Abstract
Background
Clinical trials test the efficacy of a treatment in a select patient population. We examined whether cancer clinical trial patients were similar to nontrial, “real-world” patients with respect to presenting characteristics and survival.
Methods
We reviewed the SWOG national clinical trials consortium database to identify candidate trials. Demographic factors, stage, and overall survival for patients in the standard arms were compared with nontrial control subjects selected from the Surveillance, Epidemiology, and End Results program. Multivariable survival analyses using Cox regression were conducted. The survival functions from aggregate data across all studies were compared separately by prognosis (≥50% vs <50% average 2-year survival). All statistical tests were two-sided.
Results
We analyzed 21 SWOG studies (11 good prognosis and 10 poor prognosis) comprising 5190 patients enrolled from 1987 to 2007. Trial patients were younger than nontrial patients (P < .001). In multivariable analysis, trial participation was not associated with improved overall survival for all 11 good-prognosis studies but was associated with better survival for nine of 10 poor-prognosis studies (P < .001). The impact of trial participation on overall survival endured for only 1 year.
Conclusions
Trial participation was associated with better survival in the first year after diagnosis, likely because of eligibility criteria that excluded higher comorbidity patients from trials. Similar survival patterns between trial and nontrial patients after the first year suggest that trial standard arm outcomes are generalizable over the long term and may improve confidence that trial treatment effects will translate to the real-world setting. Reducing eligibility criteria would improve access to clinical trials.
Randomized cancer clinical trials represent a final step in evaluating the efficacy of new treatments. However, few adult cancer patients participate in trials (<3%) in the United States (1,2). Reasons for low rates of clinical trial participation are numerous (3–5). Trials may not be available for patients willing to participate, or when they are available, patients are often excluded because they do not meet trial eligibility criteria (6–9).
Trial eligibility criteria must satisfy two opposing factors (10). They must be sufficiently narrow to establish a homogeneous sample, so the effect of treatment is roughly consistent across the cohort. Eligibility criteria that are too broad risk including patients for which the treatment is not optimal, which could mask the overall treatment effect. Eligibility should also be sufficiently broad that the results are generalizable. One possible difference between trial and nontrial patients is that trial eligibility criteria rule out poor-prognosis patients with prior comorbid conditions. Yet if the trial cohort is otherwise representative of the general cancer population with respect to cancer histology and stage, any differences in survival induced by ruling out poor-prognosis patients may not endure over time.
Despite attempts by clinical trialists to establish equipoise between homogeneity and generalizability, clinical trials are sometimes criticized for sacrificing generalizability (11). To assess generalizability in a systematic fashion, we evaluated whether presenting characteristics and survival outcomes for patients on the standard arms of a series of randomized phase III cancer clinical trials were representative of outcomes in patients receiving non–clinical trial treatment.
Methods
Cancer clinical trial data were from SWOG, a national clinical trials consortium sponsored by the National Cancer Institute. Nontrial data were from the Surveillance, Epidemiology, and End Results (SEER) cancer registry (12).
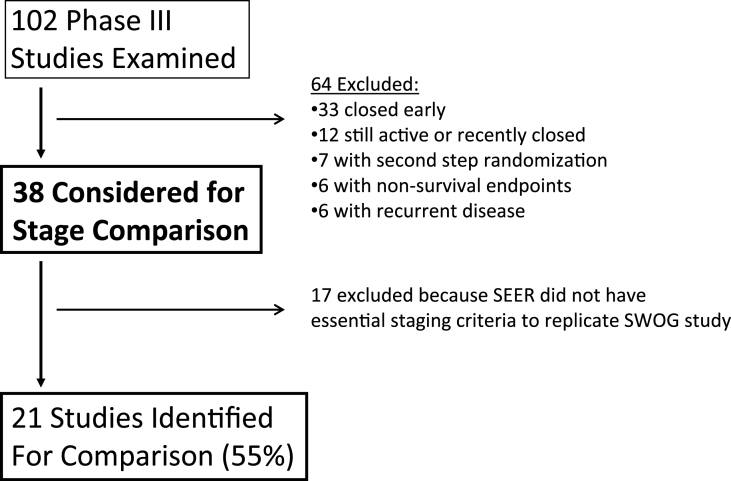
We conducted an analysis of randomized phase III studies from the SWOG historical database over a 25-year period (1987–2011). SWOG studies must have been published and must have had upfront randomization because studies with postregistration filtering of patients before receipt of standard treatment could not be reproduced using SEER. SWOG studies of recurrent disease were excluded (because SEER indexes reported case patients according to first diagnosis of a unique tumor type), as were studies with nonsurvival endpoints. Figure 1 shows how approximately two-thirds of candidate trials were excluded for these reasons.
Figure 1.
Study identification flow diagram. One hundred two phase III SWOG studies were examined over the 25-year period from 1987 to 2011. Among these, 64 were excluded from further consideration, 33 because of early closure (of which 30 were closed early because of poor accrual, two were closed early because of changed relationship with the drug manufacturer, and one was a positive study based on progression-free survival), 12 were still active or recently closed, seven did not have upfront randomization, six had nonsurvival endpoints, and six were studies for recurrent disease. Of the 38 considered for comparison with Surveillance, Epidemiology, and End Results (SEER) registry data, 17 were excluded because SEER did not have essential staging criteria to replicate the SWOG study. In the end, 21 of 38 studies (55% of those considered for stage comparison) were identified.
To be included, data to replicate the essential primary site, histology, and stage specifications from the SWOG study must have been available in SEER. Staging criteria included both TNM staging and, where appropriate, surgical and nodal staging. Studies that relied on tumor characteristics not available in SEER were excluded. We excluded positive SWOG studies for which there was also a trend toward improved survival over time in the corresponding SEER population because the standard arms for these SWOG studies likely no longer reflected community standard care at study completion. Only subjects on the standard arm were included, and corresponding SEER patients must have had a diagnosis date during the SWOG study’s enrollment period. Assuming the SWOG standard arm represented standard-of-care in the general cancer population during the study enrollment period, this allowed comparison between trial and nontrial patients with approximately similar treatments. The age limits specified in SWOG study eligibility were applied to the corresponding SEER datasets. Nearly all SWOG studies excluded patients with prior malignancies; for comparability, only SEER patients with first primaries were included.
Statistical Considerations
Comparisons between SWOG and SEER patients with respect to age (<65 years vs ≥65 years), sex, race (black vs white vs other), and stage were conducted across the panel of SWOG studies. For studies with more than one stage, stage was dichotomized into approximately equal groups to enable a consistent method of adjustment across the different studies. To test whether there was a global trend in stage or demographic rates across the panel of studies, the study-specific rates for both SEER and SWOG were converted to z scores (one for each study), and a one-sample t test was conducted on the difference in the z scores between SEER and SWOG.
For each study, Kaplan–Meier plots were generated to explore patterns of survival between SEER and SWOG patients, and Cox regression was used to estimate the hazard ratio and 95% confidence interval (CI) for the impact of trial participation, accounting for age, sex, race, stage, and year of enrollment (13,14). Studies were categorized as good (≥50%) vs poor (<50%) prognosis based on observed results using average 2-year Kaplan–Meier survival estimates.
To further explore differences in survival patterns, SWOG and, separately, SEER patients were combined by prognosis. To construct an equally weighted sample, 50 patients from each SWOG study and each corresponding SEER cohort were randomly selected. This process was averaged across 1000 repeat random samples. Kaplan–Meier plots and corresponding smoothed hazard functions (using Kernel-based methods) of the aggregate datasets were examined (15–17).
Based on the patterns observed using smoothed hazard function analysis, we applied landmark survival analysis to assess survival patterns related to trial participation given survival of the patient for a certain duration.
The contributions of cause of death to survival patterns were also investigated. SEER codes cause of death according to the International Classification of Diseases, Tenth Edition. In SWOG, a death was deemed cancer related if it followed a documented cancer progression. SWOG rates were adjusted using cause-of-death data available for a subset of patients (see Supplementary Methods, available online).
Finally, we assessed the extent to which study factors determined variation in survival outcomes. We estimated components of variation of the factors by comparing the partial log-likelihoods from nested models. We took the average of both forward and backward nesting approaches, with factors rank-ordered for model inclusion according to their χ2 statistic in a multivariable model.
All analyses were limited to survival in the first 5 years after diagnosis to emphasize outcomes related to cancer and its treatments. All statistical tests were two-sided.
Results
Study Selection
Of 102 SWOG studies examined, 64 were initially excluded (Figure 1). Seventeen of the remaining 38 studies were excluded because of inadequate SEER data on essential tumor characteristics.
Study Profiles and Eligibility
Twenty-one studies (n = 21/38; 55%) met the specified study inclusion criteria (Table 1) (18–38). The study sample included both early- and late-stage cancers from many cancer types. A total of 5190 SWOG patients and 69187 SEER patients from 1987 to 2007 were analyzed.
Table 1.
SWOG studies included for comparison with the Surveillance, Epidemiology, and End Results (SEER) registry*
| SWOG criteria | Corresponding SEER criteria | ||||||
|---|---|---|---|---|---|---|---|
| Cancer and study no. | Years of accrual | Histology | Major tumor characteristic criteria from SWOG studies† | SWOG No. | SEER No. | ICD-O-3 primary site | Histology code |
| Brain S0001 | 2001–2005 | Glioblastoma multiforme/ gliosarcoma | Biopsy or surgical resection prior to registration | 89 | 2264 | C710–725 | 9440–9444 |
| Breast S9313 | 1994–1997 | Adenocarcinoma‡ | Stage T1-3, N0, M0 (selected stages I-III; no locally advanced disease) Axillary dissection required ≥6 nodes removed and examined ≤3 positive nodes Tumor >2cm and ER/PR (-) or (+); or, 1-3 (+) axillary nodes Prior mastectomy or breast sparing surgery |
1423 | 9941 | C500–509 | 8500–8530 |
| Breast S0012 | 2001–2005 | Locally advanced or inflammatory breast carcinoma | Stage IIB–IIIB (M0) | 391 | 2855 | C500–506, C508–509 | Any |
| GI-Gastric S9008 | 1991–1998 | Adenocarcinoma§ | Stage IB–IV (M0) Prior en bloc surgery |
283 | 2487 | C150–155, 58–66, 68–69 | 8140–8800 |
| GI-Pancreas S0205 | 2004–2006 | Adenocarcinomaǁ | Locally advanced (not surgically resectable, ie, no prior surgery) or metastatic disease | 82 | 1943 | C250–254, C257–259 | 8140 |
| GU-Bladder S8710 | 1988–1997 | Transitional cell carcinoma | Stage T2–T4A (no metastasis) | 148 | 2377 | C670–679 | 8120–8124 |
| GU-Bladder S8795 | 1988–1992 | Transitional cell carcinoma (including papillary) | Stage Ta–T1 and grade I–IV Completely resected |
191 | 5059 | C670–679 | 8120–8124, 8130 |
| GU-Prostate S8894 | 1989–1994 | Adenocarcinoma | Stage D2 | 534 | 5961 | C619 | 8140 |
| GU-Renal S8949 | 1991–1998 | Carcinoma | Metastatic No nephrectomy (standard arm) |
95 | 1569 | C649 | 8312 |
| GYN-Cervix S8797 | 1990–1996 | Squamous cell carcinoma, adenocarcinoma, or adenosquamous carcinoma | Stages IA2, IB, or IIA Radical hysterectomy with total pelvic lymphadenectomy Positive pelvic or parametrial, and negative para-aortic, nodal involvement |
130 | 137 | C530–531, C538–539 | 8070–8, 8140–7, 8260–3, 8310–84, 8560–62 |
| LEUK-AML S9031 | 1991–1994 | AML | FAB classes M0–M2, M4–M7 (excluded M3s beginning in August, 1992) | 85 | 1672 | C420–1, C424 | 9801, 9840, 9861, 9866–7, 9871–74, 9891, 9896, 9910 |
| LEUK-AML S9333 | 1995–1998 | AML | FAB classes M0–M2, M4–M7 (excluded M3s) | 129 | 2320 | C420–1, C424 | 9801, 9840, 9861, 9867, 9871–74, 9891, 9896, 9910 |
| Lung-NSCLC S8738 | 1988–1990 | Squamous cell carcinoma, adenocarcinoma, and large cell carcinoma | M1 disease (including lung metastasis). Exclude patients with mets only to ipsilateral hilar nodes (N1) and/or mediastinal nodes (N2) or supraclavicular nodes (N3) ONLY | 94 | 4084 | C340–3, C348–9 | 8012, 8070–78, 8140–47 |
| Lung-NSCLC S9308 | 1993–1995 | Any NSCLC | Stage IIIB (based on positive pleural effusions or ipsilateral lung involvement) or stage IV | 178 | 4755 | C340–3, C348–9 | 8012, 8046, 8070–8, 8140–7, 8240–50, 8560, 9050–3 |
| Lung-NSCLC S9509 | 1996–1997 | Any NSCLC (except bronchioalveolar) | Stage IIIB with either 1) T4 disease due to malignant pleural effusion; 2) multiple lesions in a single lobe containing a T3 or T4 primary; or 3) lesions in multiple lobes of the ipsilateral lung for which one such lesion is T3 or T4;¶ or stage IV | 205 | 4817 | C340–3, C348–9 | 8012, 8046, 8070–8, 8140–7, 8240–9, 8560, 9050–3 |
| Lung-NSCLC S9900 | 1999–2004 | Any NSCLC | Selected stages IB (T2N0), II (T1–2, N1; or T3N0), or IIIA (T3N1) Limited to surgery type specified in protocol: lobectomy, sleeve resection, bilobectomy, or pneumonectomy (excludes limited resection or NOS) |
168 | 829 | C340–3, C348–9 | 8012, 8046, 8070–8, 8140–7, 8240–50, 8560, 9050–3 |
| Lung-NSCLC S0003# | 2000–2002 | Squamous, adeno-, large cell, or NSCLC carcinoma | Use newly diagnosed, selected stage IIIB (based on positive pleural effusions) or stage IV | 165 | 7727 | C340–3, C348–9 | 8012, 8046, 8070–8, 8140–7 |
| Lung-SCLC S0124 | 2002–2007 | Any SCLC | Extensive disease | 266 | 2790 | C340–3, C348–9 | 8041–5 |
| Melanoma S8642 | 1987–1990 | Any melanoma | Stage II (thickness ≥1.5, N0, M0) or III (any T, N1-2, M0) Complete wide-excision of tumor (≥1cm margin)** |
96 | 738 | C440–9 | 8720–72 |
| Melanoma S9035 | 1992–1996 | Any melanoma | Stage T3N0M0 (thickness 1.51–4.00mm or Clark IV if thickness unknown) Complete wide-excision of tumor (≥1cm margin)** |
299 | 1347 | C440–9 | 8720–72 |
| Myeloma S8624 | 1987–1990 | Multiple myeloma | Previously untreated | 139 | 3515 | C421 | 9732 |
| TOTAL 21 studies | 21 years (1987–2007) | 5190 | 69187 | ||||
* AML = acute myeloid leukemia; ER = estrogen receptor; FAB = French-American-British; GI = gastrointestinal; GU = genitourinary; GYN = gynecologic; LEUK = leukemia; NSCLC = non–small cell lung cancer; PR = progesterone receptor; SCLC = small cell lung cancer.
† All criteria listed in the table were explicitly accounted for in SEER. Additional tumor characteristic criteria that could not be accounted for explicitly in SEER include: Brain, S0001) Patients with three or more noncontiguous sites are ineligible; GI-Gastric, S9008) No ascites; no peritoneal seeding; no liver metastases or extra-abdominal metastases; GU-Bladder, S8710) One or more kidney and proximal ureter free of tumor and all other disease resectable; GU-Bladder, S8795) No recurrent tumor on cystoscopy within 4 weeks if first TURBT more than 4 weeks before registration; and, random biopsy or a negative urinary cytology; GU-Renal, S8949) Primary cancer must be amenable to surgery if patient did not otherwise have metastatic disease; Leukemia-AML, S9031 and S9333) Exclude blastic transformation of chronic myelogenous leukemia; Lung-NSCLC, S9509) Exclude stage IIIB tumors involving the superior sulcus; Lung-NSCLC, S9900) No patients with symptomatic tumors (T3,N0–N1) involving the superior sulcus; Melanoma, S9035) Lymphadenectomy must have resolved; patients with suspicious nodes must have regional lymph node dissection with negative nodes; Myeloma, S8642) Specific protein criteria; and, patients with immunoglobulin M myeloma not eligible.
‡ Excluding tubular, mucinous, papillary, sarcoma, lymphoma, apocrine, adenocystic, or squamous cell carcinoma; ductal or lobular carcinoma in situ allowed if one to three positive nodes. Patients with tumor greater than 1cm and ER/PR(-) excluded from both SWOG and SEER datasets because of lack of ER/PR data in SEER during the study period.
§ Stomach and esophagogastric junction.
ǁ Exclude endocrine tumors, lymphoma of pancreas, or ampullary cancer.
¶ For IIIB definition in SEER, simplified as IIIB with T3 or T4 extent-of-disease.
# Although S0003 allowed recurrent patients, these were excluded. Comparison with SEER relied on newly diagnosed patients only.
** Detailed surgical resection criteria were specified.
Table 2 summarizes additional eligibility criteria from the SWOG study that do not specifically pertain to histology or tumor characteristics. Nearly all studies had prior systemic therapy exclusions and required adequate kidney, liver, and hematologic function. The majority of studies required no current evidence or history of cardiac dysfunction. Other common exclusion criteria included other serious medical conditions, diseases, or active infections, and low patient functional status. Most of the criteria in Table 2 could not be accounted for using SEER data.
Table 2.
Eligibility criteria for SWOG clinical trials*
| Cancer type and study No. | Min (max) age | No prior cancer† | Prior treatment exclusions | Organ function criteria‡ | Max PS§ | Other PS | Pregnant/ contra- ception | Serious medical conditionǁ | HI V | Prior TX timing | Other on- study therapy | No brain mets | Study drug allergy | Scan timing | No. other criteria¶ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Brain, S0001 | 18 | X | Ch, RT | K, P | 2 | — | X | X | X | — | X | — | X | X | 0 |
| Breast, S9313 | NS | — | Ch, R, S | K, L, H, C | — | LTFU | X | X | — | X | — | — | — | X | 1 |
| Breast, S0012 | NS | X | Ch, Hr, RT, S | K, L, H, C | 2 | — | X | — | X | — | X | — | — | X | 0 |
| GI-Gastric, S9008 | NS | X | Ch, B, RT | K, L, H | 2 | — | X | X | — | X | — | — | — | X | 1 |
| GI-Pancreas, S0205 | NS | X | Ch | K, L, H, C | 2 | — | X | — | X | X | X | X | — | X | 0 |
| GU-Bladder, S8710 | NS | X | RT# | K, L, H, C | 1 | CURE | X | X | — | X | — | — | — | X | 1 |
| GU-Bladder, S8795 | NS | X | Ch | K, L, H | 2 | LE | X | — | — | X | X | — | — | X | 1 |
| GU-Prostate, S8894 | NS | X | Ch, Hr, B | K, L, H | 3 | — | — | X | — | — | X | — | — | X | 0 |
| GU-Renal, S8949 | NS | X | Ch, Hr, B, RT** | K, L, H, C | 2 | — | X | — | — | — | X | X | — | X | 0 |
| GYN-Cervix, S8797 | NS | X | Ch, Hr, B, RT# | K, L, H | 2 | — | — | X | — | X | — | — | — | X | 1 |
| LEUK-AML, S9031 | 56 | X | Ch | K, L, C | 3 | — | X | — | — | — | — | — | — | X | 0 |
| LEUK-AML, S9333 | 56 | X | Ch | K, L, C | 3 | — | X | — | — | — | — | — | — | X | 1 |
| Lung-NSCLC, S8738 | NS | X | Ch | K, H, C | 2 | LE | X | — | — | — | X | X | — | X | 0 |
| Lung-NSCLC, S9308 | 18 | X | Ch, B | K, L, H | 1 | — | X | X | — | X | — | X | — | X | 1 |
| Lung-NSCLC, S9509 | 18 | X | Ch, B | K, L, H, C | 1 | — | X | X | — | — | — | X | X | X | 1 |
| Lung-NSCLC, S9900 | 18 | X | Ch, RT | K, L, H, P | 1 | — | X | X | — | — | X | — | X | X | 1 |
| Lung-NSCLC, S0003 | NS | X | Ch, B | K, L, H | 1 | — | X | — | — | X | — | X | X | X | 0 |
| Lung-SCLC, S0124 | 18 | X | Ch, RT†† | K, L, H | 1 | — | X | — | X | X | — | — | — | X | 1 |
| Melanoma, S8642 | 18 (70) | X | Ch, Hr, B, RT | K, L, H, C | 1 | — | X | X | — | — | X | — | — | X | 2 |
| Melanoma, S9035 | 18 | X | Ch, Hr, B, RT | K, L, H, C | 1 | — | X | — | — | X | X | — | — | X | 0 |
| Myeloma, S8624 | NS | X | Ch | H‡‡, C | 3 | — | — | X | — | — | — | — | — | X | 1 |
* Only the first two criteria listed (age and prior cancer) were explicitly accounted for in the Surveillance, Epidemiology, and End Results (SEER) registry. All other criteria could not be accounted for based on SEER data. Eligibility criteria that related to comorbidity or performance status included prior treatment exclusions, prior malignancy exclusions, performance status, organ function status, human immunodeficiency virus status, serious medical conditions, brain metastases, study drug allergy, and maximum age limit. Empty cells (cells with dashes) indicate the particular eligibility criterion was not included in the study protocol.
AML = acute myeloid leukemia; B = biologic therapy; C = cardiac; Ch = chemotherapy; CURE = potentially curable; GI = gastrointestinal; GU = genitourinary; GYN = gynecologic; H = hematologic; HIV = human immunodeficiency virus; Hr = hormonal therapy; K = kidney; L = liver; LE = minimum life expectancy; LEUK = leukemia; LTFU = adequate health for long-term follow-up; NS = not specified; NSCLC = non–small cell lung cancer; P = pulmonary; PS = performance status; RT = radiation therapy; S = surgery; SCLC = small cell lung cancer; TX = treatment.
† Typically requires no prior malignancy except adequately treated non-melanoma skin cancer, in situ cervical cancer, or other cancer for which the patient has been disease free for 5 or more years.
‡ Organ function criteria were based primarily on the following tests: for kidney, creatinine clearance and/or serum creatinine; for liver bilirubin, serum glutamic oxaloacetic transaminase and/or serum glutamic pyruvate transaminase; and for hematologic, white blood count and platelets.
§ Performance status is a measure of the patient’s well-being and activity level. In SWOG, the coding scheme is: 0 = asymptomatic or fully active; 1 = symptomatic but completely ambulatory; 2 = symptomatic but in bed less than 50% of day; 3 = symptomatic, more than 50% of time in bed, but not bedbound; 4 = completely disabled or bedbound.
ǁ Including active infections.
¶ Other eligibility criteria include: Breast, S9313) Patients with breast-sparing surgery must plan RT after chemotherapy; GI-Gastric, S9008) Good caloric intake of 1500 or more calories/day required; GU-Bladder, S8710) Normal organ function required; GU-Bladder, S8795) Must be at increased risk of papillary tumor recurrence; GYN-Cervix, S8797) No pelvic inflammatory disease; Leukemia-AML, S9333) Exclude if marrow unaspirable and white blood cells and blasts + promyelocytes + promonocytes outside normal limits; Lung-NSCLC, S9308) No grade 2 or greater neuropathy; Lung-NSCLC, S9509) No grade 2 or greater neuropathy; S9900) No grade 2 or greater neuropathy; Lung-SCLC, S0124) Prior brain metastases must have been treated; Melanoma, S8642) No known seizure disorder or known central nervous system disease; no prior organ transplant; Myeloma, S8624) Patients must have objective evidence, or be symptomatic from, AML.
# Pelvic.
** Except palliative.
†† Except brain.
‡‡ Based on M-component.
The mean total number of eligibility criteria for a given study was 16.1, of which 9.8 (60%) were related to comorbidity or performance status.
Demographic Factors and Stage
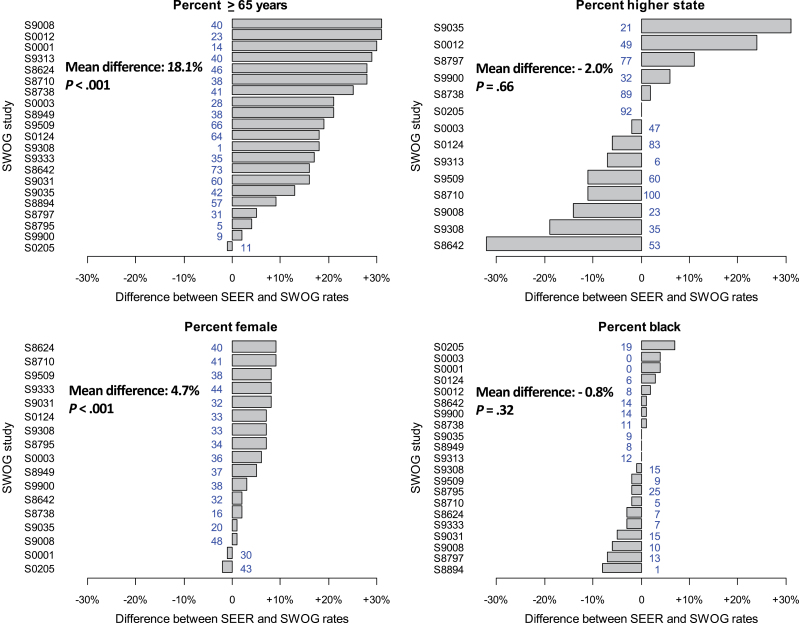
Figure 2 shows the difference between SEER and SWOG patients for each demographic and stage factor. The SEER cohort was consistently more likely to be older and, to a lesser degree, female, but there were no panel-wide trends in the proportion of patients with higher stage or black race.
Figure 2.
Horizontal barplots of the difference between Surveillance, Epidemiology, and End Results (SEER) and SWOG patients for each demographic and stage factor, in descending order of the absolute difference in percentages between SWOG and SEER cohorts. The SWOG percentage is also shown in each figure. Bars to the right of center indicate a higher proportion in SEER, and bars to the left of center indicate a higher proportion in SWOG.
Overall Survival Comparisons Between SWOG and SEER
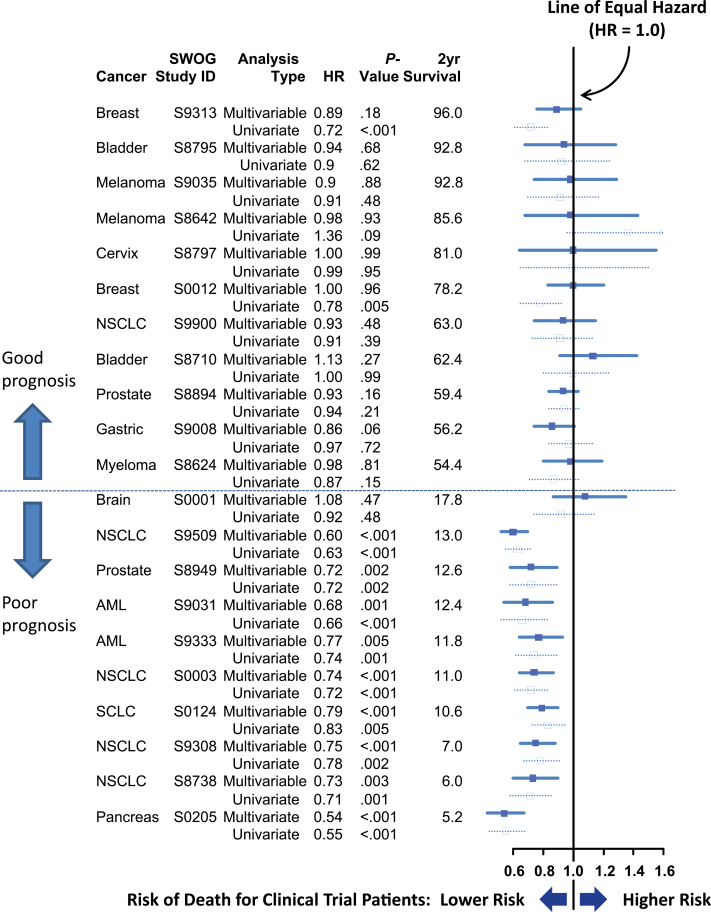
Figure 3 shows both unadjusted and multivariable (adjusted) hazard ratios comparing overall survival between SWOG and SEER cohorts in descending order of average 2-year survival. Eleven studies had average 2-year survival of 50% or greater (good prognosis) and 10 studies had 2-year survival of less than 50% (poor prognosis). For none of the good-prognosis studies did survival for SWOG patients statistically significantly differ from survival for SEER patients in multivariable analysis, whereas for nine of 10 poor prognosis studies, SWOG patients had statistically significantly lower risk of death (P < .001).
Figure 3.
Forest plot of univariate and multivariable hazard ratios (HRs) for overall survival, by study, ordered in descending order of average 2-year overall survival. In univariate analyses, two of 11 (18%) good-prognosis studies and nine of 10 (90%) poor-prognosis studies showed evidence of a survival benefit for trial patients (P = .002 by Fisher exact test). In multivariable analyses, zero of 11 good-prognosis studies and nine of 10 poor-prognosis studies showed evidence of a survival benefit for trial patients (P < .001). AML = acute myeloid leukemia; NSCLC = non–small cell lung cancer; SCLC = small cell lung cancer.
We found no evidence that the hazard ratios for trial participation differed over calendar time for either good-prognosis (P = .50) or poor-prognosis (P = .69) studies (see Supplementary Figure 1, available online). Also, results did not substantively change when a covariable for Hispanic ethnicity was added to the multivariable models (see Supplementary Methods, available online).
Differences in Aggregate Survival Patterns Between SWOG and SEER Patients
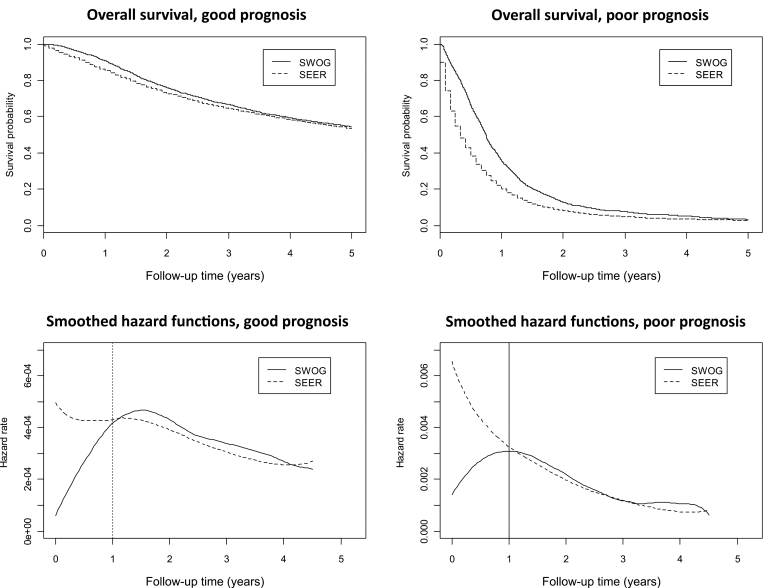
Examination of the individual study-specific survival curves (see Supplementary Figure 2, available online) indicated a frequent pattern of an early survival advantage for SWOG patients that waned over time for both good- and poor-prognosis cancers. Using aggregate data, we examined Kaplan–Meier plots of overall survival and corresponding smoothed hazard functions (Figure 4). For both good- and poor-prognosis patients, the hazard function for SWOG patients was initially much lower than the hazard function for SEER patients. But, by year 1, the hazard functions for both SEER and SWOG patients no longer differed, suggesting that trial participation was associated with better survival only in the first year. Importantly, this analysis revealed a consistent association of trial participation and survival that was not evident in the individual survival analyses for good-prognosis studies, likely because of limited power in that setting.
Figure 4.
Overall survival and corresponding hazard functions for aggregate (equally weighted) study data by prognosis.
Average Effect Accounting for the First-Year Survival Difference
For good-prognosis patients, the mean of the adjusted hazard ratios for overall survival comparing SWOG with SEER patients shown in Figure 3 was not statistically different from 1.0 (mean = 0.96; 95% CI = 0.92 to 1.01; P = .12). We analyzed the subset of patients who survived 1 year using landmark survival analysis. The results were similar (mean = 1.05; 95% CI = 0.96 to 1.14; P = .22). However, for poor-prognosis patients, the mean of the multivariable hazard ratios shown in Figure 3 was much less than 1.0 (mean = 0.74; 95% CI = 0.64 to 0.84; P < .001). Conditioning on 1-year survival, this difference was no longer evident (mean = 1.05; 95% CI = 0.95 to 1.15; P = .27), reinforcing the observation that the impact of trial participation endured for only about 1 year.
Analysis of Cancer-Specific and Non-Cancer-Specific Events
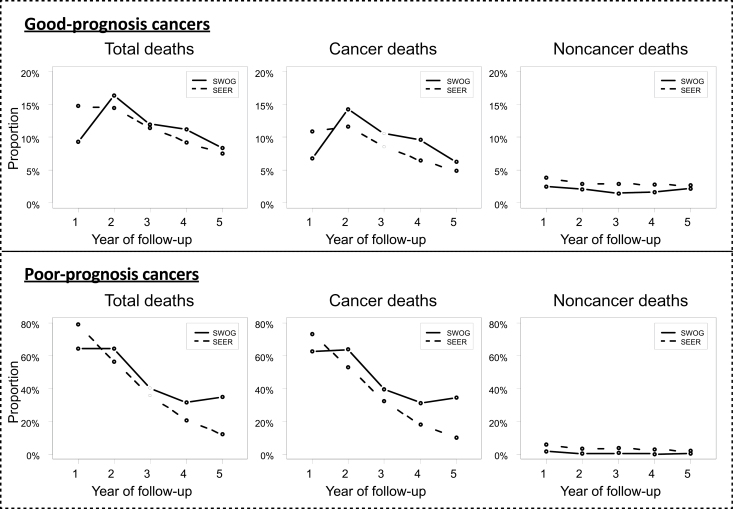
The proportion of patients experiencing cancer-related and non-cancer-related deaths relative to the number of patients at risk was analyzed by year. Non-cancer-related deaths were lower in SWOG patients, although this difference was small and relatively stable across all 5 years of follow-up (Figure 5). In contrast, cancer-related deaths were notably lower in the first year in SWOG patients but similar to SEER patients in later years. Therefore the difference in the patterns of death for trial vs nontrial patients between year 1 vs years 2 to 5 is largely attributable to different patterns of cancer-related deaths.
Figure 5.
Total, cancer-specific, and non-cancer-specific deaths by year of follow-up by prognosis. For each of the first 5 years, the proportion of patients experiencing death of any kind, cancer-specific death, and non-cancer-specific death relative to the number of patients at risk in each year is plotted for both SWOG and Surveillance, Epidemiology, and End Results (SEER) patients. Consistent with the Kaplan–Meier survival plots in Figure 4, the total event rate is notably lower in SWOG patients in the first year. In years 2 to 5, in contrast, the proportions of total events in SWOG and SEER patients are more similar and are decreasing as the risk of death decreases. For both good- and poor-prognosis patients, the pattern of a relatively lower event rate for SWOG patients in year 1 is mostly reflective of a diminished rate of cancer-related deaths in year 1; although non-cancer-related deaths are also lower in SWOG patients, this difference was small and relatively stable across all 5 years of follow-up. Indeed, in good-prognosis patients, the unweighted ratio of the rate of SEER cancer deaths to SWOG cancer deaths was 1.60 in year 1 but was less than one (only 0.78) in years 2 to 5. For non-cancer-specific deaths, the ratios are very similar whether in year 1 (1.57) or years 2 to 5 (1.52), indicating the pattern change over time occurs in cancer-related deaths only. A similar pattern held for poor-prognosis cancers. In summary, the difference in the patterns of death for trial vs nontrial patients between year 1 vs. years 2 to 5 is largely attributable to different patterns of cancer deaths.
Attributable Variation
In the non-sex-specific studies, disease and stage explained 92.2% of the relative variation in survival outcomes, followed by age (5.2%), trial participation (1.5%), race (0.6%), and sex (0.5%). In the first year only, estimate of variation in survival outcomes attributable to disease and stage was 88.4% and to trial participation was 4.9%, compared with 92.7% and 1.2%, respectively, after 1 year.
Discussion
We found that trial participation was associated with better survival only in the first year. Short-term estimates of absolute survival probabilities from clinical trials may be optimistic (Figure 4). Physicians who use clinical trial results to assist in making treatment decisions should be aware of this phenomenon. Better short-term survival for trial patients is likely related to the exclusion of sicker patients from trials through eligibility criteria pertaining to comorbidity and performance status. These exclusions also resulted in trial cohorts that were much younger and somewhat less likely to be female, consistent with prior reports (39,40).
We did not explicitly assess whether the treatment effect in a clinical trial translates (ie, generalizes) to the broader cancer population. Such a study would require a comparison between experimental and standard arm treatments occurring in the general cancer population at the same time as the clinical trial is conducted. However, similar standard arm outcomes beyond the first year may improve confidence that efficacy of treatment in a trial translates to the real-world setting. This conclusion relies on the assumption that trial participation would impact standard and experimental treatment arms similarly and would not apply in instances where new treatments have too much toxicity or poor compliance. Importantly, we found no evidence that the association of trial participation and survival increased over calendar time, which might be expected if new treatments adopted into standard care do not show the same benefit as observed in the clinical trial. This suggests that most patients may also benefit from the new treatments, even if not participating in trials.
The most reliable way to establish the causal relationship between trial participation and outcome would be to randomize patients to be offered a clinical trial vs not offered a clinical trial (41) Such a study would be practically and ethically difficult. Instead, the literature is based on observational studies, which focus on presenting characteristics and absolute survival differences between trial and nontrial patients. Identification of the appropriate nontrial control group is crucial to inference because any observational design will be limited by unmeasured confounding, whether trial patients are compared with eligible nontrial control subjects (bias with respect to factors associated with refusing trial participation), ineligible control subjects (bias with respect to prognosis), or population control subjects (multiple biases) (41). These studies most often focused on single trial vs nontrial comparisons, raising the issue of subjective study selection.
Both Peppercorn et al. (41) and Edwards et al. (42) reviewed the historical literature. Both found that a majority of comparisons from cancer studies showed evidence of better outcomes for trial patients, with no evidence of harm. Peppercorn et al. (41) concluded that there was no strong evidence of a benefit for trial patients, in part because of methodological issues with the nontrial comparator groups, whereas Edwards et al. (42) concluded that there was positive, albeit weak, evidence that participation in trials improves outcomes. Other reviews and studies also found mixed evidence (43–46).
The inconclusive picture offered by the literature could be related to the transient impact of trial participation on survival found in this study. We re-examined the cancer studies included in two prior reviews (41,42). Studies were categorized as good or poor prognosis as defined in this study. In total, there were 36 comparisons from 27 studies (see Table 3) (47–73). Fifty-six percent of good-prognosis studies showed evidence of survival benefit for trial patients, compared with 82% of poor-prognosis studies, a pattern consistent with but not as extreme as the pattern found in this study. A similar pattern was found among comparisons that included multivariable analyses only (47,49,51–58,60–62,67–70) and adult cancers only (47–57,60,61,64–66,72,73).
Table 3.
Main results and prognosis for individual studies included in reviews by Edwards et al. (42) and Peppercorn et al. (41)*
| Article† | Cancer type | Results‡ | Prognosis group§ | Evidenceǁ | |
|---|---|---|---|---|---|
| Any (U or M) | M¶,# | ||||
| Antman (E) (47)** | Sarcoma | No U result; no SS difference in M DFS (P = .15); OS not reported | Good | No | No |
| Bertelsen (E) (48) | Ovarian | Difference in OS in U setting (P < .001) but not M setting w/same TX (P = .98) | Good | Yes | No |
| Boros (P) (49) | AML | Difference in OS in U setting (P < .001) and in M setting (P = .02) | Poor | Yes | Yes |
| Burgers (P) (50) | SCLC | No SS difference in OS in U (no P value given); M not done | Poor | No | — |
| Cottin (P) (51) | SCLC | SS difference in the U (P = .01) but not M setting (unknown P value); adjusted for performance status | Poor | Yes | No |
| Dahlberg (P) (52) | Rectal | No differences between trial and nontrial pts of similar TX (surgery) | Good | No | No |
| Davis (B) (53) | NSCLC | SS difference in both U (P < .001) and M setting (P < .002) | Good | Yes | Yes |
| Dowling (P) (54) | Prostate | SS difference in U (P = .003) but not M setting after adjusting for performance status (P = .42) | Poor | Yes | No |
| Feuer (P) (55) | 1) Testicular | Minimal disease: SS difference in both U and M | Good | Yes | Yes |
| 2) Testicular | Advanced disease: No difference in U or M | Good | No | No | |
| Greil (P) (56) | Hodgkin’s | No difference in OS in either U (P = .67) or M (P = .65) settings | Good | No | No |
| Karjalainen (B) (57) | 1) Myeloma | 1979–85: SS difference in favor of trial pts | Good | Yes | Yes |
| 2) Myeloma | 1959–78: NS trend in favor of nontrial pts | Good | No | No | |
| Lennox (B) (58) | Wilms†† | SS difference in OS in both U (P < .01) and M settings (P < .001) | Good | Yes | Yes |
| Link (P) (59) | Osteo- sarcoma†† | No difference in OS in U (no P value) | Good | No | — |
| Marubini (P) (60) | Breast | SS in U setting (no P value given) but not M setting (P = .50) | Good | Yes | No |
| Mayers (P) (61) | Breast | SS in U setting (P = .02) but not M setting (P = .09) | Good | Yes | No |
| Meadows (P) (62) | ALL†† | SS differences in U (P < .001) and M (no P value) settings | Good | Yes | Yes |
| MRC (E) (63) | Leukemia†† | Difference in OS (P value not given) | Poor | Yes | — |
| Roy (P) (64) | Hodgkin’s | No P values given. OS appears worse for nontrial pts in older (≥45 y) but not younger pts | Good | Yes | — |
| Schea (P) (65) | SCLC | SS difference in U (P = .002) | Poor | Yes | — |
| Schmoor (B) (66) | Breast | Trial 2) No difference in DFS in U | Good | No | — |
| Trial 3) NS DFS trend in favor of trial pts in U | Good | No | — | ||
| Stiller (P) (67) | ALL†† | No difference in U (P = .63) | Good | No | — |
| AML†† | SS difference in U (p=.04); in M, No difference in 1984–1988, Difference in 1989–1994 | Poor | Yes | Yes | |
| Stiller (B) (68) | ALL†† | SS difference for both U (no P value given) and M (P < .0001) | Good | Yes | Yes |
| Stiller (B) (69) | AML†† | 1975–83: U not done; SS difference in M (p<.001) | Poor | Yes | Yes |
| 1984–88: U not done; No difference in M | Good | No | No | ||
| Stiller (P) (70) | ALL†† | 1980–84: U not done; No difference in M (P = .62) | Good | Yes | No |
| 1985–89: U not done; Difference in M (P = .02) | Good | Yes | Yes | ||
| 1990–94: U not done; Difference in M (P < .0001) | Good | Yes | Yes | ||
| Wagner (P) (71) | NHL†† | SPOG vs nonstudy: No SS difference in U (P = .07) | Good | No | — |
| POG vs nonstudy: SS difference in U (P < .0001) | Good | Yes | — | ||
| Ward (B) (72) | Stomach | 5/10 analyses were SS (P ≤ .05; Table III) | Poor | Yes | — |
| Winger (P) (73) | Glioma | SS difference in U (P = .00001) vs all nonstudy pts | Poor | Yes | — |
| Glioma | NS for U (P = .12) vs all nonstudy pts | Poor | No | — | |
* ACM = all-cause mortality; ALL =; AML = acute myeloid leukemia; AS = actuarial survival; DFS = disease-free survival; M = multivariable; NHL = Non-Hodgkin’s lymphoma; NS = nonsignificant; NSCLC = non–small cell lung cancer; OS = overall survival; pts = patients; SCLC = small cell lung cancer; SS = statistically significant; TX = treatment; U = univariate.
† “E” indicates article was included in Edwards et al. (42), “P” indicates article was included in Peppercorn et al. (41), and “B” indicates article was included in both reviews.
‡ Results based on overall survival for all studies except Antman et al. (47) and Schmoor et al. (66).
§ Prognosis groups: Good prognosis is defined as 50% or greater average estimated 2-year survival. Poor prognosis is defined as less than 50% average estimated 2-year survival.
ǁ Consistent with our own analysis, studies were categorized according to whether there was a statistically significant (P < .05) difference between trial and nontrial patients.
¶ Among studies where multivariable analyses were conducted.
# A dash indicates that no multivariable analyses were conducted.
** Based on full published article for the conference abstract cited by both authors.
†† Childhood cancer.
We compared trial vs nontrial patients who were similar with respect to histology, stage, age, de novo presentation, year of diagnosis, race, sex, and treatment. What remained were differences between databases that we could not account for. Trial patients could benefit from changes in behavior or outlook associated with being under observation (the “Hawthorne” effect) (74) or from care that is administered according to strict protocol (75). Alternatively, none of the eligibility criteria outlined in Table 2, the majority of which pertain to performance status and comorbidities, could be accounted for. Therefore trial patients likely exhibit better outcomes because eligibility criteria prevent sicker patients from enrolling on study. These enrollment restrictions appear to primarily limit early cancer deaths, suggesting that comorbidity and performance status identify residual variation in cancer-specific survival even after accounting for stage. Unfortunately, the extent to which the survival differences were related to patient selection or other factors cannot be estimated with these data.
This study also had some limitations. We were unable to account for the actual treatments of the nontrial control patients. It is inevitable that not all nontrial patients in SEER received standard of care for their histology and stage and may have received no treatment. The use of different databases with different methods of data collection may induce different patterns of endpoint assessment, which could impact analyses of cancer-specific events in particular. Further, SEER patients have been shown to have, on average, higher socioeconomic status; thus SEER data are not precisely representative of the US cancer population (76,77). Because trial patients also tend to have higher socioeconomic status than the general cancer population (78), these consistent biases might enable a more, rather than less, fair comparison between trial and nontrial patients with respect to survival. Unfortunately, socioeconomic status was not available for both databases. Moreover, the nature of SEER data, with respect to racial, ethnic, sociodemographic, and age distributions, has changed over time, which could impact analyses in unknown ways, although, importantly, we did not observe temporal trends toward greater or lesser generalizability over calendar time. In addition, these results may not apply to other clinical settings (ie, screening). Finally, some elements of this analysis were not prespecified, so a similar analysis in a different set of studies might reveal, in particular, a different duration of trial benefit than the 1-year effect found in this analysis.
This study also had particular strengths compared with prior studies. The approach of systematically examining an entire cooperative group phase III clinical trial database limited potential subjective selection of studies. It also provided a large panel of studies for comparison. Because these studies were from one cooperative group, other potential sources of variation (eg, data collection methods, payment methods, study designs) were implicitly controlled for. These advantages allowed us to aggregate data across studies and thus distinguish the different behaviors of the survival functions between trial and nontrial patients.
These results may serve as a stimulus to design randomized trials with less strict eligibility criteria (79). We found that eligibility pertaining to comorbid conditions comprised approximately 60% of all criteria. Despite this, histology and stage were primarily determinative of survival outcomes, even in the first year when the influence of trial participation was strongest. Eligibility criteria in clinical trials are clearly required to maintain patient safety; however, consideration should be given to relaxing or eliminating criteria where possible. For instance, laboratory cutoff values may exclude patients who are otherwise clinically appropriate for trial treatment, or the exclusion of patients with prior cancer may be less meaningful in an era in which increasingly more patients are cancer survivors. One concern is that broader eligibility will introduce heterogeneity into the clinical trial cohort, which could reduce statistical power. However, because histology and stage are the dominant predictors of outcome, sufficient homogeneity will be retained even if less impactful criteria are softened. Expanding eligibility would have the further advantage of increasing access to clinical trials for a broader cross-section of patients.
Funding
This work was supported in part by Public Health Service Cooperative Agreement grants CA32102, CA38926, CA68183, CA20319, and CA37429 awarded by the National Cancer Institute, National Institutes of Health, and Department of Health and Human Services.
References
- 1. Tejada HA, Green SB, Trimble EL, et al. Representation of African-Americans, Hispanics, and whites in National Cancer Institute cancer treatment trials. J Natl Cancer Inst. 1996;88(12):812–816 [DOI] [PubMed] [Google Scholar]
- 2. Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291(22):2720–2726 [DOI] [PubMed] [Google Scholar]
- 3. Ford JG, Howerton HW, Lai GY, et al. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer. 2008;112(2):228–242 [DOI] [PubMed] [Google Scholar]
- 4. Ross S, Grant A, Counsell C, Gillespie W, Russell I, Prescott R. Barriers to participation in randomised controlled trials: a systematic review. J Clin Epidemiol. 1999;52(12):1143–1156 [DOI] [PubMed] [Google Scholar]
- 5. Unger JM, Green S, Albain KS. Under-representation of elderly patients in cancer clinical trials: causes and remedial strategies. In: Balducci L, Lyman GH, Ershler WB, Extermann M, eds. Comprehensive Geriatric Oncology. 2nd ed. Taylor and Francis; 2004:464–491 [Google Scholar]
- 6. Begg CB, Zelen M, Carbone PP, et al. Cooperative groups and community hospitals. Measurement of impact in the community hospitals. Cancer. 1983;52(9):1760–1767 [DOI] [PubMed] [Google Scholar]
- 7. Hunter CP, Frelick RW, Feldman AR, et al. Selection factors in clinical trials: results from the Community Clinical Oncology Program Physician’s Patient Log. Cancer Treat Rep. 1987;71(6):559–565 [PubMed] [Google Scholar]
- 8. Javid SH, Unger JM, Gralow JR, et al. A prospective analysis of the influence of older age on physician and patient decision-making when considering enrollment in breast cancer clinical trials (SWOG S0316). Oncologist. 2012;17(9):1180–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Klabunde CN, Springer BC, Butler B, White MS, Atkins J. Factors influencing enrollment in clinical trials for cancer treatment. South Med J. 1999;92(12):1189–1193 [DOI] [PubMed] [Google Scholar]
- 10. Green S, Benedetti J, Crowley J. Clinical Trials in Oncology. 2nd ed. Boca Raton, FL: CRC Press; 2003 [Google Scholar]
- 11. Newhouse JP, McClellan M. Econometrics in outcomes research: The use of instrumental variables. Annu Rev Public Health. 1998;19:17–34 [DOI] [PubMed] [Google Scholar]
- 12. Ries LAG, Melbert D, Krapcho M, et al. , eds. SEER Cancer Statistics Review, 1975–2005. Bethesda, MD: National Cancer Institute; 2008 [Google Scholar]
- 13. Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–481 [Google Scholar]
- 14. Cox D. Regression models and life tables. J R Stat Assoc. 1972;34(2):187–220 [Google Scholar]
- 15. Gefeller O, Dette H. Nearest neighbor kernel estimation of the hazard function from censored data. J Statist Comput Simul. 1992;43(1–2):93–101 [Google Scholar]
- 16. Hess KR, Serachitopol DM, Brown BW. Hazard function estimators: a simulation study. Stat Med. 1999;18(22):3075–3088 [DOI] [PubMed] [Google Scholar]
- 17. Mueller HG, Wang JL. Hazard rates estimation under random censoring with varying kernels and bandwidths. Biometrics. 1994;50(1):61–76 [PubMed] [Google Scholar]
- 18. Anderson JE, Kopecky KJ, Willman CL, et al. Outcome after induction chemotherapy for older patients with acute myeloid leukemia is not improved with mitoxantrone and etoposide compared to cytarabine and daunorubicin: a Southwest Oncology Group study. Blood. 2002;100(12):3869–3876 [DOI] [PubMed] [Google Scholar]
- 19. Blumenthal DT, Wade M, Rankin C, et al. MGMT methylation in newly-diagnosed glioblastoma multiforme (GBM): from the S0001 phase III study of radiation therapy (RT) and O-benzylguanine (O BG) plus BCNU versus RT and BCNU alone for newly diagnosed GBM. J Clin Oncol. 2006;24(18S):1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eisenberger MA, Blumenstein BA, Crawford ED, et al. Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N Eng J Cancer. 1998;339(15):1036–1042 [DOI] [PubMed] [Google Scholar]
- 21. Ellis GK, Barlow WE, Gralow JR, et al. Phase III comparison of standard doxorubicin and cyclophosphamide versus weekly doxorubicin and daily oral cyclophosphamide plus granulocyte colony-stimulating factor as neoadjuvant therapy for inflammatory and locally advanced breast cancer: SWOG 0012. J Clin Oncol. 2011;29(8):1014–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Flanigan RC, Salmon SE, Blumenstein BA, et al. Nephrectomy followed by interferon alfa 2-b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Eng J Med. 2001;345(23):1655–1659 [DOI] [PubMed] [Google Scholar]
- 23. Gandara DR, Crowley J, Livingston RB, et al. Evaluation of cisplatin intensity in metastatic non-small cell lung cancer: a phase III study of the Southwest Oncology Group. J Clin Oncol. 1993;11(5):873–878 [DOI] [PubMed] [Google Scholar]
- 24. Godwin JE, Kopecky KJ, Head DR, et al. A double-blind placebo-controlled trial of granulocyte colony-stimulating factor in elderly patients with previously untreated acute myeloid leukemia: a Southwest Oncology Group study (9031). Blood. 1998;91(10):3607–3615 [PubMed] [Google Scholar]
- 25. Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Eng J Med. 2003;349(9):859–866 [DOI] [PubMed] [Google Scholar]
- 26. Kelly K, Crowley J, Bunn PA, et al. Randomized phase III trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced non-small cell lung cancer: a Southwest Oncology Group trial. J Clin Oncol. 2001;19(13):3210–3218 [DOI] [PubMed] [Google Scholar]
- 27. Lamm DL, Blumenstein BA, Crawford ED, et al. Randomized intergroup comparison of bacillus calmette-guerin immunotherapy and mitomycin C chemotherapy prophylaxis in superficial transitional cell carcinoma of the bladder. A Southwest Oncology Group study. Urol Oncol. 1995;1(3):119–126 [DOI] [PubMed] [Google Scholar]
- 28. Lara PN, Jr, Natale R, Crowley J, et al. Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive-stage small-cell lung cancer: clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol. 2009;27(15):2530–2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Linden HM, Haskell CM, Green S, et al. Sequenced compared with simultaneous anthracycline and cyclophosphamide in high-risk stage I and II breast cancer: final analysis from INT-0137 (S9313). J Clin Oncol. 2007;25(6):656–661 [DOI] [PubMed] [Google Scholar]
- 30. Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM, Martenson JA. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Eng J Med. 2001;345(10):725–730 [DOI] [PubMed] [Google Scholar]
- 31. Meyskens FL, Jr, Kopecky KJ, Taylor CW, et al. Randomized trial of adjuvant human interferon gamma versus observation in high-risk cutaneous melanoma: a Southwest Oncology Group study. J Natl Cancer Inst. 1995;87(22):1710–1713 [DOI] [PubMed] [Google Scholar]
- 32. Peters WA, III, Liu PY, Barrett RJ, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation thereapy alone as adjuvant therapy after radical surgery in high-risk early stage cancer of the cervix. J Clin Oncol. 2000;18(8):1606–1613 [DOI] [PubMed] [Google Scholar]
- 33. Philip PA, Benedetti J, Corless CL, et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. J Clin Oncol. 2010;28(22):3605–3610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pisters KM, Vallières E, Crowley JJ, et al. Surgery with or without preoperative paclitaxel and carboplatin in early-stage non-small-cell lung cancer: Southwest Oncology Group Trial S9900, an intergroup, randomized, phase III trial. J Clin Oncol. 2010;28(11):1843–1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Salmon SE, Crowley JJ, Grogan TM, Finley P, Pugh RP, Barlogie B. Combination chemotherapy, glucocorticoids, and interferon alfa in the treatment of multiple myeloma: a Southwest Oncology Group study. J Clin Oncol. 1994;12(11):2405–2414 [DOI] [PubMed] [Google Scholar]
- 36. Sondak VK, Liu PY, Tuthill RJ, et al. Adjuvant immunotherapy of resected, intermediate-thickness, node-negative melanoma with an allogeneic tumor vaccine: overall results of a randomized trial of the Southwest Oncology Group. J Clin Oncol. 2002;20(8):2058–2066 [DOI] [PubMed] [Google Scholar]
- 37. Williamson SK, Crowley JJ, Lara PN, Jr, et al. Phase III trial of paclitaxel plus carboplatin with or without tirapazamine in advanced non-small-cell lung cancer: Southwest Oncology Group trial S0003. J Clin Oncol. 2005;23(36):9097–9104 [DOI] [PubMed] [Google Scholar]
- 38. Wozniak AJ, Crowley JJ, Balcerzak SP, et al. Randomized trial comparing cisplatin with cisplatin plus vinorelbine in the treatment of advanced non-small-cell lung cancer: a Southwest Oncology Group study. J Clin Oncol. 1998;16(7):2459–2465 [DOI] [PubMed] [Google Scholar]
- 39. Hutchins LF, Unger JM, Crowley JJ, Coltman CA, Jr, Albain KS. Underrepresentation of patients 65 of age or older in cancer-treatment trials. N Eng J Med. 1999;341(27):2061–2067 [DOI] [PubMed] [Google Scholar]
- 40. Unger JM, Coltman CA, Jr, Crowley JJ, et al. Impact of the year 2000 Medicare policy change on older patient enrollment to cancer clinical trials. J Clin Oncol. 2006;24(1):141–144 [DOI] [PubMed] [Google Scholar]
- 41. Peppercorn JM, Weeks JC, Cook EF, Joffe S. Comparison of outcomes in cancer patients treated within and outside clinical trials: conceptual framework and structured review. Lancet. 2004;363(9405):263–270 [DOI] [PubMed] [Google Scholar]
- 42. Edwards SJ, Lilford RJ, Braunholtz DA, Jackson JC, Hewison J, Thornton J. Ethical issues in the design and conduct of randomised controlled trials. Health Technol Assess. 1998;2(15):1–132 [PubMed] [Google Scholar]
- 43. Elting LS, Cooksley C, Bekele BN, et al. Generalizability of cancer clinical trial results: prognostic differences between participants and nonparticipants. Cancer. 2006;106(11):2452–2458 [DOI] [PubMed] [Google Scholar]
- 44. Stiller CA. Centralised treatment, entry to trials and survival. Br J Cancer. 1994;70(2):352–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tanai C, Nakajima TE, Nagashima K, et al. Characteristics and outcomes of patients with advanced gastric cancer who declined to participate in a randomized clinical chemotherapy trial. J Oncol Pract. 2011;7(3):148–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vist GE, Hagen KB, Devereaux PJ, Bryant D, Kristoffersen DT, Oxman AD. Systematic review to determine whether participation in a trial influences outcome. BMJ. 2005;330(7501):1175–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Antman K, Amato D, Wood W, et al. Selection bias in clinical trials. J Clin Oncol. 1985;3(8):1142–1147 [DOI] [PubMed] [Google Scholar]
- 48. Bertelsen K. Protocol allocation and exclusion in two Danish randomized trials in ovarian cancer. British J Cancer. 1991;64:1172–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Boros L, Chuang C, Butler FO, Bennett JM. Leukemia in Rochester (NY). A 17-year experience with an analysis of the role of cooperative group (ECOG) participation. Cancer. 1985;56(9):2161–2169 [DOI] [PubMed] [Google Scholar]
- 50. Burgers JA, Arance A, Ashcroft L, Hodgetts J, Lomax L, Thatcher N. Identical chemotherapy schedules given on and off trial protocol in small cell lung cancer: response and survival results. Br J Cancer. 2002;87(5):562–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cottin V, Arpin D, Lasset C, et al. Small-cell lung cancer: patients included in clinical trials are not representative of the patient population as a whole. Ann Oncol. 1999;10(7):809–815 [DOI] [PubMed] [Google Scholar]
- 52. Dahlberg M, Glimelius B, Pahlman L. Improved survival and reduction in local failure rates after preoperative radiotherapy: evidence for the generalizability of the results of Swedish Rectal Cancer Trial. Ann Surg. 1999;229(4):493–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Davis S, Wright PW, Schulman SF, et al. Participants in prospective, randomized clinical trails for resected non-small cell lung cancer have improved survival compared with nonparticipants in such trials. Cancer. 1985;56(7):1710–1718 [DOI] [PubMed] [Google Scholar]
- 54. Dowling AJ, Czaykowski PM, Krahn MD, Moore MJ, Tannock IF. Prostate specific antigen response to mitoxantrone and prednisone in patients with refractory prostate cancer: prognostic factors and generalizability of a multicenter trial to clinical practice. J Urol. 2000;163(5):1481–1485 [PubMed] [Google Scholar]
- 55. Feuer EJ, Frey CM, Brawley OW, et al. After a treatment breakthrough: a comparison of trial and population-based data for advacned testicular cancer. J Clin Oncol. 1994;12(2):368–377 [DOI] [PubMed] [Google Scholar]
- 56. Greil R, Holzner B, Kemmler G, et al. Retrospective assessment of quality of life and treatment outcome in patients with Hodgkin’s disease from 1969 to 1994. Eur J Cancer. 1999;35(5):698–706 [DOI] [PubMed] [Google Scholar]
- 57. Karjalainen S, Palva I. Do treatment protocols improve end results? A study of survival of patients with multiple myeloma in Finland. BMJ. 1989;299(6707):1069–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lennox EL, Stiller CA, Jones PH, Wilson LM. Nephroblastoma: treatment during 1970–73 and the effect on survival of inclusion in the first MRC trial. Br Med J. 1979;2(6190):567–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Link MP, Goorin AM, Miser AW, et al. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity. N Engl J Med. 1986;314(25):1600–1606 [DOI] [PubMed] [Google Scholar]
- 60. Marubini E, Mariani L, Salvadori B, et al. Results of a breast-cancer-surgery trial compared with observational data from routine practice. Lancet. 1996;347(9007):1000–1003 [DOI] [PubMed] [Google Scholar]
- 61. Mayers C, Panzarella T, Tannock IF. Analysis of the prognostic effects of inclusion in a clinical trial and of myelosuppression on survival after adjuvant chemotherapy for breast carcinoma. Cancer. 2001;91(12):2246–2257 [PubMed] [Google Scholar]
- 62. Meadows AT, Kramer S, Hopsono R, Lustbader E, Jarrett P, Evans AE. Survival in childhood acute lymphocytic leukemia: effect of protocol and place of treatment. Cancer Invest. 1983;1(1):49–55 [DOI] [PubMed] [Google Scholar]
- 63. MRC Working Group on Leukaemia Duration of survival of children with acute leukemia. Report to the Medical Research Council from the Committee on Leukaemia and the Working Party on Leukaemia in Childhood. BMJ. 1971;4(5778):7–9 [PMC free article] [PubMed] [Google Scholar]
- 64. Roy P, Vaughan Hudson G, Vaughan Hudson B, Esteve J, Swerdlow AJ. Long-term survival in Hodgkin’s disease patients. A comparison of relative survival in patients in trials and those recorded in population-based cancer registries. Eur J Cancer. 2000;36(3):384–389 [DOI] [PubMed] [Google Scholar]
- 65. Schea RA, Perkins P, Allen PK, Komaki R, Cox JD. Limited-stage small-cell lung cancer: patient survival after combined chemotherapy and radiation therapy with and without treatment protocols. Radiology. 1995;197(3):859–862 [DOI] [PubMed] [Google Scholar]
- 66. Schmoor C, Olschewski M, Schumacher M. Randomized and non-randomized patients in clinical trials: experiences with comprehensive cohort studies. Stat Med. 1996;15(3): 263–271 [DOI] [PubMed] [Google Scholar]
- 67. Stiller CA, Benjamin S, Cartwright RA, et al. Patterns of care and survival for adolescents and young adults with acute leukaemia—a population-based study. Br J Cancer. 1999;79(3–4):658–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Stiller CA, Draper GJ. Treatment centre size, entry to trials, and survival in acute lymphoblastic leukaemia. Arch Dis Child. 1989;64(5):657–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Stiller CA, Eatock EM. Survival from acute non-lymphocytic leukaemia, 1971–88: a population based study. Arch Dis Child. 1994;70(3):219–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Stiller CA, Eatock EM. Patterns of care and survival for children with acute lymphoblastic leukaemia diagnosed between 1980 and 1994. Arch Dis Child. 1999;81(3):202–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wagner HP, Dingeldein-Bettler I, Berchthold W, et al. Childhood NHL in Switzerland: incidence and survival of 120 study and 42 non-study patients. Med Pediatr Oncol. 1995;24(5):281–286 [DOI] [PubMed] [Google Scholar]
- 72. Ward LC, Fielding JW, Dunn JA, Kelly KA. The selection of cases for randomised trials: a registry survey of concurrent trial and non-trial patients. The British Stomach Cancer Group. Br J Cancer. 1992;66(5):943–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Winger MJ, Macdonald DR, Schold SC, Jr, Cairncross JG. Selection bias in clinical trials of anaplastic glioma. Ann Neurol. 1989;26(4):531–534 [DOI] [PubMed] [Google Scholar]
- 74. Braunholtz DA, Edwards SJ, Lilford RJ. Are randomized clinical trials good for us (in the short term)? Evidence for a “trial effect.” J Clin Epidemiol. 2001;54(3):217–224 [DOI] [PubMed] [Google Scholar]
- 75. Grimshaw JM, Russell IT. Effect of clinical guidelines on medical practice: a systematic review of rigorous evaluations. Lancet. 1993;342(8883):1317–1322 [DOI] [PubMed] [Google Scholar]
- 76. Nattinger AB, McAuliffe TL, Schapira MM. Generalizability of the surveillance, epidemiology, and end results registry population: factors relevant to epidemiologic and health care research. J Clin Epidemiol. 1997;50(8):939–945 [DOI] [PubMed] [Google Scholar]
- 77. Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8 Suppl):IV-3–18. [DOI] [PubMed] [Google Scholar]
- 78. Sateren WB, Trimble EL, Abrams J, et al. How sociodemographics, presence of oncology specialists, and hospital cancer programs affect accrual to cancer treatment trials. J Clin Oncol. 2002;20(8):2109–2117 [DOI] [PubMed] [Google Scholar]
- 79. George SL. Reducing patient eligibility criteria in cancer clinical trials. J Clin Oncol. 1996;14(4):1364–1370 [DOI] [PubMed] [Google Scholar]