Abstract
The kinase AKT has been regarded as an obligate intermediate in the insulin signaling pathway that suppresses glucose production by inhibiting the transcription factor forkhead box O1 (FoxO1) after meals. A new study shows that, without AKT-FoxO1 signaling, insulin still contributes to postprandial responses, revealing an AKT-independent pathway for insulin action that might be exploited to treat metabolic disease (pages 388–395).
Insulin is the primary hormone controlling systemic nutrient and metabolic homeostasis in mammals. Insulin regulates many anabolic functions, including the stimulation of protein and glycogen synthesis (in muscle and liver), lipid synthesis and storage (in liver and adipose tissue) and the inhibition of fatty acid oxidation, glycogenolysis, gluconeo genesis and autophagy1. Insulin resistance leads to hyperglycemia, the hallmark of type 2 diabetes, owing at least in part to persistent hepatic glucose production2. Scientists and clinicians have sought for decades to understand what goes wrong with the insulin signaling cascade, with the hope of developing rational strategies to cure metabolic disease and prevent its progression to type 2 diabetes.
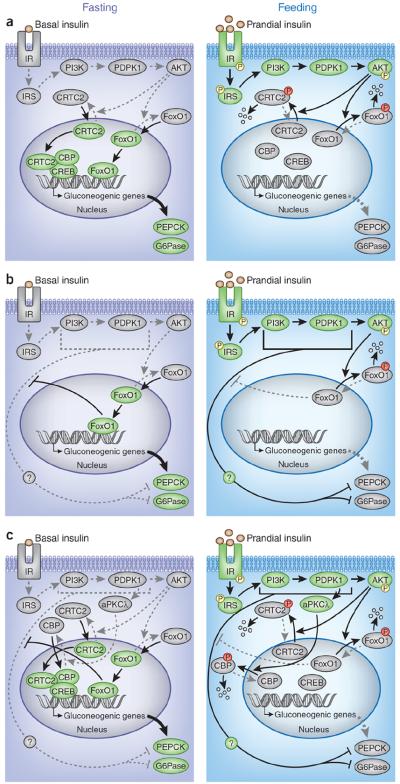
The evidence from previous studies converges on a `canonical pathway' that mediates insulin action in all tissues, including the liver. This pathway begins when insulin activates the transmembrane insulin receptor (IR) tyrosine kinase that phosphorylates the adaptor proteins insulin receptor substrate 1 (IRS1) and IRS2. Once activated by insulin receptor–mediated phosphorylation, the IRS proteins stimulate the class 1A phosphotidylinositol 3-kinase (PI3K) to generate phosphatidylinositol (3,4,5)-triphosphate (PIP3), which rec ruits 3-phosphoinositide dependent protein kinase-1 (PDPK1) and AKT to the plasma membrane, where AKT is activated by PDPK1-mediated phosphorylation1,3. During fasting, dephosphorylated FoxO1—together with a complex of cAMP response element-binding protein (CREB), CREB regulated transcription coactivator 2 (CRTC2) and CREB binding protein (CBP)—upregulates gluconeogenic genes, such as those that encode phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase, catalytic subunit (G6Pase). After a meal, insulin-stimulated AKT phosphorylates FoxO1 directly and CRTC2 indirectly to suppress gluconeogenesis (Fig. 1a)1,4.
Figure 1.
Insulin signaling pathways that control gluconeogenesis. (a) The canonical insulin receptor (IR)-IRS-PI3K-PDPK1-AKT signaling pathway regulates gluconeogenesis by inhibiting the transcription factor FoxO1 and the transcriptional regulatory CREB-CBP-CRTC2 complex. After feeding, insulin receptor–IRS-PI3K-PDPK1-AKT signaling is stimulated (green), which phosphorylates and inactivates FoxO1 and the CREB-CBP-CRTC2 complex, thus suppressing the expression of gluconeogenic genes (encoding PEPCK and G6Pase) (gray). During fasting, insulin receptor–IRS-PI3K-PDPK1-AKT signaling is less active than in feeding (gray), thus turning on the activities of FoxO1, the CREB-CBP-CRTC2 complex and gluconeogenesis (green). The P in the yellow circles denotes activating phosphorylation, and the P in the red circles denotes inhibitory phosphorylation. (b) Lu et al.8 suggest the existence of a hypothetical non-canonical pathway by which insulin controls gluconeogenesis. They suggest that the non-canonical pathway, which has yet to be identified, suppresses gluconeogenesis in parallel with the canonical pathway during feeding; however, activated FoxO1 during fasting or insulin resistance dampens the non-canonical pathway and stimulates the expression of gluconeogenic genes and glucose production. A comparison of TLKO mice and LTKOIRS mice implied that the divergent point of the non-canonical pathway from the canonical pathway could reside between IRS and AKT. (c) The PDPK1-aPKCl signaling cascade may be a potential candidate accounting for the non-canonical pathway. In response to insulin or stimuli after feeding, insulin receptor–IRS-PI3K-PDPK1 signaling activates aPKCλ, which, in turn, phosphorylates CBP and disrupts the CREB-CBP-CRTC2 complex and suppresses gluconeogenesis. However, in DLKO or TLKO mice, this pathway could remain active and contribute to the postprandial response.
Recently, emerging evidence from genetically engineered mice has placed the AKT-FoxO1 cascade at the center stage of hepatic insulin signaling. Genetic ablation of insulin receptor or IRS uncouples insulin from AKT, locking FoxO1 in its active state and leading to the dysregulated expression of a wide array of genes, including the increased expression of gluconeogenic genes5,6. Remarkably, deletion of hepatic FoxO1 largely normalizes the gene expression5,6. In addition, use of antisense oligonucleotides to suppress FoxO1 lowers the concentration of circulating glucose and the rate of basal glucose production in insulin-resistant or diabetic subjects7, suggesting that the canonical insulin receptor–IRS-PI3K-AKT-FoxO1 cascade is the principal mechanism regulating hepatic glucose production (Fig. 1a). This `linear' model of insulin action gains more traction in this issue of Nature Medicine, in which Lu et al.8 show that systemic glucose intolerance and dysregulated hepatic transcriptional responses caused by the compound deletion of Akt1 and Akt2 (termed there DLKO) in mouse liver are completely normalized by concomitant deletion of hepatic FoxO1.
However, additional experiments with liver-specific Akt1, Akt2 and FoxO1 triple knockout (TLKO) mice suggested that the Akt-FoxO1 cascade might not be the only driver of the transition from fasting to feeding (known as the postprandial transition)8 because the TLKO mice display an appropriate response to feeding and to insulin itself. Both the expression of gluco neogenic genes and the hepatic production of glucose in the TLKO mice were significantly suppressed by feeding or by insulin treatment. Lu et al.8 argue convincingly that the major role of hepatic AKT is to suppress FoxO1 after feeding and even partially suppress it in the fasting state. However, to explain how FoxO1 deletion restored insulin sensitivity in TLKO mice, the authors propose that a `non-canonical' insulin signaling cascade exists, which is suppressed by activated FoxO1 in DLKO mice but which is restored upon FoxO1 deletion and sensitive to insulin in TLKO mice (Fig. 1b)8.
It is tempting to speculate that a non-canonical insulin signaling cascade exists, although the nature of this hypothetical pathway is unknown. To narrow down the point at which the non-canonical and canonical pathways diverge, it might be informative to compare the results from the TLKO mice studied by Lu et al.8 with those obtained from previous experiments with liver-specific Irs1, Irs2 and FoxO1 triple knockout (LTKOIRS) mice5. In contrast to the TLKO mice, which were insulin responsive8, LTKOIRS mice had persistent insulin resistance compared to control mice5, suggesting that the non-canonical pathway also requires the IRS branch of insulin signaling. As such, the divergent point of the two pathways could reside between IRS and AKT (Fig. 1b,c). Atypical protein kinase C (aPKCλ) is a potential candidate that could be involved in the non-canonical pathway. aPKCλ can be activated in liver by the IRS-PI3K-PDPK1 cascade and, independently of AKT, phosphorylates CBP to disrupt the CREB-CBP-CRTC2 complex and suppress peroxisome proliferator-activated receptor-γ co-activator 1α (Ppargc1α), a key component for FoxO1-mediated and CREB-mediated gluconeogenesis4,9,10. Indeed, Ppargc1α expression is significantly reduced by feeding in DLKO and TLKO mice8 but not in Irs1 and Irs2 double knockout (LDKOIRS) and LTKOIRS mice that lack IRS-PI3K-PDPK1 signaling5. Thus, the PDPK1-aPKCλ branch of the canonical pathway could account, at least in part, for the non-canonical pathway proposed by Lu et al.8 (Fig. 1c).
Whereas FoxO1 could mediate the postprandial response through a non-canonical insulin signaling pathway, it might also contribute to the fasting-feeding transition by modulating key cellular functions. Persistent FoxO1 activity can dysregulate mitochondrial function and oxidative capacity, which changes the ratios of NAD+ to NADH and AMP to ATP in the liver11,12. These changes can dysregulate other energy and nutrient sensors, such as sirtuin 1 (Sirt1) or AMP-activated protein kinase (AMPK), that also control gluconeogenesis4,9,11. Further study to dissect the molecular links between these regulators might help to delineate a FoxO1-mediated non-canonical insulin signaling pathway.
Regardless, the findings from TLKO mice by Lu et al.8 and those from previous studies on LTKOIRS mice5 strongly suggest that inhibition of hepatic FoxO1 might be a promising strategy to reverse features of the metabolic syndrome, such as hyperglycemia and hyperinsulinemia. Particularly, ablation of FoxO1 results in a more comprehensive restoration of metabolic homeostasis in Akt-deficient mice8 than in Irs-deficient mice5, including a restoration of insulin sensitivity. However, lipid metabolism in DLKO mice and the effects of FoxO1 ablation have not been characterized8, and the uncoupled regulation of lipogenesis and gluconeogenesis by insulin resistance is not completely understood3,13. Resolving these issues will be important because humans with AKT2 defects manifest hypertriglyceridemia and hepatic steatosis in addition to insulin resistance and hyperglycemia14,15. Whether the hepatic steatosis in the face of hyperglycemia implies a role for a non-canonical insulin signaling pathway during compensatory hyper insulinemia is unknown and is worthy of further investigation.
Footnotes
COMPETING FINANCIAL INTERESTS The authors declare no competing financial interests.
References
- 1.Cheng Z, Tseng Y, White MF. Trends Endocrinol. Metab. 2010;21:589–598. doi: 10.1016/j.tem.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White MF. Science. 2003;302:1710–1711. doi: 10.1126/science.1092952. [DOI] [PubMed] [Google Scholar]
- 3.White MF. Cell Metab. 2009;9:485–487. doi: 10.1016/j.cmet.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Altarejos JY, Montminy M. Nat. Rev. Mol. Cell Biol. 2011;12:141–151. doi: 10.1038/nrm3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong XC, et al. Cell Metab. 2008;8:65–76. doi: 10.1016/j.cmet.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsumoto M, Pocai A, Rossetti L, DePinho RA, Accili D. Cell Metab. 2007;6:208–216. doi: 10.1016/j.cmet.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Samuel VT, et al. Diabetes. 2006;55:2042–2050. doi: 10.2337/db05-0705. [DOI] [PubMed] [Google Scholar]
- 8.Lu M, et al. Nat. Med. 2012;18:388–395. doi: 10.1038/nm.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He L, et al. Cell. 2009;137:635–646. doi: 10.1016/j.cell.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou XY, et al. Nat. Med. 2004;10:633–637. doi: 10.1038/nm1050. [DOI] [PubMed] [Google Scholar]
- 11.Cheng Z, et al. Nat. Med. 2009;15:1307–1311. doi: 10.1038/nm.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cantó C, Auwerx J. Curr. Opin. Lipidol. 2009;20:98–105. doi: 10.1097/MOL.0b013e328328d0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown MS, Goldstein JL. Cell Metab. 2008;7:95–96. doi: 10.1016/j.cmet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 14.George S, et al. Science. 2004;304:1325–1328. doi: 10.1126/science.1096706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Semple RK, et al. J. Clin. Invest. 2009;119:315–322. doi: 10.1172/JCI37432. [DOI] [PMC free article] [PubMed] [Google Scholar]