The Arabidopsis UV RESISTANCE LOCUS8 (UVR8) photoreceptor increases nitric oxide in response to UV-B, thus promoting stomatal closure.
Abstract
UV RESISTANCE LOCUS8 (UVR8) signaling involves CONSTITUTIVELY PHOTOMORPHOGENIC1, the ELONGATED HYPOCOTYL5 (HY5) transcription factor, and the closely related HY5 HOMOLOG. Some UV-B responses mediated by UVR8 are also regulated by nitric oxide (NO), a bioactive molecule that orchestrates a wide range of processes in plants. In this study, we investigated the participation of the UVR8 pathway and its interaction with NO in UV-B-induced stomatal movements in Arabidopsis (Arabidopsis thaliana). Stomata in abaxial epidermal strips of Arabidopsis ecotype Landsberg erecta closed in response to increasing UV-B fluence rates, with maximal closure after 3-h exposure to 5.46 μmol m–2 s–1 UV-B. Both hydrogen peroxide (H2O2) and NO increased in response to UV-B, and stomatal closure was maintained by NO up to 24 h after the beginning of exposure. Stomata of plants expressing bacterial NO dioxygenase, which prevents NO accumulation, did not close in response to UV-B, although H2O2 still increased. When the uvr8-1 null mutant was exposed to UV-B, stomata remained open, irrespective of the fluence rate. Neither NO nor H2O2 increased in stomata of the uvr8-1 mutant. However, the NO donor S-nitrosoglutathione induced closure of uvr8-1 stomata to the same extent as in the wild type. Experiments with mutants in UVR8 signaling components implicated CONSTITUTIVELY PHOTOMORPHOGENIC1, HY5, and HY5 HOMOLOG in UV-B-induced stomatal closure. This research provides evidence that the UVR8 pathway regulates stomatal closure by a mechanism involving both H2O2 and NO generation in response to UV-B exposure.
Plants, as sessile organisms that require sunlight to grow and develop, are inevitably exposed to UV wavelengths (200–400 nm), which represent almost 7% of the electromagnetic radiation emitted from the sun. UV-C and much of the UV-B radiation (280–315 nm) is absorbed by stratospheric ozone, but some UV-B is transmitted to the Earth’s surface. High doses of UV-B may damage macromolecules, including DNA, and induce the production of reactive oxygen species (ROS), affecting cell integrity and viability (Jordan, 1996; Brosché and Strid, 2003; Frohnmeyer and Staiger, 2003). However, UV-B is also a signaling stimulus that regulates various metabolic and developmental processes and modifies plant architecture (Jansen, 2002; Frohnmeyer and Staiger, 2003; Jenkins, 2009; Heijde and Ulm, 2012; Jansen and Bornman, 2012). Research undertaken in recent years has facilitated understanding of the initial mechanisms of UV-B perception and signaling in plants. The UV RESISTANCE LOCUS8 (UVR8) protein has been established as a UV-B photoreceptor that mediates photomorphogenic responses to UV-B (Rizzini et al., 2011; Christie et al., 2012; Wu et al., 2012). The initial perception of UV-B by UVR8 is followed by interaction with CONSTITUTIVELY PHOTOMORPHOGENIC1 (COP1; Favory et al., 2009; Cloix et al., 2012). Together, UVR8 and COP1 are required to stimulate transcription of the gene encoding the ELONGATED HYPOCOTYL5 (HY5) transcription factor (TF; Brown et al., 2005; Oravecz et al., 2006). HY5, sometimes acting redundantly with the closely related HY5 HOMOLOG (HYH), mediates many gene expression responses regulated by UVR8 (Brown et al., 2005; Brown and Jenkins, 2008; Favory et al., 2009). UVR8 regulates morphogenic responses to UV-B, such as inhibition of hypocotyl elongation (Favory et al., 2009), and is necessary for the UV-B-stimulated increase in stomatal index (Wargent et al., 2009).
Plants regulate stomatal movements to control CO2 influx and water loss, which critically affect whole plant growth and physiology. Stomatal movements are regulated by a variety of factors that stimulate opening and closure (Kim et al., 2010; Araújo et al., 2011; Chen et al., 2012). It has been reported that stomatal movement may be influenced by UV-B, and the response observed (opening or closure) is dependent on the fluence rate (Nogues et al., 1999; Jansen and Noort, 2000; Eisinger et al., 2003; He et al., 2005, 2013). Jansen and Noort (2000) found that UV-B may induce closure or opening in Vicia faba stomata, depending on the dose. Similarly, in Arabidopsis (Arabidopsis thaliana), very low fluence rates of UV-B stimulate stomatal opening (Eisinger et al., 2003), whereas a higher irradiance induces stomatal closure in the wild-type ecotype Wassilewskija (Ws; He et al., 2013). However, it is unknown whether UVR8 participates in the regulation of stomatal aperture by UV-B.
He et al. (2005, 2011a, 2011b) have provided convincing evidence for the participation of hydrogen peroxide (H2O2) and nitric oxide (NO) as signaling molecules in UV-B-induced stomatal closure. NO is a ubiquitous bioactive molecule, produced in plants by enzymatic and nonenzymatic routes (for review, see Wilson et al., 2008). NO orchestrates a wide range of processes in plants. For example, NO acts as a signal in disease resistance (Delledonne, 2005), regulates stomatal closure (García-Mata and Lamattina, 2001), and stimulates germination (Beligni and Lamattina, 2000). NO has also been proposed to be a broad-spectrum antistress molecule (Lamattina et al., 2003; Correa-Aragunde et al., 2007), because it confers protection against oxidative stress produced by diquat, UV-B, drought, and salt (Beligni and Lamattina, 2000; García-Mata and Lamattina, 2001; Zhao et al., 2004; Shi et al., 2005; Tossi et al., 2009, 2011, 2012). It was reported that NO increases as a consequence of UV-B irradiation in bacteria, animals, and plants (Seo et al., 2002; An et al., 2005; Qu et al., 2006). Our previous results show that NO helps to protect plants against damage by UV-B in two ways: scavenging ROS and increasing flavonoid concentration by promoting the expression of HY5 and MYB12, TF genes regulated by UVR8 (Tossi et al., 2011). However, to date, no links between UVR8-induced signaling and NO production have been reported.
In this work, we investigated the involvement of the UVR8 pathway in UV-B-induced stomatal movements in Arabidopsis. Using mutants of the UVR8, COP1, HY5, and HYH genes, we conclude that UVR8 signaling elicits both H2O2 and NO generation in response to UV-B irradiation. The NO thus produced causes the stomatal closure, limiting water loss and preventing cell injury.
RESULTS
UV-B Induces Stomatal Closure in Arabidopsis
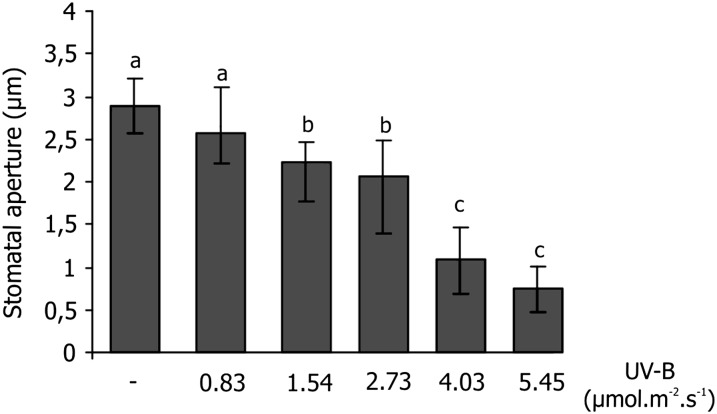
To determine the effect of UV-B on stomatal movement in Arabidopsis leaves of wild-type ecotype Landsberg erecta (Ler), epidermal strips were obtained and placed on MES-KCl buffer under white light for 3 h to induce stomatal opening. After performing time courses supplementing the white light with increasing UV-B fluence rates (Supplemental Fig. S1), we chose 3 h of irradiation as an optimal time to evaluate responses. The tested UV-B fluence rates were 0.83, 1.54, 2.73, 4.03, and 5.46 μmol m–2 s–1, which represent a broad ambient range of UV-B fluence rates reaching the Earth (Piazena, 1996; Zacher et al., 2007; Norsang et al., 2009); for comparison, the UV-B fluence rate in Glasgow, Scotland on a clear midsummer’s day measured using the same sensor was 4.5 μmol m–2 s–1.
Three hours after the start of UV-B irradiation, stomatal aperture was analyzed. As Figure 1 shows, the lowest UV-B fluence rate (0.83 μmol m–2 s–1) was not able to induce significant stomatal closure. Stomata were slightly closed at 1.54 μmol m–2 s–1, reaching the maximum closure at 5.46 μmol m–2 s–1 UV-B. Although fluence rates within the midambient range stimulate significant stomatal closure, we selected the 5.46 μmol m–2 s–1 treatment for subsequent experiments, because this fluence rate induced the strongest response.
Figure 1.
UV-B induces stomatal closure in leaves of wild-type Arabidopsis Ler. Abaxial epidermal strips of Ler Arabidopsis were kept in MES-KCl buffer in white light for 3 h to induce stomatal opening. Epidermal strips with open stomata were incubated in MES buffer under white light supplemented with the indicated fluence rates of UV-B for 3 h. After treatment, stomatal apertures were measured. Values are the means ± se of six independent experiments (n = 80). Means with different letters denote statistical differences at P < 0.05, according to one-way ANOVA.
UV-B Induced Stomatal Closure Is Sustained by NO and H2O2
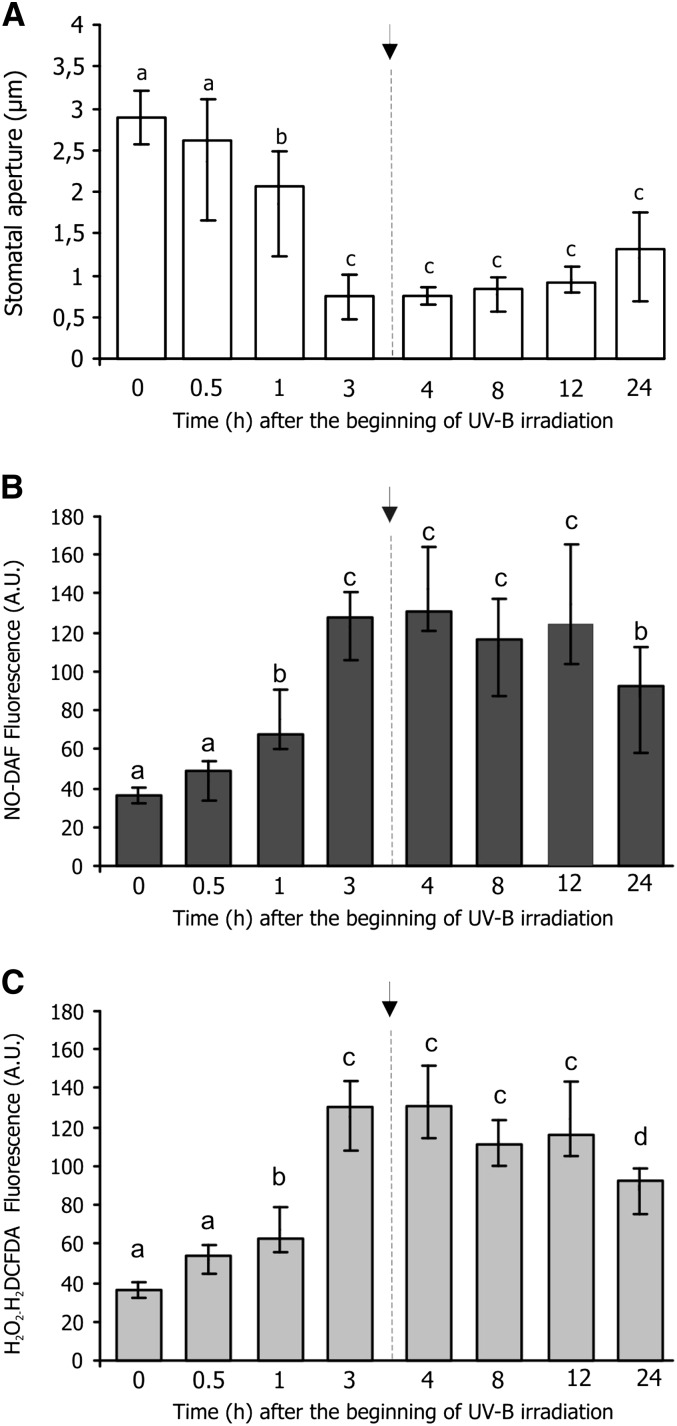
In experiments where data were collected at the end of the UV-B irradiation, it was shown that NO and H2O2 generation is required for UV-B-induced stomatal closure (He et al., 2005, 2011a, 2011b). In an alternative approach, we were interested to study the stomatal response both during and after the end of the irradiation. With this goal, epidermal strips were irradiated for 3 h with 5.46 μmol m–2 s–1 UV-B, and parameters were recorded during exposure and up to 24 h after the start of exposure. NO and H2O2 levels were measured using the NO-specific fluorescent probe 4,5-diaminofluorescein diacetate (DAF-FM-DA) and H2O2 fluorescent probe 2′,7′-dichlorofluorescin diacetate (H2DCFDA).
Figure 2A shows that the stomatal closure was maximal 3 h after the beginning of irradiation (this section is identical to Supplemental Fig. S1C but is replicated to provide a better understanding of the relationship between stomatal closure, NO, and H2O2 production). Figure 2, B and C, show that NO and H2O2 began to increase 1 h after the beginning of the UV-B radiation, reaching the maximum at 3 h. Maximal stomatal closure was coincident with the NO and H2O2 highest concentration. Thus, the maximum effects of UV-B were achieved at 3 h. Interestingly, NO and H2O2 remained strikingly high until 12 h and slightly decreased at 24 h while the stomata remained closed, suggesting that UV-B-induced stomatal closure is sustained by NO and H2O2 almost 24 h after the start of irradiation. Because viability in strips was reduced to 40% at this time, measurements were performed only in the viable stomata.
Figure 2.
Effects of UV-B on stomatal aperture and NO and H2O2 levels in Arabidopsis stomata. Epidermal strips were kept in MES-KCl buffer in white light for 3 h to induce stomatal opening. Then, light was supplemented with UV-B radiation (5.46 μmol m–2 s–1) for 3 h (arrow). A, Stomatal aperture was measured as indicated in “Materials and Methods.” B and C, Samples were preloaded with 10 μm of NO-specific fluorescent probe DAF-FM-DA (B) or 10 μm H2O2 fluorescent probe H2DCFDA (C). Fluorescence pixel intensities were measured at the indicated times. Values are the means ± se of six independent experiments (n = 80). Means with different letters are significantly different between treatments (Student’s t test, P > 0.05).
The NO scavenger 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide has been used to demonstrate that NO participates in the UV-B-induced stomatal closure (He et al., 2005). However, the use of this compound is controversial because it could be toxic depending on its concentration or exposure time. Moreover, some interference was reported between 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide and the NO probe DAF-FM-DA (Arita et al., 2007). Thus, we prefer to use an Arabidopsis transgenic line to evaluate the effect of endogenous NO on the UV-B-induced stomatal closure and the relationship between NO and H2O2. This line expresses a bacterial NO dioxygenase (NOD) under the control of a dexamethasone (DEX)-inducible promoter (Zeier et al., 2004). NOD expression in Arabidopsis results in decreased endogenous NO levels (Zeier et al., 2004).
Figure 3A shows that stomata were closed after UV-B irradiation. However, in DEX-treated plants, stomata remained open. Figure 3, B and C, show that both NO and H2O2 increased in UV-B-irradiated stomata. Nevertheless, when leaves were treated with DEX before irradiation, NO did not increase, confirming the NO degradation by NOD. Interestingly, the H2O2 levels were not affected by DEX treatment after UV-B exposure, indicating that the H2O2 rise induced by UV-B is not dependent on NO. Thus, stomata were closed when NO is present but irrespective of the H2O2 concentration. Together, these results indicate that H2O2 and NO are increased by UV-B, but only NO is necessary to induce stomatal closure.
Figure 3.
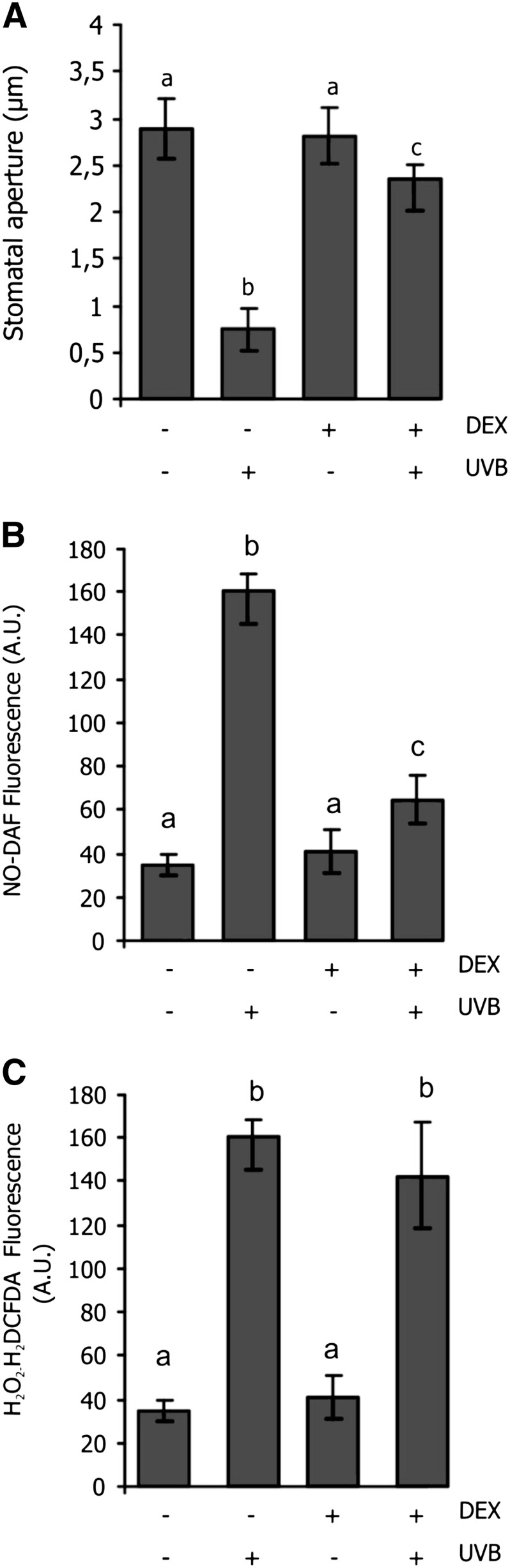
Effects of NO on UV-B-regulated stomatal aperture. Four-week-old Arabidopsis plants expressing inducible NOD were treated with 3 µm DEX to induce expression (+DEX) or 0.01% (v/v) Tween 20 as control (–DEX control). One day later, leaves were used to obtain abaxial epidermal strips. Strips were incubated in MES-KCl buffer in white light for 3 h to induce stomatal opening. Then, light was supplemented with UV-B (5.46 μmol m–2 s–1) for 3 h (+UV-B) or not in controls (–UV-B). A, Stomatal apertures were measured in epidermal strips. B, Stomatal NO was assayed as DAF-FM-DA fluorescence. C, Stomatal H2O2 was assayed as H2DCFDA fluorescence. Values are the means ± se of six independent experiments (n = 80). Means with different letters denote statistical differences at P < 0.05, according to one-way ANOVA.
The UV-B Photoreceptor UVR8 Regulates UV-B-Induced Stomatal Closure
It has been demonstrated recently that Arabidopsis UVR8 is a UV-B photoreceptor, regulating the expression of genes involved in UV-B responses (Brown et al., 2005; Favory et al., 2009; Rizzini et al., 2011; Christie et al., 2012; Wu et al., 2012). Some photomorphogenic responses driven by UVR8 influence plant morphology (Favory et al., 2009; Wargent et al., 2009). Furthermore, UVR8 regulates the stomatal index in response to UV-B (Wargent et al., 2009).
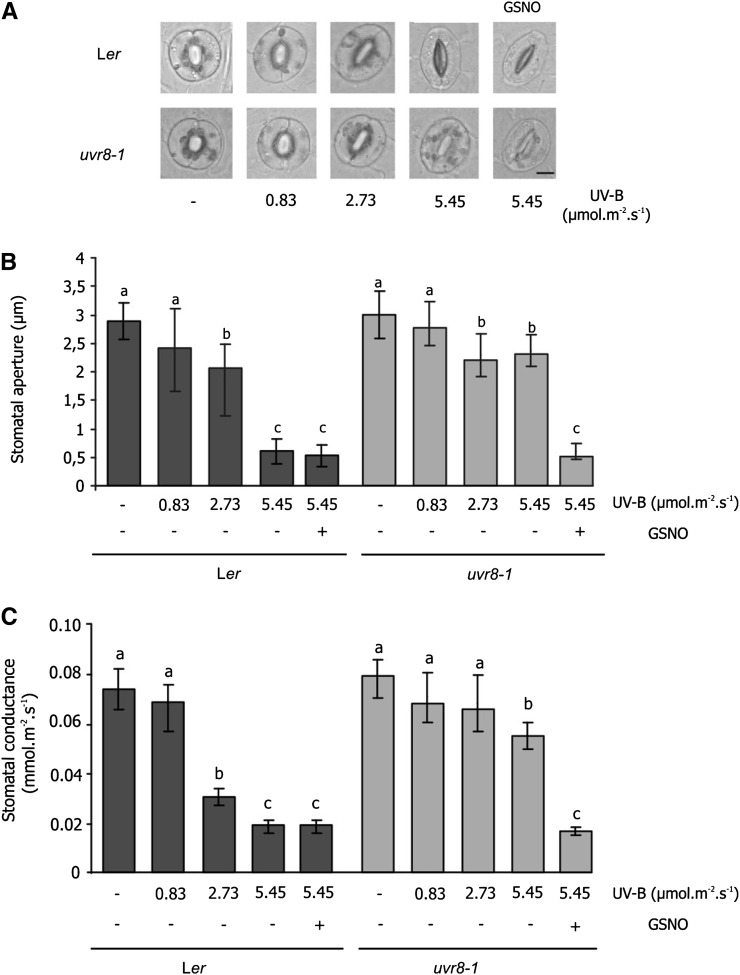
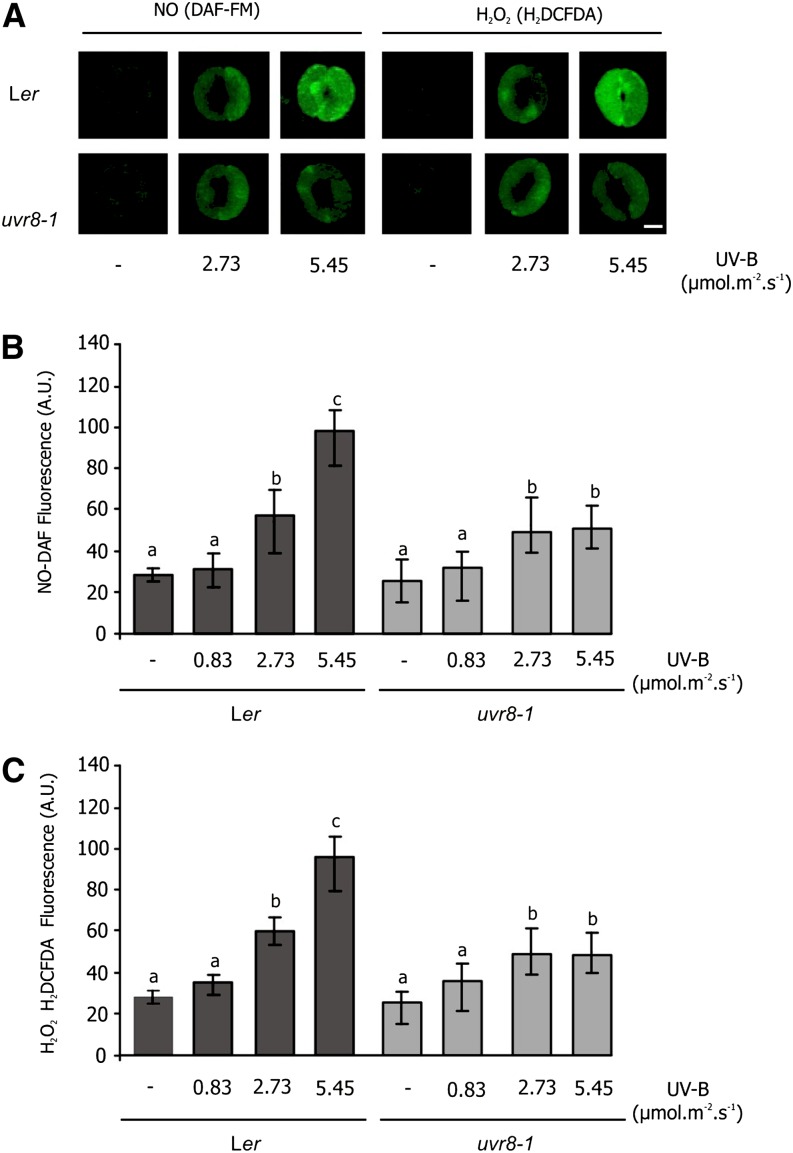
To determine whether UVR8 could be involved in regulation of stomatal movement, epidermal strips of the uvr8-1 (UVR8 null mutant; Kliebenstein et al., 2002) and wild-type (Ler ecotype) lines were exposed to low (0.83 μmol m–2 s–1), medium (2.73 μmol m–2 s–1), and high (5.46 μmol m–2 s–1) UV-B fluence rates for 3 h. Stomatal aperture and NO and H2O2 levels were measured 3 h after the end of the irradiation.
Figure 4A shows that stomata of both genotypes were similar in nonirradiated epidermal strips, where they were open. Figure 4, A and B, show that when wild-type stomata were irradiated with a low UV-B fluence rate (0.83 μmol m–2 s–1), stomata remained open, similar to the nonirradiated control. However, when the strips were exposed to medium (2.73 μmol m–2 s–1) and high (5.46 μmol m–2 s–1) UV-B fluence rates, stomatal apertures were reduced. By contrast, in uvr8-1 epidermal strips, stomata remained open even at the highest UV-B fluence rate. However, if a 100-µm concentration of the NO donor S-nitrosoglutathione (GSNO) was applied, uvr8-1 irradiated stomata closed to the same extent as the wild type. GSNO was able to induce stomatal closure at several concentrations both in wild-type and uvr8-1 epidermal strips (Supplemental Fig. S2).
Figure 4.
Effect of UV-B on stomatal closure in Ler and uvr8-1 Arabidopsis plants. Abaxial epidermal strips of Ler and uvr8-1 plants were incubated in opening buffer and white light for 3 h and then exposed to different fluence rates of UV-B for 3 h. Where indicated, 100 µm of the NO donor GSNO was applied after irradiation. A, Images of guard cells exposed to different fluence rates of UV-B radiation (0.83, 2.73, and 5.46 μmol m–2 s–1). Bar = 5 μm. B, Stomatal apertures were measured in epidermal strips. Data are the average ± se of 60 to 90 stomata measured in three independent experiments. The letters denote significant differences from control (P > 0.05). C, Stomatal conductance in Ler and uvr8-1 leaves exposed to increasing UV-B fluence rates. Four-week-old Ler and uvr8-1 plants were exposed for 3 h to the UV-B fluence rates indicated. Stomatal conductance was determined by the leaf gas exchange system QUBIT. The measurement was performed at the end of the exposure. Conductance values are expressed as millimoles of water per square meter per second and represent the mean ± se of at least 10 independent experiments. The letters indicate significant differences from control of each line (Student’s t test, P > 0.05).
Epidermal strips are currently utilized to study the signals regulating stomatal closure. While this methodology evaluates events occurring in a single cell type, some responses may depend on the interaction of various cell types. The porometer is used to analyze stomatal conductance in planta and that may be a more physiological approach than using epidermal strips. A greater conductance denotes increased stomatal opening, and conversely, a decrease in conductance indicates stomatal closure. To examine the role of UVR8 in stomatal closure induced by UV-B, we analyzed the stomatal conductance in uvr8-1 and Ler leaves in response to different UV-B fluence rates. With this aim, 4-week-old plants were exposed for 3 h to increasing UV-B fluence rates, and immediately, stomatal conductance was measured.
Figure 4C shows that in basal conditions (no UV-B), Ler plants have the same conductance as uvr8-1 mutants. The exposure of Arabidopsis to increasing UV-B fluence rates induces a gradual reduction of the conductance, down to 40% in Ler plants for the highest UV-B exposure. However, in uvr8-1 plants, the conductance stays at almost 85% at the highest UV-B fluence rate. GSNO was again able to reduce the conductance to almost 25% both in wild-type and uvr8-1 irradiated plants. These results validate those obtained in strips. Hence, we decided to continue working with epidermal strips, which have proven to be a more practical system.
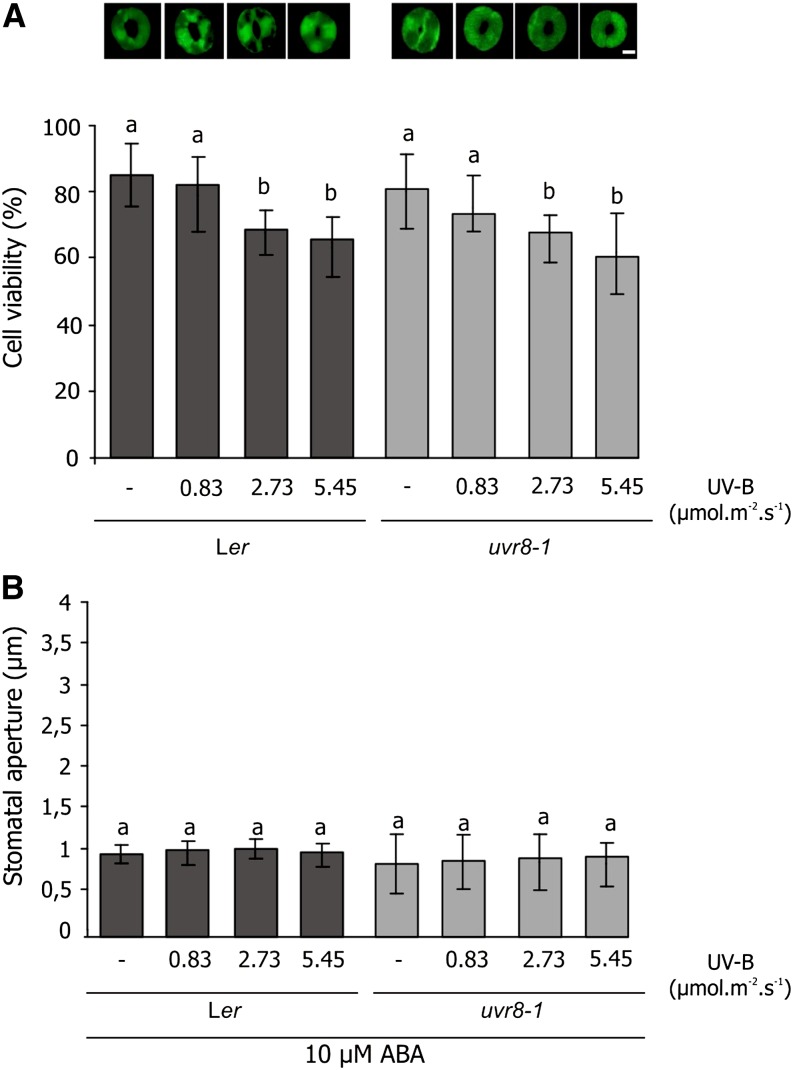
An explanation for the results obtained for stomatal closure in UV-B-exposed uvr8-1 mutant is that UVR8 is required for NO production. An alternative explanation is that stomata were damaged (for example, by UV-induced ROS), and NO production promoted recovery from this stress. To test this latter possibility, we examined the viability of irradiated guard cells as well as the ability of abscisic acid (ABA) to induce closure, a typical stomatal response (Bright et al., 2006).
Ler and uvr8-1 epidermal strips were exposed to UV-B, and viability was measured using the fluorescent probe fluorescein diacetate. Figure 5A shows that both Ler and uvr8-1 stomata had 80% viability in white light, and viability decreased to 70% after treatment with UV-B. To assess whether the stomata would be able to close in response to ABA, irradiated strips were treated with 10 µm ABA, and stomatal aperture was measured. The stomatal aperture of both Ler and uvr8-1 was reduced in response to 10 µm ABA after UV-B exposure (Fig. 5B). In addition, the dose-response relationship for stomatal closure following GSNO treatment (Supplemental Fig. S2) shows the same behavior for both the wild type and uvr8-1. These results suggest that the differential response in stomatal closure induced by UV-B in Ler and uvr8-1 was not due to cell damage or cell death. Together, these results indicate that UVR8 has an important role in the regulation of stomatal movements in response to UV-B, which could be linked to NO production.
Figure 5.
Cell viability and response to ABA in epidermal strips of Ler and uvr8-1 plants exposed to UV-B. Epidermal strips of Ler and uvr8-1 plants were incubated for 3 h in opening buffer and white light and then exposed to different fluence rates of UV-B for 3 h. A, Cell viability images and fluorescence intensity quantification. At the end of UV-B, exposure strips were incubated in 5 µm fluorescein diacetate for 5 min. Fluorescence was detected by microscopy to determine the percentage viability (fluorescent stomata/total number of stomata × 100). Bar = 5 μm. B, Stomatal closure induction by ABA, after UV-B exposure strips were incubated in 10 µm ABA for 30 min. The stomatal aperture is shown as the mean ± se. The results show the average of at least three independent experiments (n > 90). Means with different letters denote statistical differences from control (–UV-B) at P < 0.05, according to one-way ANOVA. [See online article for color version of this figure.]
The Regulatory Effect of UVR8 Is Mediated by H2O2 and NO
To further investigate the involvement of UVR8 in the UV-B-induced stomatal closure response, stomatal aperture, H2O2, and NO were measured in irradiated wild-type and uvr8-1 plants.
Figure 4B shows that increasing UV-B fluence rates induced stomatal closure in Ler plants but not in uvr8-1 mutants. NO and H2O2 were measured in the same experiments. Figure 6, A to C, shows that both NO and H2O2 increasingly accumulated in the guard cells of irradiated Ler epidermal strips at medium and high UV-B fluence rates. By contrast, the slight reduction in uvr8-1 stomatal aperture was coincident with a minor increase of NO and H2O2 even at the highest UV-B fluence rate.
Figure 6.
Effect of increasing UV-B on NO and H2O2 levels in stomata of Ler and uvr8-1 plants. Epidermal strips of Ler and uvr8-1 plants were incubated in opening buffer and white light for 3 h and then exposed to different fluence rates of UV-B for 3 h. Strips were then incubated with 10 µm of the fluorescent probe DAF-FM-DA (for NO detection) or 10 µm of the fluorescent probe H2DCFDA (for H2O2 detection) for 10 min. A, Images of stomatal closure and NO and H2O2 fluorescence intensity. B and C, Quantification of pixel intensities for NO (B) and H2O2 (C) levels. Bar = 5 μm. Data are the average ± se of 60 to 90 stomata measured in three independent experiments. The letters denote significant differences from control at P < 0.05, according to one-way ANOVA. [See online article for color version of this figure.]
These results indicate that the reduction in stomatal aperture in Ler stomata is correlated with augmented levels of NO and H2O2 and that null UVR8 mutants have impaired production of NO and H2O2 and, in consequence, diminished capacity to regulate stomatal movements in response to UV-B. We conclude that UVR8 mediates the regulation of UV-B-induced stomatal closure through a pathway involving NO and H2O2.
COP1, HY5, and HYH, Key Elements in UVR8 Signaling Pathway, Are Involved in the Regulation of UV-B-Induced Stomatal Closure
The perception of UV-B by UVR8, followed by interaction with COP1 and regulation of the TFs HY5 and HYH, has emerged as a primary mechanism of the UV-B photomorphogenic response that is crucial for UV-B acclimation and tolerance (Rizzini et al., 2011; Christie et al., 2012; Heijde and Ulm, 2012; Jansen and Bornman, 2012; Wu et al., 2012).
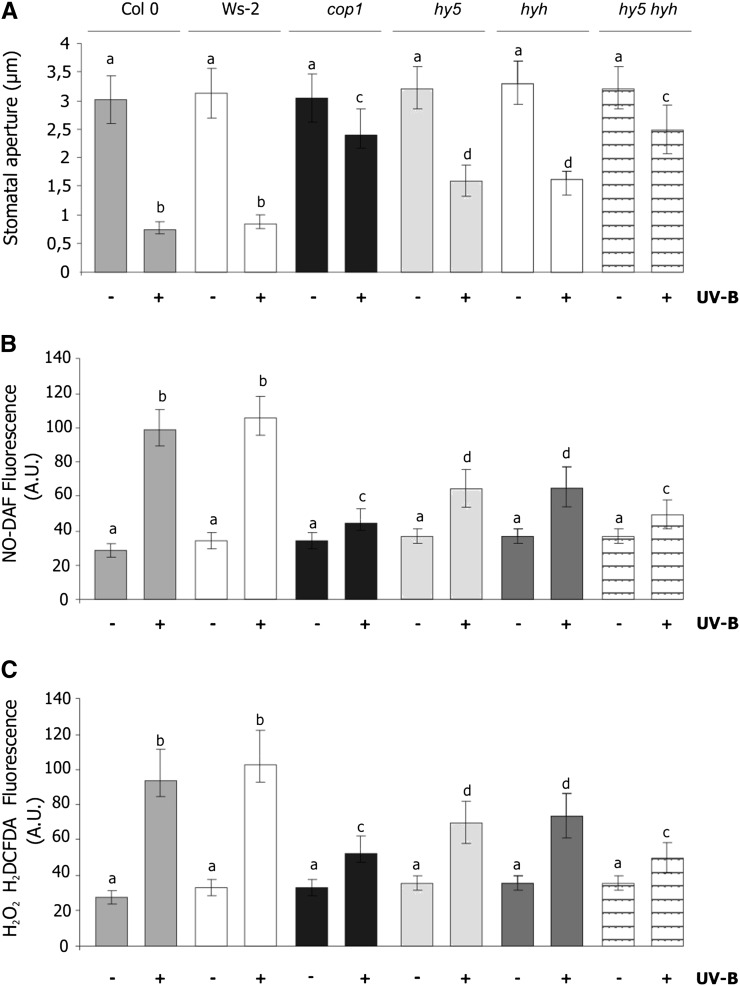
To evaluate if COP1, HY5, and HYH are also involved in stomatal closure induced by UV-B, epidermal strips of Arabidopsis mutants cop1-4, hy5-ks50 (Oyama et al., 1997), hyh, and the double mutant hy5-ks50,hyh were exposed to UV-B for 3 h. Stomatal closure and the production of NO and H2O2 were then determined. The background lines of these mutants Ws (for hy5-ks50 and hyh) and ecotype Columbia (Col-0; for cop1-4) were used as controls.
Figure 7A shows that UV-B induced a maximal reduction in stomatal aperture in strips of Ws and Col-0. By contrast, irradiated cop1-4 stomata were slightly closed. The single hy5-ks50 and hyh mutants had an intermediate response to UV-B, but in the hy5-ks50,hyh double mutant, the stomatal response was similar to that of cop1.
Figure 7.
Effect of UV-B on stomatal closure and NO and H2O2 levels in mutants of UVR8 signaling pathway. Abaxial epidermal strips of Col-0, Ws, cop1-4, hy5-ks50, hyh, and hy5-ks50,hyh were incubated in opening buffer and white light for 3 h and then exposed to 5.46 μmol m–2 s–1 UV-B for 3 h (+UV-B) or not in controls (–UV-B). A, Stomatal apertures measured after UV-B exposure. B and C, After UV-B treatment strips were incubated for 10 min with 10 µm of the fluorescent probe DAF-FM-DA for NO detection (B) or 10 µm of the fluorescent probe H2DCFDA for H2O2 detection (C). Data are the average ± se of 60 to 90 stomata measured in three independent experiments. The letters denote significant differences from control. Means with different letters are significantly different between treatments (Student’s t test, P > 0.05).
Figure 7, B and C, show that in Ws, the production of NO and H2O2 were greatly increased in strips exposed to UV-B. The cop1-4, hy5-ks50, hyh, and hy5-ks50,hyh mutants showed only small differences in NO and H2O2 production in response to UV-B. Additionally, Supplemental Fig. S3 shows that nonirradiated stomata of the wild type and the mutant lines showed increased stomatal closure and NO concentration when they were treated with 100 µm GSNO. These observations indicate that stomata of all the mutants have the same capacity to respond to GSNO as the wild type under non-UV-B conditions. Based on these results, we conclude that COP1 and HY5/HYH act with UVR8 in the UV-B signaling pathway responsible for stomatal closure and that this pathway involves NO and H2O2.
DISCUSSION
In this report, we show that the UVR8 photoreceptor is required to regulate stomatal movements in response to UV-B exposure. This finding extends the number of UV-B responses known to be mediated by UVR8. Moreover, this study has characterized key regulatory roles for the UVR8 signaling components COPI, HY5, and HYH in the stomatal response to UV-B. Furthermore, we show that the UVR8 pathway controls stomatal movement through H2O2 and NO production.
Stomatal aperture was greatly influenced by the UV-B fluence rate. The lowest fluence rate employed did not induce closure, but significant closure was observed at 1.54 μmol m–2 s–1 after 3 h of irradiation. Following 3 h exposure to the highest fluence rate (5.46 μmol m–2 s–1), closure was maintained for almost 24 h. The UV-B treatments used in this work correspond to a range of ambient UV-B fluence rates. The intensity of UV-B radiation reaching the Earth’s surface, however, varies widely around the globe, and factors such as altitude and latitude account for this variation. For example, in Lhasa (Tibet), a high-altitude area of the world, the maximum value for the UV-B irradiance was 3.96 W m–2 from 1999 to 2003 (approximately 9.4 μmol m–2 s–1; Norsanget al., 2009), and in King George Island (sea level, Antarctica), UV-B intensity was 2.4 W m–2 from 2004 to 2005 (approximately 5.7 μmol m–2 s–1; Zacher et al., 2007). By contrast, at low altitude (e.g. in Glasgow, Scotland), in winter under cloud cover, the UV-B radiation could be less than 0.1 μmol m–2 s–1. The response to UV-B is related additionally to the level of photosynthetically active radiation (PAR). Jansen and Noort (2000) reported that a UV-B irradiance similar to that used in our work (approximately 5.2 μmol m–2 s–1 for 3 h) stimulated stomatal closure when applied with low PAR but promoted opening when given at high PAR. Neither of these effects were readily reversed 24 h after the end of irradiation, consistent with our finding that stomatal closure is maintained by the high levels of NO remaining almost 24 h after UV-B exposure. It has been demonstrated that low levels of UV-B induce NO and ROS in some tissues and plant species (Zhang et al., 2003; An et al., 2005; He et al., 2005, 2013; Qu et al., 2006). However, in our work, the low UV-B radiation used did not induce NO and H2O2 production, explaining the lack of stomata closure.
It was reported that stomatal movements are regulated by NO (García-Mata and Lamattina, 2001) and that H2O2 induces NO generation in guard cells (He et al., 2005; Bright et al., 2006). Our results, obtained with the inducible NOD transgenic line, show that UV-B-irradiated stomata stay open at low levels of NO, even though H2O2 remained high. This suggests that NO regulates stomatal movements irrespective of the presence of H2O2. Nevertheless, we cannot rule out the influence of H2O2, because in the NOD plants, H2O2 is already present with NO prior to the induction of NOD, which initiates NO degradation. Thus, NO may need the H2O2 burst to be generated and even to induce the stomatal closure. Once closed, NO may be sufficient to maintain stomatal closure. These results agree in part with those of He et al. (2013). These authors presented genetic and pharmacological evidence that Arabidopsis with impaired H2O2 production neither produce NO nor regulate stomatal movements in response to UV-B. The response was rescued by the NO donor sodium nitroprusside (SNP). Moreover, mutants in NO production lacked UV-B-induced stomatal closure that could not be compensated by H2O2. Together, these results confirm the essential role of H2O2 and NO production in UV-B-induced stomatal closure.
UVR8 signaling mediates several photomorphogenic responses to UV-B, including the suppression of hypocotyl elongation, stomatal differentiation, and the synthesis of UV-protective flavonoids and anthocyanins. NO is also reported to inhibit hypocotyl elongation (Beligni and Lamattina, 2000; Lozano-Juste and León, 2011) and to induce flavonoid and anthocyanin accumulation (Tossi et al., 2011). By contrast, mutants of UVR8 and the signaling components COP1 and HY5 are impaired in the inhibition of hypocotyl elongation and induction of flavonoids and anthocyanins following UV-B irradiation (Oravecz et al., 2006; Favory et al., 2009). These mutant phenotypes are similar to NO depletion effects. In this paper, we show the first evidence of a link between the UVR8 pathway and NO-regulated processes, because Arabidopsis mutants defective in UVR8 signaling components show impaired NO production and abrogated stomatal closure. It will be very interesting to determine if the regulation of other processes mediated by UVR8 involves NO.
The network of UV-B-regulated signaling pathways is a subject of growing interest. The first evidence about the role of plant NADPH oxidase (pNOX) and NO in responses to UV-B comes from the pharmacological experiments of AH-Mackerness et al. (2001) in Arabidopsis. In that work, ROS and NO production were reported as independent events. However, further work showed that pNOX-produced H2O2 is related to NO induction (He et al., 2005). We have reported that UV-B perception by plants triggers an increase in ABA concentration, which activates pNOX and H2O2 generation. Then, through a NO synthase-like-dependent mechanism, NO production increases to maintain cell homeostasis and attenuate UV-B-induced cell damage (Tossi et al., 2009). Moreover, we suggested that ABA is a component of the UV-B response both in plant and animal cells (Tossi et al., 2012).
Regarding guard cells, the impaired NO production by ABA in nia1,nia2 nitrate reductase mutants (Bright et al., 2006) and in atrbohD/F NADPH oxidase double mutant (Desikan et al., 2006) is an indication of the crucial roles of nitrate reductase and pNOX in stomatal movements. Furthermore, He et al. (2013) provided convincing evidence for a signaling pathway leading to UV-B-induced stomatal closure in Arabidopsis, which involves G protein α-subunit (PA1)-dependent activation of H2O2 production by pNOX and subsequent Nia1-dependent NO accumulation.
Autonomous guard cell ABA synthesis in response to a reduction in relative humidity was demonstrated by Bauer et al. (2013). However, the UVR8 pathway is evidently important in the stomata response to UV-B, because, although the irradiated uvr8 mutant guard cells still induce some NO production, probably through the ABA pathway, it is insufficient to promote stomatal closure.
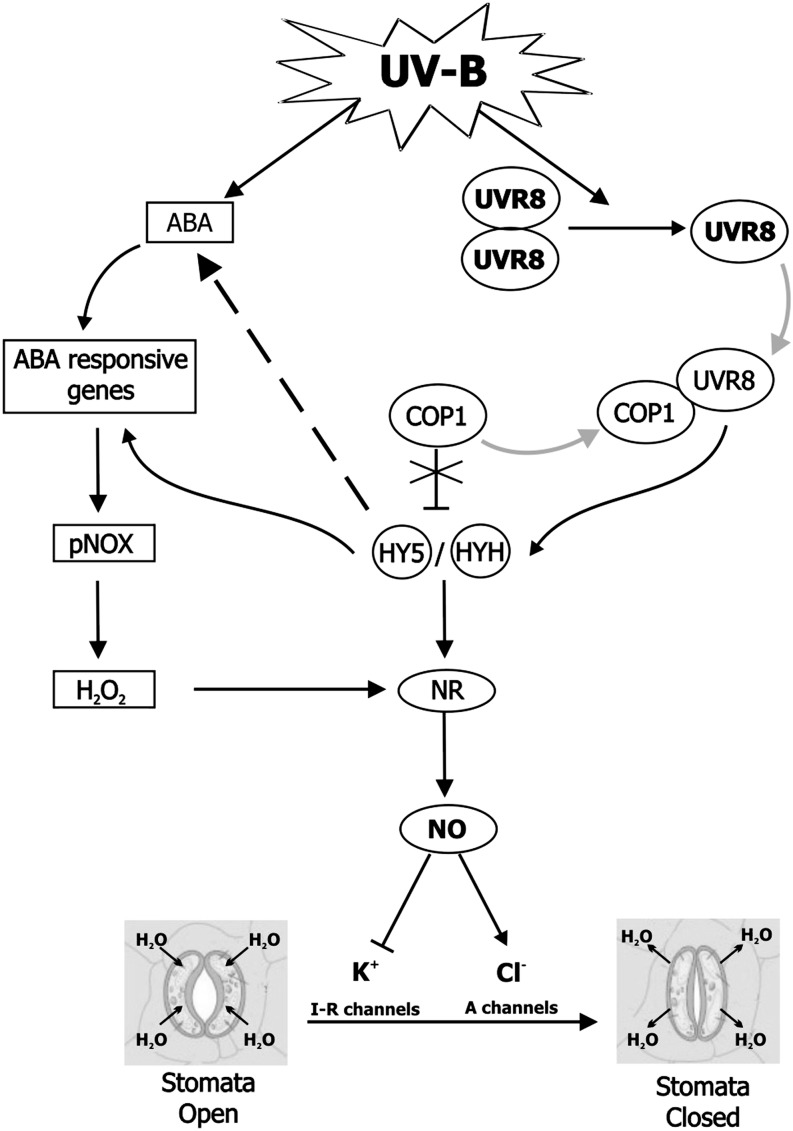
Here, we propose a model for UVR8 signaling in guard cells (Fig. 8). This model suggests that UV-B perception by the UVR8 dimer is followed by interaction of UVR8 monomer with COP1 in the nucleus, which stimulates expression of HY5/HYH TFs. In parallel, UV-B raises ABA, which could activate pNOX increasing H2O2 generation. Some ABA-responsive genes are also regulated by HY5 (Chen et al., 2008). Moreover, in chromatin immunoprecipitation and microarray experiments, it was demonstrated that some genes responsible for ABA biosynthesis are induced by HY5 (Lee et al., 2007). Stomatal NO production was mainly related to ABA-induced H2O2 produced by pNOX. However, it was reported by Jonassen et al. (2008, 2009) that HY5/HYH may also regulate the expression and activity of nitrate reductase. Finally, once produced by the UVR8 and ABA pathways, NO deactivates inward-rectifying K+ channels and activates anion channels, contributing to the loss of the turgor pressure that causes stomatal closure (García-Mata et al., 2003). Further work is required to fully understand the cross talk between ABA- and UVR8-regulated signaling pathways in stomatal movement triggered by UV-B.
Figure 8.
Simplified scheme of the UVR8 pathway in UV-B-induced stomatal closure. See text for details. Black arrows indicate induction. Black bars indicate negative regulation. Gray arrows indicate protein interaction and rearrangement and dashed line indicates hypothetical cell response. I-R, Inward-rectifying K+ channel; A, anion channel.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
The following Arabidopsis (Arabidopsis thaliana) lines were used for this study: Ler, Col-0, Ws, the COP1 mutant cop1-4 (McNellis et al., 1994), null mutants of UVR8 (uvr8-1; Kliebenstein et al., 2002), HY5 (hy5-ks50; Oyama et al., 1997), and HYH (hyh; Holm et al., 2002), the double mutant hy5-ks50,hyh (Holm et al., 2002), and Arabidopsis plants expressing the DEX-inducible NOD (Zeier et al., 2004; seeds were kindly provided by Jurgen Zeier, University of Fribourg).
Plants were grown on a mixture of soil:vermiculite (3:1, v/v) at 25°C under a 14-h-light/10-h-dark photoperiod and a relative humidity of 70%. PAR during the day period was 200 µmol m–2 s–1. Four-week-old healthy plants were used for experiments. For the NOD induction, NOD plants were sprayed with 3 mm DEX in 0.01% (v/v) Tween 20. Control samples were obtained by spraying NOD plants with 0.01% (v/v) Tween 20.
UV-B Treatments
UV-B irradiation was carried out in controlled-environment rooms at 20°C. Plants were exposed to white light supplemented with UV-B. White light was provided by Osram warm-white fluorescent tubes. Fluence rates of white light (PAR, 400–700 nm) were measured using a Skye Spectrosense-1 m equipped with a Quantum sensor (Skye Instruments).
UV-B was obtained from UVB-313 UV fluorescent tubes (Q-Panel) covered with cellulose acetate (West Design Products) to filter out UV-C radiation (<280 nm) and short-wave UV-B (<290 nm). Fluence rates of UV-B (280–315 nm) were measured either by a Skye Spectrosense-1 m equipped with a SKU 430 sensor or using a Macam spectroradiometer (model SR9910; Macam Photometrics).
Stomatal Aperture
The stomatal experiments were performed with epidermis (epidermal strips) peeled from the abaxial surface of fully expanded leaves of 4-week-old plants. The strips were preincubated at 25°C in the light for 3 h in opening buffer (10 mm MES and 10 mm KCl, pH 6.1) under white light to promote stomatal opening before treatments (Distéfano et al., 2012). Then, strips were treated with different UV-B fluence rates for different durations, as indicated in each experiment. When indicated, strips were treated with different concentrations of the NO donor GSNO for 30 min or incubated in 10 µm ABA for 30 min after UV-B exposure. All chemicals were obtained from Sigma-Aldrich.
The pore size of stomata was digitally calculated using the image analysis software ImageJ 1.3 (National Institutes of Health). The images were obtained with a confocal Eclipse Ti microscope (Nikon). Aperture values are the mean of 80 to 100 stomata measured from at least three independent experiments. Values are expressed as mean ± se. Differences between means were statistically analyzed using Sigma Plot 9 software.
NO and H2O2 Quantification
NO and H2O2 levels in stomata were detected with specific fluorescent probes DAF-FM-DA and H2DCFDA, respectively. Epidermal strips of 4-week-old Arabidopsis plants were incubated in opening buffer for 3 h in the light and then exposed to different UV-B fluence rates for different times. The strips were incubated for 30 min with 10 µm DAF-FM-DA or 10 µm H2DCFDA and washed three times with opening buffer to remove residual probe. Samples were examined by confocal microscopy (excitation, 490 nm; emission, 525 nm) in an Eclipse Ti microscope (Nikon). For quantification of fluorescence, the whole stomata areas of the micrographs were analyzed using the ImageJ 1.3 software. Fluorescence was expressed as arbitrary units.
Measurement of Stomatal Conductance
Stomatal conductance was measured in 4-week-old Ler and uvr8-1 Arabidopsis plants after 3 h of UV-B treatment, using a CO2 analyzer system (QUBIT, CO650 CO2 Plant Analysis) according to the manufacturer’s instructions.
Cell Viability
Cell viability was measured with fluorescein diacetate, which produces green fluorescence in live cells. Epidermal strips were incubated in opening buffer in the light for 3 h and then treated with 5 µm of fluorescein diacetate for 5 min. The fluorescence was observed with a Nikon Eclipse E200 microscope, with excitation at 488 nm and emission at 505 to 530 nm. The images were digitized with a Nikon Coolpix 990 camera. Fluorescence was quantified as indicated above.
Statistical Treatment of Data
Values are expressed as means with their se, being the result of at least three independent experiments. Sigma Plot 9 and Sigma Plot 11 software were used for statistical data analysis.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Effect of increasing UV-B fluence rate on stomatal closure over a time course in Arabidopsis plants.
Supplemental Figure S2. Effect of increasing NO concentrations on stomatal aperture.
Supplemental Figure S3. Effect of GSNO on stomatal closure and NO and H2O2 levels in mutants of UVR8 signaling pathway.
Supplementary Material
Acknowledgments
We thank Dr. Carlos Garcia-Mata and Denise Scuffi for helpful assistance with the porometer measurements and data analysis.
Glossary
- NO
nitric oxide
- GSNO
S-nitrosoglutathione
- Ler
ecotype Landsberg erecta
- ROS
reactive oxygen species
- TF
transcription factor
- Ws
ecotype Wassilewskija
- H2DCFDA
2′,7′-dichlorofluorescin diacetate
- DAF-FM-DA
4,5-diaminofluorescein diacetate
- DEX
dexamethasone
- ABA
abscisic acid
- Col-0
ecotype Columbia
- PAR
photosynthetically active radiation
- pNOX
plant NADPH oxidase
- H2O2
hydrogen peroxide
Footnotes
This work was supported by a European Molecular Biology Organization Fellowship (to V.T.), a research grant from the Biotechnology and Biological Sciences Research Council, and grants from the Consejo Nacional de Investigaciones Cientificas y Tecnicas, the Agencia Nacional Para Promocion de Ciencia y Tecnologia, and the Universidad Nacional de Mar del Plata, Argentina.
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
References
- AH-Mackerness S, John CF, Jordan B, Thomas B. (2001) Early signaling components in ultraviolet-B responses: distinct roles for different reactive oxygen species and nitric oxide. FEBS Lett 489: 237–242 [DOI] [PubMed] [Google Scholar]
- An L, Liu Y, Zhang M, Chen T, Wang X. (2005) Effects of nitric oxide on growth of maize seedling leaves in the presence or absence of ultraviolet-B radiation. J Plant Physiol 162: 317–326 [DOI] [PubMed] [Google Scholar]
- Araújo WL, Fernie AR, Nunes-Nesi A. (2011) Control of stomatal aperture: a renaissance of the old guard. Plant Signal Behav 6: 1305–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arita NO, Cohen MF, Tokuda G, Yamasaki H. (2007) Fluorometric detection of nitric oxide with diaminofluoresceins (DAFs): applications and limitations for plant NO research. Nitric oxide in plant growth, development and stress physiology. Plant Cell Monographs 5: 269–280 [Google Scholar]
- Bauer H, Ache P, Lautner S, Fromm J, Hartung W, Al-Rasheid KA, Sonnewald S, Sonnewald U, Kneitz S, Lachmann N, et al. (2013) The stomatal response to reduced relative humidity requires guard cell-autonomous ABA synthesis. Curr Biol 23: 53–57 [DOI] [PubMed] [Google Scholar]
- Beligni MV, Lamattina L. (2000) Nitric oxide stimulates seed germination and de-etiolation, and inhibits hypocotyl elongation, three light-inducible responses in plants. Planta 210: 215–221 [DOI] [PubMed] [Google Scholar]
- Bright J, Desikan R, Hancock JT, Weir IS, Neill SJ. (2006) ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J 45: 113–122 [DOI] [PubMed] [Google Scholar]
- Brosché N, Strid A. (2003) Molecular events following perception of ultraviolet-B radiation by plants. Physiol Plant 117: 1–10 [Google Scholar]
- Brown BA, Cloix C, Jiang GH, Kaiserli E, Herzyk P, Kliebenstein DJ, Jenkins GI. (2005) A UV-B-specific signaling component orchestrates plant UV protection. Proc Natl Acad Sci USA 102: 18225–18230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown BA, Jenkins GI. (2008) UV-B signaling pathways with different fluence-rate response profiles are distinguished in mature Arabidopsis leaf tissue by requirement for UVR8, HY5, and HYH. Plant Physiol 146: 576–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa-Aragunde N, Lanteri L, Garcia-Mata C, ten Have A, Laxalt A, Graziano M, Lamattina L. (2007) Nitric oxide functions as intermediate in auxin, abscisic acid, and lipid signaling pathways. In L Lamattina, JCE Polaco, eds, Nitric Oxide in Plant Growth, Development and Stress Physiology. Plant Cell Monographs, Vol 5 Springer, Heidelberg, Germany, pp 113–130 [Google Scholar]
- Chen C, Xiao YG, Li X, Ni M. (2012) Light-regulated stomatal aperture in Arabidopsis mol. Plant 5: 566–572 [DOI] [PubMed] [Google Scholar]
- Chen H, Zhang J, Neff MM, Hong SW, Zhang H, Deng XW, Xiong L. (2008) Integration of light and abscisic acid signaling during seed germination and early seedling development. Proc Natl Acad Sci USA 105: 4495–4500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie JM, Arvai AS, Baxter KJ, Heilmann M, Pratt AJ, O’Hara A, Kelly SM, Hothorn M, Smith BO, Hitomi K, et al. (2012) Plant UVR8 photoreceptor senses UV-B by tryptophan-mediated disruption of cross-dimer salt bridges. Science 335: 1492–1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloix C, Kaiserli E, Heilmann M, Baxter KJ, Brown BA, O’Hara A, Smith BO, Christie JM, Jenkins GI. (2012) C-terminal region of the UV-B photoreceptor UVR8 initiates signaling through interaction with the COP1 protein. Proc Natl Acad Sci USA 109: 16366–16370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delledonne M. (2005) NO news is good news for plants. Curr Opin Plant Biol 8: 390–396 [DOI] [PubMed] [Google Scholar]
- Desikan R, Last K, Harrett-Williams R, Tagliavia C, Harter K, Hooley R, Hancock JT, Neill SJ. (2006) Ethylene-induced stomatal closure in Arabidopsis occurs via AtrbohF-mediated hydrogen peroxide synthesis. Plant J 47: 907–916 [DOI] [PubMed] [Google Scholar]
- Distéfano AM, Scuffi D, García-Mata C, Lamattina L, Laxalt AM. (2012) Phospholipase Dδ is involved in nitric oxide-induced stomatal closure. Planta 236: 1899–1907 [DOI] [PubMed] [Google Scholar]
- Eisinger WR, Bogomolni RA, Taiz L. (2003) Interactions between a blue-green reversible photoreceptor and a separate UV-B receptor in stomatal guard cells. Am J Bot 90: 1560–1566 [DOI] [PubMed] [Google Scholar]
- Favory JJ, Stec A, Gruber H, Rizzini L, Oravecz A, Funk M, Albert A, Cloix C, Jenkins GI, Oakeley EJ, et al. (2009) Interaction of COP1 and UVR8 regulates UV-B-induced photomorphogenesis and stress acclimation in Arabidopsis. EMBO J 28: 591–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohnmeyer H, Staiger D. (2003) Ultraviolet-B radiation-mediated responses in plants: balancing damage and protection. Plant Physiol 133: 1420–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Mata C, Gay R, Sokolovski S, Hills A, Lamattina L, Blatt MR. (2003) Nitric oxide regulates K+ and Cl– channels in guard cells through a subset of abscisic acid-evoked signaling pathways. Proc Natl Acad Sci USA 100: 11116–11121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Mata C, Lamattina L. (2001) Nitric oxide induces stomatal closure and enhances the adaptive plant responses against drought stress. Plant Physiol 126: 1196–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Yue X, Wang R, Zhang Y. (2011a) Ethylene mediates UV-B-induced stomatal closure via peroxidase-dependent hydrogen peroxide synthesis in Vicia faba L. J Exp Bot 62: 2657–2666 [DOI] [PubMed] [Google Scholar]
- He J, Yue X, Wang R, Zhang Y. (2011b) UV-B-induced stomatal closure via ethylene-dependent NO generation in Vicia faba L. Funct Plant Biol 38: 293–302 [DOI] [PubMed] [Google Scholar]
- He JM, Ma XG, Zhang Y, Sun TF, Xu FF, Chen YP, Liu X, Yue M. (2013) Role and interrelationship of Gα protein, hydrogen peroxide, and nitric oxide in ultraviolet B-induced stomatal closure in Arabidopsis leaves. Plant Physiol 161: 1570–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He JM, Xu H, She X, Song X, Zhao W. (2005) The role and the interrelationship of hydrogen peroxide and nitric oxide in the UV-B-induced stomatal closure in broad bean. Funct Plant Biol 32: 237–247 [DOI] [PubMed] [Google Scholar]
- Heijde M, Ulm R. (2012) UV-B photoreceptor-mediated signalling in plants. Trends Plant Sci 17: 230–237 [DOI] [PubMed] [Google Scholar]
- Holm M, Ma LG, Qu LJ, Deng XW. (2002) Two interacting bZIP proteins are direct targets of COP1-mediated control of light-dependent gene expression in Arabidopsis. Genes Dev 16: 1247–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen MAK. (2002) Ultraviolet-B radiation effects on plants: induction of morphogenic responses. Physiol Plant 116: 423–429 [Google Scholar]
- Jansen MAK, Bornman JF. (2012) UV-B radiation: from generic stressor to specific regulator. Physiol Plant 145: 501–504 [DOI] [PubMed] [Google Scholar]
- Jansen MAK, Noort RE. (2000) Ultraviolet-B radiation induces complex alterations in stomatal behavior. Physiol Plant 110: 189–194 [Google Scholar]
- Jenkins GI. (2009) Signal transduction in responses to UV-B radiation. Annu Rev Plant Biol 60: 407–431 [DOI] [PubMed] [Google Scholar]
- Jonassen EM, Lea US, Lillo C. (2008) HY5 and HYH are positive regulators of nitrate reductase in seedlings and rosette stage plants. Planta 227: 559–564 [DOI] [PubMed] [Google Scholar]
- Jonassen EM, Sévin DC, Lillo C. (2009) The bZIP transcription factors HY5 and HYH are positive regulators of the main nitrate reductase gene in Arabidopsis leaves, NIA2, but negative regulators of the nitrate uptake gene NRT1.1. J Plant Physiol 166: 2071–2076 [DOI] [PubMed] [Google Scholar]
- Jordan BR. (1996) The effects of ultraviolet-B radiation on plants: a molecular perspective. Adv Bot Res 22: 97–162 [Google Scholar]
- Kaiserli E, Jenkins GI. (2007) UV-B promotes rapid nuclear translocation of the Arabidopsis UV-B specific signaling component UVR8 and activates its function in the nucleus. Plant Cell 19: 2662–2673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TH, Böhmer M, Hu H, Nishimura N, Schroeder JI. (2010) Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu Rev Plant Biol 61: 561–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliebenstein DJ, Lim JE, Landry LG, Last RL. (2002) Arabidopsis UVR8 regulates ultraviolet-B signal transduction and tolerance and contains sequence similarity to human Regulator of Chromatin Condensation 1. Plant Physiol 130: 234–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamattina L, García-Mata C, Graziano M, Pagnussat G. (2003) Nitric oxide: the versatility of an extensive signal molecule. Annu Rev Plant Biol 54: 109–136 [DOI] [PubMed] [Google Scholar]
- Lee J, He K, Stolc V, Lee H, Figueroa P, Gao Y, Tongprasit W, Zhao H, Lee I, Deng XW. (2007) Analysis of transcription factor HY5 genomic binding sites revealed its hierarchical role in light regulation of development. Plant Cell 19: 731–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano-Juste J, León J. (2011) Nitric oxide regulates DELLA content and PIF expression to promote photomorphogenesis in Arabidopsis. Plant Physiol 156: 1410–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNellis TW, von Arnim AG, Araki T, Komeda Y, Miséra S, Deng XW. (1994) Genetic and molecular analysis of an allelic series of cop1 mutants suggests functional roles for the multiple protein domains. Plant Cell 6: 487–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogues S, Allen DJ, Morison JI, Baker NR. (1999) Characterization of stomatal closure caused by ultraviolet-B radiation. Plant Physiol 121: 489–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norsang G, Kocbach L, Tsoja W, Stamnes J, Dahlback A, Nema P. (2009) Ground-based measurements and modeling of solar UV-B radiation in Lhasa, Tibet. Atmos Environ 43: 1498–1502 [Google Scholar]
- Oravecz A, Baumann A, Máté Z, Brzezinska A, Molinier J, Oakeley EJ, Adám É, Schäfer E, Nagy F, Ulm R. (2006) CONSTITUTIVELY PHOTOMORPHOGENIC1 is required for the UV-B response in Arabidopsis. Plant Cell 18: 1975–1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyama T, Shimura Y, Okada K. (1997) The Arabidopsis HY5 gene encodes a bZIP protein that regulates stimulus-induced development of root and hypocotyl. Genes Dev 11: 2983–2995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazena H. (1996) The effect of altitude upon the solar UV-B and UV-A irradiance in the tropical Chilean Andes. Sol Energy 57: 133–140 [Google Scholar]
- Qu Y, Feng H, Wang Y, Zhang M, Cheng J, Wang X, Ahn L. (2006) Nitric oxide functions as a signal in ultraviolet-B induced inhibition of pea stems elongation. Plant Sci 170: 994–1000 [Google Scholar]
- Rizzini L, Favory JJ, Cloix C, Faggionato D, O’Hara A, Kaiserli E, Baumeister R, Schäfer E, Nagy F, Jenkins GI, et al. (2011) Perception of UV-B by the Arabidopsis UVR8 protein. Science 332: 103–106 [DOI] [PubMed] [Google Scholar]
- Seo SJ, Choi HG, Chung HJ, Hong CK. (2002) Time course of expression of mRNA of inducible nitric oxide synthase and generation of nitric oxide by ultraviolet B in keratinocyte cell lines. Br J Dermatol 147: 655–662 [DOI] [PubMed] [Google Scholar]
- Shi S, Wang G, Wang Y, Zhang L, Zhang L. (2005) Protective effect of nitric oxide against oxidative stress under ultraviolet-B radiation. Nitric Oxide 13: 1–9 [DOI] [PubMed] [Google Scholar]
- Tossi V, Amenta M, Lamattina L, Cassia R. (2011) Nitric oxide enhances plant ultraviolet-B protection up-regulating gene expression of the phenylpropanoid biosynthetic pathway. Plant Cell Environ 34: 909–921 [DOI] [PubMed] [Google Scholar]
- Tossi V, Cassia R, Bruzzone S, Zocchi E, Lamattina L. (2012) ABA says NO to UV-B: a universal response? Trends Plant Sci 17: 510–517 [DOI] [PubMed] [Google Scholar]
- Tossi V, Lamattina L, Cassia R. (2009) An increase in the concentration of abscisic acid is critical for nitric oxide-mediated plant adaptive responses to UV-B irradiation. New Phytol 181: 871–879 [DOI] [PubMed] [Google Scholar]
- Wargent JJ, Gegas VC, Jenkins GI, Doonan JH, Paul ND. (2009) UVR8 in Arabidopsis thaliana regulates multiple aspects of cellular differentiation during leaf development in response to ultraviolet B radiation. New Phytol 183: 315–326 [DOI] [PubMed] [Google Scholar]
- Wilson ID, Neill SJ, Hancock JT. (2008) Nitric oxide synthesis and signalling in plants. Plant Cell Environ 31: 622–631 [DOI] [PubMed] [Google Scholar]
- Wu D, Hu Q, Yan Z, Chen W, Yan C, Huang X, Zhang J, Yang P, Deng H, Wang J, et al. (2012) Structural basis of ultraviolet-B perception by UVR8. Nature 484: 214–219 [DOI] [PubMed] [Google Scholar]
- Zacher K, Wulff A, Molis M, Hanelt D, Wiencke C. (2007) Ultraviolet radiation and consumer effects on a field-grown intertidal macroalgal assemblage in Antarctica. Glob Change Biol 13: 1201–1215 [Google Scholar]
- Zeier J, Delledonne M, Mishina T, Severi E, Sonoda M, Lamb C. (2004) Genetic elucidation of nitric oxide signaling in incompatible plant-pathogen interactions. Plant Physiol 136: 2875–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, An L, Feng H, Chen T, Chen K, Liu Y, Tang H, Chang J, Wang X. (2003) The cascade mechanisms of nitric oxide as a second messenger of ultraviolet B in inhibiting mesocotyl elongations. J Photochem 77: 219–225 [DOI] [PubMed] [Google Scholar]
- Zhao L, Zhang F, Guo J, Yang Y, Li B, Zhang L. (2004) Nitric oxide functions as a signal in salt resistance in the calluses from two ecotypes of reed. Plant Physiol 134: 849–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.