Calcium-independent protein kinase activity is important for inactivating Arabidopsis plasma membrane proton-translocating adenosine triphosphatase.
Abstract
The salt stress-induced SALT-OVERLY-SENSITIVE (SOS) pathway in Arabidopsis (Arabidopsis thaliana) involves the perception of a calcium signal by the SOS3 and SOS3-like CALCIUM-BINDING PROTEIN8 (SCaBP8) calcium sensors, which then interact with and activate the SOS2 protein kinase, forming a complex at the plasma membrane that activates the SOS1 Na+/H+ exchanger. It has recently been reported that phosphorylation of SCaBP proteins by SOS2-like protein kinases (PKSs) stabilizes the interaction between the two proteins as part of a regulatory mechanism that was thought to be common to all SCaBP and PKS proteins. Here, we report the calcium-independent activation of PKS24 by SCaBP1 and show that activation is dependent on interaction of PKS24 with the C-terminal tail of SCaBP1. However, unlike what has been found for other PKS-SCaBP pairs, multiple amino acids in SCaBP1 are phosphorylated by PKS24, and this phosphorylation is dependent on the interaction of the proteins through the PKS24 FISL motif and on the efficient activation of PKS24 by the C-terminal tail of SCaBP1. In addition, we show that Thr-211 and Thr-212, which are not common phosphorylation sites in the conserved PFPF motif found in most SCaBP proteins, are important for this activation. Finally, we also found that SCaBP1-regulated PKS24 kinase activity is important for inactivating the Arabidopsis plasma membrane proton-translocating adenosine triphosphatase. Together, these results suggest the existence of a novel SCaBP-PKS regulatory mechanism in plants.
Calcium is a ubiquitous second messenger that plays an important role in the regulation of plant growth and development. Many different types of calcium-binding proteins have been identified in plants (Harper et al., 2004), including the SALT-OVERLY-SENSITIVE3 (SOS3)-LIKE CALCIUM BINDING PROTEINS (SCaBPs; Liu and Zhu, 1998; Gong et al., 2004). Because the calcium-binding domain of these proteins shares sequence similarity with the yeast calcineurin B subunit, they have also been called CALCINEURIN B-LIKE PROTEINS (CBLs; Kudla et al., 1999; Luan et al., 2002). The founding member of this gene family, SOS3, was identified in a genetic screen from a salt-sensitive Arabidopsis (Arabidopsis thaliana) mutant (Liu and Zhu, 1998). SCaBP/CBL proteins interact with the SOS2-LIKE PROTEIN KINASES (PKSs)/CBL-INTERACTING PROTEIN KINASES (CIPKs; Shi et al., 1999; Halfter et al., 2000; Guo et al., 2001). The genetic linkage between these two families was established after identification of SOS2 from a genetic screen similar to the one that identified the sos3 mutant (Liu et al., 2000). SOS3 interacts with SOS2 in vivo and in vitro and activates SOS2 in a calcium-dependent manner in vitro (Halfter et al., 2000). The SOS3-SOS2 complex further activates SOS1, a plasma membrane (PM) Na+/H+ antiporter, by directly phosphorylating the SOS1 C terminus (Shi et al., 2000; Qiu et al., 2002; Quintero et al., 2002, 2011; Yu et al., 2010).
In addition to the calcium-dependent activation of PKSs by SCaBP calcium sensors, two other regulatory mechanisms have been identified for these protein families. First, PKSs have a conserved 21-amino acid peptide (FISL motif) in their regulatory domain that is necessary for efficient interaction with the SCaBP calcium sensors (Guo et al., 2001; Albrecht et al., 2001; Gong et al., 2004). The PKS regulatory domain interacts with its kinase domain via the FISL motif to repress PKS activity; interaction of SCaBP with the PKS FISL motif releases the kinase domain inhibition allowing for kinase activity (Guo et al., 2001; Gong et al., 2004). Second, the PKSs phosphorylate a Ser residue in the conserved C-terminal PFPF motif of the SCaBP proteins. This phosphorylation enhances the interaction between the two proteins and fully activates the complex (Lin et al., 2009; Du et al., 2011; Hashimoto et al., 2012).
In this study, we identified a novel PKS activation mechanism involving the calcium-independent activation of PKS24 by SCaBP1 and show that it requires binding of SCaBP1 to the FISL motif of PKS24 and the involvement of two Thr residues in the SCaBP1 C-terminal tail.
RESULTS
The Activation of PKS24 by SCaBP1 Is Calcium Independent
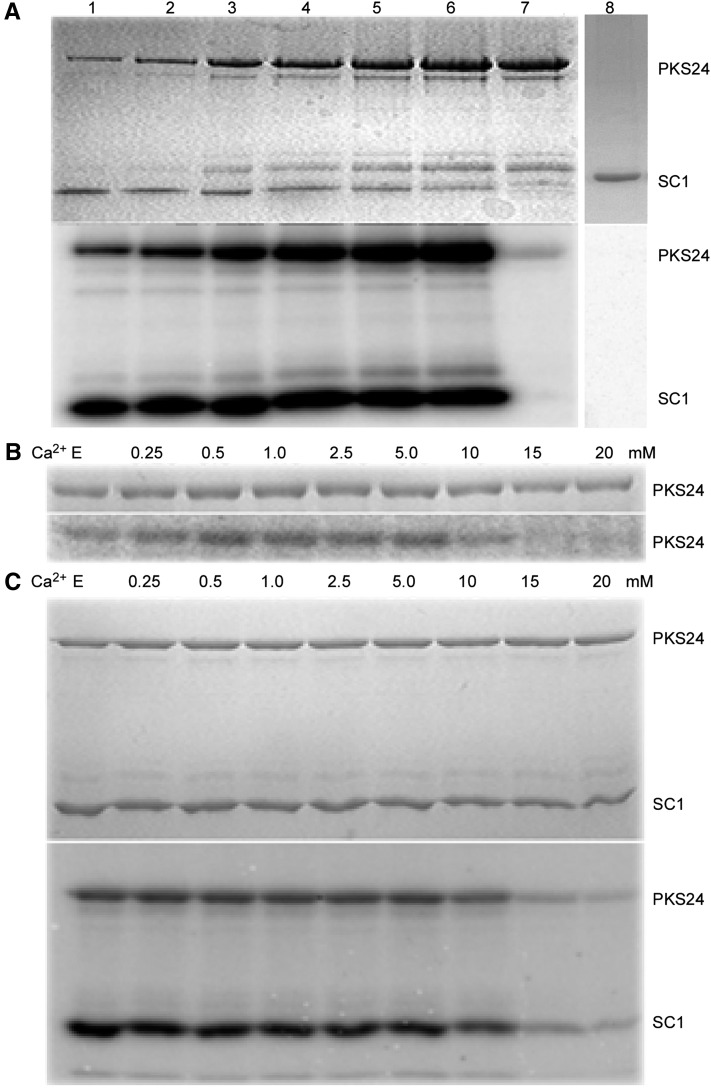
We previously showed that phosphorylation of SCaBP1 by PKS24 increases the interaction between the two proteins (Du et al., 2011). While testing the effect of SCaBP1 on PKS24 activity, we found that compared with what was seen for other SCaBP-PKS pairs (Shi et al., 1999; Halfter et al., 2000; Guo et al., 2001; Quan et al., 2007), activation of PKS24 by SCaBP1 (autophosphorylation) increased with increasing amounts of PKS24 protein and was independent of calcium (Fig. 1). Low concentrations of PKS24 (6.67 ng/μl) displayed much higher kinase activity than high concentrations (133.3 ng/μl) in the absence of SCaBP1, and there was a dramatic increase in kinase activity detected with increasing concentrations of PKS24 (Fig. 1A). Consistent with previous results, PKS24 phosphorylated SCaBP1. These results indicate that SCaBP1 plays a critical role in regulating PKS24 activity.
Figure 1.
Activation of PKS24 by SCaBP1 is calcium independent. A, Effect of SCaBP1 on PKS24 activity. Increasing concentrations of PKS24 were incubated without or with a fixed amount of SCaBP1. The concentrations of PKS24 in lanes 1 to 6 were as follows (in ng/µl): 6.7, 13.3, 33.5, 67, 100, and 133. The concentration in lane 7 was 133 ng/μl PKS24 without SCaBP1. In lane 8 (negative control), SCaBP1 (33.3 ng/μl) was incubated in reaction buffer without PKS24. Coomassie Blue staining (top) and kinase activity (bottom). B and C, Coomassie Blue-stained polyacrylamide gel (top), autophosphorylation activity of PKS24 (B), and transphosphorylation of SCaBP1 (C) in the presence of increasing concentrations of calcium (bottom). PKS24 and SCaBP1 were purified as GST-fusion proteins; the GST tag was removed from SCaBP1 by PreScission protease. E, EGTA (5 mm) was added into the reaction buffer without calcium ions. SC1, SCaBP1.
To determine whether calcium is involved in this regulation, we first assayed whether calcium has a direct effect on PKS24 activity using increasing concentrations of calcium (from 0 to 20 mM) and the calcium chelator EGTA. No difference in PKS24 autophosphorylation or SCaBP1 phosphorylation was seen in the presence of EGTA or at concentrations of calcium ranging from 0.25 to 5 mm (Fig. 1, B and C). Similar to what has been shown for kinase assays involving SOS2 (Guo et al., 2001), decreased levels of these PKS24 activities were observed at concentrations of calcium above 10 mm (Fig. 1, B and C). These results indicate that the activation of PKS24 by SCaBP1 is calcium independent in vitro. Maximum PKS24 autophosphorylation was observed when 10 mm Mg2+ was included in the assay; in comparison, only 0.5 mm Mn2+ was required to produce the maximum level of PKS24 activity, indicating a preference for Mn2+ over Mg2+ (Supplemental Fig. S1).
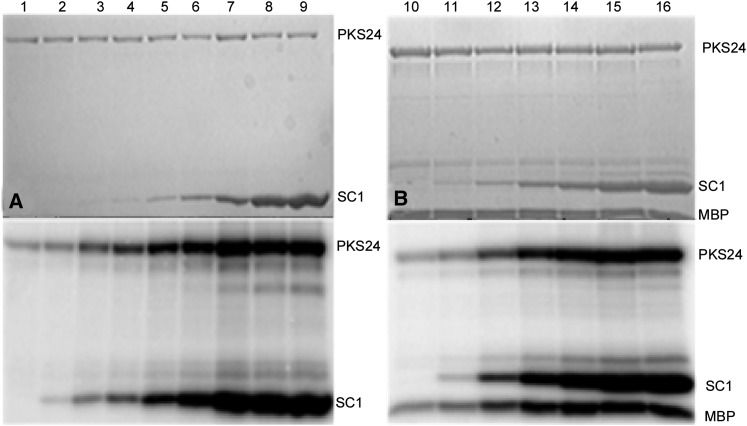
To further characterize the interaction between SCaBP1 and the kinase activity of PKS24, a fixed amount of PKS24 (1 µg) was incubated with increasing concentrations of SCaBP1 (Fig. 2A) and the ability of PKS24 to transphosphorylate SCaBP1 and myelin basic protein (MBP, 200 ng) was monitored (Fig. 2B). The results show that increasing SCaBP1 increased both the autophosphorylation and transphosphorylation activities of PKS24. We then investigated the stoichiometry of MBP phosphorylation by PKS24 in the absence or presence of SCaBP1. In the presence of 2.5 μm MBP and 10 μm ATP, MBP phosphorylation by PKS24 (0.7 µM) reached saturation within 15 min with or without 2.0 μm SCaBP1. Maximum phosphate incorporation was 0.15 ± 0.02 mol/mol of MBP without SCaBP1 but increased dramatically to 1.2 ± 0.2 mol/mol with addition of SCaBP1 (Supplemental Fig. S2).
Figure 2.
PKS24 phosphorylates SCaBP1 and SCaBP1 activates PKS24. A, Phosphorylation of SCaBP1 by PKS24. The activity of PKS24 was enhanced as the concentration of SCaBP1 was increased. Coomassie Blue-stained polyacrylamide gel (top); PKS24 autophosphorylation and SCaBP1 transphosphorylation activity (bottom). B, Phosphorylation of SCaBP1 and MBP by PKS24. Coomassie Blue-stained polyacrylamide gel (top); PKS24 autophosphorylation and SCaBP1/MBP transphosphorylation activity (bottom). The concentration of SCaBP1 in lanes 1 to 16 were as follows (in ng/µl): 0, 0.67, 3.33, 6.67, 13.4, 33.3, 66.7, 133, 266.7, 0, 6.67, 13.4, 33.3, 66.7, 133, and 266.7. PKS24 and SCaBP1 were purified as GST-fusion proteins; the GST tag was removed from SCaBP1 by PreScission protease. SC1, SCaBP1.
The FISL Motif Does Not Inhibit PKS24 Activity
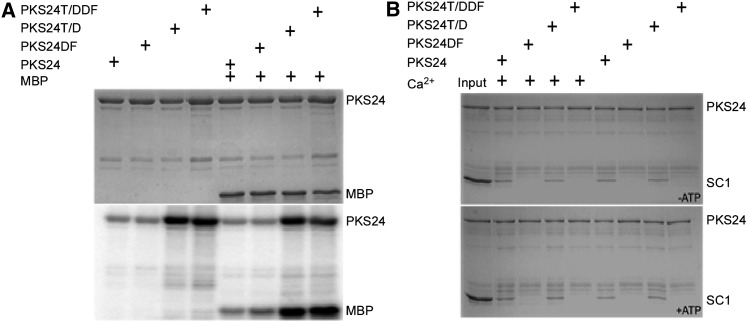
SOS2 exhibits weak autophosphorylation and transphosphorylation activity when p3 peptide is used as the substrate (Halfter et al., 2000; Quan et al., 2007). Active SOS2 can be created by changing the Thr at position 168 (T168) in the kinase activation loop to Asp (D) or by deleting the FISL motif in the regulatory domain and both of these features (a Thr in the kinase activation loop and the FISL motif) are conserved throughout the PKS family (Guo et al., 2001). To determine whether the conserved Thr and FISL motif in PKS24 affect its kinase activity, we generated three PKS24 mutants: one with the T178/D substitution (PKS24T/D), one with the FISL motif deleted (PKS24DF), and one with both mutations (PKS24T/DDF). The three mutant proteins and wild-type PKS24 were then used in in vitro kinase assays. In contrast with the results for SOS2, deleting the FISL motif did not alter the autophosphorylation or transphosphorylation activity of PKS24 when MBP was used as the substrate; however, the T178/D mutation strongly increased PKS24 activity and the kinase activity of PKS24T/DDF was similar to that of the T178/D mutant (Fig. 3A). These results suggest that the FISL motif in PKS24 does not serve as a kinase-inhibitory domain as it does in SOS2 (Guo et al., 2001).
Figure 3.
In vitro pull-down and phosphorylation of SCaBP1 by wild-type and mutant PKS24. A, Autophosphorylation and transphosphorylation activity of PKS24, PKS24T/D, PKS24DF, and PKS24T/DDF. Coomassie Blue staining (top) and phosphorylation activity (bottom). B, The interaction of PKS24 with SCaBP1 requires the FISL motif but not calcium or ATP. The pull-down experiments were performed in kinase assay buffer. Lane 1, input containing 200 ng of PKS24 and 1 μg of SCaBP1 as markers. Lane 2, the pull-down products from lane 1 after five washes. Lanes 3 to 9, SCaBP1 pull-down by PKS24, PKS24DF, PKS24T/D, and PKS24T/DDF in the presence or absence of calcium. Top, reactions in kinase buffer lacking ATP; bottom, reactions in kinase buffer containing ATP. SCaBP1, PKS24, and its variations were purified as GST-fusion proteins; the GST tag was removed from SCaBP1 by PreScission protease for the pull-down assay. SC1, SCaBP1.
SOS3 and SCaBP8 interact with the SOS2 FISL motif in a SOS2 kinase activity- and calcium-independent manner (Halfter et al., 2000; Guo et al., 2001; Quan et al., 2007). To test whether the phosphorylation of SCaBP1 by PKS24 requires an interaction between the two proteins at a site in addition to the active site, assays were performed to determine whether SCaBP1 interacts with PKS24 and PKS24T/D in the presence or absence of calcium and ATP. Neither PKS24DF nor PKS24T/DDF was able to interact with SCaBP1 (Fig. 3B), indicating that the interaction between SCaBP1 and PKS24 requires the FISL motif but is independent of calcium and PKS24 activity.
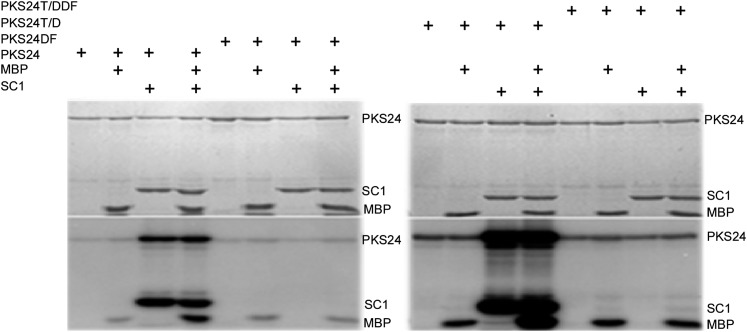
The Activation of PKS24 by SCaBP1 Is Interaction Dependent
To test whether the phosphorylation of SCaBP1 by PKS24 requires an interaction between the two proteins, kinase assays were performed using versions of PKS24, PKS24DF, PKS24T/D, and PKS24T/DDF fused to glutathione S-transferase (GST). The presence of SCaBP1 in the reactions increased both the autophosphorylation and transphosphorylation activities of PKS24 and PKS24T/D. By contrast, neither PKS24DF nor PKS24T/DDF was able to phosphorylate SCaBP1 or be activated by SCaBP1 (Fig. 4), although they could phosphorylate MBP. These results demonstrate that an interaction between the two proteins at a site in addition to the active site is required for both the phosphorylation of SCaBP1 by PKS24 and the activation of PKS24 by SCaBP1.
Figure 4.
PKS24 activation by SCaBP1 requires the interaction of PKS24 with SCaBP1. Kinase assays were performed using combinations of PKS24, PKS24DF, PKS24T/D, or PKS24T/DDF and SCaBP1, MBP, or SCaBP1 plus MBP as indicated. Coomassie Blue-stained polyacrylamide gel (top); phosphorylation activity (bottom). PKS24 and SCaBP1 were purified as GST-fusion proteins; the GST tag was removed from SCaBP1 by PreScission protease. SC1, SCaBP1.
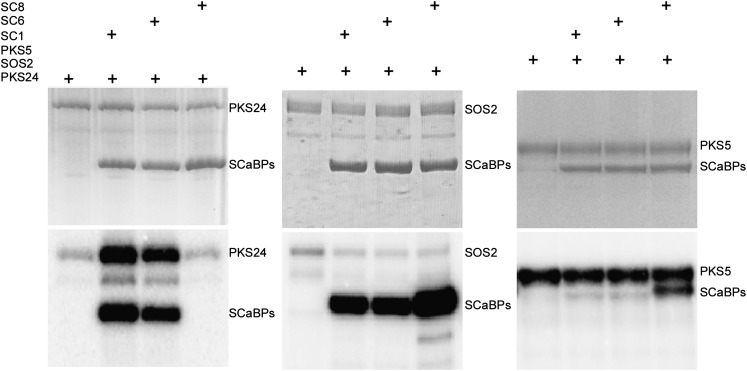
To determine whether other SCaBP proteins also activate PKS24, SCaBP8 and SCaBP6, which share 59.8% and 90.4% amino acid sequence similarity with SCaBP1, respectively, were fused to the GST and the constructs were expressed in Escherichia coli. His-PKS5, GST-PKS24, GST-SOS2, GST-SCaBP1, GST-SCaBP6, and GST-SCaBP8 fusion proteins were then purified and used in kinase assays (Fig. 5). SCaBP1 and SCaBP6 but not SCaBP8 were phosphorylated by PKS24 and activated PKS24 (Fig. 5). In vitro pull-down assays demonstrated that GST-PKS24 pulled down SCaBP1 and SCaBP6, but not SCaBP8 (Supplemental Fig. S3). Both SOS2 and PKS5 phosphorylated SCaBP1, SCaBP6, and SCaBP8. However, their autophosphorylation activities were not enhanced by the phosphorylated SCaBP proteins (Fig. 5). These results suggest that the activation of PKS24 by SCaBP1 and SCaBP6 is a specific regulatory process in the SCaBP-PKS pathway.
Figure 5.
SCaBP1 and SCaBP6 specifically activate PKS24. Kinase assays were performed using combinations of GST-PKS24, GST-SOS2, or His-PKS5 and GST-SCaBP1, GST-SCaBP6, or GST-SCaBP8 as indicated. Coomassie Blue-stained polyacrylamide gel (top); phosphorylation activity (bottom). PKS24, SOS2, SCaBP1, SCaBP6, and SCaBP8 were fused to GST tag; PKS5 was fused to His tag. SC1, SCaBP1; SC6, SCaBP6; SC8, SCaBP8.
Myc-SCaBP1 Activates Flag-PKS24 in Planta
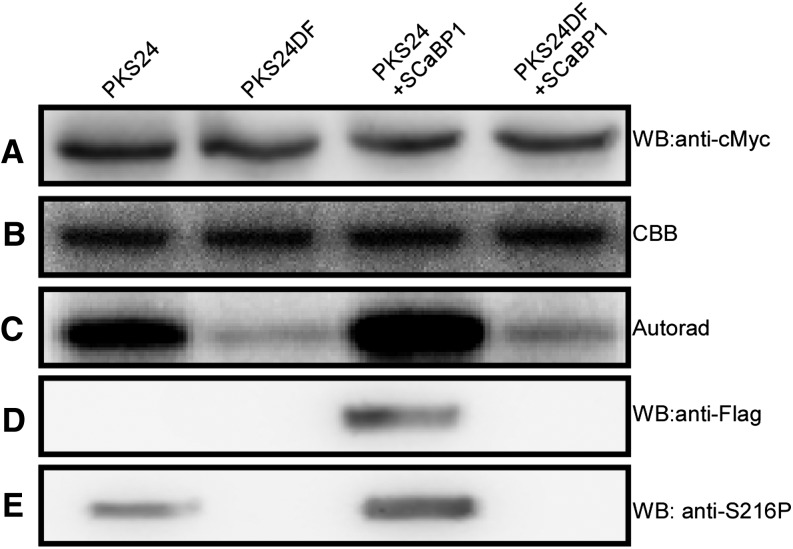
To determine the activation of PKS24 by SCaBP1 in vivo, Myc-PKS24, Myc-PKS24DF, and Flag-SCaBP1 were transformed into wild-type plants. Transgenic plants containing Myc-PKS24 and Flag-SCaBP1 or Myc-PKS24DF and Flag-SCaBP1 were generated by crossing transgenic plants harboring single transgenes. Twelve-d-old seedlings were harvested and the proteins were extracted. Anti-Myc beads were used to immunoprecipitate Myc-PKS24 or Myc-PKS24DF (Fig. 6A) and MBP was used as the substrate for the kinase assays (Fig. 6B). The results show that PKS24 from transgenic plants harboring Flag-SCaBP1 had the highest activity (Fig. 6C). MBP was very weakly phosphorylated by PKS24DF, whether the transgenic plants contained Flag-SCaBP1 or not (Fig. 6C). However, the phosphorylation of MBP by Myc-PKS24 from transgenic plants that did not contain Flag-SCaBP1 was stronger than phosphorylation by PKS24DF (Fig. 6C). These results suggest that endogenous SCaBP1 in the wild type plays a role in activating the activity of PKS24. Consistent with this, PKS24DF, which does not interact with SCaBP1, was not activated by either endogenous or overexpressed SCaBP1.
Figure 6.
SCaBP1 activates PKS24 in vivo. Coimmunoprecipitation was performed with transgenic plants as indicated, and the products were used in kinase assays or immunoblots. A, Immunoblots with anti-cMyc antibody. B, Coomassie Blue-stained polyacrylamide gel of the substrate MBP for the kinase assay. C, Autoradiography of MBP phosphorylation. D and E, Immunoblots with anti-flag antibody (D) and anti-S216P antibody (E). SCaBP1 was fused to Flag tag; PKS24 and PKS24DF were fused to Myc tag. Autorad, Autoradiography; CBB, Coomassie Brilliant Blue; SC1, SCaBP1; WB, western blot.
We previously showed that PKS24 phosphorylates Ser-216 of SCaBP1 (Du et al., 2011). Polyclonal phosphospecific antibodies (anti-S216P) detected this phosphorylation in vitro (Supplemental Fig. S4). Anti-S216P was used to detect the phosphorylation status of FLAG-SCaBP1 immunoprecipitated with anti-FLAG from the transgenic plants described above. The presence of Myc-PKS24 or Myc-PKS24DF in these immunoprecipitates was detected with anti-MYC (Fig. 6A). Flag-SCaBP1 was only detected in the Myc-PKS24 but not Myc-PKS24DF coimmunoprecipitated products (Fig. 6D). A stronger signal was detected with anti-S216P in transgenic plants harboring both Myc-PKS24 and Flag-SCaBP1 (Fig. 6E). A weaker signal was also detected in plants expressing only Myc-PKS24, suggesting that endogenous SCaBP1 was pulled down and phosphorylated by PKS24 (Fig. 6E); however, no signal was detected in the PKS24DF coimmunoprecipitated products (Fig. 6E). These results suggest that SCaBP1 is phosphorylated by PKS24 in vivo, and that phosphorylation is dependent on the interaction of SCaBP1with the FISL domain of PKS24.
Thr-211 and Thr-212 in the C Terminus of SCaBP1 Are Important for Activation of PKS24
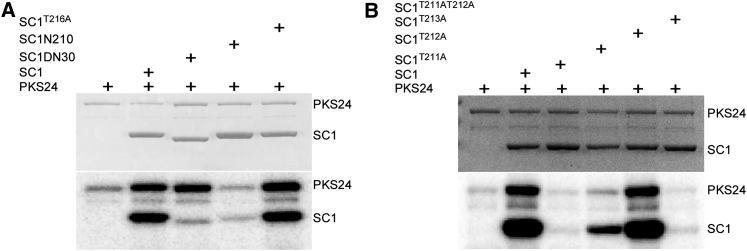
Previous results have demonstrated that PKS24 phosphorylates SCaBP1 at Ser-216 and that this phosphorylation enhances the interaction between the two proteins (Du et al., 2011). Interaction between PKS24 and SCaBP1 is also required for the activation of PKS24 (Fig. 4). To determine the region of SCaBP1 that is required for the activation of PKS24, we made two additional SCaBP1 mutant proteins by removing the last 16 amino acids (from 211 to 226, SCaBP1N210) or the first 30 amino acids from SCaBP1 (SCaBP1ND30) and used GST-fused SCaBP1, SCaBP1S216A, SCaBP1N210, and SCaBP1ND30 in kinase assays (Fig. 7A). Compared with wild-type SCaBP1, SCaBP1N210 was only weakly phosphorylated by PKS24 and did not activate PKS24. SCaBP1ND30 activated the autophosphorylation activity of PKS24 to a level similar to that of SCaBP1, but the level of SCaBP1 phosphorylation by PKS24 was dramatically reduced. SCaBP1S216A was phosphorylated by PKS24 and activated PKS24 at a level similar to that of wild-type SCaBP1 (Fig. 7A). These results indicate that the C terminus of SCaBP1 is required for the activation of PKS24 and multiple sites in the N terminus of SCaBP1 are phosphorylated by PKS24. Because Ser-216 is one of multiple phosphorylation sites in SCaBP1, additional reductions in the presence of the Ser-216 mutation were not seen.
Figure 7.
Thr-211 and Thr-212 in the C terminus of SCaBP1 are important for activation of PKS24. A, Activation of PKS24 by SCaBP1, SCaBP1S216A, and SCaBPDN30 but not by SCaBP1N210. Coomassie Blue staining (top) and kinase activity (bottom). B, Activation of PKS24 by SCaBP1, SCaBP1T212A, or SCaBP1T213A. Coomassie Blue staining (top) and kinase activity (bottom). PKS24, SCaBP1, and its variations were fused to GST tag. SC1, SCaBP1.
Interaction between PKS24 and SCaBP1 is essential for both activation of PKS24 and phosphorylation of SCaBP1 (Fig. 4). We tested whether SCaBP1N210 interacts with PKS24 and PKS24DF. The results showing that SCaBP1N210 was pulled down by PKS24 but not by PKS24DF (Supplemental Fig. S5) suggest that although the C terminus of SCaBP1 is not needed for binding to PKS24 via the FISL domain, it possesses the ability to activate PKS24. Because the phosphorylation of SCaBP1 is often coupled with the activation of PKS24, we then tested three putative phosphorylation sites within the last 16 amino acids of SCaBP1 (T211, T212, and T213) to determine whether they are important for activation of PKS24. The three Thr residues were replaced with Ala and the mutant proteins were used in kinase assays. SCaBP1T211A did not activate PKS24 and showed little evidence of phosphorylation by PKS24, whereas SCaBP1T212A activated PKS24 and was weakly phosphorylated by it. The SCaBP1T211AT212A double mutant, like SCaBP1T211A, did not activate PKS24. In comparison, SCaBP1T213A, like wild-type SCaBP1, activated PKS24 and was phosphorylated by it (Fig. 7B). These results demonstrate that both SCaBP1T211 and SCaBP1T212 are important for the activation of PKS24. In vitro pull-down assays provided evidence that these three point mutations did not change the ability of the proteins to bind to PKS24 (Supplemental Fig. S6).
PKS24 Regulates PM Proton-Translocating Adenosine Triphosphatase Activity
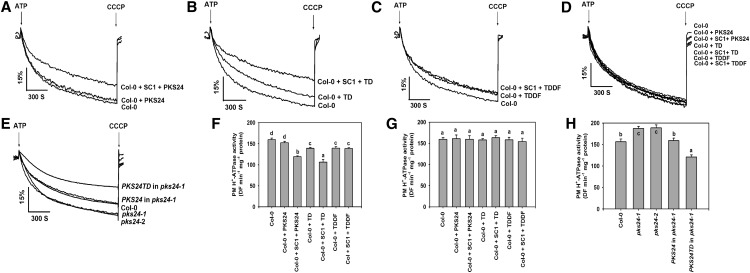
To analyze the biological function of PKS24, we obtained two transfer DNA insertion lines for PKS24 (SALK_147899 and SALK_009699, referred to as pks24-1 and pks24-2, respectively) and confirmed their status as gene knockouts (Supplemental Fig. S7). The phenotype of each mutant was monitored after exposure to Glc, abiotic stresses, and plant hormones including abscisic acid, ethylene, and auxin (data not shown). No significant differences were observed between wild-type and mutant plants with any of these treatments. PKS5 negatively regulates the PM proton-translocating adenosine triphosphatase activity (Fuglsang et al., 2007) and PKS24 shares the highest sequence similarity with PKS5 among the 25 PKS family members. SCaBP1 has been shown to activate PKS5 when expressed in yeast (Saccharomyces cerevisiae; Fuglsang et al., 2007) and to activate PKS24 in Arabidopsis (Fig. 6), suggesting that PKS24 may also play a role in regulating PM H+-ATPase activity. It has been shown that addition of activated SOS2 kinase (T/DSOS2DF) can directly stimulate the activities of the PM and tonoplast Na+/H+ antiporters (Qiu et al., 2002, 2004; Guo et al., 2004). To determine whether PKS24 plays a role in the regulation of PM H+-ATPase activity, we purified PM vesicles from wild-type plants and measured the H+-transport activity of the H+-ATPase in the presence of different combinations of PKS24 and SCaBP1 proteins. Addition of PKS24 protein had no significant effect on the H+-transport activity in wild-type vesicles; however, this activity was significantly reduced by adding SCaBP1 and PKS24 in combination (Fig. 8, A and F). H+-transport activity also decreased with the addition of active PKS24, either PKS24T/D or PKS24T/DDF (Fig. 8, B, C, and F); the level of reduction was less than that seen in the presence of SCaBP1 in combination with PKS24 (Fig. 8F). SCaBP1 in combination with PKS24T/D was more effective than the SCaBP1 and PKS24 in reducing H+-transport activity, whereas SCaBP1 in combination with PKS24T/DDF had a similar effect on H+-transport activity as PKS24T/DDF (Fig. 8, C and F). When added as a control, denatured (boiled) protein did not alter H+-transport in wild-type vesicles (Fig. 8, D and G).
Figure 8.
PKS24 negatively regulates plasma membrane H+-ATPase activity. Plasma membrane vesicles were isolated from wild-type plants, pks24 mutants, and transgenic plants treated with 250 mm sodium chloride for 3 d. Intravesicular acidification was initiated by the addition of 3 mm ATP and the pH gradient was collapsed by addition of 10 μm carbonyl cyanide m-chlorophenylhydrazone (CCCP). The H+ transport activity (ΔpH formation) was measured in wild-type vesicles (A to G), and in the presence of PKS24 (A and F), SCaBP1 and PKS24 (A and F), PKS24T/D (B and F), SCaBP1 and PKS24T/D (B and F), PKS24T/DDF (C and F), SCaBP1 and PKS24T/DDF (C and F), denatured PKS24, SCaBP1 and PKS24, PKS24T/D, SCaBP1 and PKS24T/D, PKS24T/DDF, SCaBP1 and PKS24T/DDF (D and G). The H+ transport activity was measured in vesicles isolated from wild-type plants (A, B, C, D, F, and G) or the pks24 mutants and transgenic plants (E and H). Units of H+ transport activity are ΔF/min per mg protein. All data represent means ± ses of at least three replicate experiments. Each replicate experiment was performed using independent membrane preparations. A to E, One representative experiment of three replicates is shown. The Student’s test was used for determining the statistical significance. Significant differences (P ≤ 0.05) are indicated by different lowercase letters. SCaBP1, PKS24, and its variations were fused to GST tag. Col-0, Ecotype Columbia 0 of Arabidopsis; SC1, SCaBP1; TD, PKS24T/D; TDDF, PKS24T/DDF.
To further investigate the role of PKS24 on the regulation of PM H+-ATPase activity, we purified PM vesicles from wild-type plants, the PKS24 knockout mutants and the pks24-1 mutant harboring PKS24 or PKS24T/D and measured H+-transport activity. The two mutants showed the highest activity, the transgenic plants harboring PKS24 showed activity that was the same as in wild-type and transgenic plants harboring PKS24T/D possessed the lowest activity (Fig. 8, E and H). These data suggest that PKS24 plays a role in negatively regulating PM H+-ATPase activity.
DISCUSSION
Previous data have shown that SCaBPs are calcium sensors that physically interact with and activate PKSs in a calcium-dependent manner (Luan et al., 2002; Gong et al., 2004). In this study, we found that activation of PKS24 by SCaBP1 is calcium independent but requires the C terminus of SCaBP1 and the FISL motif of PKS24, and that the phosphorylation of the SCaBP1 C terminus by PKS24 may also play an important role in PKS24 activation.
The FISL motif in the PKSs is essential for its interaction with the SCaBP calcium sensors. This is supported by analysis of the complex structures of SOS3-SOS2 (Sánchez-Barrena et al., 2007) and CBL2/SCaBP1-CIPK14/PKS24 (Akaboshi et al., 2008). The PKSs recognize their interacting calcium sensor SCaBPs by the interaction of the FISL motif with a hydrophobic cleft generated by the four calcium-binding domains (EF hands) of SCaBP.
Calcium also plays a role in regulation of PKS and SCaBP interaction. When SOS3 is not in a complex with SOS2, all four EF hands are in a Ca2+-bound form; however, when SOS3 interacts with SOS2, only EF1 and EF4 contain Ca2+. In a Ca2+-free system, the SOS3-SOS2 complex aggregates to a high Mr form, suggesting that calcium changes the conformation of the SOS2-SOS3 complex and stabilizes the interaction (Sánchez-Barrena et al., 2007). In contrast with the SOS2-SOS3 complex, Ca2+ does not affect the SCaBP1/CBL1-PKS24/CIPK14 interaction and complex stability. In both Ca2+-free and Ca2+-bound forms, this complex exists as a monomer in solution (Akaboshi et al., 2008). However, it is not understood how specificity in interaction is achieved for each SCaBP-PKS pair.
SCaBP proteins are phosphorylated by their interacting PKSs in the conserved SCaBP PFPF motif and phosphorylation increases the interaction between the two proteins (Du et al., 2011). SCaBP8S237 is the only amino acid phosphorylated by SOS2 (Lin et al., 2009); however, SCaBP1S216A can be phosphorylated by PKS24 and activated PKS24 in vitro, indicating that there are multiple PKS24-phosphorylation sites in SCaBP1. When we removed 16 amino acids from the C terminus of SCaBP1 or mutated three putative phosphorylation sites in this region (SCaBP1T211A, SCaBP1T212A, and SCaBP1T213A), the mutated proteins still interacted with PKS24, but SCaBP1T211A and SCaBP1T212A did not activate the kinase, indicating that the Thr-211 and Thr-212 are required for PKS24 activation. By contrast, Thr-213 is not required for the activation of PKS24. Our results indicate that both the interaction between PKS24 and SCaBP1 through the FISL motif and Thr-211 and Thr-212 in the C terminus of SCaBP1 are essential for the activation of PKS24.
Although we do not know whether the phosphorylation of the C terminus of SCaBP1 is required for this activation, such a multistep phosphorylation mechanism could be involved in fine-tuning the regulatory activity of SCaBP1, and the phosphorylation of these alternate sites could alter the structure of the PKS24-SCaBP1 complex and further activate PKS24. Both PKS24 and PKS24DF are able to phosphorylate MBP; however, the phosphorylation of SCaBP1 by PKS24 requires the FISL motif, suggesting that structure-based recognition between these two proteins is important for the phosphorylation of SCaBP1 by PKS24 as well as their interaction. Deletion of the FISL motif also abolishes the interaction between SOS2 and SCaBP8; however, SOS2DF still phosphorylates SCaBP8, although it is weaker than SOS2 phosphorylation (Lin et al., 2009). These results suggest that the activation of PKS24 by SCaBP1 and the activation of SOS2 by SOS3/SCaBP8 are regulated by different mechanisms.
Similar to PKS5, PKS24 is required for inactivating PM H+ transport. Activated PKS24s reduced PM H+-ATPase activity and the reduction in the level of the activity correlated with the activity of the kinases. These results suggest that the kinase activities of PKS5 and PKS24 play a central role in regulating H+-transport activity. Because SCaBP1 does not activate PKS5 in vitro, it is possible that other cofactors, additional proteins, or posttranslational modification of PKS5 are required to activate PKS5 in vivo and to further deactivate the PM H+-ATPase.
MATERIALS AND METHODS
Plasmid Construction
PKS24 and SCaBP1 complementary DNA was obtained by reverse transcription PCR from wild-type Arabidopsis (Arabidopsis thaliana Columbia ecotype) RNA. The amplified products were gel-purified, digested, and cloned into the pCAMBIA2307-6×Myc and pCAMBIA1307-3×Flag vectors, respectively. Site-directed mutagenesis was used to construct the T to D substitutions and/or a FISL motif deletion mutant of PKS24 (PKS24T/D, PKS24DF, and PKS24T/DDF). The gel-purified amplified products were digested with BamHI and SalI, cloned into the pGEX-6P-1 vector, and PKS24T/D and PKS24T/DDF were subcloned into the pCAMBIA2307-6×Myc vector. To construct the GST-SCaBP1 fusion protein and its mutant forms, SCaBP1 was extracted from pCAMBIA1307-3×Flag -SCaBP1 vector using BamHI and SalI and subcloned into pGEX-6P-1. Site-directed mutagenesis was used to construct the C-terminal deletion mutant and Ser/Thr to Ala substitutions in SCaBP1 (SCaBP1N210, SCaBP1S216A, SCaBP1T211A, SCaBP1T212A, SCaBP1T211A T212A, and SCaBP1T213A). The resulting products were then cloned into pGEX-6P-1. To construct the GST-SCaBP6 fusion plasmid, SCaBP6 complementary DNA was obtained by reverse transcription PCR from wild-type Arabidopsis RNA. The gel-purified amplified product was digested with BamHI and SalI and cloned into the pGEX-6P-1 vector. The pGEX-2TK-SOS2 and the pGEX-6P-1-SCaBP8 plasmids were described by Lin et al. (2009). The pQE-30-PKS5 plasmid was described by Yang et al. (2010). The entire insert in each of the constructs was sequenced. All primers in this study are listed in Supplemental Table 1.
Fusion Protein Expression and Purification, Pull-Down, and Kinase Assays
All GST or His fusion constructs were transformed into Escherichia coli BL21 (DE3). The transformed cells were grown at 37°C in Luria-Bertani medium with ampicillin (100 μg/mL) until Optical Density 600 = 1.0 was reached. Recombinant protein expression was induced by 1.0 mm isopropyl-β-d-thiogalactopyranoside at 16°C overnight. The recombinant proteins were affinity purified according to the manufacturer’s protocol (GE Healthcare Life Science) and analyzed by SDS-PAGE.
Kinase assays were performed as previously described (Lin et al., 2009). Kinase buffer included 20 mm Tris-HCl (pH 8.0), 5 mm MgCl2, 1 mm CaCl2, 10 μm ATP, and 1 mm dithiothreitol. The kinase reaction was performed in a total volume of 15 μL and was started by the addition of 0.1 μL of γ-32P-ATP (1μCi), and the mixtures were incubated at 30°C for 0.5 h. Reactions were terminated by adding 6× SDS loading buffer followed by incubation at 95°C for 5 min. Proteins were separated by 12% (w/v) SDS-PAGE and stained with Coomassie Brilliant Blue R-250 followed by exposure to a phosphor screen (Amersham Biosciences). After a 12-h exposure, signals were captured with a Typhoon 9410 phosphor imager (Amersham Biosciences). Enzyme activity was measured and quantified as described by Gong et al. (2002). Stoichiometry was measured and calculated as described by Halbrügge et al. (1990).
For the pull-down assay, SCaBP6, SCaBP8, SCaBP1, and its mutated versions were cleaved by PreScission protease from the GST tag; GST-PKS24 and its mutated versions were eluted from glutathione beads according to the manufacturer’s protocol (GE Healthcare Life Science). The glutathione was removed using Amicon Ultra-10 centrifugal filters (Millipore). Five μg of SCaBPs were incubated with 1 μg (or 5 μg) each of GST-PKS24 or its variations for 30 min at room temperature in 100 μL of kinase buffer (20 mm Tris-HCl, pH 8.0, 5 mm MgCl2, and 1 mm dithiothreitol); calcium or ATP were added to the buffer when testing their effects. Then 20 μL of the 50% (v/v) glutathione-bead slurry was added into the binding system and incubated for 30 min at room temperature. After centrifugation at 500g for 2 min, the supernatant was discarded. The glutathione beads were washed with 500 μL kinase buffer five times. The pull-down products were resuspended in 100 μL of SDS-PAGE loading buffer, and 20 μL was examined by 12% (w/v) SDS-PAGE gel, with visualization by staining with Coomassie Brilliant Blue R 250.
Coimmunoprecipitation Assays
Twelve-d-old seedlings were harvested and homogenized on ice in extraction buffer containing 10 mm Tris-HCl (pH7.6) 150 mm sodium chloride, 1 mm EDTA, 0.5% (v/v) Nonidet P-40. Ten μL of anti-cMyc conjugated agarose (Sigma) was incubated with the extract supernatant for 1 h at 4°C. The beads were washed five times with extraction buffer. The coimmunoprecipitation products were detected via immunoblots using anti-cMyc (Sigma), anti-Flag (Sigma), and anti-S216P antibodies.
PM Na+/H+ Antiport Assays
Na+/H+ antiport activity was measured as a Na+-induced dissipation of the pH gradient (change in pH [ΔpH]; i.e. a Na+-induced increase in quinacrine fluorescence) as described by Lin et al. (2009). Recombinant SCaBP1, PKS24, PKS24T/D, or PKS24T/DDF protein (250 ng/mL) was preincubated for 10 min at room temperature with plasma membrane vesicles isolated from plants. An inside-acid ΔpH was formed in the vesicles by the activity of the H+-ATPase and was measured as a decrease (quench) in the fluorescence of quinacrine (a pH-sensitive fluorescent probe). Assays (2 mL) contained 5 μm quinacrine, 3 mm MgSO4, 100 mm KCl, 25 mm 1,3-bis[tris(hydroxylmethyl)methylamino]propane-HEPES, pH 6.5, 250 mm mannitol, and 50 μg/mL of plasma membrane protein. Reactions were mixed by inversion several times and then placed in a dark chamber in a fluorescence spectrophotometer (Hitachi F-4500). Reactions were equilibrated in the dark with stirring for 5 min before beginning fluorescence readings. The assay was initiated by the addition of ATP to a final concentration of 3 mM, and formation of ΔpH was measured at excitation and emission wavelengths of 430 and 500 nm, respectively. When the maximum ΔpH was formed (reached steady state), sodium chloride was added to initiate Na+ transport. At the end of each reaction, 10 μm (final concentration) of the protonophore m-chlorophenylhydrazone was added to dissipate any remaining ΔpH. Specific activity was calculated by dividing the initial rate by the mass of plasma membrane protein in the reaction (Δ%F/min per mg of protein). Unless indicated, all data represent the mean ± se of at least three replicate experiments.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers ACT2, At3g18780; PKS24, AT5G01820; PKS5, AT2G30360; SCABP1, AT5G55990; SCABP6, AT4G26570; SCABP8, At4g33000; and SOS2, At5g35410.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Kinase activity of wild-type and mutant PKS24 and the effect of Mn2+ and Mg2+.
Supplemental Figure S2. Time course of MBP phosphorylation by PKS24 without (A) or with SCaBP1 (B).
Supplemental Figure S3. PKS24 interacts with SCaBP1 and SCaBP6, but not SCaBP8 in vitro.
Supplemental Figure S4. Anti-S216P antibody can be used to detect the phosphorylation of SCaBP1 by PKS24 in vitro.
Supplemental Figure S5. SCaBP1N210 interacts with PKS24, but not PKS24DF in vitro.
Supplemental Figure S6. PKS24 interacts with SCaBP1, SCaBP1S216, SCaBP1T211, SCaBP1T212, and SCaBP1T213 in vitro.
Supplemental Figure S7. Transfer DNA insertion position in pks24-1 (SALK_147899) and pks24-2 (SALK_09699) mutant lines and PKS24 expression analysis.
Supplemental Table S1. Primers used in this study.
Supplementary Material
Acknowledgments
We thank Drs. José M. Pardo and Jianmin Zhou for critical reading of the manuscript and stimulating discussions.
Glossary
- PM
plasma membrane
- MBP
myelin basic protein
- GST
glutathione S-transferase
- H+-ATPase
proton-translocating adenosine triphosphatase
- ΔpH
change in pH
Footnotes
This work was supported by the National Basic Research Program of China (grant no. 2012CB114200 to Y.G.), China National Funds for Distinguished Young Scientists (grant no. 31025003 to Y.G.), Natural Science Foundation of China International Collaborative Research Project (grant no. 31210103903 to Y.G.), Foundation for Innovative Research Group of the National Natural Science Foundation of China (grant no. 31121002), U.S. Department of Energy/Energy Biosciences (grant no. DE–FG02–04ER15616 to K.S.S.), China Postdoctoral Science Foundation (grant no. 2013M540163 to H.X.L.), and China Agricultural University (fellowship 2013BH021 to H.L.).
The online version of this article contains Web-only data.
Articles can be viewed online without a subscription.
References
- Akaboshi M, Hashimoto H, Ishida H, Saijo S, Koizumi N, Sato M, Shimizu T. (2008) The crystal structure of plant-specific calcium-binding protein AtCBL2 in complex with the regulatory domain of AtCIPK14. J Mol Biol 377: 246–257 [DOI] [PubMed] [Google Scholar]
- Albrecht V, Ritz O, Linder S, Harter K, Kudla J. (2001) The NAF domain defines a novel protein-protein interaction module conserved in Ca2+-regulated kinases. EMBO J 20: 1051–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du W, Lin H, Chen S, Wu Y, Zhang J, Fuglsang AT, Palmgren MG, Wu W, Guo Y. (2011) Phosphorylation of SOS3-like calcium-binding proteins by their interacting SOS2-like protein kinases is a common regulatory mechanism in Arabidopsis. Plant Physiol 156: 2235–2243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuglsang AT, Guo Y, Cuin TA, Qiu Q, Song CP, Kristiansen KA, Bych K, Schulz A, Shabala S, Schumaker KS, et al. (2007) Arabidopsis protein kinase PKS5 inhibits the plasma membrane H+ -ATPase by preventing interaction with 14-3-3 protein. Plant Cell 19: 1617–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong D, Gong Z, Guo Y, Chen X, Zhu JK. (2002) Biochemical and functional characterization of PKS11, a novel Arabidopsis protein kinase. J Biol Chem 277: 28340–28350 [DOI] [PubMed] [Google Scholar]
- Gong D, Guo Y, Schumaker KS, Zhu JK. (2004) The SOS3 family of calcium sensors and SOS2 family of protein kinases in Arabidopsis. Plant Physiol 134: 919–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Halfter U, Ishitani M, Zhu JK. (2001) Molecular characterization of functional domains in the protein kinase SOS2 that is required for plant salt tolerance. Plant Cell 13: 1383–1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Qiu QS, Quintero FJ, Pardo JM, Ohta M, Zhang C, Schumaker KS, Zhu JK. (2004) Transgenic evaluation of activated mutant alleles of SOS2 reveals a critical requirement for its kinase activity and C-terminal regulatory domain for salt tolerance in Arabidopsis thaliana. Plant Cell 16: 435–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbrügge M, Friedrich C, Eigenthaler M, Schanzenbächer P, Walter U. (1990) Stoichiometric and reversible phosphorylation of a 46-kDa protein in human platelets in response to cGMP- and cAMP-elevating vasodilators. J Biol Chem 265: 3088–3093 [PubMed] [Google Scholar]
- Halfter U, Ishitani M, Zhu JK. (2000) The Arabidopsis SOS2 protein kinase physically interacts with and is activated by the calcium-binding protein SOS3. Proc Natl Acad Sci USA 97: 3735–3740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JF, Breton G, Harmon A. (2004) Decoding Ca(2+) signals through plant protein kinases. Annu Rev Plant Biol 55: 263–288 [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Eckert C, Anschütz U, Scholz M, Held K, Waadt R, Reyer A, Hippler M, Becker D, Kudla J. (2012) Phosphorylation of calcineurin B-like (CBL) calcium sensor proteins by their CBL-interacting protein kinases (CIPKs) is required for full activity of CBL-CIPK complexes toward their target proteins. J Biol Chem 287: 7956–7968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudla J, Xu Q, Harter K, Gruissem W, Luan S. (1999) Genes for calcineurin B-like proteins in Arabidopsis are differentially regulated by stress signals. Proc Natl Acad Sci USA 96: 4718–4723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Yang Y, Quan R, Mendoza I, Wu Y, Du W, Zhao S, Schumaker KS, Pardo JM, Guo Y. (2009) Phosphorylation of SOS3-LIKE CALCIUM BINDING PROTEIN8 by SOS2 protein kinase stabilizes their protein complex and regulates salt tolerance in Arabidopsis. Plant Cell 21: 1607–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Ishitani M, Halfter U, Kim CS, Zhu JK. (2000) The Arabidopsis thaliana SOS2 gene encodes a protein kinase that is required for salt tolerance. Proc Natl Acad Sci USA 97: 3730–3734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhu JK. (1998) A calcium sensor homolog required for plant salt tolerance. Science 280: 1943–1945 [DOI] [PubMed] [Google Scholar]
- Luan S, Kudla J, Rodriguez-Concepcion M, Yalovsky S, Gruissem W. (2002) Calmodulins and calcineurin B-like proteins: calcium sensors for specific signal response coupling in plants. Plant Cell 14(Suppl): S389–S400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu QS, Guo Y, Dietrich MA, Schumaker KS, Zhu JK. (2002) Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3. Proc Natl Acad Sci USA 99: 8436–8441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu QS, Guo Y, Quintero FJ, Pardo JM, Schumaker KS, Zhu JK. (2004) Regulation of vacuolar Na+/H+ exchange in Arabidopsis thaliana by the salt-overly-sensitive (SOS) pathway. J Biol Chem 279: 207–215 [DOI] [PubMed] [Google Scholar]
- Quan R, Lin H, Mendoza I, Zhang Y, Cao W, Yang Y, Shang M, Chen S, Pardo JM, Guo Y. (2007) SCABP8/CBL10, a putative calcium sensor, interacts with the protein kinase SOS2 to protect Arabidopsis shoots from salt stress. Plant Cell 19: 1415–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero FJ, Martinez-Atienza J, Villalta I, Jiang X, Kim WY, Ali Z, Fujii H, Mendoza I, Yun DJ, Zhu JK, et al. (2011) Activation of the plasma membrane Na/H antiporter Salt-Overly-Sensitive 1 (SOS1) by phosphorylation of an auto-inhibitory C-terminal domain. Proc Natl Acad Sci USA 108: 2611–2616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero FJ, Ohta M, Shi H, Zhu J-K, Pardo JM. (2002) Reconstitution in yeast of the Arabidopsis SOS signaling pathway for Na+ homeostasis. Proc Natl Acad Sci USA 99: 9061–9066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Barrena MJ, Fujii H, Angulo I, Martínez-Ripoll M, Zhu JK, Albert A. (2007) The structure of the C-terminal domain of the protein kinase AtSOS2 bound to the calcium sensor AtSOS3. Mol Cell 26: 427–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Ishitani M, Kim C, Zhu JK. (2000) The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proc Natl Acad Sci USA 97: 6896–6901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Kim KN, Ritz O, Albrecht V, Gupta R, Harter K, Luan S, Kudla J. (1999) Novel protein kinases associated with calcineurin B-like calcium sensors in Arabidopsis. Plant Cell 11: 2393–2405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Qin Y, Xie C, Zhao F, Zhao J, Liu D, Chen S, Fuglsang AT, Palmgren MG, Schumaker KS, et al. (2010) The Arabidopsis chaperone J3 regulates the plasma membrane H+-ATPase through interaction with the PKS5 kinase. Plant Cell 22: 1313–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Nie J, Cao C, Jin Y, Yan M, Wang F, Liu J, Xiao Y, Liang Y, Zhang W. (2010) Phosphatidic acid mediates salt stress response by regulation of MPK6 in Arabidopsis thaliana. New Phytol 188: 762–773 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.