ABSTRACT
The present study aimed to determine the complete citrate synthase (gltA) and heat-shock protein (groEL) gene sequences of Anaplasma bovis and to infer phylogenetic relationships within the genus Anaplasma. Multiple alignments from single and concatenated sequences of the 16S rRNA, gltA and groEL genes of the genus Anaplasma were subjected to phylogenetic analyses. Percent identities of A. bovis nucleotide sequences were found highest with A. phagocytophilum in gltA (65.4%) and groEL (79.8%). Single gene phylogenetic tree results assumed similar phylogenetic positions within the genus Anaplasma, except for A. bovis. However, consensus and concatenated sequence phylogenetic trees showed similar results, revealing 2 subgroups within the genus.
Keywords: 16S rRNA gene, Anaplasma bovis, citrate synthase gene (gltA), heat-shock operon gene (groEL), phylogeny
The genus Anaplasma currently recognizes 6 distinct species: A. phagocytophilum, A. platys, A. marginale, A. centrale, A. ovis and A. bovis. The reclassification was mainly based on the phylogenetic information derived from 16S rRNA (complete representation of all member species) and heat-shock operon or groEL (only selected member species) gene data sequences [6]. A recent study identified a potentially novel Anaplasma sp. in Japan (herein provisionally referred to as Anaplasma sp. Japan), which revealed phylogenetic divergence in the 16S rRNA, gltA and groEL genes from any recognized Anaplasma spp. [21]. However, the previous studies present paucity of information on whether the use of secondary structures was employed in their phylogenetic analyses, which is known to produce better tree resolution [15]. In addition, the widely accepted 16S rRNA gene based phylogenies are sometimes inconsistent [16], which is probably due to the propensity of the 16S rRNA gene to recombination/horizontal or lateral gene transfer phenomenon [1]. Therefore, other genes like the gltA [12] and groEL [11, 13] can be alternatively used to clarify phylogenetic relationships.
A. bovis infects circulating monocytes [5]. This particular species has been mainly analyzed only using the 16S rRNA gene [6]. Previous phylogenetic analyses of the genus Anaplasma using the groEL gene did not include yet A. bovis [6, 21] due to the unavailability of the data sequence during the time of analyses. The present study generally aimed to molecularly characterize and analyze A. bovis based on gltA and groEL genes and to infer phylogenetic relationships within the genus Anaplasma using individual and multi-locus approach (including the 16S rRNA gene). Phylogenetic analyses were performed with or without the consideration of secondary structures, using maximum likelihood (ML) and Bayesian Inference (BI) methods.
Blood sample from a feral raccoon (Procyon lotor) [18] in Hokkaido, herein referred to as R499, was used. The sample was previously tested to be 16S rRNA-positive for A. bovis (1,387 bp; GenBank accession number GU937020) and was stored at −30°C. The DNA was extracted and stored as previously described [21].
The designing of primers, determination of the partial gltA and groEL sequences of A. bovis by PCR, genome walking and DNA sequencing strategies were performed as described previously [21]. Primers used in the present study are shown in Table 1. The negative control used was double distilled water. Instead of using an A. bovis DNA, the positive control used was A. platys.
Table 1. Oligonucleotide sequences of primers used in this study.
| Primer name | Oligonucleotide (5’→3’) | Reference |
|---|---|---|
| PCR amplification for a partial gltA sequence | ||
| CS7F2 | ATGR*TAGAAAAW*GCTGTTTT | [21] |
| HG1085R | ACTATACCK*GAGTAAAAGTC | [10] |
| F1b | GATCATGAR*CAR*AATGCTTC | [9] |
| AnaCS1085R1 | ACTATACCK*GAGTAAAAR*TC | This study |
| PCR amplification for a partial groELsequence | ||
| EEgro1F | GAGTTCGACGGTAAGAAGTTCA | [3] |
| Anagro712R | CCGCGATCAAACTGCATACC | [21] |
| Anagro122F | AAATACGGTW*GTCACGGG | This study |
| Anagro649R | CTTTCTTCR*ACAGTTATAAG | This study |
| Genome walker gene-specific primers (gltA) | ||
| ABgl-46R1 | AATGCAGCTGCTCCCGCACTTAAGCAAGTA | This study |
| ABgl-1R2 | GTTGAGCCCACCATTCTTACTGTAGATGTA | This study |
| ABgl-307F1 | CAATATAGCGATCGCTATGGAAGAAATAGC | This study |
| ABgl-338 F2 | TTGCAGGATGAATACTTCATAGAGAGGAAG | This study |
| Genome walker gene-specific primers (groEL) | ||
| ABgr-361F1 | TAAAGGCAAAAGAGGCTGTTCTTACGG | This study |
| ABgr-385F2 | CGGC TCTT ATGT CCAT GAGA CGTG AA | This study |
| ABgr-1010GRF3 | ACAGTGCATCTTCCAGCATAGAAAGTAG | This study |
| ABgr-1117F4 | AGCTTTCAGGTGGTGTGGCTGTGCTGAAAG | This study |
| ABgr-1132F5 | TGGCTGTGCTGAAAGTTGGTGGATCAAGTGA | This study |
| ABgr-96R1 | TTAGGTCCAGCAGTACATCCAACAGCA | This study |
| ABgr-30R2 | GGATGTGTACTATCTCCCTAATGGCTTA | This study |
| ABgr-25R3 | TCTCCCTAATGGCTTTATCCAACTTCTC | This study |
*Degenerate primers: R=A or G, W=A or T, K=G or T
The gltA and groEL sequences were translated into deduced amino acids (dAA) and were manually trimmed to include only the sequence of interest (generally from the start to stop codon). Percent identities were computed as previously described [21]. Multiple sequence alignments (MSA) were performed as suggested by Hall [7] or by using PROMALS3D [15], which considers secondary structures for protein coding genes. Subsequent analyses with and without using the secondary structure information were performed using raxmlGUI [19] by general time reversible (GTR) model. Analyses by ML with prior best model testing using MEGA 5 [20] and by BI using MrBayes 3.2 [17] were also employed. For the protein coding genes, ML analyses were performed using MEGA 5 with prior best model testing, while BI was performed using MrBayes 3.2 guided by the prior best model test results from MEGA 5. All analyses utilizing MEGA 5 were estimated using 100 bootstrap replications. Selected representative sequences from species which had available information on the 3 different genes were concatenated (herein referred to as “supermatrix”). Supermatrices from sequences were analyzed using MrBayes 3.2. Tree results from MrBayes and raxmlGUI were viewed using the FigTree v1.3.1 (http://tree.bio.ed.ac.uk/software/figtree/). Phylogenetic tree inputs were generated from each MSA (with secondary structure guidance) that were concatenated for the supermatrix by BI and were used to construct of a majority-rule consensus tree using the Dendrosope [9]. Using the SplitsTree4 [8], the supermatrix was subjected to phylogenetic network analysis (by NeighborNet method) and was tested for the presence of recombination phenomenon using the PHI test.
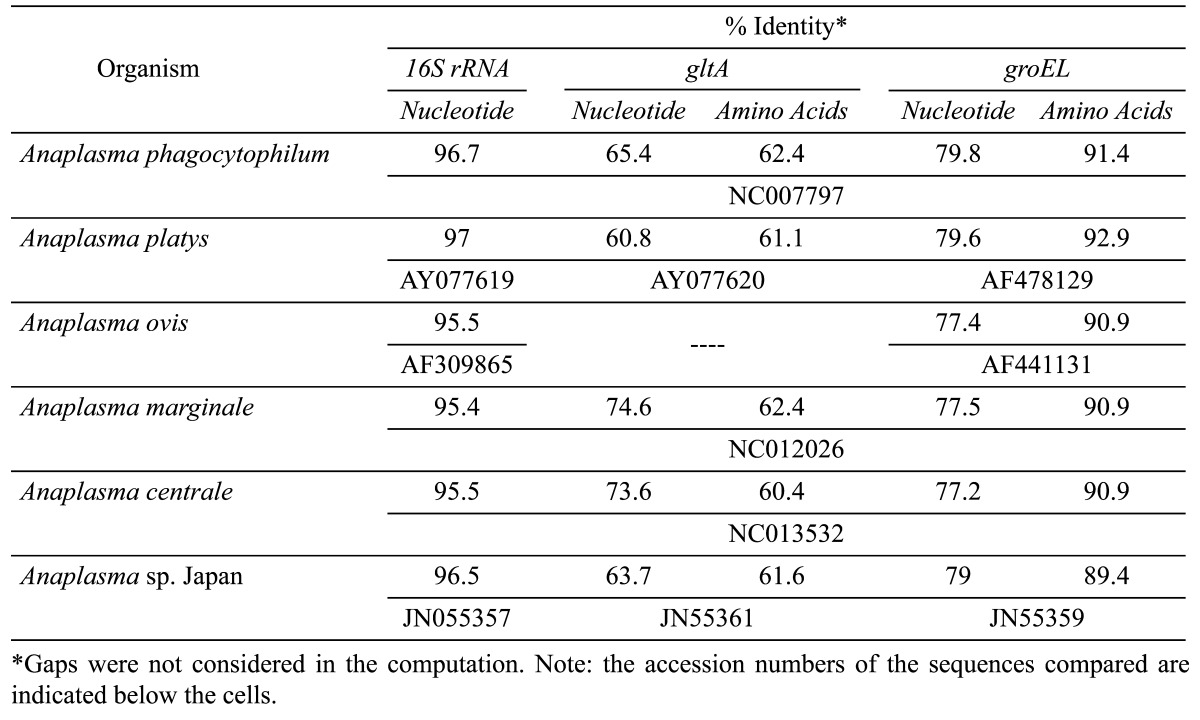
Initially, partial gltA (382 bp) and groEL (385 bp) sequences of A. bovis from R499 were obtained. After the genome walking procedure and sequencing, the complete gltA (1,239 bp; JN588561) and groEL (1,644 bp; JN588562) sequences were determined. In comparison with other Anaplasma species, the gltA and groEL sequences of A. bovis were found closest to A. phagocytophilum (Table 2). Within the genus Anaplasma, species shared at least 60.8 and 60.4% identities in the nucleotide and dAA sequences of the gltA gene, respectively and 77.2 and 89.4% identities in the nucleotide and dAA of the groEL gene, respectively (data not shown).
Table 2. Percent identities of Anaplasma bovis from R499 with other Anaplasma spp. based on 16S rRNA, gltA and groEL gene sequences.
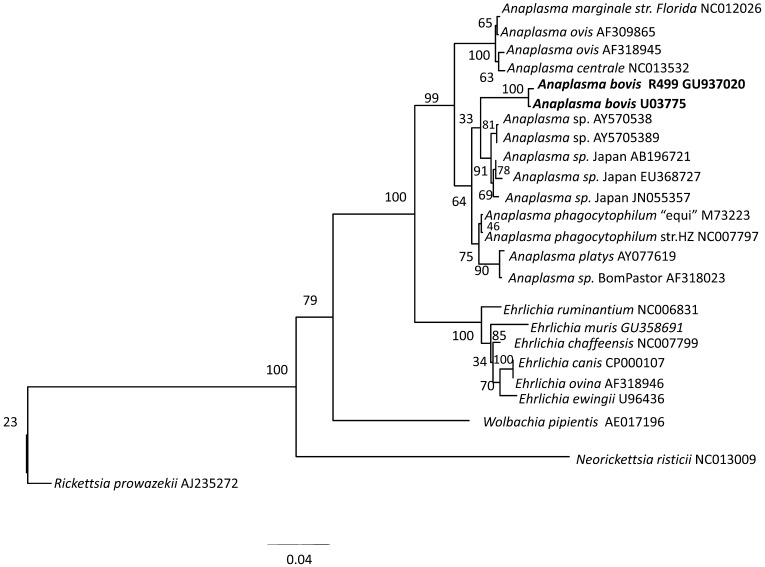
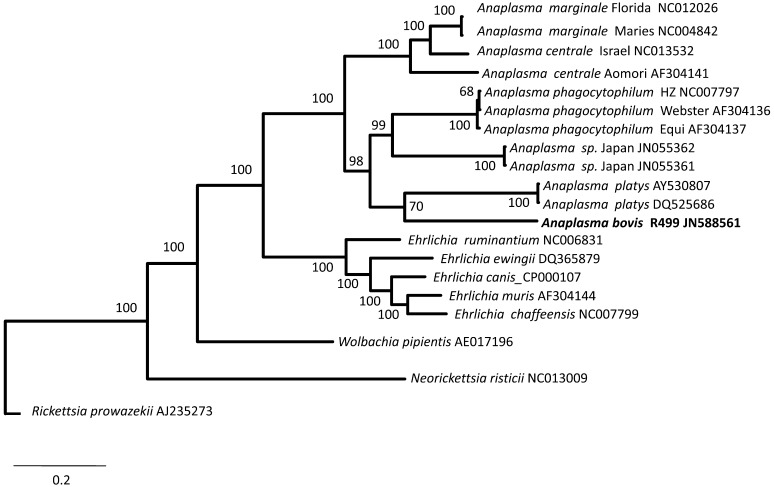
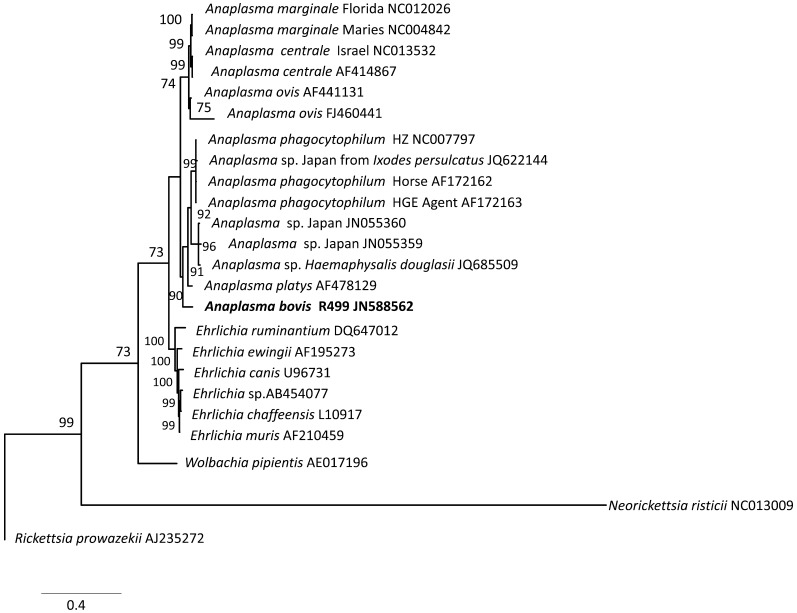
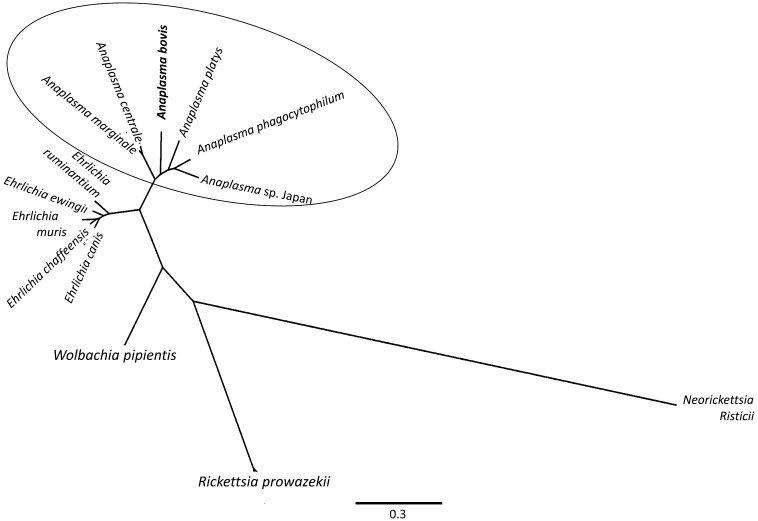
In the 16S rRNA phylogenetic analyses, 2 subclades were seen: (1) a subclade containing A. marginale, A. centrale and A. ovis and (2) a subclade containing A. phagocytophilum, A. platys, A. bovis and Anaplasma sp. Japan (Fig. 1). A. bovis also frequently formed a cluster with Anaplasma sp. Japan. In the gltA phylogenetic analyses (Fig. 2), topologies revealed the 2 subclades observed in the 16S rRNA trees. In the groEL phylogenetic analyses, some positions within the genus Anaplasma changed depending on whether nucleotide or dAA sequences were used, but the 2 subclades were still frequently observed (Fig. 3). On the other hand, trees generated from the supermatrix also revealed the 2 subclades (Fig. 4).
Fig. 1.
Phylogenetic trees based on the 16S rRNA gene. Analyses were performed by maximum likelihood (general time reversible model) using 100 bootstrap replications employed in raxmlGUI [18]. Rickettsia prowazekii was set as the outgroup.
Fig. 2.
Phylogenetic trees based on gltA with consideration of the protein secondary structures. Analyses were performed by the Bayesian method (Jones-Taylor-Thornton model) employed in MrBayes 3.2 [16]. Values in the nodes represent posterior probability values expressed in percent. Rickettsia prowazekii was set as the outgroup.
Fig. 3.
Phylogenetic trees based on groEL genes with consideration of the protein secondary structures. Analyses were performed by the Bayesian method (Jones-Taylor-Thornton model) employed in MrBayes 3.2 [16]. Values in the nodes represent posterior probability values expressed in percent. Rickettsia prowazekii was set as the outgroup.
Fig. 4.
Results of the concatenation approach by the Bayesian method using MrBayes 3.2 [16]. All nodes in the figure revealed posterior probability values of 1 or 100%. Rickettsia prowazekii was set as the outgroup.
A. bovis consistently formed a cluster with A. phagocytophilum, A. platys and Anaplasma sp. Japan in the 16S rRNA phylogenetic trees. This finding varied from the tree results of Dumler et al. [6], in which their 16S rRNA phylogenetic analysis placed A. bovis closer to A. centrale and A. ovis, but was similar to that of Ooshiro et al. [14] and Doan et al. [4], in which A. bovis formed a cluster with A. phagocytophilum and A. platys.
For the gltA and groEL gene phylogenetic analyses, the subclade groupings of the different taxa appear to be consistent when protein secondary structures were considered in the MSA construction, than when nucleotide sequences were used. The groEL-based trees generated in the present study also varied from those of Dumler et al. [6] as sequences of A. bovis, A. ovis, A. centrale and A. platys were not yet included in their analyses. The groEL sequences of A. centrale, A. ovis [13] and A. platys [12] were only determined at a later time. Dumler et al. [6] pointed out the ambiguities among Anaplasma spp. and the arbitrary position of A. bovis within the Anaplasma species clade in the various phylogenetic analyses they performed.
Comparing the single gene or the multi-loci phylogenetic trees, the consistently observed result was the formation of the 2 subclades when secondary structures were considered. Moreover, the resulting topologies corroborated with our previous findings [20], in which the Anaplasma sp. Japan was found to be a potentially novel species. The absence of statistical evidence of recombination (using PHI test) and the subsequent result of the phylogenetic network analysis (by NeighborNet method) on the concatenated alignment also supported the reliability of the tree results. PHI tests are used to test MSAs for the presence of recombination, which can obscure the results of phylogenetic analyses [2].
The present study documented the first molecular analyses of A. bovis based on complete groEL and gltA gene sequences and inferred phylogenetic relationships within the genus Anaplasma with the inclusion of new sequence data. Results clarified the phylogenetic position of A. bovis and established the existence of 2 subclades within the genus Anaplasma. This information can serve as a guide to future phylogenetic studies using the same genus.
ACKNOWLEDGMENTS
This study was supported by a grant for Research on Emerging and Re-emerging Infectious Diseases (H21-Shinkou-Ippan-06) from the Ministry of Health, Labor and Welfare, Japan. The authors also thank Mr. Fujisawa, T., Mr. Angeles, J. Ma. M., Dr. Hakimi, H. and Ms. Ybañez, R. H. D. for their technical assistance.
REFERENCES
- 1.Brayton K. A., Palmer G. H., Lundgren A., Yi J., Barbet A. F.2002. Antigenic variation of Anaplasma marginale msp2 occurs by combinatorial gene conversion. Mol. Microbiol. 43: 1151–1159. doi: 10.1046/j.1365-2958.2002.02792.x [DOI] [PubMed] [Google Scholar]
- 2.Bruen T. C., Philippe H., Bryant D.2006. A Simple and robust statistical test for detecting the presence of recombination. Genetics 172: 2665–2681. doi: 10.1534/genetics.105.048975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chae J. S., Foley J. E., Dumler J. S., Madigan J. E.2000. Comparison of the nucleotide sequences of 16S rRNA, 444 Ep-ank, and groESL heat shock operon genes in naturally occurring Ehrlichia equi and human granulocytic ehrlichiosis agent isolates from Northern California. J. Clin. Microbiol. 38: 1364–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doan H. T., Noh J. H., Choe S. E., Yoo M. S., Kim Y. H., Reddy K. E., Van Quyen D., Nguyen L. T., Nguyen T. T., Kweon C. H., Jung S. C., Chang K. Y., Kang S. W.2013. Molecular detection and phylogenetic analysis of Anaplasma bovis from Haemaphysalis longicornis feeding on grazing cattle in Korea. Vet. Parasitol. 196: 478–481. doi: 10.1016/j.vetpar.2013.03.025 [DOI] [PubMed] [Google Scholar]
- 5.Donatien A., Lestoquard F.1936. Rickettsia bovis, novelle espece pathogene pour le boeuf. Bull. Soc. Pathol. Exot. 29: 1057–1061 [Google Scholar]
- 6.Dumler J. S., Barbet A. F., Bekker C. P., Dasch G. A., Palmer G. H., Ray S. C., Rikihisa Y., Rurangirwa F. R.2001. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int. J. Syst. Evol. Microbiol. 51: 2145–2165. doi: 10.1099/00207713-51-6-2145 [DOI] [PubMed] [Google Scholar]
- 7.Hall B. G.2011. Phylogenetic Trees Made Easy: A How-To Manual, 4th ed., Sinauer Associates, Sunderland. [Google Scholar]
- 8.Huson D. H., Bryant D.2006. Application of phylogenetic networks in evolutionary studies. Mol. Biol. Evol. 23: 254–267. doi: 10.1093/molbev/msj030 [DOI] [PubMed] [Google Scholar]
- 9.Huson D. H., Richter D. C., Rausch C., Dezulian T., Franz M., Rupp R.2007. Dendroscope: an interactive viewer for large phylogenetic trees. BMC Bioinformatics 8: 460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inokuma H., Brouqui P., Drancourt M., Raoult D.2001. Citrate synthase gene sequence: a new tool for phylogenetic analysis and identification of Ehrlichia. J. Clin. Microbiol. 39: 3031–3039. doi: 10.1128/JCM.39.9.3031-3039.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inokuma H., Terada Y., Kamio T., Raoult D., Brouqui P.2001. Analysis of the 16S rRNA gene sequences of Anaplasma centrale and its phylogenetic relatedness to other Ehrlichiae. Clin. Diag. Lab. Immunol. 8: 241–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inokuma H., Fujii K., Okuda M., Onishi T., Beaufils J. P., Raoult D., Brouqui P.2002. Determination of the nucleotide sequences of heat shock operon (groESL) and the citrate synthase gene (gltA) of Anaplasma (Ehrlichia) platys for phylogenetic and diagnostic studies. Clin. Diagn. Lab. Immunol. 9: 1132–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lew A. E., Gale K. R., Minchin C. M., Shkap V., de Waal D. T.2003. Phylogenetic analysis of the erythrocytic Anaplasma species based on 16S rDNA and GroEL (HSP60) sequences of A. marginale, A. centrale, and A. ovis and the specific detection of A. centrale vaccine strain. Vet. Microbiol. 92: 145–160. doi: 10.1016/S0378-1135(02)00352-8 [DOI] [PubMed] [Google Scholar]
- 14.Ooshiro M., Zakimi S., Matsukawa Y., Katagiri Y., Inokuma H.2008. Detection of Anaplasma bovis and Anaplasma phagocytophilum from cattle on Yonaguni Island, Okinawa, Japan. Vet. Parasitol. 154: 360–364. doi: 10.1016/j.vetpar.2008.03.028 [DOI] [PubMed] [Google Scholar]
- 15.Pei J., Kim B. H., Grishin N. V.2008. PROMALS3D: a tool for multiple protein sequence and structure alignments. Nucleic Acids Res. 36: 2295–2300. doi: 10.1093/nar/gkn072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reischl U., Feldmann K., Naumann L., Gaugler B. J. M., Ninet B., Hirschel B., Emler S.1998. 16S rRNA sequence diversity in Mycobacterium celatum strains caused by presence of two different copies of 16S rRNA gene. J. Clin. Microbiol. 36: 1761–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ronquist F., Teslenko M., van der Mark P., Ayres D. L., Darling A., Höhna S., Larget B., Liu L., Suchard M. A., Huelsenbeck J. P.2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61: 539–542. doi: 10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sashika M., Abe G., Matsumoto K., Inokuma H.2011. Molecular survey of Anaplasma and Ehrlichia infections of feral raccoons (Procyon lotor) in Hokkaido, Japan. Vector Borne Zoonotic Dis. 11: 349–354. doi: 10.1089/vbz.2010.0052 [DOI] [PubMed] [Google Scholar]
- 19.Silvestro D., Michalak I.2012. raxmlGUI: a graphical front-end for RAxML. Org. Divers. Evol. 12: 335–337 [Google Scholar]
- 20.Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S.2011. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28: 2731–2739. doi: 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ybañez A. P., Matsumoto K., Kishimoto T., Inokuma H.2012. Molecular analyses of a potentially novel Anaplasma species closely related to Anaplasma phagocytophilum detected in sika deer (Cervus nippon yesoensis) in Japan. Vet. Microbiol. 157: 232–236. doi: 10.1016/j.vetmic.2011.12.001 [DOI] [PubMed] [Google Scholar]