thus, transmembrane water transport and, possibly, aquaporin water channels might be crucial in pollen biology (Pina et al., 2005; Bock et al., 2006; Soto et al., 2008).
Plant aquaporins can be divided into four to five subfamilies (Wudick et al., 2009), with the plasma membrane intrinsic proteins and the tonoplast intrinsic proteins (TIPs) forming the largest ones. Six (out of 35) aquaporins have been repeatedly found to be expressed in Arabidopsis pollen: nodulin26-like intrinsic protein (NIP)7;1 (AtNIP7;1), small basic intrinsic protein (SIP)1;1 (AtSIP1;1), and AtSIP2;1 are also expressed in other tissues (Ishikawa et al., 2005; Pina et al., 2005; Li et al., 2011), whereas AtNIP4;1 and AtTIP1;3, and to a lesser extent AtTIP5;1, are restricted to pollen (Pina et al., 2005; Borges et al., 2008; Qin et al., 2009; Loraine et al., 2013). Expression of AtNIP4;1 and the two AtTIPs peaks during PT elongation and in mature pollen grains, respectively (Qin et al., 2009), while AtNIP7;1 was shown to be expressed in microspores (Li et al., 2011). Interestingly, transcriptomic and promoter analyses have shown that AtTIP1;3 is expressed in the vegetative cell (Pina et al., 2005; Bock et al., 2006; Borges et al., 2008), whereas AtTIP5;1 expression would be restricted to the sperm cells (Borg et al., 2008, 2011). A boron-induced expression of AtTIP5;1 was also uncovered in the Arabidopsis hypocotyl (Pang et al., 2010).
Because TIPs are primarily localized at the tonoplast, the occurrence of pollen-specific TIPs has initially pointed to specialized roles of vacuolar water transport in this organ. Plant aquaporins can also facilitate the bidirectional transport of small solutes or gases across cell membranes. In these respects, expression in Xenopus laevis oocytes revealed that AtTIP1;3 and AtTIP5;1 transport urea in addition to water, while excluding boric acid and glycerol (Soto et al., 2008). Based on bioinformatic predictions and expression of a GFP-TIP5;1 fusion in pollen vegetative cell, Soto et al. (2010) recently proposed that AtTIP5;1 is not targeted to the vacuole but rather to pollen mitochondria, where it would play a role in nitrogen remobilization. By contrast, an AtTIP5;1-GFP fusion was localized in the vacuole upon overexpression in Arabidopsis mesophyll protoplasts (Pang et al., 2010), and a role in tolerance to boron toxicity was suggested. Thus, the expression and function properties of AtTIP5;1 have remained unclear.
The primary objective of this work was to characterize the expression and role of AtTIP1;3 and AtTIP5;1 in pollen. For this, we characterized the in vivo temporal and spatial expression pattern of the two aquaporins and showed that they can be employed as unique tonoplast markers to investigate the vacuolar dynamics of the vegetative and sperm cells from pollen maturation to fertilization. Loss-of-function analyses revealed that the two aquaporins act in concert and positively contribute to plant reproduction under water and nutrient stress conditions.
RESULTS
Chemical Staining of Vacuoles in Mature and Germinating Pollen Grains
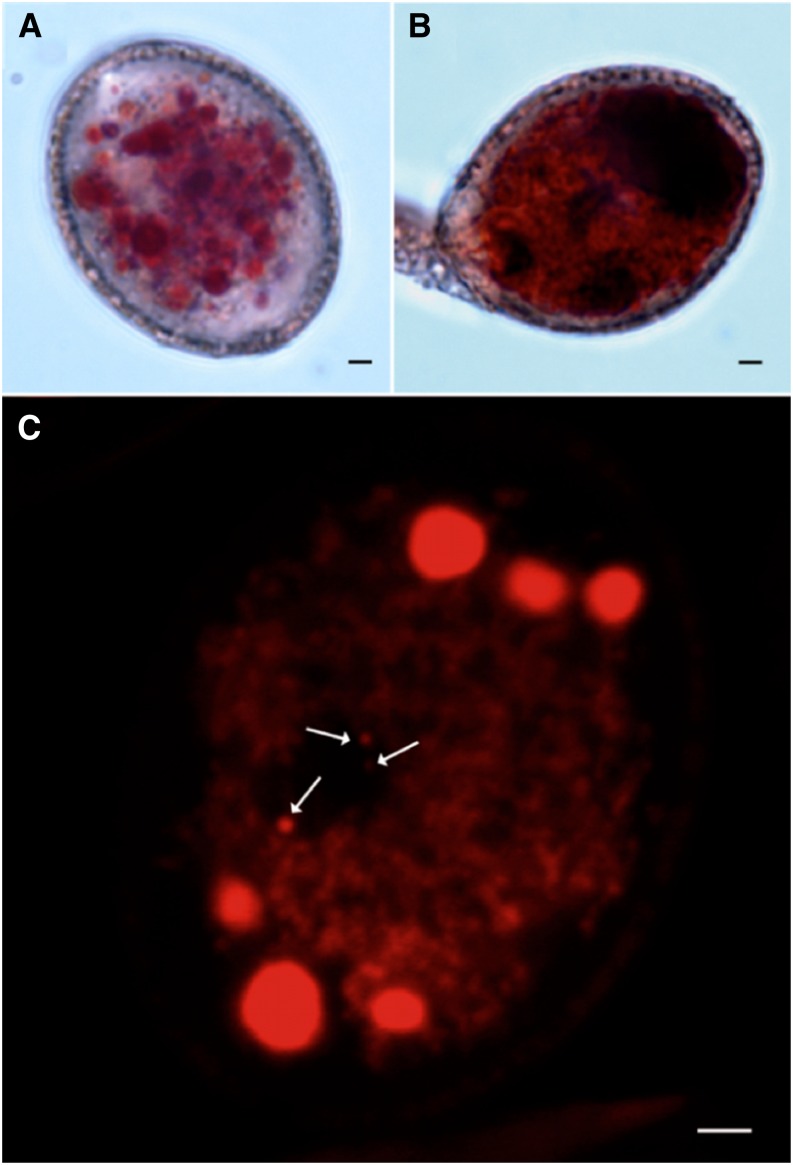
Neutral red was used to visualize the vacuolar equipment of mature and germinating Arabidopsis pollen. Under light transmission, a multitude of vacuoles of 0.5 to 4 µm in diameter was revealed throughout the whole vegetative cell (Fig. 1A). During pollen germination, a bigger vacuole became visible, distal to the PT emergence side, while smaller vacuoles were present proximal to the emergence side (Fig. 1B). To possibly get a better resolution, we also used confocal laser scanning microscopy. The z-stack projection of a series of optical sections obtained in the vegetative cell showed a pattern and amount of stained structures similar to those observed under transmission light (Fig. 1C; Supplemental Movie S1). Small spherical vesicles were also fluorescently labeled in the sperm cells of mature pollen (Fig. 1C). These vesicles probably represent the sperm vacuoles.
Figure 1.
Staining by neutral red of pollen vacuoles. Labeling of vacuoles in mature (A and C) and germinating (B) pollen grains using light transmission (A and B) or confocal laser scanning microscopy (C). Arrows indicate the labeling of small spherical structures that probably correspond to sperm cell vacuoles (as deduced from three-dimensional stacks of the respective pollen; Supplemental Movie S1). Note that the fluorescence image (C) represents an optical section, while the bright-field images (A and B) represent the totality of transmitted lights. Bar = 2 µm.
Temporal Expression Analysis of AtTIP1;3 and AtTIP5;1 in Maturating Pollen Grains
C-terminal fusions of AtTIP1;3 and AtTIP5;1 with GFP and mCherry, respectively, were placed under the control of their native promoter and introduced into transgenic Arabidopsis, and their expression was imaged in pollen. The DNA dye 4′,6′-diamino-2-phenylindole (DAPI) was used to define the different developmental stages.
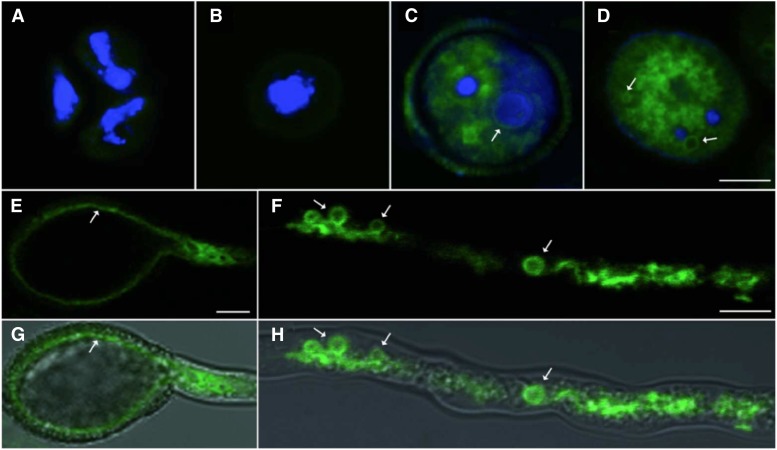
In accordance with transcriptomic data (Honys and Twell, 2004), no or low expression of AtTIP1;3-GFP was observed in tetrads or released microspores (Fig. 2, A and B). Expression became visible after first pollen mitosis, exclusively in the vegetative cell of bicellular pollen grains, and with early expression in a perinuclear ring (Fig. 2C). Soon afterward, AtTIP1;3-GFP labeled fuzzy structures and a multitude of small, dispatched vesicles (Fig. 2D), similar to those visualized using neutral red (Fig. 1A). A mitochondria-targeted red fluorescent protein (RFP) under the control of a vegetative cell-specific Late Anther Tomato52 (LAT52) promoter (VC-mtRFP; Matsushima et al., 2008) did not show any colocalization with AtTIP1;3-GFP (Fig. 3A). By contrast, the fuzzy structures were partially costained with ER-Tracker Blue-White DPX (Supplemental Fig. S1). Thus, these structures probably correspond to multiple compartments of the secretory system. Because their labeling by AtTIP1;3-GFP diminished over the course of pollen maturation and was almost totally lacking in germinating pollen grains (Fig. 2E), this localization may reflect the anterograde trafficking of AtTIP1;3-GFP to the vacuole. The AtTIP1;3-GFP fusion also intensely labeled the vegetative cell during pollen germination and PT emergence, revealing the high degree of vacuolation associated with these processes (Fig. 2, E and G). Tubular vacuoles (Hicks et al., 2004) and bulb-like structures (Saito et al., 2002) were also strongly highlighted by the fusion protein in the PT (Fig. 2, F and H).
Figure 2.
Temporal expression pattern of AtTIP1;3-GFP in maturating pollen and germinating PT. The expression of AtTIP1;3-GFP (green) was analyzed during pollen grain maturation (A–D) and germination (E–H). DAPI staining of nuclei (blue) was used to define stages of pollen development as tetrad (A), released microspore (B), bicellular (C), and tricellular (D) pollen. Arrows indicate labeling by AtTIP1;3-GFP of a perinuclear ring (C) and of small vacuoles (D) in maturating pollen grains, as well as the labeling of the tonoplast (E and G) and vacuolar bulbs (F and H) in the vegetative cell and the PT of germinating pollen, respectively. The vegetative nucleus in D was detected with DAPI staining but at a different optical section. It is therefore not visible in this image. G and H represent the superposition of an AtTIP1;3-GFP fluorescence image (E and F) with the corresponding transmission image. Residual green fluorescence in the cytosol (A) and at the pollen walls (B–D) is due to autofluorescence, as it can be also found in nontransformed pollen (not shown). Bars = 10 µm.
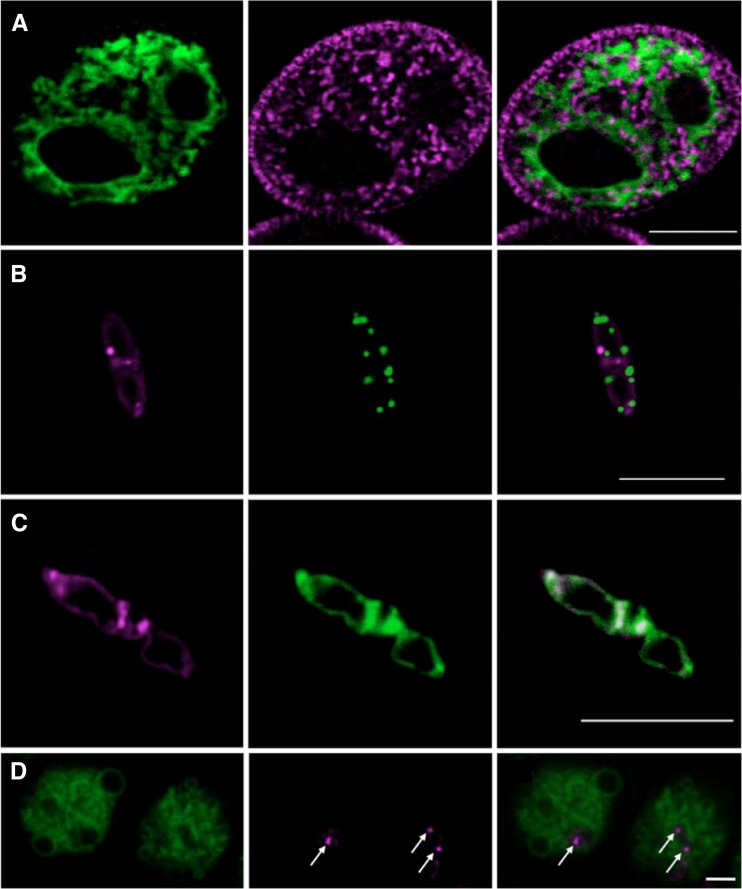
Figure 3.
Colabeling of mature pollen grains with AtTIP1;3-GFP, AtTIP5;1-mCherry, and mitochondrial or vacuolar markers. A, Representative mature pollen grain coexpressing AtTIP1;3-GFP (left) and a vegetative cell-specific mitochondria fluorescent reporter (VC-mtRFP; middle). The merged image is shown on the right. B, Coexpression of AtTIP5;1-mCherry with a sperm cell-specific mitochondria fluorescent reporter (SC-mtGFP; middle). The merged image is shown on the right. C, Coexpression of AtTIP5;1-mCherry (left) with the E1 subunit of the vacuolar H+-ATPase fused to GFP (VHA-E1-GFP; middle). Note that the latter is specifically enriched in the two sperm cells. Same conventions as in B. D, Coexpression of AtTIP1;3-GFP (left) and AtTIP5;1-mCherry (middle) in mature pollen grains. The merged image is shown on the right. Arrows indicate the labeling of small spherical vesicles by AtTIP5;1-mCherry in the sperm cells. Bars = 10 µm.
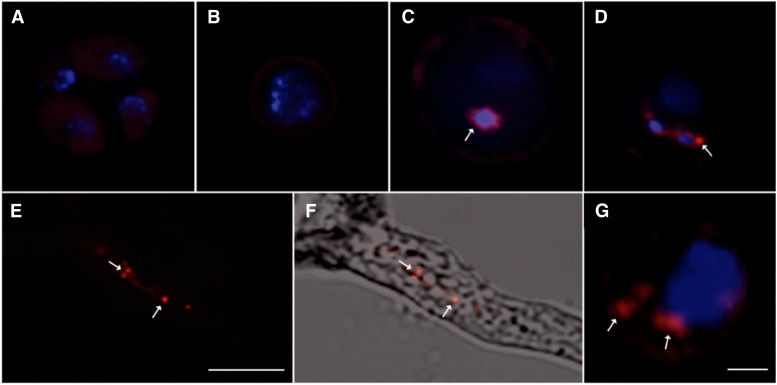
The expression of AtTIP5;1-mCherry appeared at the binuclear pollen state, simultaneously to that of AtTIP1;3-GFP (Fig. 4). By contrast, AtTIP5;1-mCherry showed expression exclusively in the sperm cells. Expression first appeared as a perinuclear ring surrounding the generative nucleus (Fig. 4, A–C). The protein then accumulated in the membrane of minute spherical structures (Fig. 4, D–F). An observation of sperm cells released from mature pollen grains confirmed the sperm-specific expression of AtTIP5;1-mCherry (Fig. 4G; Supplemental Fig. S2A). Each of the sperm cells examined (n > 40) disposed of one to six of these structures that typically ranged between 0.1 and 1 µm in size (Fig. 4E). Because these structures were comparable to those seen with neutral red (Fig. 1C) but could also correspond to mitochondria, we assessed their nature using coexpression of AtTIP5;1-mCherry with well-identified subcellular markers (Fig. 3, B and C). SC-mtGFP, a mitochondria-targeted GFP fusion protein under the control of the sperm cell-specific Duo Pollen1 (DUO1) promoter (Matsushima et al., 2008), revealed intensely stained oblong structures of approximately 0.5 µm in length (10–22 per cell; n = 11 cells). Thorough observations in dry (n = 17) and hydrated (n = 17) pollen did not indicate, however, any colocalization of SC-mtGFP with AtTIP5;1-mCherry (Fig. 3B; Supplemental Movie S2). VHA-E1, one of three E soluble subunits of the vacuolar H+-ATPase, is highly abundant in sperm cells (Strompen et al., 2005). Upon coexpression, colocalization, in both spherical and fuzzy structures, was observed between AtTIP5;1-mCherry and VHA-E1-GFP (Fig. 3C), which could be confirmed in three-dimensional image reconstructions (Supplemental Movie S3) and in released sperm cells (Supplemental Fig. S2). A colocalization in sperm cells of AtTIP5;1-mCherry with another soluble subunit of the vacuolar H+-ATPase (VHA-E3-GFP), which localizes to both vegetative and sperm vacuoles (Strompen et al., 2005), confirmed this finding (Supplemental Fig. S3). Our overall data indicate that AtTIP5;1-mCherry was localized to organelles of the secretory pathway, including vacuoles, but not to the mitochondria of sperm cells. This pattern of labeling was sustained during pollen germination when the sperm cell migrated into the PT (Fig. 4, E and F). It could be followed up to the micropyle penetration of the PT into the embryo sac (Supplemental Fig. S4).
Figure 4.
Temporal expression of AtTIP5;1-mCherry in maturating pollen and in germinating PT. The expression of AtTIP5;1-mCherry (red) was analyzed during pollen grain maturation as tetrad (A), released microspore (B), bicellular pollen (C), tricellular pollen (D), and during pollen germination (E and F). DAPI staining of nuclei (blue) was used to define stages of pollen development. Arrows indicate AtTIP5;1-mCherry labeling of a perinuclear ring (C) and small spherical structures in the sperm cells during pollen maturation (D). Similar structures are also labeled during PT growth (E) and in a released sperm cell (G; see arrows). F represents the superposition of a AtTIP5;1-mCherry fluorescence image (E) with the corresponding transmission image. Bars = 10 µm (A–F) and 1 µm (G).
Because an earlier study proposed that AtTIP5;1 localizes to the mitochondria of the vegetative cell (Soto et al., 2010), we wondered whether this protein may not show distinct subcellular localization depending on its cell expression context. We therefore generated the construct (LAT52::GFP-TIP5;1) previously used for ectopic expression of GFP-TIP5;1 in the vegetative cell and analyzed its coexpression with the VC-mtRFP mitochondrial marker (Supplemental Fig. S5). The TIP5;1 fusion protein showed a fuzzy expression across the whole vegetative cell, with a more pronounced accumulation in the plasma membrane and small punctuate structures (Supplemental Fig. S5, A and D). Yet, no colocalization with the mitochondrial marker was observed in any of these locations (Supplemental Fig. S5, C and F).
To further establish the expression specificity of AtTIP1;3-GFP and AtTIP5;1-mCherry, we analyzed the pollen of plants coexpressing the two fusion proteins (Fig. 3D). While expression of AtTIP1;3-GFP was exclusively found throughout the vegetative cell, expression of AtTIP5;1-mCherry was restricted to the sperm cell. The overall data establish the expression specificity of AtTIP1;3 and AtTIP5;1 in the vacuoles of the vegetative and sperm cells, respectively.
Isolation and Characterization of Single AtTIP1;3 and AtTIP5;1 Transfer DNA (T-DNA) Insertion Mutants
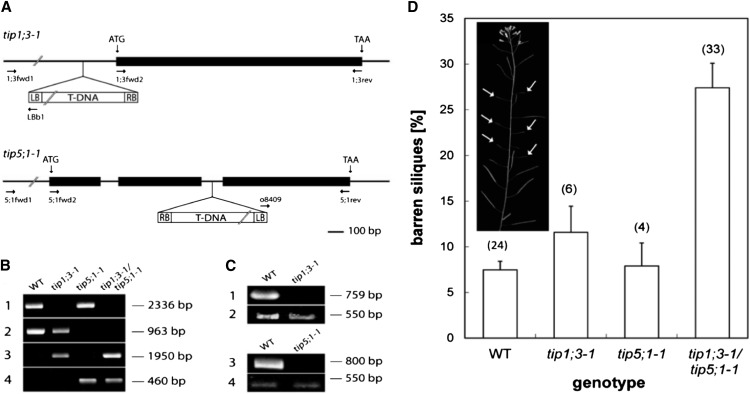
Transgenic Arabidopsis lines carrying a T-DNA insertion in the promoter of AtTIP1;3 (SALK_088276) and in the second intron of AtTIP5;1 (GABI_171E08; Fig. 5A) were used to produce homozygous knockout lines hereafter referred to as tip1;3-1 and tip5;1-1 (Fig. 5B) and lacking any transcript for AtTIP1;3 and AtTIP5;1, respectively (Fig. 5C). tip5;1-1 represents a knockout allele different from that described by Soto et al. (2010).
Figure 5.
Molecular characterization and reproduction phenotype of Arabidopsis AtTIP1;3 and AtTIP5;1 T-DNA insertion lines. A, Schematic gDNA structure of AtTIP1;3 and AtTIP5;1 with indicated open reading frame (ATG to TAA). Exons and introns are displayed as black rectangles and black bars, respectively. Note the lack of introns in AtTIP1;3. The insertion site of the T-DNA (white rectangle) and its orientation is indicated for both tip1;3-1 and tip5;1-1. Horizontal arrows indicate the orientation and position of primers used for PCR analyses of the gDNA and cDNA as indicated below. LB, Left border; RB, right border. B, PCR analyses of gDNA of wild-type (WT), tip1;3-1, tip5;1-1, and tip1;3-1/tip5;1-1 plants using a pair of AtTIP1;3-specific primers (1;3fwd1/1;3rev; 1), a pair of AtTIP5;1-specific primers (5;1fwd2/5;1rev; 2), and primers specific for AtTIP1;3 (1;3fwd1) or the T-DNA (LBb1; 3) or specific for AtTIP5;1 (5;1rev) or the T-DNA (o8409; 4). C, Detection by reverse transcription-PCR of AtTIP transcripts on total RNA from flowers of wild-type, tip1;3-1, and tip5;1-1 plants. Expression of AtTIP1;3 and AtTIP5;1 cDNA was probed using gene-specific primer pairs (1;3fwd2/1;3rev [1], 5;1fwd2/5;1rev [3]). The amplification of EF1α cDNA (2 and 4) served as a control for cDNA integrity. D, Proportion (in percentage) of barren siliques in plants of the indicated phenotype grown in soil (See “Materials and Methods”). The number of plants studied is indicated in brackets. The inset shows the phenotypic appearance of the shoots of tip1;3-1/tip5;1-1 plants with short siliques, devoid of seeds (arrows).
Neither tip1;3-1 nor tip5;1-1 showed any macroscopic shoot growth phenotype (data not shown), in accordance with the quasiexclusive expression of these TIPs in generative pollen tissues. Although the loss of AtTIP1;3 or AtTIP5;1 might cause a defect in pollen maturation or pollen vacuole functions, there was no major reduction in fertility in the mutant plants (not shown). To investigate slight alterations in male transmission of the mutated allele, we carried out crosses in which pollen from heterozygous +/tip1;3-1 or +/tip5;1-1 plants were manually deposited on pistils from sister wild-type plants. Such crosses create a competition for male transmission between the mutated and the wild-type alleles. A reciprocal cross in which pollen from the same wild-type plant was deposited on stigma of the respective +/tip1;3-1 or +/tip5;1-1 plant was performed as a control (Table I). Transmission of the mutated allele in the offspring seeds was screened using PCR (tip3;1-1) or T-DNA-encoded antibiotic resistance (tip5;1-1).
Table I. Transmission efficiency of the tip1;3-1 and tip5;1-1 alleles.
Pollen from heterozygous mutant plants (+/tip1;3-1 or +/tip5;1-1) was crossed onto stigma of wild-type (+/+) plants. In a control assay, the reciprocal crosses were accomplished on the same plants. Crosses were performed in six to seven plant pairs, with at least six individual flowers per plant, yielding more than 200 seeds for each cross. The table shows the number of wild-type and heterozygous plants and the percentage of heterozygous plants in the corresponding progenies. A χ2 test was applied comparing the transmission of the mutated allele to the theoretically expected percentage of transmission (50%) and, in the case of a heterozygous male, to the percentage of transmission observed in the reciprocal cross. χ2 values are in all cases below the given threshold value of 3.84 (P = 0.05 for a difference). The plants used for the crossings were sister lines obtained in the progeny of self-crossed heterozygous mutant plants. Dashes indicate that data are not available.
| Cross (Male × Female) |
Progeny |
χ2 |
||||
|---|---|---|---|---|---|---|
| +/+ | +/tip1;3-1 |
+/tip5;1-1 |
||||
| n | % | n | % | |||
| +/+ × +/tip1;3-1 | 120 | 107 | 47.1 | – | – | 0.744a |
| +/tip1;3-1 × +/+ | 111 | 111 | 50.0 | – | – | 0a/0.728b |
| +/+ × +/tip5;1-1 | 902 | – | – | 838 | 48.2 | 2.354a |
| +/tip5;1-1 × +/+ | 928 | – | – | 870 | 48.4 | 1.871a/0.0368b |
With respect to the theoretically expected percentage of transmission (50%). bWith respect to the percentage of transmission observed in the reciprocal cross.
Control crosses (with wild-type pollen) yielded 47.1% and 48.2% of +/tip1;3-1 and +/tip5;1-1 seeds (Table I). These values are slightly below but not significantly different (χ2 test) from the theoretically expected value (50%). In the reciprocal crosses (with heterozygous mutant pollen), the transmission efficiency of the tip1;3-1 and tip5;1-1 mutant alleles was 50% and 48.8%, respectively (Table I). These percentages were not significantly different from the respective control crosses or from the theoretically expected percentage (50%). Thus, there was no significant defect in male transmission of either one of the tip1;3-1 or tip5;1-1 alleles, in agreement with the work by Soto et al. (2010).
Phenotype of Double Knockout Plants
A homozygous double knockout line, tip1;3-1/tip5;1-1, that lacks both AtTIP1;3 and AtTIP5;1 was prepared (Fig. 5B) and characterized for pollen function and reproduction. Vitality assay with Alexander’s stain (Alexander, 1969) did not reveal any abnormalities when comparing tip1;3-1/tip5;1-1 with wild-type pollen (Supplemental Fig. S6). Wild-type and tip1;3-1/tip5;1-1 plants grown in soil at 20°C under regular irrigation had similar silique number. We noticed, however, that 7.5% ± 0.9% (n = 24 plants) and 27.4% ± 2.7% (n = 33 plants) of their siliques were shorter than fully developed siliques and mostly devoid of seeds (Fig. 5D; Supplemental Fig. S7). These siliques contained instead numerous nonfertilized ovules (Supplemental Fig. S7). With respect to wild-type plants, the proportion of barren siliques was significantly higher (P < 0.01) in tip1;3-1/tip5;1-1 but not in tip1;3-1 and tip5;1-1 plants (Fig. 5D). When grown in hydroponic, nutrient-replete conditions, wild-type and double knockout plants had virtually no barren siliques (<0.5%) and comparable mean silique numbers (wild type: number of siliques = 682 ± 31, n = nine plants; tip1;3-1/tip5;1-1: number of siliques = 709 ± 36, n = 10 plants). When grown in hydroponic conditions, but under nitrate deprivation, plants showed a dramatically reduced number of siliques, with more pronounced effects in tip1;3-1/tip5;1-1 (number of siliques = 25 ± 2, n = 10 plants) than in wild-type plants (number of siliques = 62 ± 5, n = 10 plants). When plants were grown in soil but subjected to a severe heat treatment (32°C), the number of siliques was reduced by 2-fold in the wild type (20°C, 596 ± 48; 32°C, 328 ± 34) and approximately 22-fold in tip1;3-1/tip5;1-1 (20°C, 534 ± 62; 32°C, 24 ± 2).
Altogether, the data point to a defect of tip1;3-1/tip5;1-1 plants to achieve proper seed development and silique growth, that is, a true reproduction phenotype. This phenotype was the most pronounced under environmental stress conditions.
DISCUSSION
Cell-Specific Expression of AtTIP1;3 and AtTIP5;1
Previous transcriptomic analyses have indicated that whereas most of plant aquaporin isoforms possess a broad tissue expression pattern, only a handful shows significant expression in pollen, AtTIP1;3 and AtTIP5;1 being quasi exclusively expressed in this organ (Pina et al., 2005; Bock et al., 2006; Borges et al., 2008). To address the supposedly important role of these two close homologs in male fertility, we first performed a precise expression analysis using two fluorescently tagged fusion proteins, AtTIP1;3-GFP and AtTIP5;1-mCherry, under the control of their native promoter. Microscopic data showed for both of them an onset of expression in bicellular pollen, in agreement with the overlapping temporal expression pattern of the corresponding AtTIP1;3 and AtTIP5;1 transcripts (Honys and Twell, 2004; Qin et al., 2009). Most importantly, the two fusion proteins showed strictly distinct spatial expression patterns, AtTIP1;3-GFP being expressed throughout the vegetative cell, whereas expression of AtTIP5;1-mCherry was restricted to the sperm cells. The latter pattern confirms the transcriptome analysis of Arabidopsis sperm cells by Borges et al. (2008) and the recent finding that expression of AtTIP5;1 is under the control of DUO1, a transcriptional activator specific to sperm cells (Borg et al., 2011).
AtTIP1;3 and AtTIP5;1 Are Both Vacuolar Aquaporins
Because of the somewhat contradictory localization of AtTIP5;1, in mesophyll vacuoles and mitochondria of pollen vegetative cells (Pang et al., 2010; Soto et al., 2010), we carefully examined the subcellular expression of the AtTIP5;1-mCherry fusion. In contrast to previous studies, the native AtTIP5;1 promoter provided a proper cell specificity and level of expression to the fluorescent reporter, whose pattern could be compared to that of well-established fluorescent markers of sperm cell vacuoles (VHA-E1-GFP, VHA-E3-GFP) and mitochondria (SC-mtGFP). Our data suggest that, in sperm, a localization of AtTIP5;1-mCherry in mitochondria, if any, must be minor, whereas AtTIP5;1-mCherry showed a marked colocalization with both VHA-GFP fusion proteins analyzed. Thus, AtTIP5;1 represents the first integral membrane protein reported in sperm vacuoles. This result is at variance with the earlier study of Soto et al. (2010), who concluded on the mitochondrial localization of a TIP5;1-GFP fusion protein after expression in the vegetative cell. Yet, this conclusion has to be considered with caution due to several possible experimental pitfalls. Firstly, expression of AtTIP5;1 under the strong LAT52 promoter forces the presence of a sperm-specific protein in the vegetative cell. Secondly, the use of a chemical mitochondrial marker (MitoTrackerRed) and its visualization by means of epifluorescence microscopy might not have allowed a sufficient resolution for properly interpreting a mitochondrial colocalization. Thirdly, the authors used bioinformatic software that predicts a localization in mitochondria for AtTIP5;1 (and an AtTIP5;1-mCherry fusion) but not for the N-terminal GFP-TIP5;1 fusion used in their work. For instance, MITOPROT yields probabilities of export to mitochondria of 0.92 and 0.045 for AtTIP5;1 and GFP-TIP5;1, respectively.
To address these issues, we generated a LAT52::GFP-TIP5;1 construct identical to that of Soto et al. (2010) and analyzed its pollen expression by means of confocal microscopy. A fuzzy and punctate intracellular staining by the AtTIP5;1 construct was observed, together with a localization in the plasma membrane. Coexpression with the VC-mtRFP mitochondrial marker did not reveal any colocalization, neither in mature pollen nor in PTs. Thus, our data indicate that GFP-TIP5;1 did not localize to mitochondria but rather throughout the secretory system, including endosomes and plasma membrane. This pattern suggests that ectopic expression in pollen vegetative cells leads to mistargeting of the fusion protein. We note, however, that seed-specific TIP3;1 and TIP3;2 transiently localize to the plasma membrane during early seed germination (Gattolin et al., 2011).
In a similar approach as for AtTIP5;1, we localized an AtTIP1;3-GFP fusion protein driven by the native AtTIP1;3 promoter. A labeling pattern consistent with vacuolar structures revealed by neutral red staining indicated that AtTIP1;3 is localized in the tonoplast of vegetative cells. In agreement with earlier work using a LAT52::TIP2;1-GFP construct (Hicks et al., 2004), AtTIP1;3-GFP revealed the existence of a complex and dynamic vacuolar apparatus in the vegetative cell.
Overall, this work establishes AtTIP1;3-GFP and AtTIP5;1-mCherry as two valuable markers for studying the vacuolar dynamics in pollen vegetative and sperm cells, respectively. In the latter cells, minute vacuoles could be visualized by fluorescence microscopy after neutral red staining. Yet, with respect to this staining or earlier studies using a VHA-E1-GFP reporter fusion protein (Strompen et al., 2005), AtTIP5;1-mCherry labeling provided an unprecedented resolution of the fragmented structure, remodeling, and fate of sperm vacuoles over the course of pollen maturation, pollen germination, and even fecundation.
Pollen TIPs and Plant Reproduction
A reverse genetics approach was also undertaken to investigate a general role of the two aquaporins in plant reproduction. Transmission of mutated alleles in reciprocal crossings of wild-type and heterozygous single knockout plants was investigated to possibly reveal defects that may occur during pollen maturation, rehydration, and germination or PT growth and female gametophyte fertilization. Our failure to detect any segregation distortion of the tip1;3-1 or tip5;1-1 alleles suggested, however, that, if any, these defects can be overcome in the environmental conditions used in our experiments.
Nevertheless, we observed that a double tip1;3-1 × tip5;1-1 mutant displayed an increased proportion of barren siliques. This result establishes a true reproduction phenotype for the two aquaporins, likely due to a defect in male fertility. Because the two aquaporins are not expressed in the same territory, this phenotype reveals, rather than a true genetic redundancy of AtTIP1;3 and AtTIP5;1, a functional interplay between the vegetative and sperm cells during plant reproduction. Soto et al. (2010) have described a role for both AtTIP1;3 and AtTIP5;1 in PT growth that could contribute to this phenotype. Yet, a similar growth defect was observed in PT of single and double knockout plants, and other defects in male function are surely involved.
Importantly, the reproduction phenotype uncovered in this work was not detected in water and nutrient-replete conditions, that is, under hydroponic conditions, but rather in several nonoptimal growth conditions or conditions that are known to affect pollen viability, robustness, and fitness, such as heat. Such phenotype was not strictly linked to nitrogen deficiency, as previously shown for PT growth in AtTIP1;3 and AtTIP5;1 knockouts (Soto et al., 2010).
Cellular Functions of Pollen TIPs
AtTIP1;3 and AtTIP5;1 transport water, urea (Soto et al., 2008), and potentially other solutes (Hove and Bhave, 2011). Our work indicates several nonexclusive, hypothetical cellular functions. Firstly, the two TIPs may facilitate cellular water equilibration during pollen de- and/or rehydration. A fine-tuning by AtTIP1;3 of water permeability in the dispatched vacuoles of the vegetative cell may be particularly critical during revacuolation and other vacuolar morphology adjustments that accompany pollen maturation and germination (Pacini et al., 2011). Revacuolation of the vegetative cell likely provides the motive force for turgor-driven pollen germination and PT growth. Furthermore, the vacuolation of the PT results in the displacement of the metabolically active cytoplasm and the sperm cells toward the tip of the growing, and AtTIP1;3 may contribute to the overall PT vacuolar dynamics. The role of AtTIP5;1 in water transport might be similarly important for dynamic vacuolar rearrangements in the sperm cells. Such changes, which may occur during the course of sperm cell delivery and fertilization, will have to be investigated in detail. Whereas molecular evidence links AtTIP5;1 function to nitrogen transport and metabolism (Soto et al., 2010), it cannot be excluded that AtTIP1;3 and AtTIP5;1 transport, in addition to urea, other nitrogen compounds such as ammonia and work in concert with plasma membrane-localized ammonium transporters (Yuan et al., 2009). Electrophysiological analyses (C. Tournaire-Roux and C. Maurel, unpublished data) have shown, however, that, in contrast to mammalian intracellular Aquaporin6 (Yasui et al., 1999), the two AtTIPs are not permeable to nitrate. Finally, hydrogen peroxide is possibly transported by both AtTIPs (Ludewig and Dynowski 2009) and has been recently linked to PT growth (Potocký et al., 2007) and pollen/stigma signaling (Hiscock et al., 2007).
CONCLUSION
This work describes the spatial and temporal expression pattern of AtTIP1;3 and AtTIP5;1 in pollen and identifies them as vegetative and sperm cell-specific aquaporins, respectively. By establishing the tonoplast localization of these two aquaporins, the work also allowed to highlight the vacuolar equipment of maturating and germinating pollen grains. While AtTIP1;3-GFP expression revealed the highly dynamic morphology of pollen vacuoles in the vegetative cell and during PT growth, a AtTIP5;1-mCherry allowed, for the first time, to monitor the in vivo localization of sperm cell vacuoles up to PT penetration in the ovule. The abnormal rate of barren siliques shown by a double knockout indicates that vacuoles of vegetative and sperm cells functionally interact and contribute to male fertility in adverse environmental conditions.
MATERIALS AND METHODS
Plant Material, Cloning, and Plant Transformation
All plants used in this work were Arabidopsis (Arabidopsis thaliana) accession Columbia-0. The complementary DNAs (cDNAs) of enhanced green fluorescent protein (EGFP) and mCherry were PCR amplified with primers XFPfwd/XFPrev (Supplemental Table S1), allowing for the addition of a short partial Ser/Gly4-linker sequence at their 5′ extremity. The resulting fragments were cloned into the XhoI/XbaI sites of a pBluescript SK(+)-based vector (Stratagene) containing the EcoRI/ClaI pKYLX71 expression cassette (Schardl et al., 1987).
Plant genomic DNA (gDNA) was used as a template for PCR amplification of AtTIP1;3 (At4g01470) and AtTIP5;1 (At3g47440), using the primer pairs 1;3fwd1/1;3rev and 5;1fwd1/5;1rev, respectively (Supplemental Table S1). Fragments comprising the open reading frames of interest and approximately 1.5 kb of the corresponding promoter region upstream the initiating ATG were thus amplified with the addition of a short partial Gly4/Ser-linker sequence at the 3′ end (Supplemental Table S1). The amplicon was then cloned into the EcoRI/XhoI sites of the modified pBluescript SK. This resulted in an in-frame fusion of AtTIP1;3 or AtTIP5;1 with a Gly4/Ser2/Gly4-linker and EGFP (AtTIP1;3) or mCherry (AtTIP5;1). The two chimaeras were followed by a ribulose-1,5-bisphosphate carboxylase/oxygenase small subunit terminator cloned within XbaI/ClaI sites.
The whole cassette was subsequently cloned into the EcoRI/ClaI sites of a pGreen-0029 (AtTIP1;3) or a pGreenII-0179 (AtTIP5;1) vector. The LAT52::GFP-AtTIP5;1 construct was made using Gateway cloning technology (Invitrogen) according to the manufacturer’s instructions. The cDNA of AtTIP5;1 was PCR amplified with primers allowing the addition of attB recombination sites and cloned into a pDONR 207 vector using a Gateway BP Clonase enzyme mix. The cDNA was then transferred into the binary destination vectors pK7WGF[LAT52::GFP] (gift from Dr. José A. Feijó) by using a Gateway LR Clonase enzyme mix. The pK7WGF[LAT52::GFP] vector allows fusion of GFP with the aquaporin N terminus (GFP-AtTIP5;1), and expression of the resulting cDNA is placed under the control of a LAT52 promoter and of the 35S Cauliflower mosaic virus terminator. All plasmid constructs were checked by DNA sequencing (GATC Biotech AG) and transformed into Agrobacterium tumefaciens strain GV3101 to allow transformation of Arabidopsis ecotype Columbia-0 by the floral-dip method (Clough and Bent, 1998). Transformants were screened on one-half-strength Murashige and Skoog (MS/2) medium (Murashige and Skoog, 1962) containing either 50 mg L–1 kanamycin (AtTIP1;3::AtTIP1;3-GFP; LAT52::GFP-AtTIP5;1) or 40 mg L–1 hygromycin (AtTIP5;1::AtTIP5;1-mCherry).
Plants expressing fluorescent reporter proteins specific for mitochondria of the pollen vegetative cell (VC-mtRFP) or sperm cells (SC-mtGFP) were as described (Matsushima et al., 2008). Expression of VHA-E1-GFP and of VHA-E3-GFP was monitored using transgenic plants described by Strompen et al. (2005).
Plant Growth Conditions
Reciprocal crosses were accomplished with plants cultured in soil (Neuhaus Humin Substrat N2, Klasman-Deilmann) in a controlled growth chamber at 20°C with regular irrigation and a relative humidity of 70% and under an 8-h-light (180 µE m–2 s–1)/16-h-dark cycle. Antibiotic resistance of the tip5;1-1 × wild-type offspring was screened on MS/2 medium (Murashige and Skoog, 1962) containing sulfadiazine (5 mg L–1). Silique formation in wild-type and tip1;3 and tip5;1 mutant genotypes was monitored in plants grown in soil as described above. Hydroponic cultures were carried out as described earlier (Javot et al., 2003). In brief, cultures were maintained at 70% relative humidity with a 16-h light (250 µmol photons m–2 s–1) at 22°C/8-h dark at 21°C cycle in a culture medium containing 1.25 mm KNO3, 0.75 mm MgSO4, 1.5 mm Ca(NO3)2, 0.5 mm KH2PO4, 50 µm FeEDTA, 50 µm H3BO3, 12 µm MnSO4, 0.70 µm CuSO4, 1 µm ZnSO4, 0.24 µm MoO4Na2, and 100 µm Na2SiO3. In nitrate deprivation experiments, 15-d-old plants where transferred in a same solution but with 1.25 mm KNO3 and 1.5 mm Ca(NO3)2 substituted by 1.25 mm KSO4, 1.5 mm CaSO4, and 0.05 mm Ca(NO3)2. For heat treatments, 15-d-old soil-grown plants were transferred from growth at 20°C to growth at 32°C under the same relative humidity and light conditions as above.
DNA Extraction
Extraction of gDNA was accomplished using a simplified cetyltrimethylammonium bromide method as described in Javot et al. (2003) but without performing the second precipitation step. In brief, the collected plant material was ground with the help of a bead blender after liquid nitrogen freezing. Each sample was then incubated in 500 μL of extraction buffer (1.4 m NaCl, 20 mm EDTA, 2% [w/v] cetyltrimethylammonium bromide, 100 mm Tris-HCl, pH 8.0, and 0.4% [v/v] β-mercaptoethanol added extemporaneously). After 30 min at 65°C, each sample was chloroform extracted, and DNA was precipitated once with isopropanol and finally washed with 70% ethanol, dried, and dissolved in 50 μL water. Three to five microliters of DNA were used for each PCR reaction.
Molecular Characterization of AtTIP1;3 and AtTIP5;1 T-DNA Insertion Mutants
The Arabidopsis T-DNA insertion lines SALK_088276 (AtTIP1;3) and GABI_171E08 (AtTIP5;1) were obtained from the Nottingham Arabidopsis Stock Center. The T-DNA insertions in these lines were confirmed by PCR on gDNA using a combination of primers specific for the T-DNA left border and genomic sequence of AtTIP1;3 (LBb1/1;3fwd1, Supplemental Table S1) and AtTIP5;1 (o8409/5;1rev, Supplemental Table S1), respectively. The neomycine phosphotransferaseII (NPTII) selectable marker of the SALK_088276 line was probably silenced, because no kanamycin resistance was observed in this line.
Homozygous mutant lines were identified from SALK_088276 and GABI_171E08, and named tip1;3-1 and tip5;1-1, respectively. The genotype of these lines was established by PCR on gDNA. A tip1;3-1/tip5;1-1 double knockout line was obtained by crossing the two homozygous single knockout lines, and the offspring was amplified. A homozygous double knockout line was identified within the F3 generation and confirmed by PCR on gDNA using the primer pairs described above.
The absence of full-length transcript in tip1;3-1 and tip5;1-1 was checked by reverse transcription-PCR using pairs of AtTIP1;3- and AtTIP5;1-specific primers (1;3fwd2/1;3rev and 5;1fwd2/5;1rev, respectively). The cDNA matrix was obtained from RNAs isolated from inflorescences using a spin or vacuum total RNA isolation system (Promega) and after reverse transcription with a M-MLV Reverse Transcriptase (Promega), according to the manufacturer’s instructions. Expression of the Elongation Factor1α (EF1α) gene was used as a control as described (Javot et al., 2003).
Microscopy Analysis and Pollen Staining
For temporal expression analyses, flower buds were dissected at different developmental stages, and pollen grains were mounted on slides in a liquid MS/2 medium. The DNA dye DAPI was added directly to the mounting media at a final concentration of 2 µg mL–1. Pollen vacuoles were stained with 0.01% (w/v) neutral red for up to 2 h. ER-Tracker Blue-White DPX (Molecular Probes) was applied to the mounting media at a final concentration of 10 µm. Pollen vitality staining was accomplished by incubating pollen for 15 min in the dark in a 1:25 dilution of a stock solution of Alexander’s stain (Alexander, 1969) containing 10% (v/v) ethanol, 0.05% (w/v) fuchsin acid, 0.005% (w/v) Orange G, 0.05% (v/v) malachite green, 2% (v/v) acetic acid, and 25% (v/v) glycerol. Microscopic observations were carried out as described (Boursiac et al., 2005) using an Olympus BX610 microscope equipped with an epifluorescence condenser or using a confocal laser scanning microscope (Zeiss LSM510 AX70, Olympus Fluoview FV10i-W).
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers: AtTIP1;3, At4g01470; and AtTIP5;1, At3g47440.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Staining of pollen expressing AtTIP1;3-GFP with an endoplasmic reticulum-specific dye.
Supplemental Figure S2. Colabeling of released sperm cells with AtTIP5;1-mCherry and a VHA-E1-GFP vacuolar marker.
Supplemental Figure S3. Colabeling of mature pollen grains with AtTIP5;1-mCherry and a VHA-E3-GFP vacuolar marker.
Supplemental Figure S4. Embryo penetration by a PT delivering the sperm cells.
Supplemental Figure S5. Coexpression in a germinating pollen of LAT52::GFP-TIP5;1 and a mitochondrial marker.
Supplemental Figure S6. Viability assay on pollen from different genotypes.
Supplemental Figure S7. Morphology of barren siliques.
Supplemental Table S1. Sequence of primers used for amplification of AtTIP1;3, AtTIP5;1, mCherry, EGFP, and T-DNA.
Supplemental Movie S1. Three-dimensional reconstruction of a mature pollen grain stained with neutral red.
Supplemental Movie S2. Three-dimensional reconstruction of sperm cells coexpressing AtTIP5;1-mCherry (magenta) and SC-mtGFP (green).
Supplemental Movie S3. Three-dimensional reconstruction of pollen expressing AtTIP5;1-mCherry (magenta) and VHA-E1-GFP (green) and showing colocalization of both proteins (white).
Supplementary Material
Acknowledgments
We thank Dr. José A. Feijó, Dr. Erwan Michard, and Dr. Hannetz Roschzttardtz for scientific advice and helpful discussion on the manuscript and Dr. Karin Schumacher for kindly providing plant lines expressing VHA-E1-GFP and VHA-E3-GFP.
Glossary
- PT
pollen tube
- DAPI
4',6'-diamino-2-phenylindole
- T-DNA
transfer DNA
- cDNA
complementary DNA
- gDNA
genomic DNA
Footnotes
This work was supported by the European Union Marie Curie Research Training Network (Vacuolar Transport Equipment for Growth Regulation in Plants, grant no. MRTN–CT–2006–035833 to M.M.W.).
The online version of this article contains Web-only data.
References
- Alexander MP. (1969) Differential staining of aborted and nonaborted pollen. Stain Technol 44: 117–122 [DOI] [PubMed] [Google Scholar]
- Bock KW, Honys D, Ward JM, Padmanaban S, Nawrocki EP, Hirschi KD, Twell D, Sze H. (2006) Integrating membrane transport with male gametophyte development and function through transcriptomics. Plant Physiol 140: 1151–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg M, Brownfield L, Khatab H, Sidorova A, Lingaya M, Twell D. (2011) The R2R3 MYB transcription factor DUO1 activates a male germline-specific regulon essential for sperm cell differentiation in Arabidopsis. Plant Cell 23: 534–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges F, Gomes G, Gardner R, Moreno N, McCormick S, Feijó JA, Becker JD. (2008) Comparative transcriptomics of Arabidopsis sperm cells. Plant Physiol 148: 1168–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boursiac Y, Chen S, Luu DT, Sorieul M, van den Dries N, Maurel C. (2005) Early effects of salinity on water transport in Arabidopsis roots. Molecular and cellular features of aquaporin expression. Plant Physiol 139: 790–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewbaker JL. (1967) The distribution and phylogenetic significance of binucleate and trinucleate pollen grains in the angiosperms. Am J Bot 54: 1069–1083 [Google Scholar]
- Clough SJ, Bent AF. (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Dettmer J, Liu TY, Schumacher K. (2010) Functional analysis of Arabidopsis V-ATPase subunit VHA-E isoforms. Eur J Cell Biol 89: 152–156 [DOI] [PubMed] [Google Scholar]
- Dettmer J, Schubert D, Calvo-Weimar O, Stierhof YD, Schmidt R, Schumacher K. (2005) Essential role of the V-ATPase in male gametophyte development. Plant J 41: 117–124 [DOI] [PubMed] [Google Scholar]
- Firon N, Nepi M, Pacini E. (2012) Water status and associated processes mark critical stages in pollen development and functioning. Ann Bot (Lond) 109: 1201–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattolin S, Sorieul M, Frigerio L. (2011) Mapping of tonoplast intrinsic proteins in maturing and germinating Arabidopsis seeds reveals dual localization of embryonic TIPs to the tonoplast and plasma membrane. Mol Plant 4: 180–189 [DOI] [PubMed] [Google Scholar]
- Heslop-Harrison J. (1979) An interpretation of the hydrodynamics of pollen. Am J Bot 66: 737–743 [Google Scholar]
- Hicks GR, Rojo E, Hong S, Carter DG, Raikhel NV. (2004) Geminating pollen has tubular vacuoles, displays highly dynamic vacuole biogenesis, and requires VACUOLESS1 for proper function. Plant Physiol 134: 1227–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscock SJ, Bright J, McInnis SM, Desikan R, Hancock JT. (2007) Signaling on the stigma: potential new roles for ROS and NO in plant cell signaling. Plant Signal Behav 2: 23–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honys D, Twell D. (2004) Transcriptome analysis of haploid male gametophyte development in Arabidopsis. Genome Biol 5: R85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hove RM, Bhave M. (2011) Plant aquaporins with non-aqua functions: deciphering the signature sequences. Plant Mol Biol 75: 413–430 [DOI] [PubMed] [Google Scholar]
- Ishikawa F, Suga S, Uemura T, Sato MH, Maeshima M. (2005) Novel type aquaporin SIPs are mainly localized to the ER membrane and show cell-specific expression in Arabidopsis thaliana. FEBS Lett 579: 5814–5820 [DOI] [PubMed] [Google Scholar]
- Javot H, Lauvergeat V, Santoni V, Martin-Laurent F, Güçlü J, Vinh J, Heyes J, Franck KI, Schäffner AR, Bouchez D, et al. (2003) Role of a single aquaporin isoform in root water uptake. Plant Cell 15: 509–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Choi WG, Wallace IS, Baudry J, Roberts DM. (2011) Arabidopsis thaliana NIP7;1: an anther-specific boric acid transporter of the aquaporin superfamily regulated by an unusual tyrosine in helix 2 of the transport pore. Biochemistry 50: 6633–6641 [DOI] [PubMed] [Google Scholar]
- Loraine A, McCormick S, Estrada A, Patel K, Qin P. (2013) High thoughput sequencing of Arabidopsis thaliana pollen cDNA uncovers novel transcription and alternative splicing. Plant Physiol 162: 1092–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludewig U, Dynowski M. (2009) Plant aquaporin selectivity: where transport assays, computer simulations and physiology meet. Cell Mol Life Sci 66: 3161–3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima R, Hamamura Y, Higashiyama T, Arimura S, Sodmergen, Tsutsumi N, Sakamoto W. (2008) Mitochondrial dynamics in plant male gametophyte visualized by fluorescent live imaging. Plant Cell Physiol 49: 1074–1083 [DOI] [PubMed] [Google Scholar]
- Mogensen HL. (1992) The male germ unit: concept, composition, and significance. Int Rev Cytol 140: 129–148 [Google Scholar]
- Murashige T, Skoog F. (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–497 [Google Scholar]
- Owen HA, Makaroff CA. (1995) Ultrastructure of microsporogenesis and microgametogenesis in Arabidopsis thaliana (L.) Heynh. ecotype Wassilewskija (Brassicaceae). Protoplasma 185: 7–21 [Google Scholar]
- Pacini E, Jacquard C, Clément C. (2011) Pollen vacuoles and their significance. Planta 234: 217–227 [DOI] [PubMed] [Google Scholar]
- Pang Y, Li L, Ren F, Lu P, Wei P, Cai J, Xin L, Zhang J, Chen J, Wang X. (2010) Overexpression of the tonoplast aquaporin AtTIP5;1 conferred tolerance to boron toxicity in Arabidopsis. J Genet Genomics 37: 389–397, 1–2 [DOI] [PubMed] [Google Scholar]
- Pina C, Pinto F, Feijó JA, Becker JD. (2005) Gene family analysis of the Arabidopsis pollen transcriptome reveals biological implications for cell growth, division control, and gene expression regulation. Plant Physiol 138: 744–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potocký M, Jones MA, Bezvoda R, Smirnoff N, Zárský V. (2007) Reactive oxygen species produced by NADPH oxidase are involved in pollen tube growth. New Phytol 174: 742–751 [DOI] [PubMed] [Google Scholar]
- Qin Y, Leydon AR, Manziello A, Pandey R, Mount D, Denic S, Vasic B, Johnson MA, Palanivelu R. (2009) Penetration of the stigma and style elicits a novel transcriptome in pollen tubes, pointing to genes critical for growth in a pistil. PLoS Genet 5: e1000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell SD, Strout GW. (2005) Microgametogenesis in Plumbago zeylanica (Plumbaginaceae). 2. Quantitative cell and organelle dynamics of the male reproductive cell lineage. Sex Plant Reprod 18: 113–130 [Google Scholar]
- Saito C, Ueda T, Abe H, Wada Y, Kuroiwa T, Hisada A, Furuya M, Nakano A. (2002) A complex and mobile structure forms a distinct subregion within the continuous vacuolar membrane in young cotyledons of Arabidopsis. Plant J 29: 245–255 [DOI] [PubMed] [Google Scholar]
- Sarker RH, Elleman CJ, Dickinson HG. (1988) Control of pollen hydration in Brassica requires continued protein synthesis, and glycosylation in necessary for intraspecific incompatibility. Proc Natl Acad Sci USA 85: 4340–4344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schardl CL, Byrd AD, Benzion G, Altschuler MA, Hildebrand DF, Hunt AG. (1987) Design and construction of a versatile system for the expression of foreign genes in plants. Gene 61: 1–11 [DOI] [PubMed] [Google Scholar]
- Soto G, Alleva K, Mazzella MA, Amodeo G, Muschietti JP. (2008) AtTIP1;3 and AtTIP5;1, the only highly expressed Arabidopsis pollen-specific aquaporins, transport water and urea. FEBS Lett 582: 4077–4082 [DOI] [PubMed] [Google Scholar]
- Soto G, Fox R, Ayub N, Alleva K, Guaimas F, Erijman EJ, Mazzella A, Amodeo G, Muschietti J. (2010) TIP5;1 is an aquaporin specifically targeted to pollen mitochondria and is probably involved in nitrogen remobilization in Arabidopsis thaliana. Plant J 64: 1038–1047 [DOI] [PubMed] [Google Scholar]
- Strompen G, Dettmer J, Stierhof YD, Schumacher K, Jürgens G, Mayer U. (2005) Arabidopsis vacuolar H-ATPase subunit E isoform 1 is required for Golgi organization and vacuole function in embryogenesis. Plant J 41: 125–132 [DOI] [PubMed] [Google Scholar]
- Tang LY, Nagata N, Matsushima R, Chen Y, Yoshioka Y, Sakamoto W. (2009) Visualization of plastids in pollen grains: involvement of FtsZ1 in pollen plastid division. Plant Cell Physiol 50: 904–908 [DOI] [PubMed] [Google Scholar]
- Van Aelst AC, Pierson ES, Van Went JL, Cresti M. (1993) Ultrastructural changes of Arabidopsis thaliana pollen during final maturation and rehydration. Zygote 1: 173–179 [DOI] [PubMed] [Google Scholar]
- Weber M. (1988) Formation of sperm cells in Galium mollugo (Rubiaceae), Trichodiadema setuliferum (Aizoaceae), and Avena sativa (Poaceae). Plant Syst Evol 161: 53–64 [Google Scholar]
- Winship LJ, Obermeyer G, Geitmann A, Hepler PK. (2010) Under pressure, cell walls set the pace. Trends Plant Sci 15: 363–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wudick MM, Luu DT, Maurel C. (2009) A look inside: localization patterns and functions of intracellular plant aquaporins. New Phytol 184: 289–302 [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Nishimura M, Hara-Nishimura I, Noguchi T. (2003) Behavior of vacuoles during microspore and pollen development in Arabidopsis thaliana. Plant Cell Physiol 44: 1192–1201 [DOI] [PubMed] [Google Scholar]
- Yasui M, Hazama A, Kwon TH, Nielsen S, Guggino WB, Agre P. (1999) Rapid gating and anion permeability of an intracellular aquaporin. Nature 402: 184–187 [DOI] [PubMed] [Google Scholar]
- Yu HS, Hu SY, Russel SD. (1992) Sperm cells in pollen tubes of Nicotiana tabacum L.: three-dimensional reconstitution, cytoplasmic diminution, and quantitative cytology. Protoplasma 168: 172–183 [Google Scholar]
- Yuan L, Graff L, Loqué D, Kojima S, Tsuchiya YN, Takahashi H, von Wirén N. (2009) AtAMT1;4, a pollen-specific high-affinity ammonium transporter of the plasma membrane in Arabidopsis. Plant Cell Physiol 50: 13–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.