Abstract
Background
The effect of prostate-specific antigen (PSA) screening on prostate cancer mortality remains debated, despite evidence from randomized trials. We investigated the association between prostate cancer incidence, reflecting uptake of PSA testing, and prostate cancer mortality.
Methods
The study population consisted of all men aged 50 to 74 years residing in eight counties in Sweden with an early increase in prostate cancer incidence and six counties with a late increase during two time periods. Incidence of metastatic prostate cancer was investigated in the period from 2000 to 2009, and prostate cancer–specific mortality and excess mortality were investigated in the period from 1990 to 1999 and the period from 2000 to 2009 by calculating rate ratios for high- vs low-incidence counties and rate ratios for the period from 2000 to 2009 vs the period from 1990 to 1999 within these two groups. All statistical tests were two-sided.
Results
There were 4528134 person-years at risk, 1577 deaths from prostate cancer, and 1210 excess deaths in men with prostate cancer in high-incidence counties and 2471373 person-years at risk, 985 prostate cancer deaths, and 878 excess deaths in low-incidence counties in the period from 2000 to 2009. Rate ratios in counties with high vs low incidence adjusted for time period were 0.81 (95% confidence interval [CI] = 0.73 to 0.90) for prostate cancer– specific mortality and 0.74 (95% CI = 0.64 to 0.86) for excess mortality, and the rate ratio of metastatic prostate cancer was 0.85 (95% CI = 0.79 to 0.92).
Conclusions
The lower prostate cancer mortality in high-incidence counties reflecting a high PSA uptake suggests that more-intense as compared with less-intense opportunistic PSA screening reduces prostate cancer mortality.
The use of prostate-specific antigen (PSA) screening for detection of prostate cancer remains controversial. Two large, population-based, randomized clinical trials—the European Randomized Study of Screening for Prostate Cancer (ERSPC) and the Göteborg trial—demonstrated 21% to 44% statistically significant decreases in prostate cancer–specific mortality after 11 to 14 years of follow-up in screened vs unscreened men (1,2). In contrast, the prostate arm of the US Prostate, Lung, Colorectal, and Ovarian cancer screening trial (PLCO) found no benefit from systematic screening compared with opportunistic screening (3).
Thus, a controversy on PSA screening still remains, and in 2012 the US Preventive Services Task Force recommended against screening, stating “there is moderate to high certainty that the benefits of PSA-based screening for prostate cancer [in terms of reduced prostate cancer mortality] do not outweigh the harm [in terms of overdiagnosis]” (4). This recommendation has been criticized (5–7), and more data on the association between PSA screening and prostate cancer mortality are needed.
In addition to extended data from ERSPC and the Göteborg trial and the awaited results from the ongoing UK ProtecT trial, carefully designed and conducted observational population-based studies may provide valuable information on the association between PSA testing, early diagnosis, and treatment and prostate cancer mortality (8,9). Results from previous observational studies have been inconsistent; some studies have shown decreased prostate cancer mortality in areas with high PSA testing (10,11), whereas others have found no such difference (12,13).
In Sweden, as in many other Western countries, the introduction of PSA testing in the 1990s resulted in an increase in prostate cancer incidence. Except for a randomized trial on systematic screening conducted in the city of Göteborg, the introduction of PSA testing was in the form of opportunistic screening (14). Among the 24 Swedish counties, there were large differences in prostate cancer incidence, with an estimated fourfold variation in the proportion of men undergoing PSA testing despite a uniform, equal-access health-care system (15).
To capitalize on this natural experiment, we retrieved outcome data from three nation-wide, population-based registries in Sweden and assessed the incidence of metastatic prostate cancer, prostate cancer–specific mortality, and excess mortality in counties with an early increase in the incidence of prostate cancer and in counties with a late increase, reflecting differences in uptake of PSA testing, to investigate the association between PSA testing and prostate cancer mortality.
Methods
The Swedish Cancer Register, Cause of Death Register, and National Prostate Cancer Register
Swedish law mandates and regulates the registration of incident cancer cases in the Swedish Cancer Register and deaths in the Cause of Death Register. The Cancer Register contains each patient’s 10-digit personal identity number, date of diagnosis, county of residence, and cancer site. The Cause of Death Register contains the personal identity number, date of death, and underlying and contributory causes of death. Since 1998, approximately 98% of all incident prostate cancer cases in the Swedish Cancer Register were also registered in the National Prostate Cancer Register, including the reason for the work-up that led to diagnosis, tumor stage, Gleason score, serum PSA at time of diagnosis, and primary treatment (16,17).
Identification of Prostate Cancer Case Patients and Endpoints
We used the Swedish Cancer Register to identify prostate cancer case patients diagnosed from January 1, 1980, to December 31, 2009, and linked by personal identity numbers (18) to the National Prostate Cancer Register to obtain information on metastases at the time of diagnosis, evidenced by radiographic evaluation (bone scans) for the large majority of men or serum PSA levels greater than 100ng/mL (16). We obtained date and cause of death by linkage to the Swedish Cause of Death Register for death attributed to prostate cancer when it was coded as “underlying cause of death.” The study was approved by the Research Ethics Board at Umeå University Hospital.
Statistical Analysis
We calculated the predicted prostate cancer incidence—that is, the incidence that would be expected if no PSA testing had occurred. Because PSA testing was introduced in clinical practice in Sweden in the 1990s, the prediction for each year up to 2009 was based on the observed incidence for the period from 1980 to 1990. We used a linear regression model with a common slope for calendar year but separate intercepts for each county. Age-adjusted prostate cancer incidence for men aged 50 to 74 years was calculated by direct standardization with weights from the Swedish population census of 2000 (19). We then calculated the cumulative difference between the observed and predicted age-adjusted prostate cancer incidence in men aged 50 to 74 years starting from 1995 and found a range of −126 per 100000 to 1634 per 100000. We used this difference to categorize counties as having high, intermediate, or low prostate cancer incidence and chose the cutoffs of 100 per 100000 between low- and intermediate-incidence counties and 800 per 100000 between intermediate- and high-incidence counties.
We used two measures of prostate cancer mortality—prostate cancer–specific mortality, which was based on the underlying cause of death given in the Cause of Death Register (20,21), and excess mortality (22), based on the excess number of deaths (observed minus expected) regardless of cause of death among men with prostate cancer. We calculated the expected number of deaths as the product of person-years among the incident prostate cancer case patients and the total mortality rate in the population, calculated per year and per attained age in groups of 5 years. We determined prostate cancer mortality during two calendar periods, 1990 to 1999 and 2000 to 2009. We calculated incidence-based mortality for each of the mortality measures based on deaths among men diagnosed with prostate cancer during the study period (20,21). In addition, we calculated the 2000 to 2009 incidence of metastatic prostate cancer (23). To determine incidence and mortality rates, we used person-years based on population statistics by year and by 5-year age group. All rates were measured at the population level (with the male population, not the number of men with prostate cancer as denominator).
We calculated rate ratios (RRs) for high- vs low-incidence counties and the rate ratios for the period from 2000 to 2009 vs the period from 1990 to 1999 within these two groups. Finally we also determined rate ratios for high- vs low-incidence counties adjusted for time period by dividing by the corresponding rate ratio in the period from 1990 to 1999. We calculated rate ratios for men aged 50 to 74 years and the subgroup aged 55 to 69 years, which was the core age group in the ERSPC study (24). We based confidence intervals (CIs) on the assumption of a Poisson distribution of the number of events and calculated variances using the delta method on the logarithm of the estimates followed by normal approximation (25).
Results
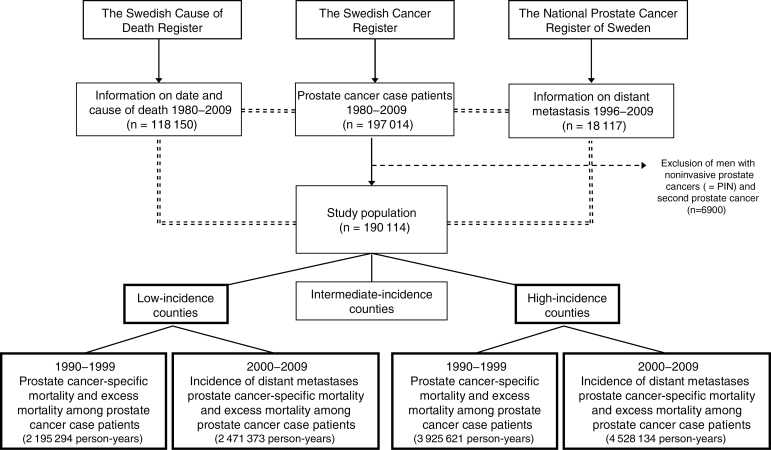
Between 1980 and 2009, 197014 Swedish men aged 50 to 74 years were diagnosed with prostate cancer, and of those, 6900 men with noninvasive or secondary prostate cancers were excluded from the study. Figure 1 presents a flow chart of the study cohort.
Figure 1.
Flow chart of linkages between the Swedish Cancer Register, the Swedish Cause of Death Register, and the National Prostate Cancer Register of Sweden and final study population. * Distant metastasis defined as M1 and/or prostate-specific antigen ≥ 100ng/mL.
======= indicates registry linkage.
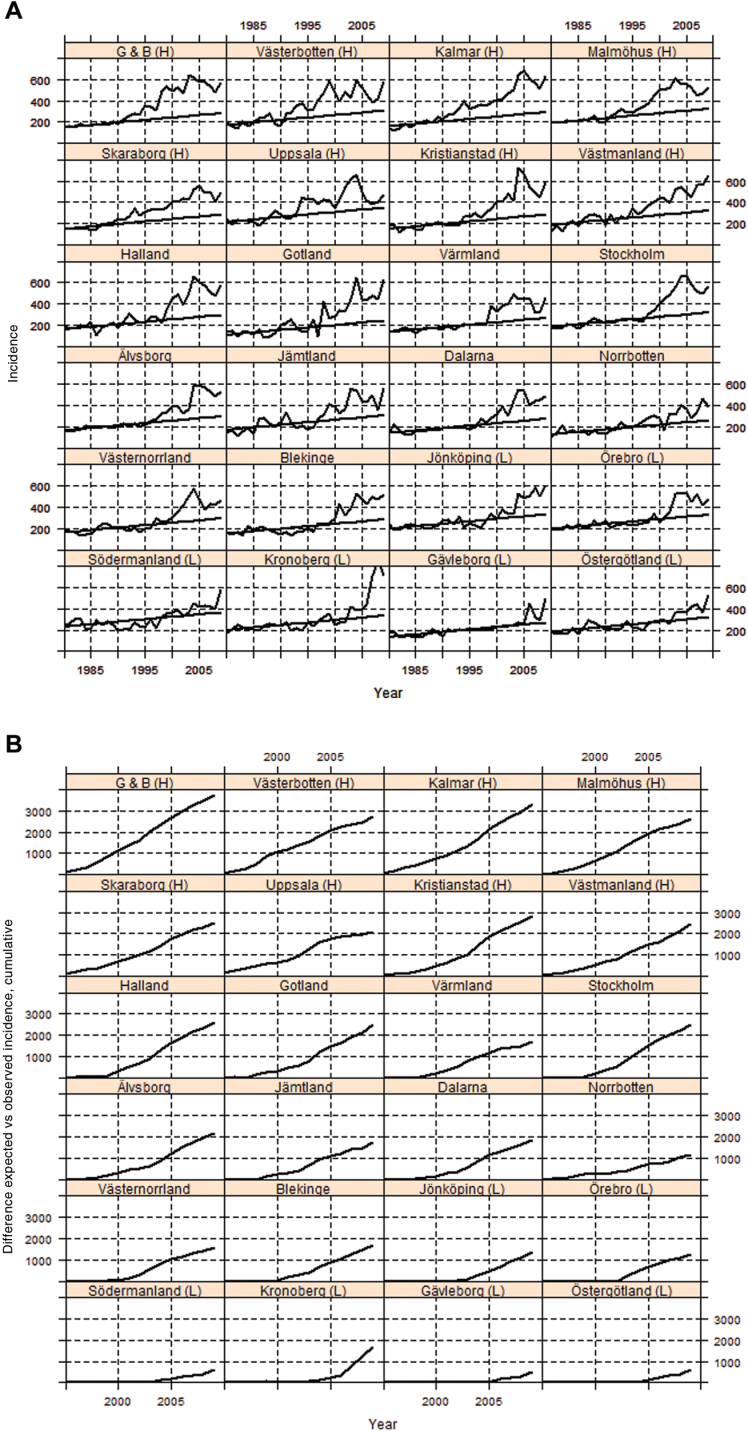
There were 4528134 person-years at risk, 1577 deaths from prostate cancer, and 1210 excess deaths in men with prostate cancer in high-incidence counties and 2471373 person-years, 985 prostate cancer deaths, and 878 excess deaths in low-incidence counties in the period from 2000 to 2009. A rapid increase in prostate cancer incidence began in some counties in 1990 but not until 10 years later in other counties (Figure 2A). Figure 2B shows the cumulative difference between observed and predicted prostate cancer incidence in each county from 1995 to 2009. The difference in incidence between the high- and low-incidence counties was largest in 2005 and decreased thereafter, disappearing in 2009 (Supplementary Figure 1, available online).
Figure 2.
Counties ranked by the cumulative difference between observed and predicted prostate cancer incidence per 100000 from 1995 through 2002. A) Observed and predicted age-standardized prostate cancer incidence in men aged 50–74 years in 24 Swedish counties during the period from 1980 to 2009. Steady line is predicted incidence, and undulating line is observed incidence. B) Cumulative difference between observed and predicted incidence of prostate cancer during the period from 1995 to 2009. Negative differences resulting from the predicted incidence being higher than the observed incidence in low-incidence counties were set to zero. G & B = Göteborg and Bohus county; H = high-incidence county; L = low incidence county.
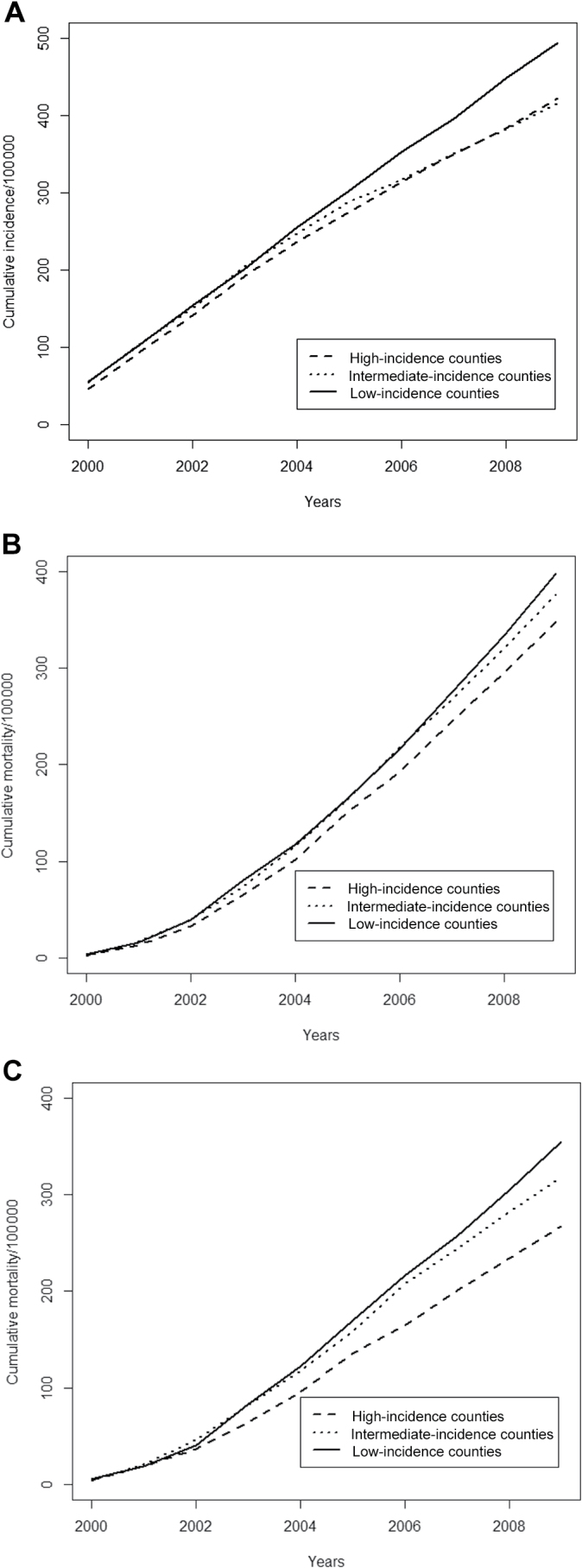
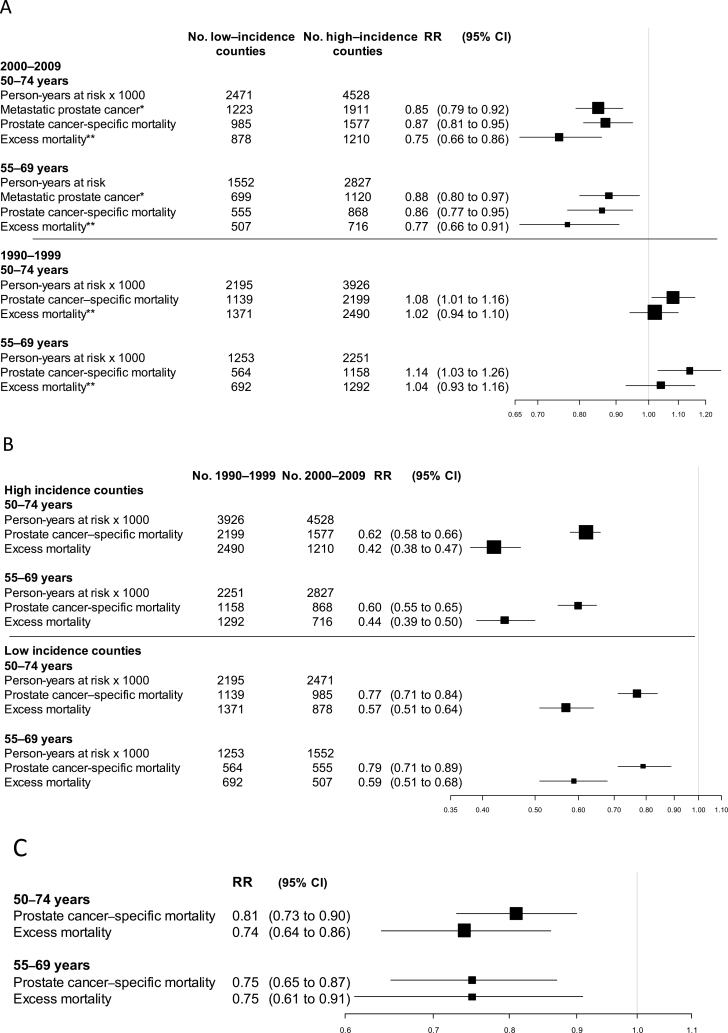
In the period from 2000 to 2009, the cumulative incidence of metastatic disease, prostate cancer–specific mortality, and excess mortality was statistically significantly lower in high-incidence counties than in low incidence counties (Figure 3, A–C), with rate ratios of 0.85 (95% = 0.79 to 0.92) for metastatic disease, 0.87 (95% CI = 0.81 to 0.95) for prostate cancer–specific mortality, and 0.75 (95% CI = 0.66 to 0.86) for excess mortality (Figure 4A).
Figure 3.
Prostate cancer incidence and mortality in men in Sweden aged 50 to 74 years, 2000 to 2009.
A) Cumulative incidence of metastatic disease. B) Prostate cancer-specific mortality.
C) Excess mortality.
Figure 4.
Risk of prostate cancer mortality according to county of residency (in groups of counties with high and low incidence) and time period in groups of counties with high, intermediate and low incidence of prostate cancer A) Rate ratio (RR) of incidence of metastatic prostate cancer, prostate cancer–specific mortality, and excess mortality in high- vs low-incidence counties. B) Rate ratio of prostate cancer–specific mortality and excess mortality in the period from 2000 to 2009 vs the period from 1990 to 1999. C) Rate ratio for high- vs low-incidence group adjusted for time period. * Metastatic prostate cancer defined as M1 and/or prostate-specific antigen ≥ 100ng/mL at diagnosis. ** Excess mortality defined as the excess number of deaths (observed minus expected), regardless of cause of death among men with prostate cancer. CI = confidence interval.
In high-incidence counties, prostate cancer–specific mortaliy and excess mortality were statistically significantly lower during the period from 2000 to 2009 than during the period from 1990 to 1999, and we observed similar, albeit somewhat weaker differences in low-incidence counties (Figure 4B). When taking both county group and time period into consideration, the differences in cancer-specific mortality and excess mortality between high- vs low-incidence counties remained statistically significant (Figure 4C). The rate ratios adjusted for time period for high- vs low-incidence counties were 0.81 (95% CI = 0.73 to 0.90) for prostate cancer–specific mortality and 0.74 (95% CI = 0.64 to 0.86) for excess mortality, and the rate ratios for the subgroup of men aged 55 to 69 years were similar. The estimated rate ratio of prostate cancer specific mortality of 0.81 would correspond to an annual absolute reduction at 0.23 per 1000 men when applied to the Swedish prostate cancer mortality year 2000 in the age group 55 to 79 years.
Data in the National Prostate Cancer Register from 2000 to 2009 indicated that diagnostic and therapeutic activity was higher in high-incidence counties than in low-incidence counties (Table 1). In the high-incidence counties, median age at diagnosis was lower, a higher proportion of men had low-risk cancer (clinical stage T1–T2, Gleason score 2–6, and PSA < 10ng/mL at diagnosis), a higher proportion underwent radical prostatectomy, and median serum PSA at diagnosis was lower. The difference between high- vs low-incidence counties in diagnostic PSA levels was largest in 2000 (10.0 vs 17.6ng/mL) and decreased steadily after that (Supplementary Table 1, available online). The use of radiotherapy and radical prostatectomy showed similar temporal trends with the highest use in high-incidence counties and with a decreasing difference over time (Supplementary Figure 2, available online).
Table 1.
Characteristics of men aged 50 to 74 years with prostate cancer in the National Prostate Cancer Register of Sweden, 2000 to 2009*
| Characteristic | County Incidence | ||
|---|---|---|---|
| High (n = 33780) | Intermediate (n = 37624) | Low (n = 16377) | |
| Age at diagnosis, y | |||
| Median (IQR) | 70 (63–77) | 70 (63–77) | 72 (65–79) |
| Mean (SD) | 70.0 (9.3) | 70.2 (9.2) | 71.7 (9.1) |
| Serum PSA level, ng/mL | |||
| Median (IQR) | 10.8 (6.0–27.0) | 12.0 (6.6–32.0) | 15.0 (7.6–43.0) |
| No. missing (%) | 506 (1.5) | 1114 (3.0) | 385 (2.4) |
| Mode of detection, No. (%) | |||
| PSA testing as a part of health check-up | 10684 (31.6) | 10101 (26.8) | 3646 (22.3) |
| Lower urinary tract symptoms | 10533 (31.2) | 10668 (28.4) | 5793 (35.4) |
| Other symptoms/unknown | 12563 (37.2) | 16855 (44.8) | 6938 (42.4) |
| Planned treatment, No. (%)† | |||
| Surveillance | 8937 (26.5) | 8613 (22.9) | 4079 (24.9) |
| Radical prostatectomy | 8444 (25.0) | 8425 (22.4) | 2419 (14.8) |
| Radiation therapy | 4198 (12.4) | 4891 (13.0) | 2501 (15.3) |
| Hormonal therapy | 10931 (32.4) | 12600 (33.5) | 6620 (40.4) |
| Other/missing | 1270 (3.8) | 3095 (8.2) | 758 (4.6) |
| Risk category, No. (%)‡ | |||
| Low risk | 9874 (29.2) | 9593 (25.5) | 3366 (20.6) |
| Intermediate risk | 8651 (25.6) | 8997 (23.9) | 3867 (23.6) |
| High risk | 7908 (23.4) | 9917 (26.4) | 4570 (27.9) |
| Regionally metastatic | 2097 (6.2) | 2735 (7.3) | 1407 (8.6) |
| Distant metastases | 4524 (13.4) | 5158 (13.7) | 2891 (17.7) |
| Missing | 726 (2.1) | 1224 (3.3) | 276 (1.7) |
* IQR = interquartile range; PSA = prostate-specific antigen; SD = standard deviation.
† Initiated or planned within the 6 months after diagnosis.
‡ Risk groups according to modification of the National Comprehensive Cancer Network. Low risk: T1 to 2, Gleason score 2 to 6, and PSA < 10ng/mL. Intermediate risk: T1 to 2, Gleason score 7, and/or PSA 10 to <20ng/mL. High risk: T3, and/or Gleason score 8 to 10, and/or PSA 20 to <50ng/mL. Regionally metastatic disease: T4 and/or N1 and/or PSA 50 to <100ng/mL in the absence of distant metastases (M0 or Mx). Distant metastases: M1 and/or PSA ≥100ng/mL.
Discussion
In this register-based, population-based study in Sweden, incidence of metastatic prostate cancer was 15% lower, and prostate cancer–specific mortality and excess mortality adjusted for time period were 19% and 26% lower, respectively, in counties with high vs low incidence of prostate cancer, reflective of early vs late uptake of PSA testing. These results suggest that opportunistic PSA screening decreases prostate cancer mortality.
The strength of our study lies in its population-based design, its magnitude, the completeness of the registers, and the equal access health care in the two study groups. It covered nearly 7 million person-years at risk and 2562 prostate cancer deaths registered between 2000 and 2009 and had the power to detect moderately strong associations between PSA testing and prostate cancer death. Furthermore, PSA testing is likely to be particularly effective in Sweden because prostate cancer mortality is higher in Sweden than in other countries, with a lifetime risk of prostate cancer death of 5% to 6%, so Swedish men with prostate cancer are at high risk of disease progression (26). Other strengths of our study were the use of incidence-based mortality, which enabled us to avoid diluting risk estimates by including deaths among men diagnosed before the introduction of PSA testing, and the use of three separate endpoints—incidence of metastatic prostate cancer, prostate cancer-specific mortality, and excess mortality.
The Swedish Cancer Register captures 96% of all cancer diagnoses, and the capture rate is particularly high for solid tumors and in subjects aged less 70 years (27). The validity of the Cause of Death Register is high for prostate cancer. For example, in the Göteborg screening trial, there was a 96% agreement between a chart review of death certificates and the Cause of Death Register (28), and in another study with a wider range in stage and grade, the agreement was 86% (29). Besides prostate cancer–specific mortality, we also investigated the incidence of metastatic prostate cancer, which was the first indication of the efficacy of PSA screening in the European trials (1,2). We assessed the occurrence of metastatic disease at date of diagnosis by use of data on the presence of bone metastases or a serum level greater than 100ng/mL available from 2000 in the National Prostate Cancer Register.
Our study also had some limitations because we were unable to directly measure the extent of PSA testing in the population. Instead, we used the difference between the observed and predicted cumulative incidence of prostate cancer under the assumption that a high incidence indicated an early introduction and a high prevalence of PSA testing with ensuing early prostate cancer diagnosis and treatment. This assumption was corroborated by data in the National Prostate Cancer Register on distribution of risk categories with lower median serum PSA levels at diagnosis, higher proportion of clinically localized low-risk cancers, higher proportion of curative treatments, and a lower age at diagnosis in high- vs low-incidence counties.
Geographical comparisons can be hampered by differences in baseline risk, and prior observational studies comparing high and low prostate cancer incidence areas in the United States reported no difference in prostate cancer mortality (12,13). In the first time period of our study, prostate cancer–specific mortality was higher in high-incidence counties than in low-incidence counties, showing that the subsequently lower prostate cancer mortality in high-incidence counties was not simply the result of differences in baseline risk. Temporal comparisons can be hampered by changes in diagnostic criteria over time and thus can also be affected by bias. To address these issues, we made separate geographical and temporal comparisons and used a combined approach as well, including adjustment for time periods in the analysis of geographical differences.
Our risk estimates were affected by other sources of bias. The decrease in excess mortality was consistently larger than the decrease in prostate cancer–specific mortality. Excess mortality likely overestimates the benefit of screening because it reflects a lower mortality from causes other than prostate cancer. There may be selection bias for healthy Swedish men with a long life expectancy who undergo PSA testing and early detection, as suggested in a previous study in the National Prostate Cancer Register, which showed lower 10-year all-cause mortality among men with low- and intermediate-risk prostate cancer compared with the background population, indicating a healthy screenee effect (30). In contrast, prostate cancer–specific mortality underestimates the risk reduction because it is affected by attribution bias; death from an uncertain cause is more likely attributed to prostate cancer in men with a prostate cancer diagnosis than in other men (31). Furthermore, our follow-up time was 10 years at maximum, which is likely too short a time to reap the full effect of early detection. Finally, confounding by unknown factors cannot be ruled out. Despite these shortcomings, a higher incidence of prostate cancer was consistently associated with lower risk estimates in all 18 risk analyses.
The ERSPC study, the largest randomized screening trial to date with 761 prostate cancer deaths, showed virtually the same reduction (21%) in mortality observed after 11 years of follow-up as our study (1). In the Göteborg screening trial in Sweden, based on 122 prostate cancer deaths, a larger reduction in mortality was observed (44%), likely because of the longer median follow-up of 14 years. Speculatively, the larger effect in the Göteborg trial compared with our observations may, in addition to a longer follow-up, also be because of a more stringent work-up of men with elevated serum PSA in a trial setting and to a superior diagnostic and therapeutic level of care in a high-volume setting as compared with our results that were based on routine clinical practice among all health-care providers in 14 Swedish counties.
Our results from a population-based, real-life study indicate that more-intense as compared with less-intense opportunistic PSA screening decreases prostate cancer mortality, which reconciles the findings of the two largest trials on PSA screening to date, namely ERSPC (organized vs no screening) and PLCO (organized vs opportunistic screening) and is congruent with the reduction of prostate cancer mortality that has occurred in the United States during the last decades, during which time period early diagnosis and early treatment has increased drastically (32).
However, opportunistic screening as it is currently implemented in real life is inefficient and is implemented too frequently in the wrong age groups. In a recent Swedish study, 6% of men aged 40 to 49 years, 16% of men age 50 to 59 years, 27% of men aged 60 to 69 years, 30% of men aged 70 to 79 years, and 23% of men age 80 to 89 years had an annual PSA test (33), whereas in the United States, 45% of men aged 75 years and older have a yearly PSA test (34). The frequent PSA testing of older men leads to overdetection and overtreatment, and to maximize the benefits while at the same time minimizing the adverse effects of screening and ensuing treatment, risk-stratified screening with regular but infrequent PSA testing of middle-aged men holds promise (35).
In conclusion, in our population-based study we observed lower incidence of metastatic prostate cancer, lower prostate cancer–specific mortality, and lower excess mortality in counties with high vs low incidence of prostate cancer, reflecting PSA uptake. This indicates that more-intense as compared with less-intense opportunistic PSA screening reduces prostate cancer mortality.
Funding
This work was funded by The Swedish Research Council 825-2008-5910 and the Swedish Cancer Society 11 0471, Västerbotten County Council, and Lions Cancer Research Foundation at Umeå University, Sweden. HL is supported by grants from the National Cancer Institute (R33 CA 127768-03, P50-CA92629); the Swedish Cancer Society (11–0624); the Sidney Kimmel Center for Prostate and Urologic Cancers; David H. Koch through the Prostate Cancer Foundation; the National Institute for Health Research, Oxford Biomedical Research Centre, Oxford University Hospitals NHS Trust and University of Oxford; and Fundación Federico SA. SC is supported by grants from the Swedish Cancer Society, the Sweden America Foundation, the Swedish Council for Working Life and Social Research, and the Swedish Society for Medical Research.
None of the study sponsors had any role in the design of the study, the data collection, analysis, interpretation of the data, manuscript writing, or decision to submit the manuscript for publication.
The project was made possible by the continuous work of the National Prostate Cancer Register of Sweden steering group: Pär Stattin (chairman), Anders Widmark, Camilla Thellenberg, Ove Andrén, Anna Bill-Axelsson, Ann-Sofi Fransson, Magnus Törnblom, Stefan Carlsson, Marie Hjälm-Eriksson, Bodil Westman, Bill Pettersson, David Robinson, Mats Andén, Jan-Erik Damber, Jonas Hugosson, Ingela Franck-Lissbrant, Maria Nyberg, Göran Ahlgrén, Ola Bratt, René Blom, Lars Egevad, Calle Waller, Jan-Erik Johansson, Olof Akre, Per Fransson, Eva Johansson, Fredrik Sandin, Hans Garmo, Mats Lambe, Karin Hellström, Annette Wigertz, and Erik Holmberg. Miriam Bloom, PhD (SciWrite Biomedical Writing & Editing Services), provided linguistic editing.
References
- 1. Schroder FH, Hugosson J, Roobol MJ, et al. Prostate-cancer mortality at 11 years of follow-up. N Engl J Med. 2012;366(11):981–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hugosson J, Carlsson S, Aus G, et al. Mortality results from the Goteborg randomised population-based prostate-cancer screening trial. Lancet Oncol. 2010;11(8):725–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Andriole GL, Crawford ED, Grubb RL, 3rd, et al. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104(2):125–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moyer VA, US Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157(2):120–134 [DOI] [PubMed] [Google Scholar]
- 5. Volk RJ, Wolf AM. Grading the new US Preventive Services Task Force prostate cancer screening recommendation. JAMA. 2011;306(24):2715–2716 [DOI] [PubMed] [Google Scholar]
- 6. Carlsson S, Vickers AJ, Roobol M, et al. Prostate cancer screening: facts, statistics, and interpretation in response to the US Preventive Services Task Force Review. J Clin Oncol. 2012;30(21):2581–2584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McNaughton-Collins MF, Barry MJ. One man at a time—resolving the PSA controversy. N Engl J Med. 2011;365(21):1951–1953 [DOI] [PubMed] [Google Scholar]
- 8. Benson K, Hartz AJ. A comparison of observational studies and randomized, controlled trials. N Engl J Med. 2000;342(25):1878–1886 [DOI] [PubMed] [Google Scholar]
- 9. Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med. 2000;342(25):1887–1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bartsch G, Horninger W, Klocker H, et al. Tyrol Prostate Cancer Demonstration Project: early detection, treatment, outcome, incidence and mortality. BJU Int. 2008;101(7):809–816 [DOI] [PubMed] [Google Scholar]
- 11. van Leeuwen PJ, Connolly D, Gavin A, et al. Prostate cancer mortality in screen and clinically detected prostate cancer: estimating the screening benefit. Eur J Cancer. 2010;46(2):377–383 [DOI] [PubMed] [Google Scholar]
- 12. Lu-Yao G, Albertsen PC, Stanford JL, Stukel TA, Walker-Corkery E, Barry MJ. Screening, treatment, and prostate cancer mortality in the Seattle area and Connecticut: fifteen-year follow-up. J Gen Intern Med. 2008;23(11):1809–1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lu-Yao G, Albertsen PC, Stanford JL, Stukel TA, Walker-Corkery ES, Barry MJ. Natural experiment examining impact of aggressive screening and treatment on prostate cancer mortality in two fixed cohorts from Seattle area and Connecticut. BMJ. 2002;325(7367):740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bray F, Lortet-Tieulent J, Ferlay J, Forman D, Auvinen A. Prostate cancer incidence and mortality trends in 37 European countries: an overview. Eur J Cancer. 2010;46(17):3040–3052 [DOI] [PubMed] [Google Scholar]
- 15. Jonsson H, Holmstrom B, Duffy SW, Stattin P. Uptake of prostate-specific antigen testing for early prostate cancer detection in Sweden. Int J Cancer. 2011;129(8):1881–1888 [DOI] [PubMed] [Google Scholar]
- 16. Van Hemelrijck M, Wigertz A, Sandin F, et al. Cohort profile: The National Prostate Cancer Register of Sweden and Prostate Cancer data Base Sweden 2.0. Int J Epidemiol. 2013;42(4):956–967 [DOI] [PubMed] [Google Scholar]
- 17. National Prostate Cancer Registry Annual report. http://npcr.se Accessed October 17, 2013
- 18. Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, Ekbom A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24(11):659–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. National Board of Health and Welfare Cancer Incidence in Sweden 2010. Stockholm: National Board of Health and Welfare; 2010 [Google Scholar]
- 20. Jonsson H, Bordas P, Wallin H, Nystrom L, Lenner P. Service screening with mammography in Northern Sweden: effects on breast cancer mortality—an update. J Med Screen. 2007;14(2):87–93 [DOI] [PubMed] [Google Scholar]
- 21. Swedish Organised Service Screening Evaluation Reduction in breast cancer mortality from organized service screening with mammography: 1. further confirmation with extended data. Cancer Epidemiol Biomarkers Prev. 2006;15(1):45–51 [DOI] [PubMed] [Google Scholar]
- 22. Lenner P, Jonsson H. Excess mortality from breast cancer in relation to mammography screening in northern Sweden. J Med Screen. 1997;4(1):6–9 [DOI] [PubMed] [Google Scholar]
- 23. Mohler J, Bahnson RR, Boston B, et al. NCCN clinical practice guidelines in oncology: prostate cancer. J Natl Compr Canc Netw. 2010;8(2):162–200 [DOI] [PubMed] [Google Scholar]
- 24. Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360(13):1320–1328 [DOI] [PubMed] [Google Scholar]
- 25. Davison AC. Statistical Models. Cambridge, UK: Cambridge University Press; 2003 [Google Scholar]
- 26. International Agency for Research on Cancer GLOBOCAN. http://globocan.iarc.fr/factsheet.asp
- 27. Barlow L, Westergren K, Holmberg L, Talback M. The completeness of the Swedish Cancer Register: a sample survey for year 1998. Acta Oncol. 2009;48(1):27–33 [DOI] [PubMed] [Google Scholar]
- 28. Godtman R, Holmberg E, Stranne J, Hugosson J. High accuracy of Swedish death certificates in men participating in screening for prostate cancer: a comparative study of official death certificates with a cause of death committee using a standardized algorithm. Scand J Urol Nephrol. 2011;45(4):226–232 [DOI] [PubMed] [Google Scholar]
- 29. Fall K, Stromberg F, Rosell J, Andren O, Varenhorst E, South-East Region Prostate Cancer Group Reliability of death certificates in prostate cancer patients. Scand J Urol Nephrol. 2008;42(4):352–357 [DOI] [PubMed] [Google Scholar]
- 30. Stattin P, Holmberg E, Johansson JE, et al. Outcomes in localized prostate cancer: National Prostate Cancer Register of Sweden follow-up study. J Natl Cancer Inst. 2010;102(13):950–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Baker SG, Kramer BS, Prorok PC. Statistical issues in randomized trials of cancer screening. BMC Med Res Methodol. 2002;2:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nordström T, Aly M, Clements MS, Weibull CE, Adolfsson J, Grönberg H. Prostate specific antigen (PSA) testing is prevalent and increasing in Stockholm County, Sweden, despite no recommendations for PSA screening: results from a population-based study, 2003–2011. Eur Urol. 2013;63(3):419–425 [DOI] [PubMed] [Google Scholar]
- 33. Etzioni R, Gulati R, Cooperberg MR, Penson DM, Weiss NS, Thompson IM. Limitations of basing screening policies on screening trials: the US Preventive Services Task Force and Prostate Cancer Screening. Med Care 2013;51(4):295–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Prasad SM, Drazer MW, Huo D, Hu JC, Eggener SE. 2008 US Preventive Service Task Force recommendations and prostate cancer screening rates. JAMA. 2012;307(16):1692–1694 [DOI] [PubMed] [Google Scholar]
- 35. Vickers AJ, Ulmert D, Sjoberg DD, et al. Strategy for detection of prostate cancer based on relation between prostate specific antigen at age 40–55 and long term risk of metastasis: case–control study. BMJ. 2013;346:f2023. [DOI] [PMC free article] [PubMed] [Google Scholar]