ABSTRACT
We describe a case of human Becker muscular dystrophy (BMD)-like myopathy that was characterized by the declined stainability of dystrophin at sarcolemma in a pig and the immunostaining for dystrophin on the formalin-fixed, paraffin-embedded (FFPE) tissue. The present case was found in a meat inspection center. The pig looked appeared healthy at the ante-mortem inspection. Muscular abnormalities were detected after carcass dressing as pale, discolored skeletal muscles with prominent fat infiltrations and considered so-called “fatty muscular dystrophy”. Microscopic examination revealed following characteristics: diffused fat infiltration into the skeletal muscle and degeneration and regeneration of the remaining skeletal muscle fibers. Any lesions that were suspected of neurogenic atrophy, traumatic muscular degeneration, glycogen storage disease or other porcine muscular disorders were not observed. The immunostaining for dystrophin was conducted and confirmed to be applicable on FFPE porcine muscular tissues and revealed diminished stainability of dystrophin at the sarcolemma in the present case. Based on the histological observations and immunostaining results, the present case was diagnosed with BMD-like myopathy associated with dystrophin abnormality in a pig. Although the genetic properties were not clear, the present BMD-like myopathy implied the occurrence of dystrophinopathy in pigs. To the best of our knowledge, this is the first report of a natural case of myopathy associated with dystrophin abnormalities in a pig.
Keywords: Becker muscular dystrophy, dystrophinopathy, fatty muscular dystrophy, swine
Muscular steatosis (MS), also known as so-called “fatty muscular dystrophy”, is a muscular lesion detected in meat inspection centers by its characteristics, such as abnormally fat infiltrated and discolored skeletal muscle [24, 36]. The so-called “fatty muscular dystrophy” has not been thought to be related to human muscular dystrophy (MD). This term has been applied to the macroscopic appearance of afflicted skeletal muscle that is characterized by excessive fat deposition within muscles, especially in meat inspection centers in Japan [9, 16]. MS results in an economic loss to pig farms, because MS-afflicted muscle or the afflicted whole body is disposed of according to regulation. However, effective prevention has not yet been established, because the pathogenesis of MS is not clear.
One of the possible pathogenesis of MS is the inherited muscular disorder. Inherited muscular disorders have been reported in various animal species, including horse [23], cow [11], chicken [20], dog [8, 30] and cat [7, 26, 28]. In particular, the disorders that possess similar gene deficiencies to human MD are important as a human disease models resource.
MD is a hereditary primary skeletal muscular disorder characterized by progressive muscular degeneration and gradual loss of muscle fibers [10]. Duchenne muscular dystrophy (DMD) and Becker muscular dystrophy (BMD) are X-linked MDs caused by dystrophin gene mutation [6, 12] and called dystrophinopathies [33]. The gene codes for dystrophin, which is a cytoskeletal-associated protein of 427 kDa in human and of 425 kDa in pig and connects cytoskeletal and membrane proteins, such as actin, dystroglycan and dystrophin-associated glycoproteins [17]. DMD is the most severe and common MD in humans [25]. BMD is a milder MD than DMD, and the incidence is approximately one-third of that of DMD [5]. The histological characteristics of MDs are varied muscular fiber diameters, degeneration, necrosis, regeneration and emergence of round-shaped muscular fibers in transverse sections. In MDs, these changes are heterogeneously distributed in the affected skeletal muscle, not homogeneously distributed like massive necrosis of the affected muscle in the white muscle disease. Endomysial fibrosis is profound especially in DMD in accord with its rapid disease progression and hypertrophic fibers, whereas internal nuclear dispositions are profound especially in BMD in accord with its gradual progression. The difference between DMD and BMD is explained by the reading frame thesis with some exceptions; DMD patients have an out-of-frame deletion in the dystrophin gene, which results in a lacked dystrophin at the sarcolemma in skeletal muscle, while BMD patients have an in-frame deletion in the gene, which results in faint and/or patchy stainability of dystrophin at the sarcolemma [1, 19, 22, 34].
We encountered a case, which macroscopically diagnosed with MS in a meat inspection center. The histological features of the examined muscle resembled human BMD. For further diagnosis of the present case, we conducted and evaluated the immunofluorescence staining for dystrophin on formalin-fixed paraffin-embedded (FFPE) tissue and sex determination by polymerase chain reaction (PCR) analysis of FFPE samples. Herein, we report a case of BMD-like myopathy in a pig and the useful application of immunofluorescent staining on FFPE tissue in the diagnosis of porcine dystrophin-associated myopathy.
MATERIALS AND METHODS
Case history and pathological examination: The pig was brought into a slaughterhouse in Japan. Its sex, breed and age were unknown at the time. The pig had no clinical symptoms and an average physique at the ante mortem inspection in the meat inspection center. Pale, yellowish-red, discolored muscles with fat infiltration were found after carcass dressing in the rectus abdominis muscle and muscles of the femoral region. The other muscles appeared normal. No significant lesions were found in the other organs. The rectus abdominis muscle was collected, fixed in 10% neutral-buffered formalin and embedded in paraffin for the pathological examinations. The paraffin sections were stained with hematoxylin and eosin (HE), phosphotungstic acid hematoxylin (PTAH), Bodian-Luxol fast blue (B-LFB) and periodic acid-Schiff reaction (PAS).
In addition to the present case, the following 2 samples were used as controls for comparison: normal muscular tissue from a 4-week-old mixed-breed male pig, which was purchased from a pig farm in Japan (Shinasawa pig farming union, Saitama, Japan) and necrotic muscular tissue due to an infraction in a slaughtered pig of unknown sex. These 2 samples were fixed, embedded, sectioned, stained with HE and subjected to the following immunofluorescence examinations. In addition, frozen normal muscular tissue was collected for confirming the cross-reactivity of an anti-dystrophin antibody. All research procedures involving animals were approved and were in accordance with the guidelines of the animal research committee of the National Veterinary Assay Laboratory.
Immunofluorescence staining on FFPE samples: The indirect immunofluorescence antibody technique was applied using an anti-dystrophin mouse monoclonal antibody (clone 1808; 1 in 100 dilution; Abcam, Cambridge, MA, U.S.A.) as the primary antibody [29]. To unmask the epitope, tissue sections were boiled in 1 mM EDTA solution (pH 8.0) for 20 min. A fluorescence-labeled anti-mouse IgG goat polyclonal antibody (1 in 500 dilution; Life Technologies Corporation, Carlsbad, CA, U.S.A.) was used as the secondary antibody. Vectashield mounting medium with DAPI (Vector Laboratories, Burlingame, CA, U.S.A.) was used for mount.
Cross-reactivity of the primary antibody: The cross-reactivity of the primary antibody with porcine dystrophin was confirmed by western blotting using a lysate obtained from the normal muscle tissue and compared to the anti-dystrophin mouse monoclonal antibody with previously confirmed cross-reactivity (Dys1; clone Dy4/6D3; 1 in 250 dilution; Leica Biosystems Newcastle, Newcastle, U.K.) [12].
Sex identification by polymerase chain reaction from FFPE sample: To determine the sex of the present pig, PCR analysis was performed in order to amplify the 163 base pairs of the DNA fragment with the porcine SRY gene DNA primer pair (sense: 5’-TGAACGCTTTCATTGTGTGGTC-3’ and anti-sense: 5’-GCCAGTAGTCTCTGTGCCTCCT-3’) designed by Pomp et al. [27] for sex identification of a raw embryo. Template DNA was extracted from FFPE samples of the present case and the control samples of both sexes. The male control sample was prepared from the normal muscular tissue, and the female sample was prepared from the spleen of female pig, which was one of the previous case collections of our laboratory. In brief, these samples were cut into 50-µm sections, collected in sample tubes, conjugated with 0.5 ml of DNA Isolator PS-Rapid reagent (Wako Pure Chemical Industries, Osaka, Japan), boiled 10 min, transferred to a centrifuge tube with 0.2 µm filter unit and centrifuged. The filtrated samples were collected for the PCR templates. Each template was mixed with a Platinum Pfx DNA Polymerase kit (Life Technologies Corporation) according to the manufacturer’s instructions. After incubation at 95°C for 4.5 min, 40 cycles of 95°C for 25 sec, 55°C for 40 sec and 68°C for 30 sec were performed. The amplicons were resolved by electrophoresis in a non-denaturing 2.0% agarose gel and then stained with ethidium bromide.
RESULTS
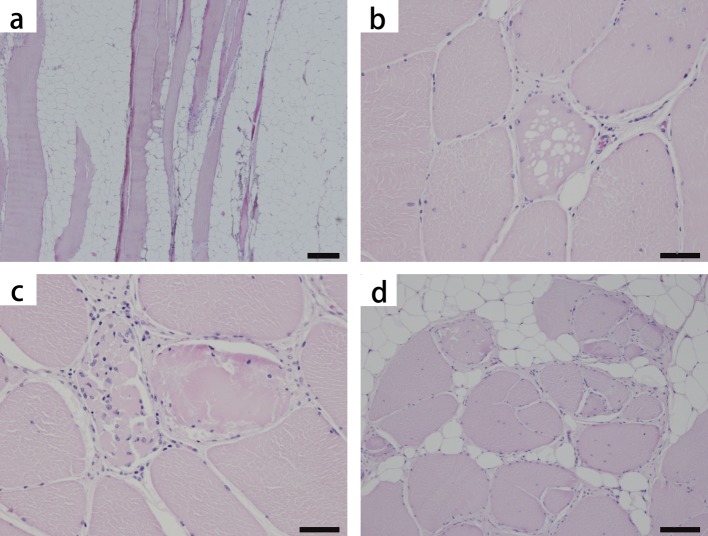
Histological findings: The most characteristic histological alteration was fat infiltration; up to approximately 50% of the sectioned tissue was adipose tissue (Fig. 1a). In a cross-section of muscle, the remaining muscular fibers varied in fiber diameters with mildly rounded shapes and the emergence of hypertrophic fibers, sometimes accompanied by vacuolar degeneration (Fig. 1b). Hyalinized fibers and myophagy were scattered (Fig. 1c). The internal disposition of nuclei and fiber splitting were scattered as well (Fig. 1d). Slight endomysial fibrosis and intra-adipose tissue fibrosis were also observed.
Fig. 1.
Pig. Skeletal muscle (rectus abdominis), hematoxylin and eosin stain (HE). Severe fat infiltration (a), vacuolar degeneration (b), hyalinized fiber and myophagy (c) and fiber splitting (d) are observed. The internal disposition of the nuclei is also seen in (b–d). Bar scales: 200 µm (a), 50 µm (b), 50 µm (c) and 100 µm (d).
In the longitudinal sections, almost all of the remaining muscular fibers showed internal disposition of nuclei, and a few fibers showed nuclear chains. Scattered segmental necrosis of myofibrils was observed as partial hyalinization, sometimes flocculated or accompanied with accumulated macrophages. The cross-striations of the fibers were focally obscure in HE-stained sections, and those foci were ascertained to be Z-disc streaming with PTAH staining. Regenerated, small and pale basophilic fibers were not evident in cross-section and longitudinal section. Other fiber alterations, such as calcification, target fiber or sarcoplasmic masses, were not observed. There were no small angular fibers or grouping atrophy as well. B-LFB revealed that the peripheral nerves were normal. PAS-positive materials were not found in the vacuoles of the degenerated muscular fibers.
Immunofluorescence: The muscles of the present case showed focal and faint stainability of dystrophin at the sarcolemma in most areas (Fig. 2f). By contrast, the healthy pig muscles (Fig. 2d) and necrotic muscular tissue showed intense and continuous stainability at the sarcolemma (Fig. 2e).
Fig. 2.
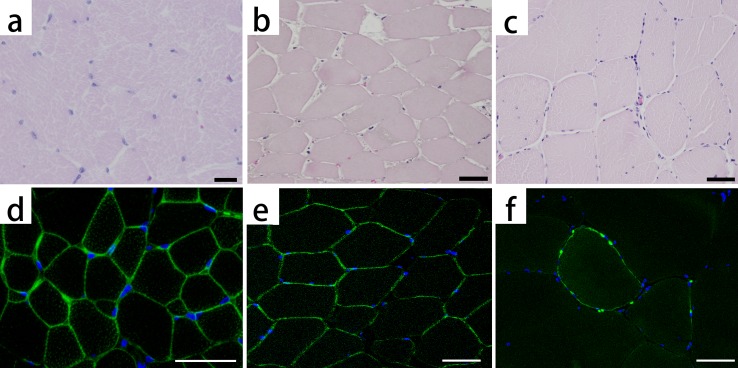
Hematoxylin and eosin staining (HE) and immunofluorescence staining for dystrophin: healthy pig (a, d), necrotic muscle (b, e) and the present pig (c, f). Dystrophin is stained light green. The nuclei are stained blue. The healthy pig shows small fibers (a), because of its young age and clear stainability at the sarcolemma (d). The necrotic muscle, which consists of muscle fibers that are massively necrotized because of an infarct, shows strongly eosinophilic pale cytoplasm and declined muscle nucleus stainability in HE (b), whereas dystrophin is stained at the sarcolemma (e). In the present pig, dystrophin is faintly and partially stained at the sarcolemma (f). Bar scales: 20 µm (a) and 50 µm (b–f).
Cross-reactivity: To confirm the cross-reactivity of the primary antibody used in the present immunofluorescent examination, total muscular lysates from a healthy pig were examined by western blot analysis with the primary antibody. A closely spaced doublet band of 425 kDa of porcine dystrophin was detected in porcine muscular homogenates below the 500-kDa marker (Fig. 3), which was similar to the findings of a previous report [13]. Nonspecifically reacted bands were not detected. The same bands were also detected by a western blot analysis using Dys1 antibody.
Fig. 3.
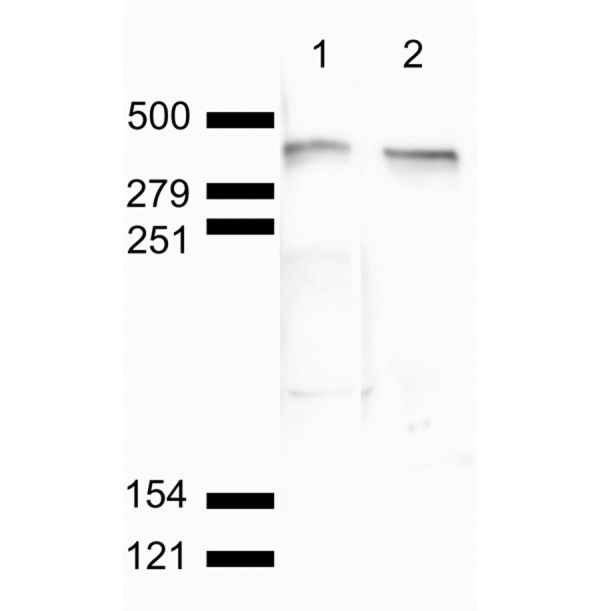
Western blot analysis of normal porcine muscle. Lane 1 is stained using pan-dystrophin antibody Dys1 (clone Dy4/6D3). Lane 2 is stained using the primary antibody (clone 1808) used in the present immunofluorescent examination. Closely spaced doublet bands of approximately 425 kDa were visualized on both lanes below the 500-kDa marker. Non-specific reactivity was not detected.
Sex identification: The SRY detection method by PCR was applicable to DNA extract from FFPE samples. The electrophoretic analysis revealed a primer-specific band, which nearly corresponded to 160 bp, in the positive male control lane and the present case lane (Fig. 4). The band was not observed in the female control lane.
Fig. 4.
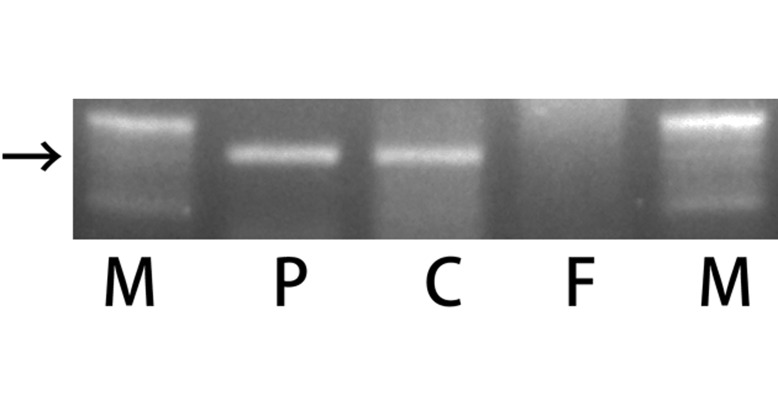
Agarose gel showing ethidium bromide-stained electrophoresis of PCR products of the SRY gene fragment, which was amplified from DNA isolated from paraffin-embedded tissue. Lane M is a ladder. Lane P is a positive control from the spleen of a male pig. Lane C is a sample from the muscular tissue of the present pig. Lane F is a sample from the spleen of a female pig. The arrow indicates 160 bp. A positive band was observed in lane P and lane C.
DISCUSSION
Histological features of the examined skeletal muscle were profound diffused fat infiltration into the skeletal muscle and heterogeneously scattered various types of degenerative and regenerative changes in the remaining muscles. The stainability of the dystrophin in the muscular tissue of the present case was revealed to be declined and patchy at the sarcolemma by immunofluorescent staining. Based on the results of the histological and immunofluorescent analyses, the present case was diagnosed with BMD-like myopathy associated with dystrophin abnormality in a pig. This is the first report of a natural case of myopathy associated with dystrophin abnormality in a pig. The results in this study implied an inherited factor as one of the causes of MS and the possible occurrence of the human BMD homolog in pigs. Furthermore, the immunostaining for dystrophin on the porcine FFPE tissue was evaluated and demonstrated in this study.
An immunostaining for dystrophin is the most sensitive detection method for dystrophinopathy among the several types of detection methods, such as the detection of mutations in the dystrophin gene by PCR or sequence analysis and western blot analysis of dystrophin [15, 21]; however, an immunostaining for dystrophin is limitedly applicable on frozen sections with some exceptions of the immunostaining methods for dystrophin used in human [14]. We suspected an association of dystrophin abnormality with the present myopathy, but we had not obtained frozen specimens. Thus, we tested an immunofluorescence staining for dystrophin on porcine FFPE tissue. The present immunostaining revealed an intense and a continuous stainability at the sarcolemma in a normal control skeletal muscle and a necrotized skeletal muscle, and it detected the declined stainability, specifically in the skeletal muscle of the present case. The antibody used in the present immunostaining was also confirmed to react with porcine dystrophin without non-specific reactivity in the western blotting. The present immunostaining for dystrophin was revealed to be applicable on porcine FFPE tissue, and this may be useful for the detection and diagnosis of dystrophin abnormality-associated myopathy since frozen sections are not always available, especially in field cases.
Considering the similar declination of dystrophin stainability in human BMD, these findings suggested that the present case was a homolog of human BMD, which is an X-linked muscular disorder in humans. On the other hand, the declination of dystrophin expression is reported in female carriers of DMD as a mosaic expression of dystrophin [2]. The possibility that the present pig was a female carrier of dystrophinopathy could not be ruled out, because the sex of the case was not noted. For this reason, we attempted to identify the sex by SRY gene amplification of FFPE tissue. The SRY detection method by PCR, developed for raw materials, was applicable to DNA extracts from FFPE tissue and showed that the present case was male. This result was consistent with the possibility that the present case was a human BMD homolog. Provided that a BMD homolog occurs in pigs, and the homolog results in abnormally fat-infiltrated muscle, namely MS, the detection and removal of a carrier sow is a feasible preventative measure that can be used to avoid the economic loss due to MS. The possible occurrence of a BMD homolog also suggested the possibility of BMD disorder model in a pig. In dystrophinopathy, experimental DMD models have been developed in mouse [4, 32] and dog [31]. In particular, the experimental dog DMD model, originated from naturally occurring DMD in golden retriever dog [30, 31, 35], is valuable for the further study of DMD pathogenesis in humans, because its clinical features are similar to those of human DMD. In other animals, a genetically engineered dystrophin-deficient DMD pig model has been reported recently [18]. In contrast to DMD, there is no report of animal BMD cases, including dystrophin-declined myopathy or the establishment of an animal model of BMD. The cDNA of porcine dystrophin is sequenced, and the deduced amino-acid sequence of porcine dystrophin shows a high degree of sequence similarity to human dystrophin with a 94% overall amino-acid identity [3]. In addition, a pig is not only a meat-producing livestock but also a possible candidate animal model for biomedical research addressing regenerative medicine or preclinical investigations in pharmacology [37]. Taking into account the high sequence similarity of porcine dystrophin to human dystrophin and the pig’s aptitude as an animal model, the present BMD-like myopathy suggested a possibility of establishing a valuable disease model of human BMD using a pig.
The muscular disorders characterized by abnormal fat infiltrate and discoloration in macroscopic findings may be routinely diagnosed as MS or so-called “fatty muscular dystrophy” without detailed histopathological examinations. Considering the fat infiltration is not a particular lesion in the skeletal muscle but is sequel after the muscular disorder, MS may consist of diverse collection of muscular disorders including the BMD-like myopathy. A lot of issues, such as the prevalence, genetic factors, pedigree and the systemic analysis of muscles, including heart, still remain unclear in our understanding of the BMD-like myopathy in a pig. Further studies on the BMD-like myopathy and MS with the present immunostaining method as one of the keys for examining muscular disorders are required in order to prevent economic loss and to ascertain the possibility of a BMD model using a pig.
ACKNOWLEDGMENTS
We thank Ms. Mayumi Shibayama for her excellent technical assistance.
REFERENCES
- 1.Arahata K., Ishiura S., Ishiguro T., Tsukahara T., Suhara Y., Eguchi C., Ishihara T., Nonaka I., Ozawa E., Sugita H.1988. Immunostaining of skeletal and cardiac muscle surface membrane with antibody against Duchenne muscular dystrophy peptide. Nature 333: 861–863. doi: 10.1038/333861a0 [DOI] [PubMed] [Google Scholar]
- 2.Arahata K., Ishihara T., Kamakura K., Tsukahara T., Ishiura S., Baba C., Matsumoto T., Nonaka I., Sugita H.1989. Mosaic expression of dystrophin in symptomatic carriers of Duchenne’s muscular dystrophy. N. Engl. J. Med. 320: 138–142. doi: 10.1056/NEJM198901193200302 [DOI] [PubMed] [Google Scholar]
- 3.Bordais A., Bolaños-Jimenez F., Fort P., Varela C., Sahel J. A., Picaud S., Rendon A.2005. Molecular cloning and protein expression of Duchenne muscular dystrophy gene products in porcine retina. Neuromuscul. Disord. 15: 476–487. doi: 10.1016/j.nmd.2005.03.011 [DOI] [PubMed] [Google Scholar]
- 4.Bulfield G., Siller W. G., Wight P. A., Moore K. J.1984. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc. Natl. Acad. Sci. U.S.A. 81: 1189–1192. doi: 10.1073/pnas.81.4.1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bushby K. M., Thambyayah M., Gardner-Medwin D.1991. Prevalence and incidence of Becker muscular dystrophy. Lancet 337: 1022–1024. doi: 10.1016/0140-6736(91)92671-N [DOI] [PubMed] [Google Scholar]
- 6.Carlson C. G.1998. The dystrophinopathies: an alternative to the structural hypothesis. Neurobiol. Dis. 5: 3–15. doi: 10.1006/nbdi.1998.0188 [DOI] [PubMed] [Google Scholar]
- 7.Carpenter J. L., Hoffman E. P., Romanul F. C., Kunkel L. M., Rosales R. K., Ma N. S., Dasbach J. J., Rae J. F., Moore F. M., McAfee M. B., Pearce L. K.1989. Feline muscular dystrophy with dystrophin deficiency. Am. J. Pathol. 135: 909–919 [PMC free article] [PubMed] [Google Scholar]
- 8.Deitz K., Morrison J. A., Kline K., Guo L. T., Shelton G. D.2008. Sarcoglycan-deficient muscular dystrophy in a Boston Terrier. J. Vet. Intern. Med. 22: 476–480. doi: 10.1111/j.1939-1676.2008.0080.x [DOI] [PubMed] [Google Scholar]
- 9.Doi H.1995. Fatty muscular dystrophy of a pig. Rinsho-Juui (J. Clin. Vet. Med.) 13: 65–66 (in Japanese). [Google Scholar]
- 10.Emery A. E.2002. The muscular dystrophies. Lancet 359: 687–695. doi: 10.1016/S0140-6736(02)07815-7 [DOI] [PubMed] [Google Scholar]
- 11.Furuoka H., Doi T., Nakamura N., Inada I., Osame S., Matsui T.1995. Hereditary myopathy of the diaphragmatic muscles in Holstein-Friesian cattle. Acta Neuropathol. 90: 339–346. doi: 10.1007/BF00315007 [DOI] [PubMed] [Google Scholar]
- 12.Hoffman E. P., Brown R. H., Jr, Kunkel L. M.1987. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 51: 919–928. doi: 10.1016/0092-8674(87)90579-4 [DOI] [PubMed] [Google Scholar]
- 13.Hoffman E. P., Hudecki M. S., Rosenberg P. A., Pollina C. M., Kunkel L. M.1988. Cell and fiber-type distribution of dystrophin. Neuron 1: 411–420. doi: 10.1016/0896-6273(88)90191-2 [DOI] [PubMed] [Google Scholar]
- 14.Hoshino S., Ohkoshi N., Watanabe M., Shoji S.2000. Immunohistochemical staining of dystrophin on formalin-fixed paraffin-embedded sections in Duchenne/Becker muscular dystrophy and manifesting carriers of Duchenne muscular dystrophy. Neuromuscul. Disord. 10: 425–429. doi: 10.1016/S0960-8966(99)00116-9 [DOI] [PubMed] [Google Scholar]
- 15.Hu X. Y., Burghes A. H., Ray P. N., Thompson M. W., Murphy E. G., Worton R. G.1988. Partial gene duplication in Duchenne and Becker muscular dystrophy. J. Med. Genet. 25: 369–376. doi: 10.1136/jmg.25.6.369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamikawa S., Hasebe K., Kaneko N., Okano Y.1991. Fatty muscular dystrophy in cattle and hogs. Shokuhin Eisei Kenkyu (Food Sanit. Stud.) 41: 65–78 (in Japanese). [Google Scholar]
- 17.Kanagawa M., Toda T.2006. The genetic and molecular basis of muscular dystrophy: roles of cell-matrix linkage in the pathogenesis. J. Hum. Genet. 51: 915–926. doi: 10.1007/s10038-006-0056-7 [DOI] [PubMed] [Google Scholar]
- 18.Klymiuk N., Blutke A., Graf A., Krause S., Burkhardt K., Wuensch A., Krebs S., Kessler B., Zakhartchenko V., Kurome M., Kemter E., Nagashima H., Schoser B., Herbach N., Blum H., Wanke R., Aartsma-Rus A., Thirion C., Lochmüller H., Walter M. C., Wolf E.2013. Dystrophin-deficient pigs provide new insights into the hierarchy of physiological derangements of dystrophic muscle. Hum. Mol. Genet. 22: 4368–4382. doi: 10.1093/hmg/ddt287 [DOI] [PubMed] [Google Scholar]
- 19.Koenig M., Beggs A. H., Moyer M., Scherpf S., Heindrich K., Bettecken T., Meng G., Müller C. R., Lindlöf M., Kaariainen H., de la Chapelle A., Kiuru A., Savontaus M. L., Gilgenkrantz H., Récan D., Chelly J., Kaplan J. C., Covone A. E., Archidiacono N., Romeo G., Liechti-Gallati S., Schneider V., Braga S., Moser H., Darras B. T., Murphy P., Francke U., Chen J. D., Morgan G., Denton M., Greenberg C. R., Wrogemann K., Blonden L. A. J., van Paassen H. M. B., van Ommen G. J. B., Kunkel L. M.1989. The molecular basis for Duchenne versus Becker muscular dystrophy: correlation of severity with type of deletion. Am. J. Hum. Genet. 45: 498–506 [PMC free article] [PubMed] [Google Scholar]
- 20.Matsumoto H., Maruse H., Inaba Y., Yoshizawa K., Sasazaki S., Fujiwara A., Nishibori M., Nakamura A., Takeda S., Ichihara N., Kikuchi T., Mukai F., Mannen H.2008. The ubiquitin ligase gene (WWP1) is responsible for the chicken muscular dystrophy. FEBS lett. 582: 2212–2218. doi: 10.1016/j.febslet.2008.05.013 [DOI] [PubMed] [Google Scholar]
- 21.Monaco A. P., Bertelson C. J., Coletti-Feener C., Kunkel L. M.1987. Localization and cloning of Xp2l deletion breakpoints involved in muscular dystrophy. Hum. Genet. 75: 221–227. doi: 10.1007/BF00281063 [DOI] [PubMed] [Google Scholar]
- 22.Monaco A. P., Bertelson C. J., Liechti-Gallati S., Moser H., Kunkel L. M.1988. An explanation for the phenotypic differences between patients bearing partial deletions of the DMD locus. Genomics 2: 90–95. doi: 10.1016/0888-7543(88)90113-9 [DOI] [PubMed] [Google Scholar]
- 23.Montagna P., Liguori R., Monari L., Strong P. N., Riva R., Di Stasi V., Gandini G., Cipone M.2001. Equine muscular dystrophy with myotonia. Clin. Neurophysiol. 112: 294–299. doi: 10.1016/S1388-2457(00)00511-3 [DOI] [PubMed] [Google Scholar]
- 24.Monlux W. S., Monlux A. W.1972. Miscellaneous diseases that are frequently submitted for diagnostic clarification. pp. 139–140. In: Atlas of Meat Inspection Pathology, United States Department of Agriculture, Washington. [Google Scholar]
- 25.Moser H.1984. Duchenne muscular dystrophy: pathogenetic aspects and genetic prevention. Hum. Genet. 66: 17–40. doi: 10.1007/BF00275183 [DOI] [PubMed] [Google Scholar]
- 26.O’Brien D. P., Johnson G. C., Liu L. A., Guo L. T., Engvall E., Powell H. C., Shelton G. D.2001. Laminin a2 (merosin)-deficient muscular dystrophy and demyelinating neuropathy in two cats. J. Neurol. Sci. 189: 37–43. doi: 10.1016/S0022-510X(01)00559-7 [DOI] [PubMed] [Google Scholar]
- 27.Pomp D., Good B. A., Geisert R. D., Corbin C. J., Conley A. J.1995. Sex identification in mammals with polymerase chain reaction and its use to examine sex effects on diameter of day-10 or -11 pig embryos. J. Anim. Sci. 73: 1408–1415 [DOI] [PubMed] [Google Scholar]
- 28.Salvadori C., Vattemi G., Lombardo R., Marini M., Cantile C., Shelton G. D.2009. Muscular dystrophy with reduced beta-sarcoglycan in a cat. J. Comp. Pathol. 140: 278–282. doi: 10.1016/j.jcpa.2008.12.003 [DOI] [PubMed] [Google Scholar]
- 29.Sealock R., Butler M. H., Kramarcy N. R., Gao K. X., Murnane A. A., Douville K., Froehner S. C.1991. Localization of dystrophin relative to acetylcholine receptor domains in electric tissue and adult and cultured skeletal muscle. J. Cell Biol. 113: 1133–1144. doi: 10.1083/jcb.113.5.1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharp N. J., Kornegay J. N., Van Camp S. D., Herbstreith M. H., Secore S. L., Kettle S., Hung W. Y., Constantinou C. D., Dykstra M. J., Roses A. D., Bartlett R. J.1992. An error in dystrophin mRNA processing in golden retriever muscular dystrophy, an animal homologue of Duchenne muscular dystrophy. Genomics 13: 115–121. doi: 10.1016/0888-7543(92)90210-J [DOI] [PubMed] [Google Scholar]
- 31.Shimatsu Y., Katagiri K., Furuta T., Nakura M., Tanioka Y., Yuasa K., Tomohiro M., Kornegay J. N., Nonaka I., Takeda S.2003. Canine X-linked muscular dystrophy in Japan (CXMDJ). Exp. Anim. 52: 93–97. doi: 10.1538/expanim.52.93 [DOI] [PubMed] [Google Scholar]
- 32.Sicinski P., Geng Y., Ryder-Cook A. S., Barnard E. A., Darlison M. G., Barnard P. J.1989. The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science 244: 1578–1580. doi: 10.1126/science.2662404 [DOI] [PubMed] [Google Scholar]
- 33.Sinnreich M.2010. Dystrophinopathies. pp. 205–229 In: Disorders of Voluntary Muscle, 8th ed. (Karpati, G., Hilton-Jones, D., Bushby, K. and Griggs, R. C. ed.), Cambridge University Press, Cambridge. [Google Scholar]
- 34.Uchino M., Araki S., Miike T., Teramoto H., Nakamura T., Yasutake T.1989. Localization and characterization of dystrophin in muscle biopsy specimens from Duchenne muscular dystrophy and various neuromuscular disorders. Muscle Nerve 12: 1009–1016. doi: 10.1002/mus.880121209 [DOI] [PubMed] [Google Scholar]
- 35.Valentine B. A., Cooper B. J., de Lahunta A., O’Quinn R., Blue J. T.1988. Canine X-linked muscular dystrophy. An animal model of Duchenne muscular dystrophy: clinical studies. J. Neurol. Sci. 88: 69–81. doi: 10.1016/0022-510X(88)90206-7 [DOI] [PubMed] [Google Scholar]
- 36.Van Vleet J. F., Valentine B. A.2007. Muscle and tendon. pp. 185–280. In: Pathology of Domestic Animals, 5th ed. (Maxie, M. G. ed.), Elsevier, Saunders, Philadelphia. [Google Scholar]
- 37.Vodicka P., Smetana K., Jr, Dvoránková B., Emerick T., Xu Y. Z., Ourednik J., Ourednik V., Motlík J.2005. The miniature pig as an animal model in biomedical research. Ann. N.Y. Acad. Sci. 1049: 161–171. doi: 10.1196/annals.1334.015 [DOI] [PubMed] [Google Scholar]