ABSTRACT
The present study evaluated and compared the oxidative stress status of dogs undergoing laparoscopic or open ovariectomy. Twelve healthy female dogs were divided into two groups according to the type of the surgical procedure, laparoscopic or open ovariectomy. Plasma total oxidant status (TOS), total antioxidant status (TAS) and oxidative stress index (OSI) levels for the evaluation of oxidative stress were determined. Increases in plasma TOS and OSI levels and decreases in TAS levels were observed in both groups after surgery. The TOS level was significantly lower in the laparoscopic ovariectomy group compared with the open surgery group. Laparoscopic ovariectomy is a safe and beneficial surgical alternative to traditional ovariectomy with respect to oxidative stress status in dogs.
Keywords: canine, laproscopic ovariectomy, oxidative stress index, total antioxidant status, total oxidant status
Laparoscopic surgery is becoming widely accepted, because of some advantages in terms of postoperative pain, patient mobilization and recovery and systemic immune-metabolic response, compared with open surgery [3, 4, 18, 19]. On the other hand, there are concerns for adverse hemodynamic changes associated with laparoscopic surgery, such as reduced venous return and increased systemic vascular resistance that are compromised by prolonged carbon dioxide (CO2) insufflations and increased intra-abdominal pressure (IAP) [11]. In addition, mesenteric hypoxia and related gut ischemia-reperfusion injury, occurring during and after pneumoperitoneum, could be an important cause of oxidative stress during laparoscopic surgical procedures [17].
Ovariectomy is a neutering method for healthy dogs, and several surgical approaches have been described [14]. Numerous studies have been evaluated in the bitch, including operative time, complication, postoperative pain and systemic stress parameters according to the type of surgical techniques [6, 8, 10, 16]. However, no controlled studies have been conducted to evaluate oxidant-antioxidant status of laparoscopic ovariectomy in bitches.
In the present clinical study, we investigated and compared the oxidative stress status of dogs undergoing laparoscopic or open ovariectomy.
This study was approved by the Chungnam National University Animal Care and Use Committee (Approval No. CNU-00038). Twelve healthy female dogs, weighing 3.3 to 5.4 kg, were collected from an Animal Shelter House, Korean Animal Welfare Association. Experimental dogs were kept in individual pens and received standard balanced diet throughout the experiment. Dogs were returned to the Shelter House after experiment. The study was carried out 14 days after procuring the dogs. The dogs were kept in a quiet room to avoid any stress-inducing factors during this period. The dogs were fasted for 8–12 hr before the experiments, and water was withheld 2 hr before anesthesia in order to prevent any potential adverse effects, such as vomiting or regurgitating stomach contents during anesthesia or during the recovery period. The dogs had received given intravenous (IV) fluid therapy with Hartmann’s Solution (Hartmann Solution®, Daihan Pharm Co., Ltd., Seoul, Korea) at the infusion rate of 60 ml/kg/24 hr for 12 hr until starting the experiment. The dogs were divided into two groups according to the type of the surgical procedure, laparoscopic (n=6) or open ovariectomy (n=6).
The dogs were premedicated with a subcutaneous injection of atropine sulfate (0.04 mg/kg: Atropine Sufate Injection®, Daihan Pharm), an intramuscular injection of butorphanol (0.2 mg/kg: Butophan Injection®, Myungmoon Pharm, Seoul, Korea) and cefazolin (20 mg/kg, IV: Falexin®, Dong Wha Pharm, Seoul, Korea). Afterwards, propofol (4 to 6 mg/kg: Aanepol Injection®, Hana Pharm, Seoul, Korea) was administered for tracheal intubation. Anesthesia was maintained with 2% of end-tidal concentration of isoflurane (Forane Sol®, Choongwae Pharm, Seoul, Korea) delivered with pure oxygen. During the surgical operation, the dogs were given intravenous fluid (Hartmann’s solution, 10 ml/kg/hr).
Bilateral laparoscopic ovariectomy was performed through two 5 mm ports placed on midline approximately 3 cm cranial to the umbilicus and 2 cm caudal to the umbilicus. Insufflations with CO2 were provided via automatic insufflators with pressure set a 10 to 12 mmHg. Each ovary was elevated with grasping forceps and suspended from the body wall by passing a percutaneous needle and suture through the body wall and through the tissue adjacent to the proper ovarian ligament. A Harmonic ACE 5 mm ultrasonic scalpel (Ethicon Endosurgery, Cincinnati, OH, U.S.A.) was used to coagulate and cut the suspensory ligament, ovarian pedicle and fallopian tube. Each ovary was removed from one of port sites, and then, the port sites were closed with sutures in the body wall, subcutaneous tissue and skin.
A standard ventral midline incision was made from 2 cm caudal to the umbilicus toward the pubis. After ventral median celiotomy, the ovarian pedicles were clamped using forceps, double ligated with suture and transected. The fallopian tube and proper ovarian ligament were ligated with suture, and the ovary was removed. The abdominal, subcutaneous and skin layers were sutured routinely.
Heart rate (HR), mean arterial pressure (MAP), rectal temperature (RT), end-tidal carbon dioxide (EtCO2), peripheral oxygen saturation (SpO2) and respiratory rates (RR) were monitored continuously during surgery by using a patient monitor (Pulscan-Component; Scionic, Seoul, Korea). Total anesthesia and surgical times were recorded each dog. Total anesthesia time was the time from the injection of propofol to when dogs achieved sternal recumbency. Total surgical time was the time from the first port placement (laparoscopic group) or skin incision with blade (open group) to the time of the port sites (laparoscopic group) or skin layers (open group) were closed. Blood samples were obtained for arterial blood gases analysis before anesthesia and the end of surgery. After surgery, each animal was monitored until they were ambulatory. Dogs were given butorphanol 0.2 mg/kg intramuscularly at the end of the surgical procedure for postoperative analgesia. A second dose of butorphanol 0.1 mg/kg was given intramuscularly 6 hr after surgery. Water and moistened dog food were offered 12 hr after surgery.
Blood samples were collected via venipuncture from the jugular vein before the first port placement or skin incision (preoperative) and after closing the port sites or skin layers (postoperative). Approximately 3 ml blood was collected into a plasma separation tube containing heparin for analysis of oxidative stress markers. Blood samples were centrifuged at 3,000 rpm for 10 min to separate plasma, and the plasma samples were stored at −80°C until analysis. Plasma total oxidant status (TOS) and total antioxidant status (TAS) levels for the evaluation of oxidative stress were determined using a commercially available kit developed by Erel [12, 13]. The results of TOS and TAS are expressed in micromolar hydrogen peroxide equivalents per liter (µmol H2O2 equiv/l) and mmol Trolox equiv/l, respectively. The ratio of TOS to TAS provided the oxidative stress index (OSI), an indicator of the degree of oxidative stress.
All statistical analyses were performed using SPSS version 19.0 (Chicago, IL, U.S.A.). Results are expressed as median (inter-quartile range). A Mann–Whitney U-test was applied with a P-value<0.05 being considered significant.
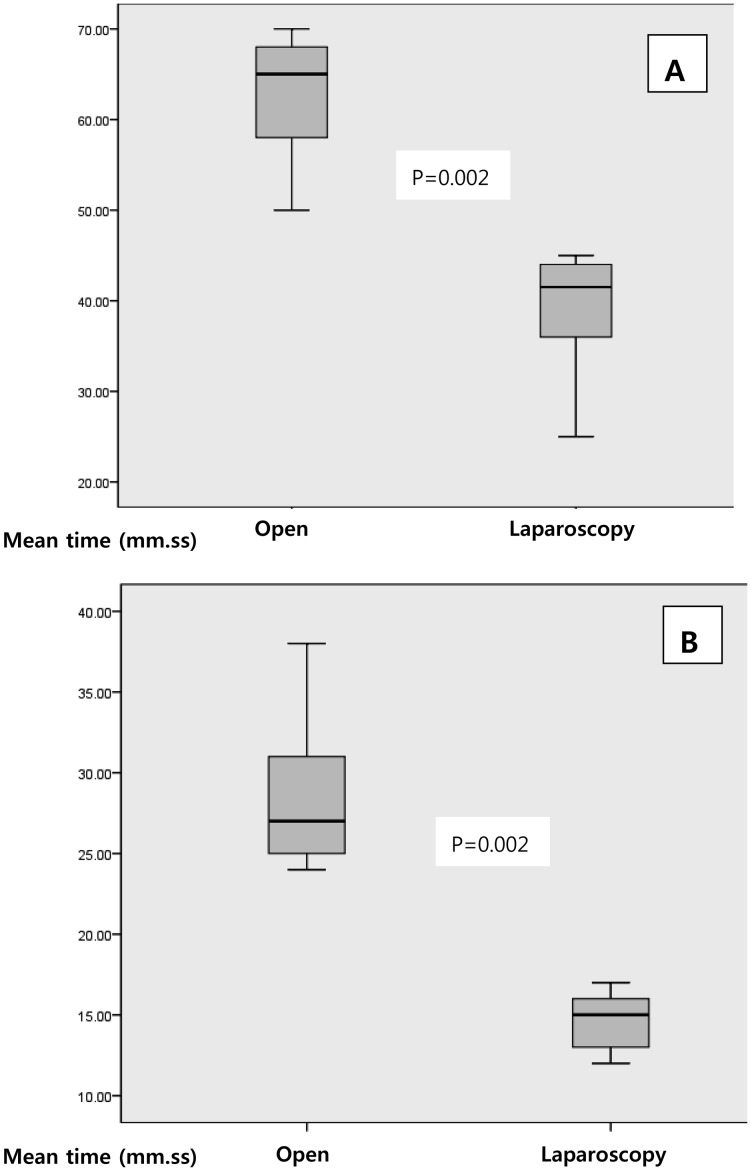
All dogs were hemodynamically stable during anesthesia and surgery. There was no significant worsening in HR, MAP, RT, EtCO2, SpO2 and RR (data not shown). In additions, there was no statistically significant change in arterial blood gases values in both groups (Table 1). Total anesthesia and surgical time were significantly shorter in laparoscopic ovariectomy group compared with open surgery group (Fig. 1). Increases in plasma TOS and OSI levels and decreases in TAS levels were observed in both groups after surgery (Table 1). Significant difference in TOS and OSI levels was observed as compared with baseline in both groups, but significant change in the plasma TAS levels was not observed in both groups. Significant difference in TOS levels was found when the groups were compared. The TOS level was significantly lower in the laparoscopic ovariectomy group compared with the open surgery group.
Table 1. Pre- and postoperative levels of ETCO2, PCO2, PO2, Ph, TOS, TAS and OSI in dogs undergoing open or laparoscopic ovariectomy.
| Parameters | Group | Preoperative | Postoperative | P |
|---|---|---|---|---|
| ETCO2 (mm Hg) | Open | 46 (2.54) | 48 (3.04) | NS |
| Laparoscopy | 44 (2.05) | 47.5 (3.47) | NS | |
| PCO2 (mm Hg) | Open | 35.9 (2.22) | 38.6 (3.31) | NS |
| Laparoscopy | 38.1 (2.16) | 40.5 (2.48) | NS | |
| PO2 (mm Hg) | Open | 504.6 (15.96) | 510.3 (21.34) | NS |
| Laparoscopy | 546.3 (36.58) | 593.3 (27.26) | NS | |
| pH | Open | 7.36 (0.05) | 7.34 (0.08) | NS |
| Laparoscopy | 7.38 (0.06) | 7.36 (0.05) | NS | |
| TOS (μmol H2O2/l) | Open | 11.5 (2.62) | 34.52 (8.60) | 0.002* |
| Laparoscopy | 10.9 (2.24) | 22.34 (3.69) | 0.002*, 0.009** | |
| TAS (mmol Trolox equiv/l) | Open | 4.3 (1.17) | 3.38 (1.15) | NS |
| Laparoscopy | 4.27 (1.30) | 3.12 (1.36) | NS | |
| OSI (arbitrary unit) | Open | 2.7 (0.48) | 10.3 (1.74) | 0.002* |
| Laparoscopy | 2.9 (1.47) | 9.21 (5.93) | 0.009* | |
Median (inter-quartile range), Open, open ovariectomy group; Laparoscopy, laparoscopic ovariectomy group. ETCO2, end-tidal CO2; TOS, total oxidant status; TAS, total antioxidant status; OSI, oxidative stress index; NS, not significant. *Statistically difference compared to “preoperative” (n=6). **Statistically difference compared to open surgery group (n=6).
Fig. 1.
Total anesthesia (A) and total surgical time (B) in dogs. Data are expressed as median (inter-quartile range) (n=6). Open, open ovariectomy group; Laparoscopy, laparoscopic ovariectomy group.
In small animal surgery, many laparoscopic approaches with different techniques have been used for ovariectomy [6, 8, 10, 16], and these techniques have been conducted to minimizing of surgical complication. This study represents the first clinical trial that compares oxidative stress parameters between open and laparoscopic ovariectomy in bitches. The results of this study showed that oxidative stress induced by anesthesia and surgical trauma and the changes in oxidative stress parameters were lower in laparoscopic surgery compared with open surgery. In addition, total anesthesia and surgical times were comparable in both groups.
Oxidative stress in the body represents an imbalance between the production of reactive oxygen species (ROS) and the ability of the antioxidant defense mechanisms to detoxify the reactive intermediates [20]. The greater the oxidative stress, the more severe the resulting cellular damage during surgery may cause poor outcome in patients [20], and the minimization of oxidative stress is therefore very important.
Plasma TOS and TAS levels have been used to reflect overall oxidative stress and actions against oxidative stress, respectively. An evaluation of these oxidative stress parameters can indirectly reflect changes in organ microcirculation during surgery [12]. The blood contains many antioxidant molecules that prevent and inhibit the harmful effects of ROS. The effects of total antioxidant species reflect the antioxidative status of plasma. The cooperative actions of these various antioxidants in plasma protect organs against oxidative stress [12].
Surgery results in a wide spectrum of unfavorable alteration in normal body homeostasis, which is collectively referred to as surgical stress [1, 2]. In the present study, oxidative stress was induced by anesthesia and surgical trauma in both groups. TOS levels of both groups were significantly increased after surgery. In a similar manner, a recent study on surgical patients reported that surgical trauma alone raises TOS [15].
Laparoscopic surgery has many advantages including less operative pain, minimal incision and short hospital day. But, several studies demonstrated the adverse effects of laparoscopic surgery. During laparoscopic operation, CO2 pneumoperitoneum needs to be induced. This increases abdominal pressure, which may be responsible for splanchnic hypoperfusion with oxidative damage [17]. These phenomena raise reasonable doubts about the safety and advantages of laparoscopic surgery compared with open operations. Problems of the CO2 laparoscopy, such as hemodymic and metabolic effects including CO2 absorption and CO2 intravasation, coupled with an increase in PCO2 have been described [7]. The result can be a metabolic acidosis and a hypercapnia as well as a hypoxemia in the tissue [21]. However, the clinical consequences of these events are uncertain and partially speculative. The results of this study showed no significant difference in PO2, PCO2, pH and ETCO2 between open and laparoscopic ovariectomy in dogs. Moreover, the changes in TOS were significantly lower in laparoscopic surgery compared with open surgery. In contrast to the previous data that showed laparoscopy-induced ischemia-reperfusion injury, the results of the present trial suggest that laparoscopic ovariectomy in dogs is associated with beneficial effects on oxidative stress status compared with open procedures during the operation.
The occurrence of possible side effects and complications is more significant in longer operations. In this study, total anesthesia and surgical times were significantly shorter in laparoscopic groups compared with open surgery group. Davidson et al. (2004) showed that surgical time and complication rate were greater in laparoscopic ovariohyterectomy in dogs [9]. On the other hand, another study showed that operation time was found lower in patients who underwent laparoscopic cholecystectomy [5]. Although the surgical time may be dependent on the surgeon’s skills, type of surgery and surgical techniques, the operation time in laparoscopic ovariectomy was significantly shorter than that of open ovariectomy in this study. Therefore, these advantages of laparoscopic ovariectomy, such as less oxidative stress and shorter surgical time, might be useful to reduce oxidative injury in dogs.
In conclusion, changes in oxidative stress parameters were evident during open or laparoscopic ovariectomy in dogs. Less change of TOS level was observed in laparoscopic ovariectomy group compared with open ovariectomy group. Moreover, total anesthesia and surgical times were significantly shorter in laparoscopic surgery compared with open surgery. Therefore, laparoscopic ovariectomy is a safe and beneficial surgical alternative to traditional ovariectomy on oxidative stress status in dogs.
ACKNOWLEDGMENTS
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (No.2010-0024553).
References
- 1.Anup R., Balasubramanian K. A.2000. Surgical stress and the gastrointestinal tract. J. Surg. Res. 92: 291–300. doi: 10.1006/jsre.2000.5874 [DOI] [PubMed] [Google Scholar]
- 2.Anup R., Aparna V., Pulimood A., Balasubramanian K. A.1999. Surgical stress and the small intestine: role of oxygen free radicals. Surgery 125: 560–569. doi: 10.1016/S0039-6060(99)70209-6 [DOI] [PubMed] [Google Scholar]
- 3.Araujo-Teixeira J. P., Rocha-Reis J., Costa-Cabral A., Barros H., Saraiva A. C., Araujo-Teixeira A. M.1999. Laparoscopy or laparotomy in acute cholecystitis (200 cases). Comparison of the results and factors predictive of conversion. Chirurgie 124: 529–535. doi: 10.1016/S0001-4001(00)88276-8 [DOI] [PubMed] [Google Scholar]
- 4.Avrutis O., Friedman S. J., Meshoulm J., Haskel L., Adler S.2000. Safety and success of early laparoscopic cholecystectomy for acute cholecystitis. Surg. Laparosc. Endosc. Percutan. Tech. 10: 200–207. doi: 10.1097/00129689-200008000-00003 [DOI] [PubMed] [Google Scholar]
- 5.Bukan M. H., Bukan N., Kaymakcioglu N., Tufan T.2004. Effects of open vs. laparoscopic cholecystectomy on oxidative stress. Tohoku J. Exp. Med. 202: 51–56. doi: 10.1620/tjem.202.51 [DOI] [PubMed] [Google Scholar]
- 6.Case J. B., Marvel S. J., Boscan P., Monnet E. L.2011. Surgical time and severity of postoperative pain in dogs undergoing laparoscopic ovariectomy with one, two, or three instrument cannulas. J. Am. Vet. Med. Assoc. 239: 203–208. doi: 10.2460/javma.239.2.203 [DOI] [PubMed] [Google Scholar]
- 7.Cherniack N. S., Longobardo G. S., Staw I., Heymann M.1966. Dynamics of carbon dioxide stores changes following an alteration in ventilation. J. Appl. Physiol. 21: 785–793 [DOI] [PubMed] [Google Scholar]
- 8.Culp W. T., Mayhew P. D., Brown D. C.2009. The effect of laparoscopic versus open ovariectomy on postsurgical activity in small dogs. Vet. Surg. 38: 811–817. doi: 10.1111/j.1532-950X.2009.00572.x [DOI] [PubMed] [Google Scholar]
- 9.Davidson E. B., Moll H. D., Payton M. E.2004. Comparison of laparoscopic ovariohysterectomy and ovariohysterectomy in dogs. Vet. Surg. 33: 62–69. doi: 10.1111/j.1532-950X.2004.04003.x [DOI] [PubMed] [Google Scholar]
- 10.Dupré G., Fiorbianco V., Skalicky M., Gültiken N., Ay S. S., Findik M.2009. Laparoscopic ovariectomy in dogs: comparison between single portal and two-portal access. Vet. Surg. 38: 818–824. doi: 10.1111/j.1532-950X.2009.00601.x [DOI] [PubMed] [Google Scholar]
- 11.Eleftheriadis E., Kotzampassi K., Botsios D., Tzartinoglou E., Farmakis H., Dadoukis J.1996. Splanchnic ischemia during laparoscopic cholecystectomy. Surg. Endosc. 10: 324–326. doi: 10.1007/BF00187381 [DOI] [PubMed] [Google Scholar]
- 12.Erel O.2004. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 37: 277–285. doi: 10.1016/j.clinbiochem.2003.11.015 [DOI] [PubMed] [Google Scholar]
- 13.Erel O.2005. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 38: 1103–1111. doi: 10.1016/j.clinbiochem.2005.08.008 [DOI] [PubMed] [Google Scholar]
- 14.Howe L. M.2006. Surgical methods of contraception and sterilization. Theriogenology 66: 500–509. doi: 10.1016/j.theriogenology.2006.04.005 [DOI] [PubMed] [Google Scholar]
- 15.Koksal H., Kurban S.2010. Total oxidant status, total antioxidant status, and paraoxonase and arylesterase activities during laparoscopic cholecystectomy. Clinics 65: 285–290. doi: 10.1590/S1807-59322010000300008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manassero M., Leperlier D., Vallefuoco R., Viateau V.2012. Laparoscopic ovariectomy in dogs using a single-port multiple-access device. Vet. Rec. 171: 69. doi: 10.1136/vr.100060 [DOI] [PubMed] [Google Scholar]
- 17.Nesek-Adam V., Vnuk D., Rasić Z., Rumenjak V., Kos J., Krstonijević Z.2009. Comparison of the effects of low intra-abdominal pressure and pentoxifylline on oxidative stress during CO2 pneumoperitoneum in rabbits. Eur. Surg. Res. 43: 330–337. doi: 10.1159/000237747 [DOI] [PubMed] [Google Scholar]
- 18.Pessaux P., Regenet N., Tuech J. J., Rouge C., Bergamaschi R., Arnaud J. P.2001. Laparoscopic versus open cholecystectomy: a prospective comparative study in the elderly with acute cholecystitis. Surg. Laparosc. Endosc. Percutan. Tech. 11: 252–255. doi: 10.1097/00129689-200108000-00005 [DOI] [PubMed] [Google Scholar]
- 19.Schietroma M., Carlei F., Liakos C., Rossi M., Carloni A., Enang G. N., Pistoia M. A.2001. Laparoscopic versus open cholecystectomy. An analysis of clinical and financial aspects. Panminerva Med. 43: 239–242 [PubMed] [Google Scholar]
- 20.Sies H.1997. Oxidative stress: oxidants and antioxidants. Exp. Physiol. 82: 291–295 [DOI] [PubMed] [Google Scholar]
- 21.Taura P., Lopez A., Lacy A. M., Anglada T., Beltran J., Fernandez-Cruz L., Targarona E., Garcia-Valdecasas J. C., Marin J. L.1998. Prolonged pneumoperitoneum at 15 mmHg causes lactic acidosis. Surg. Endosc. 12: 198–201. doi: 10.1007/s004649900633 [DOI] [PubMed] [Google Scholar]