Gibberellins and expression levels of their biosynthesis genes decrease in developing anthers on exposure to moderate low temperatures, disrupting pollen development and reducing grain yields.
Abstract
Microsporogenesis in rice (Oryza sativa) plants is susceptible to moderate low temperature (LT; approximately 19°C) that disrupts pollen development and causes severe reductions in grain yields. Although considerable research has been invested in the study of cool-temperature injury, a full understanding of the molecular mechanism has not been achieved. Here, we show that endogenous levels of the bioactive gibberellins (GAs) GA4 and GA7, and expression levels of the GA biosynthesis genes GA20ox3 and GA3ox1, decrease in the developing anthers by exposure to LT. By contrast, the levels of precursor GA12 were higher in response to LT. In addition, the expression of the dehydration-responsive element-binding protein DREB2B and SLENDER RICE1 (SLR1)/DELLA was up-regulated in response to LT. Mutants involved in GA biosynthetic and response pathways were hypersensitive to LT stress, including the semidwarf mutants sd1 and d35, the gain-of-function mutant slr1-d, and gibberellin insensitive dwarf1. The reduction in the number of sporogenous cells and the abnormal enlargement of tapetal cells occurred most severely in the GA-insensitive mutant. Application of exogenous GA significantly reversed the male sterility caused by LT, and simultaneous application of exogenous GA with sucrose substantially improved the extent of normal pollen development. Modern rice varieties carrying the sd1 mutation are widely cultivated, and the sd1 mutation is considered one of the greatest achievements of the Green Revolution. The protective strategy achieved by our work may help sustain steady yields of rice under global climate change.
Grain yields in rice plants (Oryza sativa) are often reduced by exposure to moderate low temperature (LT), which is termed cool-temperature damage. It is estimated that the net effect of cool-temperature damage is an annual loss of at least three to five million tons of rice in East Asia (Li and Guo, 1993). Unexpected climate change, such as abnormally hot or cool summer temperatures, has occurred repeatedly during recent years due to the El Niño/La Niña-Southern Oscillation (Intergovernmental Panel on Climate Change, 2007). Shifts in population demographics result in the abandonment of agricultural fields in rapidly industrialized areas and the establishment of new fields in mountainous areas. Studies on cool-temperature damage in rice have a long history and have identified physiological responses to LT, including abnormal enlargement of anther wall cells and tapetal cells, reduction in the numbers of mature pollen, and increased male sterility (Sakai, 1943; Nishiyama, 1976, 1982). These studies show that microsporogenesis is the most susceptible stage to LT during pollen development in rice. However, the mechanisms underlying these physiological changes have not been completely elucidated.
Research on plants during the last decade has identified numerous cellular pathways that respond to abiotic environmental stresses. Several phytohormones are involved in the regulation of homeostasis, stress responses, and cross talk in different signaling pathways (Qin et al., 2011). Abscisic acid is a typical phytohormone that responds to abiotic stress (Raghavendra et al., 2010; Qin et al., 2011). GA is generally regarded as a growth-promoting phytohormone that positively regulates processes such as seed germination, vegetative growth, flowering, and fruit development (Olszewski et al., 2002; Sun and Gubler, 2004). GA functions to mediate both tolerance and intolerance pathways involved in the responses to different abiotic stresses. Application of exogenous GA to plant seeds reverses the salt stress- and heat stress-induced inhibition of germination and seedling establishment (Kabar and Baltepe, 1990; Kaur et al., 1998; Nasri et al., 2011). By contrast, salt stress in Arabidopsis (Arabidopsis thaliana) seedlings leads to a decrease of GA levels, the accumulation of DELLA proteins, and the suppression of plant growth, which ultimately confers tolerance to stress (Achard et al., 2006). A quadruple DELLA mutant lacking Gibberellic Acid Insensitive (GAI), Repressor of GA (RGA), RGA-Like1 (RGL1), and RGL2 is more sensitive to salt stress than the wild-type plant. In barley (Hordeum vulgare) seedlings, treatment with GA inhibitors leads to higher tolerance to heat and oxidative stresses (Sarkar et al., 2004). Barley seedlings, which have the largest concentrations of endogenous GAs, are most susceptible to these abiotic stresses. Although stress tolerance response pathways mediated by GA are complex, it is well known that GA-deficient and GA-insensitive mutants in several plant species display abnormal anther development (Nester and Zeevaart, 1988; Jacobsen and Olszewski, 1991; Cheng et al., 2004; Aya et al., 2009). The typical defect of abnormal tapetal cell enlargement observed in rice GA mutants (Aya et al., 2009) is quite similar to the observed effects induced by LT injury in wild-type cultivated rice plants (Nishiyama, 1976; Oda et al., 2010).
In this paper, we studied the relationship between GA and LT damage of anther development in rice. The endogenous GA levels and expression of genes involved in GA biosynthesis were measured in developing anthers with or without exposure to LT. The sensitivity of the GA biosynthesis mutant semidwarf1 (sd1; Asano et al., 2011) to LT damage was monitored. The sd1 mutant is considered one of the greatest achievements of the Green Revolution (Monna et al., 2002; Sasaki et al., 2002). In addition, we studied the response to LT in other GA biosynthesis mutants, including dwarf Tan-Ginbozu (d35; Suge, 1975; Itoh et al., 2004), the gain-of-function slender rice1 (slr1-d) mutant (Asano et al., 2009), and the GA-insensitive gibberellin insensitive dwarf1 (gid1) mutant (Ueguchi-Tanaka et al., 2007). We also explored potential remedial strategies for LT damage by examining the effects of GA application on pollen development under cool-temperature conditions.
RESULTS
LT Reduces the Endogenous Levels of Bioactive GAs in Developing Anthers
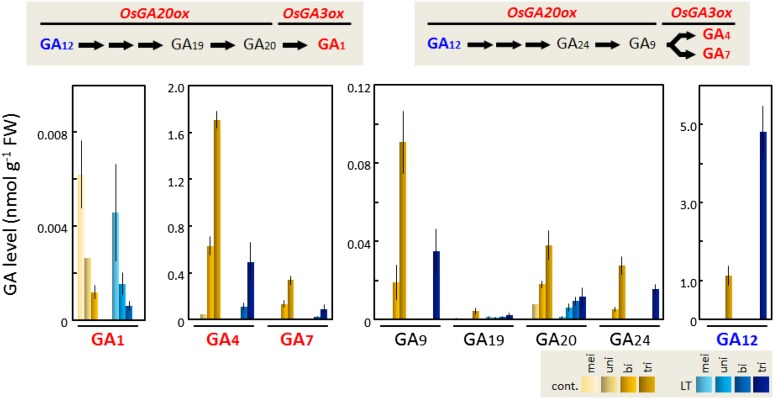
GA-deficient, GA-insensitive, and gamyb mutants display aborted pollen development with abnormal enlargement of tapetal cells (Aya et al., 2009). This resembles the effects induced by cool-temperature damage in cultivated rice plants (Nishiyama, 1976; Oda et al., 2010). Therefore, we measured alterations in the levels of endogenous GAs in developing anthers of the japonica cv Sasanishiki grown under normal and LT (average 19°C) conditions in a field at Furukawa Agricultural Experiment Station, Miyagi Prefecture, Japan (see “Materials and Methods”; Supplemental Fig. S1). LT treatment starting from the panicle developmental stage until the heading stage leads to a drastic reduction in mature pollen and a severe decline in the seed-setting rate (Fig. 1). Anthers were sampled and dissected at each developmental stage, including meiotic, uninucleate, binucleate, and trinucleate pollen, and the endogenous GA levels were quantified with ultra-performance liquid chromatography (UPLC)-tandem mass spectrometry (Kojima et al., 2009). The results indicated that the dominant bioactive GAs in pollen were GA4 and GA7 and that the levels significantly increased during pollen maturation under normal conditions (Fig. 2). A different bioactive GA, GA1, had levels that were much lower and gradually decreased during pollen development (Fig. 2). Other Gas, such as GA9, GA19, GA20, and GA24, were at minor levels, but the levels increased at the binucleate and trinucleate pollen stages (Fig. 2). As the decline in GA1 content was matched with an accumulation of GA20 under both normal and LT conditions, GA3β-hydroxylase1 (GA3ox1), which was dominantly expressed in developing anthers (Oda et al., 2010), could more efficiently convert GA9 to GA4 than GA20 to GA1. GA3, GA8, GA19, GA44, and GA53 were hardly detected in anthers under normal conditions. Under LT conditions, the levels of essentially all GAs were reduced by 50% to 70% compared with those under normal-temperature conditions (Fig. 2). Only the level of GA12, which was the common precursor of bioactive GAs, drastically increased at the trinucleate pollen stage in response to LT conditions (Fig. 2). These results suggest that the synthesis of bioactive GAs is suppressed under LT conditions.
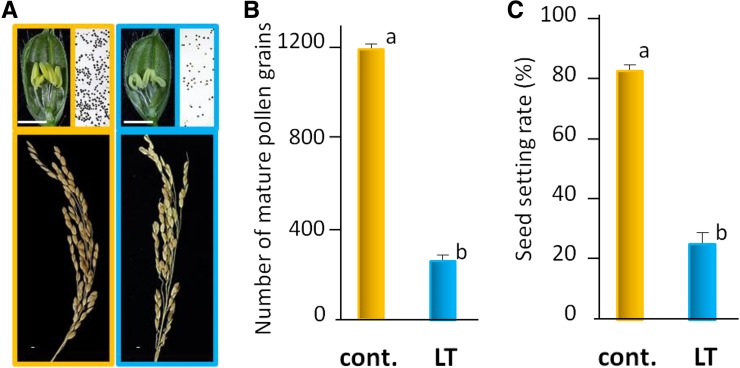
Figure 1.
Severe abortion of pollen formation and reduction of seed setting occur in response to LT. The japonica cv Sasanishiki was cultured under normal (orange frame; as a control, an experimental paddy field at average maximum 27°C and minimum 19.4°C) and LT (blue frame; using a running cold-water system at 19°C, 25 cm deep) conditions from the panicle primordial stage to the completion of heading at the Furukawa Agricultural Experiment Station, Miyagi Prefecture, Japan (Supplemental Fig. S1). A, Representative features (pistil, anthers, and pollen) are shown in cv Sasanishiki spikelets that were dissected at the heading period with or without LT treatment. The pollen at the heading period was stained with iodine solution. The bottom images reveal the representative structures of mature ears after LT treatment. Bars = 2 mm. B, Number of pollen grains in anthers cultured at normal temperature and LT (n = 18 anthers tested in each plant). C, Seed-setting percentage was calculated after grain filling in cv Sasanishiki (n = approximately 70 ears in six plants with or without LT treatment). Values are means ± se. Letters denote statistical differences with Tukey’s test (P < 0.05). Orange columns, Control samples (cont.); blue columns, LT-treated samples.
Figure 2.
Quantification of GAs (nmol g−1 fresh weight [FW]) in developing anthers using UPLC-tandem mass spectrometry. Concentrations and identities of endogenous GAs in the developing anthers of the japonica cv Sasanishiki at meiotic, uninucleate, binucleate, and trinucleate pollen stages with or without LT treatment were determined. Data are means ± sd (n = 3).
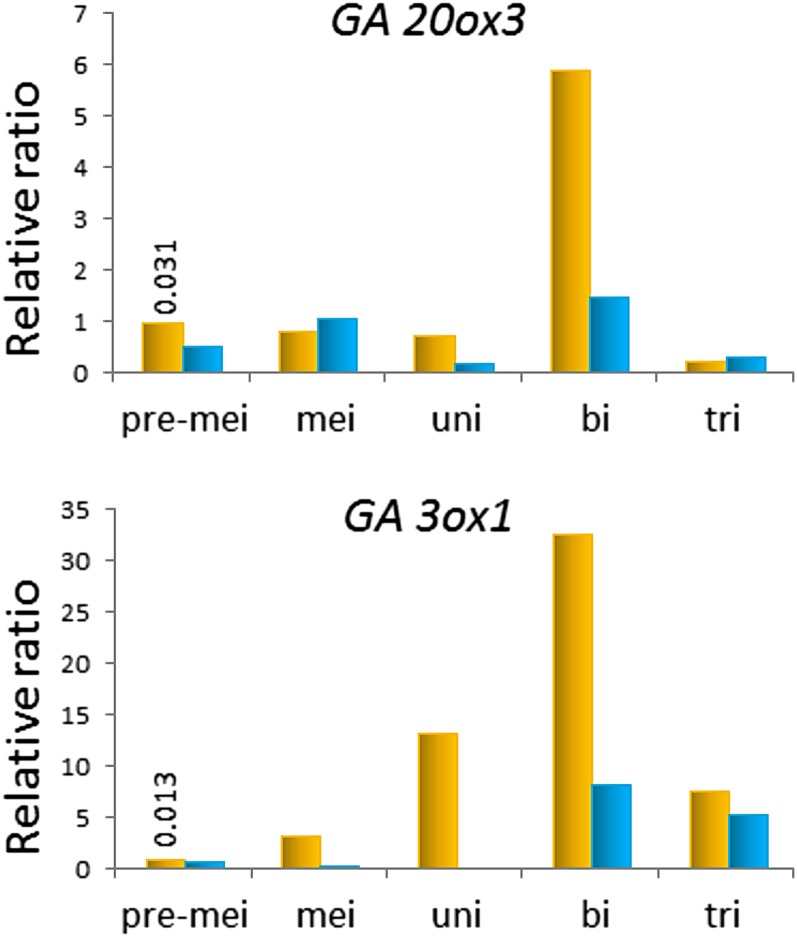
We monitored the transcription of the GA biosynthesis genes GA3ox1 and GA20ox3 (expressed dominantly in anthers; Oda et al., 2010) during pollen development at the premeiotic, meiotic, uninucleate, binucleate, and trinucleate stages. The expression of GA20ox3 was significantly up-regulated at the binucleate pollen stage in the control (Fig. 3). The expression of GA3ox1 gradually increased during anther development under normal conditions and peaked at the binucleate pollen stage, similar to that of GA20ox3 (Fig. 3). Under LT conditions, the levels of GA20ox3 expression from the uninucleate to the binucleate stage, and the levels of GA3ox1 expression from the meiotic to binucleate stage, were drastically reduced (Fig. 3). We also investigated the transcription levels of the GA2ox GA catabolic genes. One of them, GA2ox1, was expressed feebly at the uninucleate stage and increased at the binucleate stage, but these levels were further suppressed by LT treatment (Supplemental Fig. S2). The other GA2oxs are barely expressed in developing anthers of rice (Oda et al., 2010; http://ricexpro.dna.affrc.go.jp/). Taken together, these results indicate that LT severely reduces the endogenous levels of bioactive GAs through transcriptional repression of the GA biosynthetic genes GA20ox3 and GA3ox1, which are strongly up-regulated during normal anther development.
Figure 3.
LT reduces the expression levels of the GA biosynthesis genes GA20ox3 and GA3ox1, which are up-regulated during anther development. Expression levels of GA20ox3 (Os07g0169700) and GA3ox1 (Os05g0178100) in anthers of cv Sasanishiki at premeiotic, meiotic, uninucleate, binucleate, and trinucleate pollen stages were determined by real-time quantitative RT-PCR analysis. The expression level of ACTIN1 (Os03g718100) was used as an internal standard, and each numeral in the control indicates the average expression level. A set of expression levels of GA biosynthesis genes was calculated as a ratio relative to the control at the premeiotic stage. Orange columns, Control samples; blue columns, LT treatment samples.
LT Causes Up-Regulation of DREB2B and SLR1/DELLA Expression in Developing Anthers
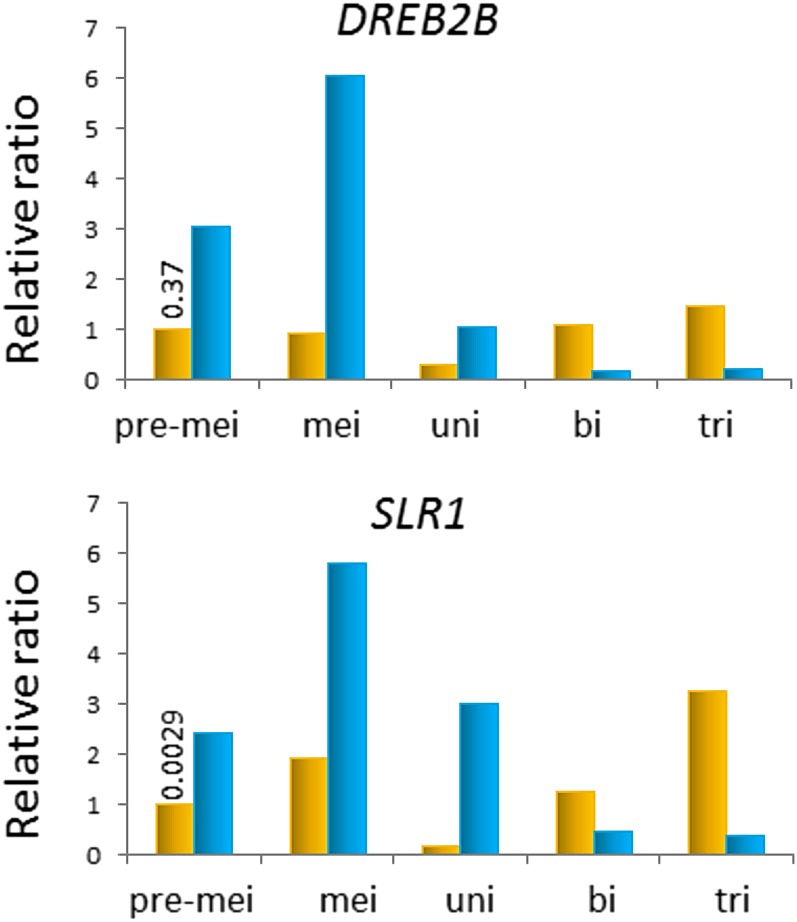
In Arabidopsis, a member of the DELLA gene family called RGA-LIKE3 is up-regulated by the dehydration-responsive element-binding protein DREB1b/C-repeat/DRE Binding Factor1 (CBF1), which is induced by exposure to LT (Achard et al., 2008). The DELLA protein functions as a key repressor of GA-responsive growth and development (Fleet and Sun, 2005; Davière et al., 2008). To evaluate alterations in DREB and DELLA gene expression during rice anther development under LT conditions, we monitored the levels of DREB2B and SLR1/DELLA expression by real-time quantitative reverse transcription (RT)-PCR. In the rice genome, SLR1/DELLA is present as a single copy, whereas DREB2B is present as four ubiquitously expressed copies (http://ricexpro.dna.affrc.go.jp/). The expression levels of both DREB2B and SLR1/DELLA strongly increased in response to LT treatment in developing anthers from the early premeiotic stage to the uninucleate pollen stage (Fig. 4). These results suggest the possibility that LT could disrupt GA-responsive pollen development through the transcriptional activation of SLR1/DELLA, which would be up-regulated by higher levels of DREB2B.
Figure 4.
DREB2B and SLR1/DELLA are up-regulated in the early-developing anther exposed to LT. Expression levels of DREB2B (Os05g0346200) and SLR1/DELLA (Os03g0707600) in cv Sasanishiki anthers at premeiotic, meiotic, uninucleate, binucleate, and trinucleate pollen stages were determined by real-time quantitative RT-PCR analysis. The expression level of ACTIN1 (Os03g718100) was used as an internal standard, and each numeral in the control indicates the average expression level. A set of expression levels for DREB2B and SLR1/DELLA was calculated as a ratio relative to the control at the premeiotic stage. Orange columns, Control samples; blue columns, LT treatment samples.
Semidwarf Mutants sd1 and d35 Are Hypersensitive to LT
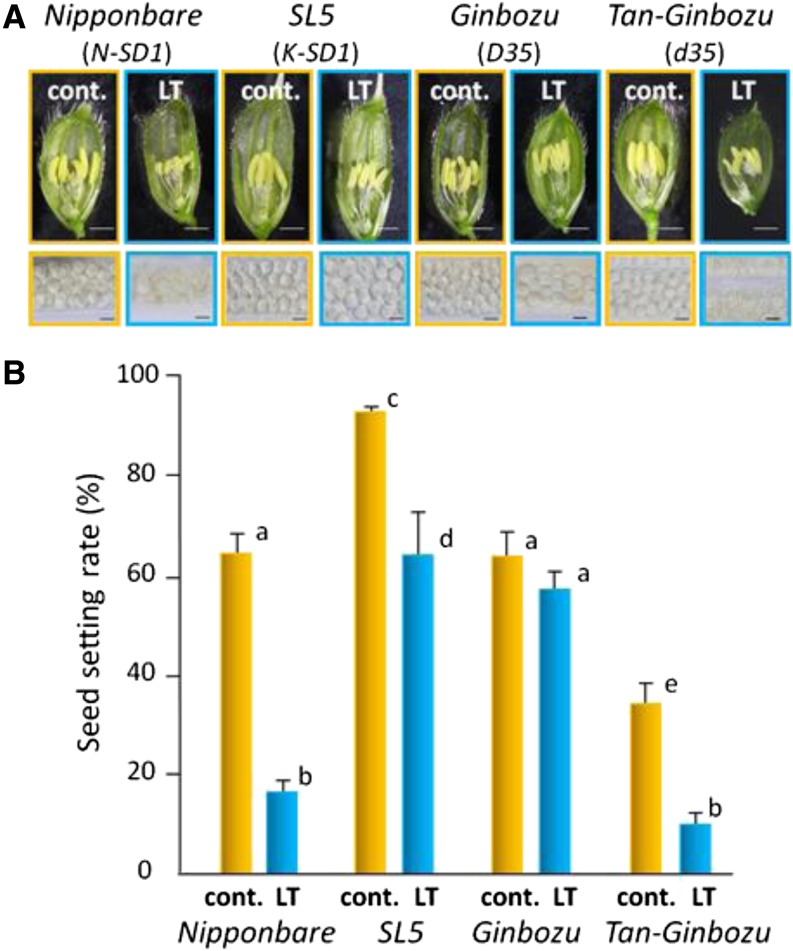
The rice cv Nipponbare is characterized by a short culm length with functional single-nucleotide polymorphisms in a GA20ox2 gene, which is responsible for the sd1 mutant (Asano et al., 2011). A backcross-inbred line, SL5, which was generated from a cross between cv Nipponbare and the indica variety Kasalath, was substituted in qCL1a (the quantitative trait locus for culm length on chromosome 1) that included the Kasalath-type sd1 (Asano et al., 2011). Therefore, SL5 was much taller than cv Nipponbare (Asano et al., 2011). Under LT conditions, cv Nipponbare anthers were smaller and abnormally curved due to a drastic reduction in the number of pollen grains and hypertrophy of tapetal cells. The percentage of seed setting in cool-stressed cv Nipponbare was significantly reduced compared with that under normal temperatures. By contrast, SL5 displayed strong tolerance to LT (Fig. 5). The semidwarf rice cv Tan-Ginbozu, which has a mutation in an ent-kaurene oxidase d35 gene involved in GA biosynthesis (Suge, 1975; Itoh et al., 2004), also was hypersensitive to cool-temperature damage compared with the wild-type (D35) parental cv Ginbozu (Fig. 5).
Figure 5.
Severe disruption of pollen development and reduction of seed setting occur in response to LT in varieties that harbor mutations in sd1 and ent-kaurene oxidase (d35) genes. A, Representative features (pistil, anthers, and pollen) are shown in spikelets dissected at the heading period with (LT; blue frames) or without (cont.; orange frames) LT treatment. Bars = 1 mm (top) and 50 μm (bottom). B, Seed setting was calculated after grain filling for each plant variety (n = 18 ears from the main stem of independent plants). Values are means ± se. Letters denote statistical differences with Tukey’s test (P < 0.05).
GA-Insensitive Mutants Are Hypersensitive to LT Stress
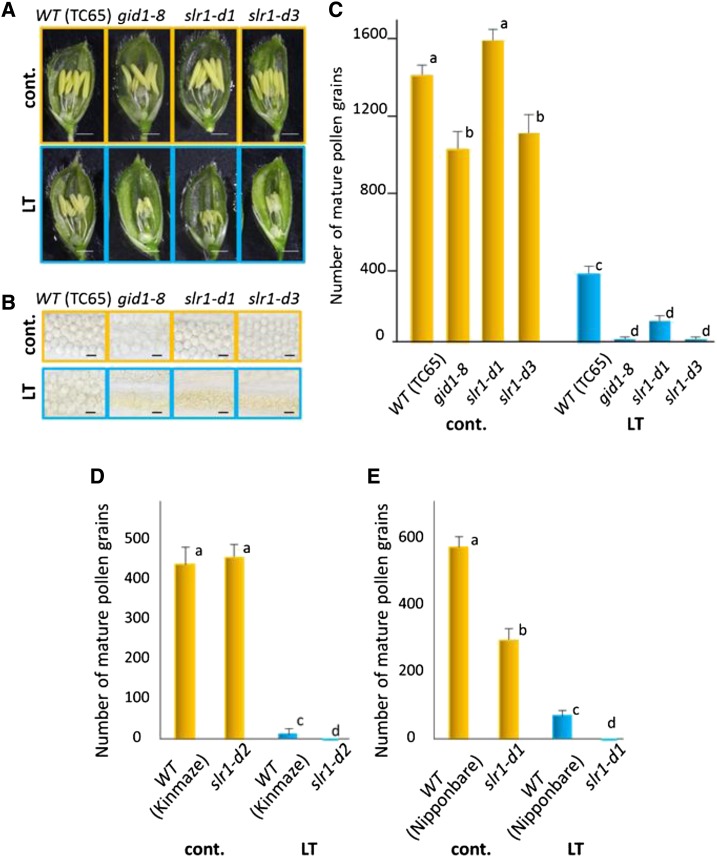
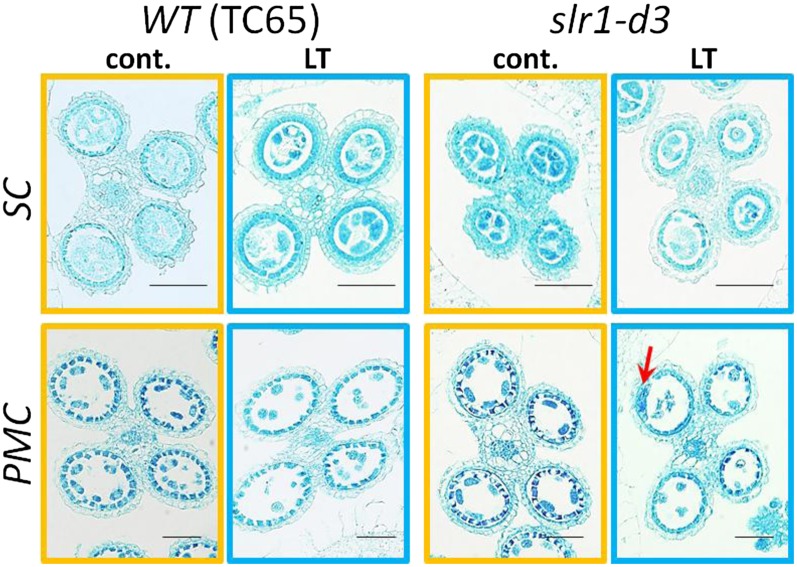
We characterized LT sensitivity in GA-insensitive rice mutants isolated previously. All GA-insensitive mutants, including gid1-8 (Ueguchi-Tanaka et al., 2007) and the gain-of-function SLR1 mutants (slr1-d1, slr1-d2, slr1-d3, and slr1-d4; Asano et al., 2009), were hypersensitive to cool temperatures and exhibit severe disruptions of pollen development at LT. Figure 6 shows the results using gid1-8, slr1-d1, and slr1-d3 mutants in the Taichung 65 (TC65) background (Fig. 6, A–C). Similar hypersensitivity of slr1-d2 in cv Kinmaze and slr1-d4 in cv Nipponbare to LT was observed (Fig. 6D). In the slr1-d mutants, LT induced a severe decrease in the number of sporogenous cells and hypertrophy of tapetal cells (Fig. 7). The average number of sporogenous cells in each transverse section (n > 100) of a single locule was 4.31 ± 0.04 (TC65 at control temperature), 4.03 ± 0.09 (slr1-d at control temperature), 3.17 ± 0.03 (TC65 at LT), and 2.19 ± 0.04 (slr1-d at LT). The average anther length at the tetrad stage of pollen mother cells was 1.03 ± 0.15 mm (TC65 at control temperature), 1.10 ± 0.14 mm (slr1-d at control temperature), 0.62 ± 0.17 mm (TC65 at LT), and 0.46 ± 0.14 mm (slr1-d at LT). These results indicate that LT significantly inhibits the proliferation of sporogenous cells, and this inhibition was much more severe in the GA-insensitive mutant.
Figure 6.
GA-insensitive mutants are hypersensitive to LT. A, Representative features (pistil, anthers, and pollen) are shown in spikelets of the wild-type (WT) TC65, in spikelets of the GA-insensitive gid1-8 mutant, and in spikelets of the SLR1 gain-of-function slr1-d1 and slr1-d3 mutants in the TC65 genetic background. Spikelets were dissected at the heading period with (LT; blue frames) or without (cont.; orange frames) LT treatment. Bars = 1 mm. B, The number of mature pollen grains was severely reduced in the gid1 and slr1-d mutants compared with their parental wild-type TC65 plants. Bars = 50 μm. C, Quantification of the number of mature pollen grains in the mutants and the TC65 control with (blue columns) and without (orange columns) LT treatment (n = 36 anthers tested in each). D and E, Quantification of the number of mature pollen grains in the slr1-d2 mutant and its parental cv Kinmaze (D), and in the slr1-d4 mutant and its parental cv Nipponbare (E), with (blue columns) and without (orange columns) LT treatment (n = 36 anthers tested in each). Values are means ± se. Letters denote statistical differences with Tukey’s test (P < 0.05).
Figure 7.
The GA-insensitive slr1-d mutant has a hypersensitive response to LT and a severe reduction in the number of sporogenous cells (SC) and pollen mother cells (PMC). Representative sections of anthers at the sporogenous cell and pollen mother cell stages are indicated for the slr1-d3 mutant and its parental TC65. At the pollen mother cell stage, hypertrophy of tapetal cells (indicated by the red arrow) is often observed in the slr1-d3 mutant exposed to LT.
Male Sterility Can Be Rescued by the Application of Exogenous GA
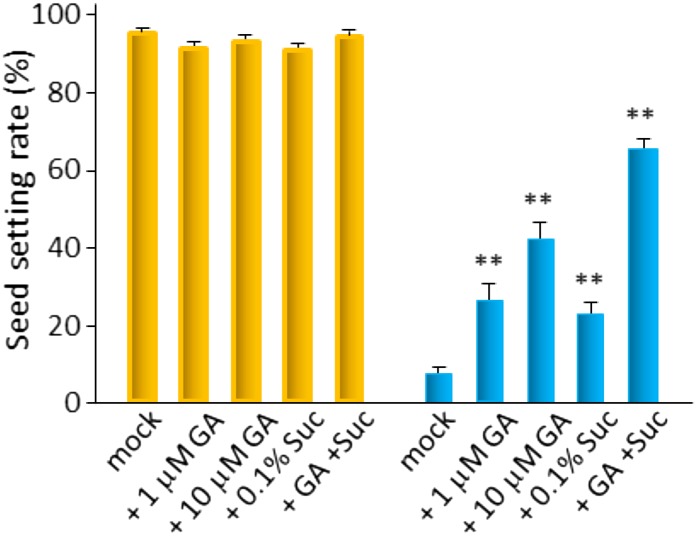
To assess how an exogenous application of GA affects cool-temperature disruption of anther development, we applied 1 or 10 μm GA3 during LT treatment to japonica rice cv Sasanishiki and cv Nipponbare grown in cool-temperature treatment units installed in a natural field. The results showed that exogenous GA significantly restored the seed-setting rate in a dose-dependent manner in cv Sasanishiki and cv Nipponbare (Fig. 8; Supplemental Fig. S3). By contrast, treatment with the GA biosynthesis inhibitor uniconazole potentiated the disruption of pollen development under LT conditions (Supplemental Fig. S3). Simultaneous application of 10 μm GA3 with 0.1% (w/v) Suc substantially improved the restoration of pollen development and seed set (Fig. 8). These results indicate that Suc had a synergistic effect with GA to reverse male sterility under LT. Cool temperature most likely led to a decline in energy production because decreasing temperatures directly influence photosynthetic activity, carbon metabolism, and the allocation of carbon to developing sink tissues (Holaday et al., 1992).
Figure 8.
Application of GA and/or simultaneous application of Suc substantially improved seed setting in cv Sasanishiki exposed to LT in cool-temperature treatment units installed in the experimental field (Supplemental Fig. S4). During 2 weeks of LT treatment, plants were treated with submersion water containing 1 μm GA3, 10 μm GA3, 0.1% (w/v) Suc, or 10 μm GA3 + 0.1% (w/v) Suc under LT conditions (blue columns) or normal-temperature conditions (orange columns). The percentage of seed set was measured after grain filling for plants of each treatment condition (n = 16 ears from the main stem of independent plants). Values are means ± se. Asterisks shows statistical significance at P < 0.01 (unpaired Student’s two-tailed t test).
DISCUSSION
Studies on cool-weather damage in rice have a long history, and have identified physiological responses including abnormal abortion of developing pollen followed by male sterility (Sakai, 1943; Nishiyama, 1976; 1982). The establishment of rice lines that are tolerant to cool temperatures has been a goal of breeders around the world. However, identification of the factors underlying temperature tolerance poses particular challenges. Cool-temperature tolerance in rice appears to be a highly complex trait that involves divergent loci in different rice lines (Takeuchi et al., 2001; Saito et al., 2001; Andaya and Mackill, 2003; Kuroki et al., 2007; Zeng et al., 2009; Saito et al., 2010; Zhou et al., 2010). These quantitative trait loci have been mapped on all twelve chromosomes. However, the responsible genes, their protein functions, the biological mechanisms, and the reasons for so many tolerant traits remain unclear.
GAs play important roles in seed germination and anther development in several plant species. GA deficiency or insensitivity causes abnormal development of anthers and leads to male sterility in tomato, petunia, Arabidopsis, and rice (Nester and Zeevaart, 1988; Izhaki et al., 2002; Jacobsen and Olszewski, 1991; Cheng et al., 2004; Aya et al., 2009). All these studies suggest that GA is essential for normal development of tapetal cells and pollen. The abnormal enlargement of tapetal cells in GA-deficient rice mutants is similar to the typical morphology induced by cool-temperature damage of developing anthers (Nishiyama 1976; Oda et al., 2010). However, evidence for the correlation between phytohormonal regulation (including GAs) and cool-temperature damage during anther development has not been reported.
We monitored the alteration of endogenous levels of GAs in developing rice anthers exposed to LT. The results clearly indicate that LT significantly reduced endogenous levels of bioactive GAs (GA4 and GA7) and raised the levels of the precursor GA12 (Fig. 2). The expression levels of the GA biosynthesis genes GA20ox3 and GA3ox1 were reduced in the developing anthers in response to LT treatment (Fig. 3). Our results clearly indicate that GA has a positive functional role in LT stress tolerance in rice plants. GA biosynthesis mutants are hypersensitive to LT (Fig. 5). Application of exogenous GA suppressed the cool-temperature damage, increased the number of mature pollen grains, and led to higher seed set (Fig. 8; Supplemental Fig. S3). It has been reported that GA functions in diametrically opposed capacities in response to abiotic stresses. GA is involved in stress tolerance during germination and seedling establishment (Kabar and Baltepe, 1990; Kaur et al., 1998; Nasri et al., 2011), whereas it is involved in stress intolerance in established vegetative tissues (Sarkar et al., 2004; Achard et al., 2006). In the tissues of Arabidopsis, DELLA/RGA-LIKE3 is induced by the transcriptional activator AtCBF1/DREB1B in response to cold and water deprivation (Achard et al., 2008). DELLA proteins are stabilized in the absence of bioactive GAs, which leads to the suppression of plant growth and confers tolerance to several environmental stresses (Fleet and Sun, 2005; Achard et al., 2006, 2008, 2009; Davière et al., 2008). Application of bioactive GAs stimulates DELLA degradation by the ubiquitin-proteasome system and results in the loss of stress tolerance (Achard et al., 2006, 2008, 2009; Davière et al., 2008).
We found that the transcription of DREB2B and SLR1/DELLA was up-regulated in early developing anthers in response to LT stress (Fig. 4). The endogenous levels of bioactive GAs and expression levels of GA biosynthesis genes were oppositely reduced (Fig. 3). These may lead toward the accumulation and stabilization of SLR1/DELLA proteins, and it would result in the arrest of sporogenous cell proliferation at early developmental stages and abnormal tapetal cell enlargement at later developmental stages. Therefore, application of exogenous GAs reverses the arrest of sporogenous cell proliferation and promotes normal tapetal cell degeneration by destabilizing SLR1/DELLA proteins. This working hypothesis is supported by the results that the GA-insensitive mutant gid1 (Ueguchi-Tanaka et al., 2007) and the gain-of-function mutant slr1-d (Asano et al., 2009) become hypersensitive to LT (Fig. 6). When SLR1/DELLA proteins are induced and stabilized by the gid1 and slr1-d mutations, they are more resistant to ubiquitin degradation after the GA levels increase and/or after the removal of stress conditions (Hirano et al., 2010).
The development and differentiation of anthers, microspores, and pollen generally proceed synchronously and in a known sequence of developmental stages (Sakata and Higashitani, 2008). However, it may be difficult to resume pollen development and differentiation after abiotic stress disrupts cell proliferation and growth. Therefore, cell proliferation and growth must be well coordinated through the activation and repression of GA signaling via DELLA stabilization to establish stress tolerance in the whole plant and in specific organs. In rice, wheat (Triticum aestivum), barley, and other commercially important crops, the early phase of anther development is more susceptible to LT or high temperature (Sakata and Higashitani, 2008). Crop yields are threatened by global climate change (Intergovernmental Panel on Climate Change, 2007). Our previous work identified a correlation between high-temperature injury to anther development in barley and Arabidopsis and depletion of the phytohormone auxin and showed that exogenous application of auxin reversed plant male sterility caused by high temperatures (Sakata et al., 2010).
In this study, we found that application of the phytohormone GA suppressed LT-induced male sterility in the modern rice cv Sasanishiki and cv Nipponbare (Fig. 8; Supplemental Fig. S3). The Green Revolution refers to a series of research and frontier technologies that were applied to modern cereal breeding during the last 50 years, which increased global agricultural yields and saved more than one billion people from starvation. The rice semidwarf sd1 recessive mutation is one of the greatest achievements of the Green Revolution. It reduces rice plant height, improves plant stature, and significantly increases grain yields (with the aid of fertilizers) without causing the plants to topple over (Hargrove and Cabanilla, 1979; Monna et al., 2002; Sasaki et al., 2002). Essentially all japonica rice cultivars including cv Nipponbare and cv Sasanishiki are introgressed with a semidwarf (sd1) character selected artificially, which has single-nucleotide polymorphisms on the GA20ox2 (sd1) gene (Hargrove and Cabanilla., 1979; Asano et al., 2011). Asano et al. (2011) also defined sd1 as the responsible gene for qCL1a in japonica rice. A cool-temperature tolerance qCT-1 has been mapped to the region of qCL1a (Takeuchi et al., 2001). In this study, we report that cv Nipponbare carrying japonica-type sd1 is more susceptible to LT stress than the chromosomal substitution line SL5 carrying the phenotypically taller indica sd1 (Fig. 5). These results suggest that Green Revolution rice may be at greater risk of developing plant male sterility in response to cool-temperature damage. We show that the application of GA and/or GA plus Suc can rescue LT stress-induced male sterility, which will be an important technique for sustainable agriculture in the face of global climate change.
MATERIALS AND METHODS
Plant Materials
Rice (Oryza sativa) varieties used in this study included cv Sasanishiki (Sasaki et al., 2004; Oda et al., 2010), cv Nipponbare (Asano et al., 2011), the cv Nipponbare backcross inbred line SL5 (Asano et al., 2011), cv Ginbozu (Suge, 1975; Itoh et al., 2004), cv Tan-Ginbozu (Suge, 1975; Itoh et al., 2004), TC65 (SLR1, GID1; Ueguchi-Tanaka et al., 2007; Asano et al., 2009), cv Kinmaze (SLR1, GID1; Ueguchi-Tanaka et al., 2007; Asano et al., 2009), a series of hypomorphic SLR1 gain-of-function mutants (slr1-d1, slr1-d2, slr1-d3, and slr1-d4; Asano et al., 2009), and the GID1 mutant gid1-8 (Ueguchi-Tanaka et al., 2007).
Growth Conditions
The cv Sasanishiki was used and grown primarily in an experimental field at the Miyagi Prefecture Furukawa Agricultural Experiment Station. Basal fertilizer, with slow release at rates of 40, 60, and 46 kg ha−1 (nitrogen, PO5, and potassium), was applied 5 to 7 d before transplanting. Cool-temperature stress was applied using a running cold water system (19°C, 25 cm deep) from the primordial stage to the completion of heading (approximately 2 months, from early July to early September) at the experimental station (38°36.1′N, 140°54.7′E; Supplemental Fig. S1). For control plants, the average maximum and minimum temperatures were 27°C and 19.4°C from early July to early September 2013. Anther development was divided into the following five consecutive stages according to cytological observations of microspores and pollen: (1) premeiotic sporogenous cell stage; (2) meiotic stage; (3) uninucleate pollen stage; (4) binucleate pollen stage; and (5) trinucleate pollen stage.
To study the effect of exogenous GA application, we installed cool-temperature treatment units in the experimental field (Supplemental Fig. S4). Twelve cv Sasanishiki seedlings were cultured until the stage of just-completed panicle formation (6–8 mm panicles) in a 1:5,000 Wagner pot at normal (control) temperature. Plants at stages from just-completed panicle formation to uninucleate pollen stage were treated with cool temperature (19°C) or control-temperature water at a depth of approximately 25 cm from the surface of the pots for 2 weeks (Supplemental Fig. S4). The pots were covered with 150 L containing 1 or 10 μm GA3 and 0.1% (w/v) Suc or 0.001 μg mL−1 uniconazole and placed into the 200-L plastic container. The treatment water was exchanged with fresh treatment water once per week. Following the treatment, every plant was removed from the plastic container and cultured under normal conditions.
Other series of experiments using mutant cultivars were performed in a 1:10,000 Wagner pot with vermiculite and fertilized soil containing 0.7 g of nitrogen, 1.2 g of PO5, and 0.6 g of potassium per kg (vermiculite:soil, 1:1, v/v) and then placed in a large growth cabinet (Espec) with a 12-h photoperiod, day/night temperatures of 27°C/22°C, and 80% humidity. Visible light in the growth cabinet was supplied by a combination of metal-halide lamps (MT 400 L/BUD; Iwasaki Electric) and high-pressure sodium lamps (NH360DL; Iwasaki Electric). A heat-absorbing filter was present that eliminated radiation below 350 nm. The spectral distribution of the visible radiation in the growth chamber was described previously (Kang et al., 1998). For LT treatments during the stages from early panicle development to the heading period, the pots were placed into a 200-L plastic container, covered with water to a depth of approximately 25 cm from the surface of the pots, and kept at 21.5°C by a circulated cooling system (LT treatment) or kept at the ambient temperature without a cooling system (control treatment).
The morphology of spikes and pollen in the first panicle of the main stem was observed at the heading period (Aya et al., 2009; Sakata et al., 2010). Then, the seed-setting percentage in the first panicle was counted in each plant variety. Each experiment contained more than 10 independent samples, and reproducibility was confirmed in duplicate experiments. One-way ANOVA with Tukey’s method was performed to test for statistical significance in each experiment.
Quantification of GAs
GAs were extracted from 100 mg of cv Sasanishiki anthers dissected at meiotic, uninucleate, binucleate, and trinucleate pollen stages with or without LT treatment, and their identities were determined using UPLC-tandem mass spectrometry (AQUITY UPLC System/XEVO-TQS; Waters) with an octadecylsilyl column (AQUITY UPLC BEH C18, 1.7 µm, 2.1 × 100 mm; Waters) as described previously (Kojima et al., 2009). Each series of experiments was performed in biological triplicate.
Expression Analyses with Real-Time Quantitative RT-PCR
The cv Sasanishiki cultivated in the field was used in these analyses. Total RNA was extracted with Trizol reagent (Invitrogen) from approximately 500 anthers at premeiotic sporogenous cell and meiotic stages and from approximately 300 anthers at uninucleate, binucleate, and trinucleate pollen stages, with or without LT treatment. The anther tissues and cells were broken by a microhomogenizing system using 1-mm-diameter zirconium beads at 4,300 rpm for 2 min at 4°C (Micro Smash homogenizer; TOMY). The first-strand complementary DNA was synthesized with PrimeScript reverse transcriptase (PrimeScript RT Reagent Kit; TaKaRa Bio). RT-PCR was performed with SYBR Premix Ex Taq II (Perfect Real-Time; TaKaRa Bio) with the following sets of gene-specific primers: Os07g0169700 (GA20ox3), 5ʹ-AATACCGCCACATGGGGGAGGT-3ʹ and 5ʹ-GTAGTGGTTCAGCCGCATCACC-3ʹ; Os05g0178100 (GA3ox1), 5ʹ-GACGATTCACCTCAACATGTTCCCT-3ʹ and 5ʹ-GGCTCTGCAGGATGAAGGTGAA-3ʹ; Os05g0158600 (GA2ox1), 5′-TTGGTGATGTCCTCCAGGCTCTG-3′ and 5′-CGGAGTGAGTACATTGTCGTCTTGT-3′; Os05g0346200 (DREB2B), 5ʹ-GTGGAGGCGAGGAAAGTACTGGA-3ʹ and 5ʹ-CCTGTGGATCAAGCTCCTGC-3ʹ; Os03g0707600 (SLR1/DELLA), 5ʹ-GTGCAGCAGGAGAACTTCG-3ʹ and 5ʹ-CGGCGAAGGCGGCGTCG-3ʹ; and Os03g718100 (ACTIN1), 5ʹ-CCTTCAACACCCCTGCTATGTACG-3ʹ and 5ʹ-GACGAAGGATAGCATGGGGGAGAG-3ʹ. Each expression level was normalized with respect to the level of ACTIN1 and was determined in biological duplicate samples (n = 2) with technical triplication.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Cool-temperature treatment was applied using a running cold-water system from the panicle primordial stage to the completion of heading in the experimental field.
Supplemental Figure S2. LT reduces the expression levels of the GA catabolic gene GA2ox1, which is up-regulated during anther development.
Supplemental Figure S3. Exogenous GA application rescues pollen development in cv Nipponbare under LT conditions.
Supplemental Figure S4. Schematic illustration and photographs of japonica rice tested in the cool-temperature treatment units in the experimental field.
Supplementary Material
Acknowledgments
We thank Drs. Tadashi Sato, Nahoko Higashitani, Jun Hidema, Mika Teranishi, Shusei Sato, Hiroshi Suge, and Hideyuki Takahashi for helpful suggestions.
Glossary
- LT
low temperature
- UPLC
ultra-performance liquid chromatography
- RT
reverse transcription
- TC65
Taichung 65
Footnotes
This work was supported by the Ministry of Education, Culture, Sports, Science, and Technology (grant nos. 20678001, 21370076, and 23113006), the Japan Society for the Promotion of Science (Grant-in-Aid for Fellows no. 25–6166), and the Ministry of Agriculture, Fisheries, and Food (grant nos. IPG–0003, IPG–0018, and IPG–0019).
The online version of this article contains Web-only data.
Articles can be viewed online without a subscription.
References
- Achard P, Cheng H, De Grauwe L, Decat J, Schoutteten H, Moritz T, Van Der Straeten D, Peng J, Harberd NP. (2006) Integration of plant responses to environmentally activated phytohormonal signals. Science 311: 91–94 [DOI] [PubMed] [Google Scholar]
- Achard P, Gong F, Cheminant S, Alioua M, Hedden P, Genschik P. (2008) The cold-inducible CBF1 factor-dependent signaling pathway modulates the accumulation of the growth-repressing DELLA proteins via its effect on gibberellin metabolism. Plant Cell 20: 2117–2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achard P, Gusti A, Cheminant S, Alioua M, Dhondt S, Coppens F, Beemster GT, Genschik P. (2009) Gibberellin signaling controls cell proliferation rate in Arabidopsis. Curr Biol 19: 1188–1193 [DOI] [PubMed] [Google Scholar]
- Andaya VC, Mackill DJ. (2003) QTLs conferring cold tolerance at the booting stage of rice using recombinant inbred lines from a japonica × indica cross. Theor Appl Genet 106: 1084–1090 [DOI] [PubMed] [Google Scholar]
- Asano K, Hirano K, Ueguchi-Tanaka M, Angeles-Shim RB, Komura T, Satoh H, Kitano H, Matsuoka M, Ashikari M. (2009) Isolation and characterization of dominant dwarf mutants, Slr1-d, in rice. Mol Genet Genomics 281: 223–231 [DOI] [PubMed] [Google Scholar]
- Asano K, Yamasaki M, Takuno S, Miura K, Katagiri S, Ito T, Doi K, Wu J, Ebana K, Matsumoto T, et al. (2011) Artificial selection for a green revolution gene during japonica rice domestication. Proc Natl Acad Sci USA 108: 11034–11039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aya K, Ueguchi-Tanaka M, Kondo M, Hamada K, Yano K, Nishimura M, Matsuoka M. (2009) Gibberellin modulates anther development in rice via the transcriptional regulation of GAMYB. Plant Cell 21: 1453–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Qin L, Lee S, Fu X, Richards DE, Cao D, Luo D, Harberd NP, Peng J. (2004) Gibberellin regulates Arabidopsis floral development via suppression of DELLA protein function. Development 131: 1055–1064 [DOI] [PubMed] [Google Scholar]
- Davière JM, de Lucas M, Prat S. (2008) Transcriptional factor interaction: a central step in DELLA function. Curr Opin Genet Dev 18: 295–303 [DOI] [PubMed] [Google Scholar]
- Fleet CM, Sun TP. (2005) A DELLAcate balance: the role of gibberellin in plant morphogenesis. Curr Opin Plant Biol 8: 77–85 [DOI] [PubMed] [Google Scholar]
- Hargrove TR, Cabanilla VL. (1979) The impact of semidwarf rice varieties on Asian rice-breeding programs. Bioscience 29: 731–735 [Google Scholar]
- Hirano K, Asano K, Tsuji H, Kawamura M, Mori H, Kitano H, Ueguchi-Tanaka M, Matsuoka M. (2010) Characterization of the molecular mechanism underlying gibberellin perception complex formation in rice. Plant Cell 22: 2680–2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holaday AS, Martindale W, Alred R, Brooks AL, Leegood RC. (1992) Changes in activities of enzymes of carbon metabolism in leaves during exposure of plants to low temperature. Plant Physiol 98: 1105–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intergovernmental Panel on Climate Change (2007) Fourth Assessment Report. Cambridge University Press, Cambridge, UK [Google Scholar]
- Itoh H, Tatsumi T, Sakamoto T, Otomo K, Toyomasu T, Kitano H, Ashikari M, Ichihara S, Matsuoka M. (2004) A rice semi-dwarf gene, Tan-Ginbozu (D35), encodes the gibberellin biosynthesis enzyme, ent-kaurene oxidase. Plant Mol Biol 54: 533–547 [DOI] [PubMed] [Google Scholar]
- Izhaki A, Borochov A, Zamski E, Weiss D. (2002) Gibberellin regulates post-microsporogenesis processes in petunia anthers. Physiol Plant 115: 442–447 [DOI] [PubMed] [Google Scholar]
- Jacobsen SE, Olszewski NE. (1991) Characterization of the arrest in anther development associated with gibberellin deficiency of the gib-1 mutant of tomato. Plant Physiol 97: 409–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabar K, Baltepe S. (1990) Effects of kinetin and gibberellic acid in overcoming high temperature and salinity (NaCl) stresses on the germination of barley and lettuce seeds. Phyton 30: 65–74 [Google Scholar]
- Kang HS, Hidema J, Kumagai T. (1998) Effects of light environment during culture on UV-induced cyclobutyl pyrimidine dimers and their photorepair in rice (Oryza sativa L.). Photochem Photobiol 68: 71–77 [Google Scholar]
- Kaur S, Gupta AK, Kaur N. (1998) Gibberellin A3 reverses the effect of salt stress in chickpea (Cicer arietinum L.) seedlings by enhancing amylase activity and mobilization of starch in cotyledons. Plant Growth Regul 26: 85–90 [Google Scholar]
- Kojima M, Kamada-Nobusada T, Komatsu H, Takei K, Kuroha T, Mizutani M, Ashikari M, Ueguchi-Tanaka M, Matsuoka M, Suzuki K, et al (2009) Highly sensitive and high-throughput analysis of plant hormones using MS-probe modification and liquid chromatography-tandem mass spectrometry: an application for hormone profiling in Oryza sativa. Plant Cell Physiol 50: 1201–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroki M, Saito K, Matsuba S, Yokogami N, Shimizu H, Ando I, Sato Y. (2007) A quantitative trait locus for cold tolerance at the booting stage on rice chromosome 8. Theor Appl Genet 115: 593–600 [DOI] [PubMed] [Google Scholar]
- Li TG, Guo WM (1993) Identification and study on tolerance in main stresses of China cultivated rice germplasm resource. In CS Ying, ed, Rice Germplasm Resources in China. China Agricultural Science Technology Press, Beijing, pp 71–75 [Google Scholar]
- Monna L, Kitazawa N, Yoshino R, Suzuki J, Masuda H, Maehara Y, Tanji M, Sato M, Nasu S, Minobe Y. (2002) Positional cloning of rice semidwarfing gene, sd-1: rice “green revolution gene” encodes a mutant enzyme involved in gibberellin synthesis. DNA Res 9: 11–17 [DOI] [PubMed] [Google Scholar]
- Nasri N, Kaddour R, Rabhi M, Plassard C, Lachaâl M. (2011) Effect of salinity on germination, phytase activity and phytate content in lettuce seedling. Acta Physiol Plant 33: 935–942 [Google Scholar]
- Nester JE, Zeevaart JAD. (1988) Flower development in normal tomato and a gibberellin-deficient (ga-2) mutant. Am J Bot 75: 45–55 [Google Scholar]
- Nishiyama I. (1976) Male sterility caused by cooling treatment at the young microspore stage in rice plants. XIII. Ultrastructure of tapetal hypertrophy without primary wall. Proc Crop Sci Soc Jpn 45: 270–278 [Google Scholar]
- Nishiyama I. (1982) Male sterility caused by cooling treatment at the young microspore stage in rice plants. XXIII. Anther length, pollen number and the difference in susceptibility to coolness among spikelets on the panicle. Jpn J Crop Sci 51: 462–469 [Google Scholar]
- Oda S, Kaneko F, Yano K, Fujioka T, Masuko H, Park JI, Kikuchi S, Hamada K, Endo M, Nagano K, et al. (2010) Morphological and gene expression analysis under cool temperature conditions in rice anther development. Genes Genet Syst 85: 107–120 [DOI] [PubMed] [Google Scholar]
- Olszewski N, Sun TP, Gubler F. (2002) Gibberellin signaling: biosynthesis, catabolism, and response pathways. Plant Cell (Suppl) 14: S61–S80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin F, Shinozaki K, Yamaguchi-Shinozaki K. (2011) Achievements and challenges in understanding plant abiotic stress responses and tolerance. Plant Cell Physiol 52: 1569–1582 [DOI] [PubMed] [Google Scholar]
- Raghavendra AS, Gonugunta VK, Christmann A, Grill E. (2010) ABA perception and signalling. Trends Plant Sci 15: 395–401 [DOI] [PubMed] [Google Scholar]
- Saito K, Hayano-Saito Y, Kuroki M, Sato Y. (2010) Map-based cloning of the rice cold tolerance gene Ctb1. Plant Sci 179: 97–102 [Google Scholar]
- Saito K, Miura K, Nagano K, Hayano-Saito Y, Araki H, Kato A. (2001) Identification of two closely linked quantitative trait loci for cold tolerance on chromosome 4 of rice and their association with anther length. Theor Appl Genet 103: 862–868 [Google Scholar]
- Sakai K. (1943) Cyto-histological investigations on the sterility mechanism in rice plants in Hokkaido caused by cold injury in 1941. Rep Agric Res St Hokkaido 40: 1–17 [Google Scholar]
- Sakata T, Higashitani A. (2008) Male sterility accompanied with abnormal anther development in plants: genes and environmental stresses with special reference to high temperature injury. Intl J Plant Dev Biol 2: 42–51 [Google Scholar]
- Sakata T, Oshino T, Miura S, Tomabechi M, Tsunaga Y, Higashitani N, Miyazawa Y, Takahashi H, Watanabe M, Higashitani A. (2010) Auxins reverse plant male sterility caused by high temperatures. Proc Natl Acad Sci USA 107: 8569–8574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Perras MR, Falk DE, Zhang R, Pharis RP, Fletcher RA. (2004) Relationship between gibberellins, height, and stress tolerance in barley (Hordeum vulgare L.) seedlings. J Plant Growth Regul 42: 125–135 [Google Scholar]
- Sasaki A, Ashikari M, Ueguchi-Tanaka M, Itoh H, Nishimura A, Swapan D, Ishiyama K, Saito T, Kobayashi M, Khush GS, et al (2002) Green revolution: a mutant gibberellin-synthesis gene in rice. Nature 416: 701–702 [DOI] [PubMed] [Google Scholar]
- Sasaki K, Nagano K, Chiba B, Endo T. (2004) Characteristics of eating quality of principal rice cultivars developed at Miyagi Prefectural Furukawa Agricultural Experiment Station. Tohoku J Crop Sci 47: 57–58 [Google Scholar]
- Suge H. (1975) Complementary genes for height inheritance in relation to gibberellin production in rice plants. Jpn J Genet 50: 121–131 [Google Scholar]
- Sun TP, Gubler F. (2004) Molecular mechanism of gibberellin signaling in plants. Annu Rev Plant Biol 55: 197–223 [DOI] [PubMed] [Google Scholar]
- Takeuchi Y, Hayasaka H, Chiba B, Tanaka I, Shimano T, Yamagishi M, Nagano K, Sasaki T, Yano M. (2001) Mapping quantitative trait loci controlling cool-temperature tolerance at booting stage in temperate japonica rice. Breed Sci 51: 191–197 [Google Scholar]
- Ueguchi-Tanaka M, Nakajima M, Katoh E, Ohmiya H, Asano K, Saji S, Hongyu X, Ashikari M, Kitano H, Yamaguchi I, et al (2007) Molecular interactions of a soluble gibberellin receptor, GID1, with a rice DELLA protein, SLR1, and gibberellin. Plant Cell 19: 2140–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Yang S, Cui H, Yang X, Xu L, Du J, Pu X, Li Z, Cheng Z, Huang X. (2009) QTLs of cold tolerance-related traits at the booting stage for NIL-RILs in rice revealed by SSR. Genes Genomics 31: 143–154 [Google Scholar]
- Zhou L, Zeng Y, Zheng W, Tang B, Yang S, Zhang H, Li J, Li Z. (2010) Fine mapping a QTL qCTB7 for cold tolerance at the booting stage on rice chromosome 7 using a near-isogenic line. Theor Appl Genet 121: 895–905 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.