ABSTRACT
The purpose of this study was to evaluate the gene expression of growth factors and growth factor receptors of primary hepatic masses, including hepatocellular carcinoma (HCC) and nodular hyperplasia (NH), in dogs. Quantitative real-time reverse transcriptase-polymerase chain reaction was performed to measure the expression of 18 genes in 18 HCCs, 10 NHs, 11 surrounding non-cancerous liver tissues and 4 healthy control liver tissues. Platelet-derived growth factor-B (PDGF-B), transforming growth factor-α, epidermal growth factor receptor, epidermal growth factor and hepatocyte growth factor were found to be differentially expressed in HCC compared with NH and the surrounding non-cancerous and healthy control liver tissues. PDGF-B is suggested to have the potential to become a valuable ancillary target for the treatment of canine HCC.
Keywords: canine, hepatic nodular hyperplasia, hepatocellular carcinoma, platelet-derived growth factor-B, targeted therapy
Hepatocellular carcinoma (HCC) is the most common primary hepatic tumor in dogs. Canine HCC arises from the uncontrolled proliferation of hepatocytes. Viral infections have been associated with HCC in humans [3], but no causal link with canine HCC has yet been established. In humans, HCC pathogenesis is a multistep process involving sequential events, such as chronic inflammation, hyperplasia and dysplasia, and ultimately, malignant transformation [3]. Several epigenetic and genetic alterations are involved in HCC, which ultimately lead to alterations of molecular pathways.
Recent discoveries in the complex networks involved in HCC proliferation, progression and survival have created many opportunities for the development of targeted drugs and new therapeutic approaches to this disease [5, 18].These new targets include signal transduction pathways, oncogenes and growth factors and their receptors. The key signal transduction pathways that have been implicated in the pathogenesis of HCC include those mediated by vascular endothelial growth factor (VEGF)/VEGF receptor (VEGFR), platelet-derived growth factor (PDGF)/PDGF receptor (PDGFR), epidermal growth factor (EGF)/transforming growth factor-α (TGF-α)/heparin-binding EGF-like growth factor (HB-EGF)/EGF receptor (EGFR), insulin-like growth factor (IGF)/IGF receptor (IGFR), hepatocyte growth factor (HGF)/MET and angiopoietin (Ang)/tyrosine kinases with immunoglobulin and epidermal growth factor homology domains 2 (Tie2) signaling [4, 24]. Activation of these pathways will eventually lead to resistance to apoptosis, cell proliferation, stimulation of angiogenesis, invasiveness and metastasis [4, 24].
It has been demonstrated that mutations in c-kit could lead to constitutive phosphorylation and activation of the receptor in the absence of ligand binding and that such alterations could induce the growth factor-independent proliferation of canine mast cell tumor (MCT) [16]. In addition, imatinib (Gleevec®) and masitinib (Masivet®) are clinically used for the treatment of canine MCT [8, 12]. These drugs compete with adenosine triphosphate (ATP) for the ATP binding site of protein-tyrosine kinase and prevent downstream signaling. For the prediction of the tumor response to these drugs, the detection of a mutation in c-kit is likely to be valuable; however, the expression of molecules in dogs with HCC is still unknown.
The identification of molecules that are overexpressed in dogs with HCC not only increases understanding of tumorigenesis, but also helps to develop therapeutic targets for the treatment of affected dogs. The objectives of this study were to measure the expression of these molecules in dogs with primary hepatic masses and to evaluate the clinical utility of these molecules in the treatment of canine HCC.
Twenty-two client-owned canine patients with primary hepatic masses were used for this study. Each patient underwent a hepatectomy at the Animal Medical Center of Nihon University between March 2010 and July 2012 (Table 1). The hepatic masses and surrounding tissues were histopathologically diagnosed and stored at −80°C until mRNA extraction. Control hepatic tissues were obtained from four sacrificed Beagles and stored at −80°C prior to mRNA extraction. The experimental protocol was performed in accordance with the “Guide for the Experiment of Animals” published by the College of Bioresource Sciences, Nihon University.
Table 1. Samples from Dogs with Hepatocellular Carcinoma and Hepatic Nodular Hyperplasia Used for qRT-PCR.
| Case | Breed | Sex | Age (year) | Body weight (kg) | Histopathology |
Non- cancerous liver | |
|---|---|---|---|---|---|---|---|
| HCCa) | NHb) | ||||||
| 1 | Chihuahua | Castrated male | 10 | 7.15 | 1 | 0 | 1 |
| 2 | Mix | Castrated male | 11 | 24.2 | 1 | 0 | 1 |
| 3 | Shiba | Male | 12 | 11.76 | 1 | 1 | 0 |
| 4 | Siberian Husky | Spayed female | 13 | 19.45 | 1 | 0 | 1 |
| 5 | Golden Retriever | Spayed female | 11 | 30.1 | 1 | 0 | 0 |
| 6 | Beagle | Spayed female | 10 | 8.35 | 1 | 1 | 0 |
| 7 | Shiba | Male | 13 | 13.7 | 1 | 0 | 1 |
| 8 | Shiba | Spayed female | 11 | 8.0 | 1 | 1 | 0 |
| 9 | Mix | Spayed female | 13 | 13.74 | 1 | 1 | 0 |
| 10 | Shetland Sheepdog | Female | 14 | 13.0 | 1 | 0 | 1 |
| 11 | Miniature Dachshund | Spayed female | 10 | 5.94 | 1 | 0 | 1 |
| 12 | Shih Tzu | Female | 13 | 4.62 | 1 | 0 | 1 |
| 13 | Shih Tzu | Spayed female | 11 | 7.02 | 1 | 0 | 1 |
| 14 | Yorkshire Terrier | Spayed female | 12 | 2.9 | 1 | 0 | 1 |
| 15 | Welsh Corgi | Female | 10 | 12.0 | 1 | 1 | 0 |
| 16 | Labrador Retriever | Female | 10 | 27.58 | 1 | 0 | 1 |
| 17 | Beagle | Male | 9 | 17.05 | 1 | 1 | 0 |
| 18 | Shih Tzu | Castrated male | 10 | 8.15 | 1 | 0 | 1 |
| 19 | Toy Poodle | Female | 7 | 4.64 | 0 | 1 | 1 |
| 20 | Mix | Spayed female | 13 | 12.4 | 0 | 1 | 1 |
| 21 | Miniature Dachshund | Castrated male | 12 | 6.45 | 0 | 1 | 0 |
| 22 | Mix | Spayed female | 13 | 6.45 | 0 | 1 | 0 |
a) Hepatocellular carcinoma, b) Nodular hyperplasia.
Liver tissue sections prepared from the surgically resected hepatic tumors and non-cancerous tissues were immediately frozen in liquid nitrogen. The frozen samples were stored at −80°C until use after the homogenization with Trizol reagent (Life Technologies Corporation, Tokyo, Japan). Total RNA was isolated from homogenized samples. In brief, frozen liver tissue (10 mg) was homogenized in an RNase-free homogenizer with 1.0 ml Trizol and then mixed with chloroform. After the samples were centrifuged at 12,000 × g for 15 min, an equal volume of isopropanol was added to the supernatant. After mixing, the RNA was pelleted by centrifugation, washed with cold 75% ethanol and then resuspended in RNase-free water. RNA was isolated with Trizol and then purified with the RNeasy Plus Mini Kit (Qiagen, Tokyo, Japan), according to the RNA Cleanup Protocol. RNA integrity was checked using absorptiometer (NanoDrop 1000, LMS Co., Ltd., Tokyo, Japan).
The cDNA was synthesized from 500 µg of total RNA and oligo dT primer by using a PrimeScript RT reagent kit with gDNA Eraser (TaKaRa Bio Inc., Otsu, Japan), according to the manufacturer’s protocol. Quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) was performed for each sample using a Thermal Cycler Dice Real Time System II device (TaKaRa).
Primers were designed using the Perfect Real Time Primer Support System (TaKaRa) for canines. Two reference genes, glucuronidase beta (GUSB) and TATA-box binding protein (TBP), were measured for normalization based on their stable expression in the liver. Primers for reference genes and genes of interest, including their optimum temperatures, are listed in Table 2.
Table 2. Primers Used for qRT-PCR.
| Gene | Primer setID | F/R | Sequence | Tm (°C) | Amplicon Size (bp) |
|---|---|---|---|---|---|
| GUSB | DA021273 | F | 5’-ACATCGACGACATCACCGTCA-3’ | 76 | 90 |
| R | 5’-GGAAGTGTTCACTGCCCTGGA-3’ | ||||
| TBP | DA049514 | F | 5’-ATGGTGTGTACGGGAGCCAAG-3’ | 76 | 184 |
| R | 5’-ACTGTTGGTGGGTCAGCACAAG-3’ | ||||
| VEGF-A | DA027420 | F | 5’-TCAGGACACTGCTGTACTTTGAGG-3’ | 79.1 | 133 |
| R | 5’-GGCTTGTCAGGAGCAAGTGAA-3’ | ||||
| VEGFR-1 | DA035194 | F | 5’-CACCTGGGCTGAGAGCAAAC-3’ | 77.2 | 117 |
| R | 5’-CCACACCTGGAATGGCAGAA-3’ | ||||
| VEGFR-2 | DA022900 | F | 5’-CTTGGACAGCATCACCAGTAGTCAG-3’ | 75 | 131 |
| R | 5’-TGAGATGCTCCAAGGTCAGGAA-3’ | ||||
| VEGFR-3 | DA049880 | F | 5’-GGATGGAGTTCCTGGCCTCA-3’ | 77.9 | 142 |
| R | 5’-TTTCGCACATAGTCAGGGTCTTTG-3’ | ||||
| PDGF-B | DA006888 | F | 5’-TACGAGATGCTGAGCGACCAC-3’ | 79.1 | 112 |
| R | 5’-ATCGGGTCAAATTCAGGTCCAA-3’ | ||||
| PDGFR-β | DA076806 | F | 5’-TGAGGGCAAGCTGGTCAAGA-3’ | 77.7 | 155 |
| R | 5’-ACACGTCGCTCAGGGTGGTA-3’ | ||||
| EGF | DA074730 | F | 5’-CTATGGCCCTCAAGGATGGTG-3’ | 75.1 | 125 |
| R | 5’-GCAGCCTTGCTCTGTGTCCTTA-3’ | ||||
| TGF-α | DA057929 | F | 5’-GTGGTGTCCCACTTCAACGACT-3’ | 77 | 85 |
| R | 5’-TGTCCTCCTGCACCAGAAACC-3’ | ||||
| HB-EGF | DA044315 | F | 5’-GCTCTGGCCACACCCAGTAA-3’ | 75.3 | 113 |
| R | 5’-CCATGGATGCAGAAGTCCTTGTA-3’ | ||||
| EGFR | DA000951 | F | 5’-TGCATTTGCCAAGCCCTACA-3’ | 75.1 | 90 |
| R | 5’-GGTACTCGTCAGCATCCACAACA-3’ | ||||
| HGF | DA071494 | F | 5’-GGCTACTGCTCCCAAATTCCA-3’ | 71.9 | 124 |
| R | 5’-CCCACATTGAACATGTTAGTCCAGA-3’ | ||||
| MET | DA075812 | F | 5’-ACCAGTGAAGTGGATGGCTTTAGAA-3’ | 75.5 | 135 |
| R | 5’-AAGGTGTTGACGTCAGGATAAGGTG-3’ | ||||
| IGF1 | DA020266 | F | 5’-GATAGAGCCTGCGCAATGGAA-3’ | 71.3 | 120 |
| R | 5’-CTGGAAATGAATTGGTTAGCAGGAA-3’ | ||||
| IGF1R | DA026006 | F | 5’-ATGGTGGCCGAAGATTTCACA-3’ | 75.1 | 120 |
| R | 5’-AGGTGACATCCAGCGCACAG-3’ | ||||
| IGF2R | DA044849 | F | 5’-CACAGTGCGTGACTTACGGAAAC-3’ | 76.2 | 84 |
| R | 5’-CCAGAGCAGGAATCCCAAGTG-3’ | ||||
| Ang-2 | DA059135 | F | 5’-TGGGTGGACGGTCATTCAG-3’ | 74.7 | 106 |
| R | 5’-CAGCCAGTGTTCGCCAGAAG-3’ | ||||
| Tie2 | DA084660 | F | 5’-GCGGGAATGACTTGCCTGA-3’ | 76.9 | 146 |
| R | 5’-AGGGCCAGAGTTCCTGAGTTGA-3’ |
The initial qRT-PCR reaction was carried out for 30 sec at 95°C in SYBR Premix Ex Taq (TaKaRa), followed by 40 two-temperature cycles of 5 sec at 95°C and 30 sec at 60°C. After 40 cycles, a dissociation curve was generated to verify the specificity of each primer. All reactions were performed in duplicate. The specificity of the amplification of the expected DNA fragments was confirmed by 2% agarose gel electrophoresis. Expression was normalized to that of canine TBP. A sample of normal liver tissue was used for calibration, and the ΔΔCt method was used to determine relative gene expression levels. All data were integrated and analyzed using TaKaRa Multiplate RQ (TaKaRa).
Differences in quantitative values were analyzed using the Kruskal–Wallis test with the post-hoc Dunn’s procedure. A P-value of less than 0.05 was considered significant. All analyses were performed using StatMate IV software (ATMS Co., Ltd., Tokyo, Japan).
In this study, 18 HCC samples, 10 hepatic nodular hyperplasia (NH) samples and 11 samples of non-cancerous liver tissue (normal) were obtained from 22 dogs with primary hepatic masses. The expression levels of 18 genes that were differentially expressed in dogs with NH and HCC were measured using qRT-PCR. For technical reasons, no qRT-PCR data could be obtained for IGF2. Only 5 genes proved to be differentially expressed in HCC samples compared with the NH, normal and healthy control samples. The qRT-PCR amplified products were analyzed by agarose gel electrophoresis, and each one band was shown in the expected molecular length (Fig. 1).
Fig. 1.
Analysis by agarose gel electrophoresis of 18 genes real-time PCR amplification.
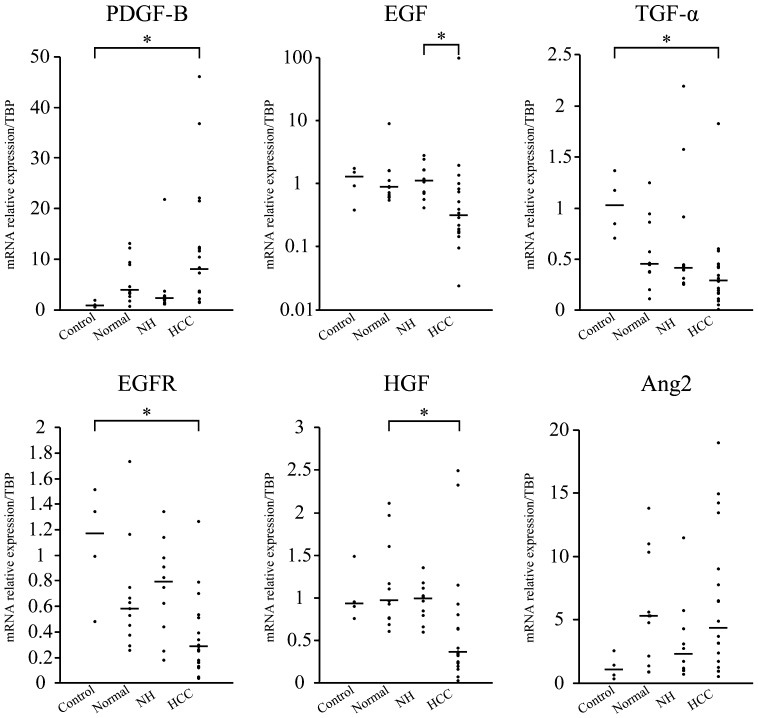
PDGF-B was upregulated, and TGF-α and EGFR were downregulated in HCC compared with healthy controls (P<0.05). EGF was downregulated in HCC compared with NH (P<0.05). HGF was downregulated in HCC compared with normal samples (P<0.05; Fig. 2). The expression of Ang-2 in HCC was not significantly different from that in dogs with NH and normal samples, although it was considered to be upregulated in normal samples (Fig. 2).
Fig. 2.
Distribution of the mRNA relative expression/TBP expression in control, normal, NH and HCC samples. Each bar represents the median value. The PDGF-B gene was upregulated, and TGF-α and EGFR genes were downregulated in HCC compared with controls (*P<0.05). EGF was downregulated in HCC compared with NH (*P<0.05). HGF was downregulated in HCC compared with the normal samples (*P<0.05). Ang-2 in HCC was not significantly different between dogs with NH and normal samples, although its expression was considered to be upregulated in normal samples.
This study showed that canine PDGF-B mRNA is significantly increased in HCC, although levels varied widely in comparison to those measured in healthy controls. PDGFR-β signaling is critical for the recruitment of pericytes or perivascular progenitor cells during developmental and tumor angiogenesis [27]. Endothelial cells-derived PDGF-BB, as the classical PDGFR-β ligand, is essential for embryonic pericytes recruitment [17], and a requisite role of local extracellular PDGF-BB concentration gradients for the proper integration of PDGFR-β-positive pericytes into the wall of tumor vessels has been demonstrated [1]. In humans, angiogenesis and disruption of liver vascularity have been linked to progression to cirrhosis and HCC in chronic liver diseases [7]. Interference with angiogenesis may prevent or delay the development of HCC, considering that angiogenesis plays a key role in the growth of tumors. Therefore, we expected to find increased PDGF-B mRNA expression in dogs with HCC. PDGF-B represents a molecule of potential value in tumor angiogenesis in dogs as well as humans.
In this study, the median expression level of canine Ang-2 mRNA in normal samples was higher than that in HCC samples, but the expression level of canine Ang-2 mRNA had no significant difference between normal and HCC samples. The generally accepted view is that Ang-1 is the major agonist for Tie2, whereas Ang-2 may act as an antagonist or a partial agonist [19]. Although Ang-1 functions in stabilizing the vasculature, Ang-2 destabilizes vessel formation. Recent evidence indicates that, unexpectedly, Ang-2 plays a positive role, at least in tumor angiogenesis [22]. Taken together, these studies suggest that Ang2 plays critical physiological and pathological roles in clinical HCC in vivo in murine tumor models [30]. Administration of Ang-2 inhibitors to tumor-bearing mice has been reported to result in delayed tumor growth accompanied by reduced endothelial cell proliferation, which is consistent with an antiangiogenic mechanism. Therefore, Ang-2 may be an attractive candidate target for the antiangiogenic treatment of HCC [22]; however, this study didn’t demonstrate that the mRNA expression of canine Ang-2 in normal liver tissues was significantly different from that in HCC tissues. Then, further investigations on the angiogenesis mechanism of canine HCC including not only Ang-2 but also Ang-1 are needed for the promising targeted therapy.
In contrast, canine TGF-α and EGFR mRNA expressions were significantly reduced in HCC compared to healthy controls. In addition, EGF mRNA expression was significantly lower in HCC than in NH. TGF-α is a member of the EGF superfamily of polypeptide mitogens and binds to the EGFR [6, 20]. TGF-α is generally thought to be involved in hepatocarcinogenesis [10, 14]. Expression of TGF-α has been reported to be associated with hepatocyte proliferation and hepatocarcinogenesis in humans [10] and mice [13, 23]. In addition, EGFR/ErbB1 is the EGFR that has been most widely studied in HCC. EGFR is frequently overexpressed in HCC [10], suggesting that the EGFR signaling pathway plays a role in hepatocarcinogenesis. Therefore, the EGFR signaling pathway represents a good potential molecular target for biological therapy of HCC. However, the clinicopathological significance of the expressions of EGFR and human epidermal growth factor receptor 2 in HCC remains controversial [2, 9, 11]. Activating mutations of EGFR, the signature markers for the sensitivity of non-small cell lung cancer to small-molecule EGFR inhibitors, were not found in HCC tissues [28]. In addition, increased expressions of TGF-α and EGFR were shown to occur as part of regenerative processes in adjacent non-tumorous liver tissues [13]. An immunohistochemical analysis revealed that TGF-α was overexpressed, equally expressed and downregulated in 17, 21 and 62% of HCC tissues, respectively, compared to the surrounding hepatic tissue [15]. Therefore, our results suggest that TGF-α, EGF and EGFR mRNAs do not reflect the progression of HCC in dogs. In addition, TGF-α and EGFR expressions may be associated with an increased level of proliferation of normal hepatocytes.
HGF is the most potent mitogen for mature hepatocytes. HGF is scarcely expressed in the normal liver, its expression is dramatically increased after liver injury [21] and it stimulates albumin secretion in primary cultures of rat hepatocytes [29]. In addition, HGF inhibits the growth of HCC cells [26] and decreased the incidence of HCC caused by TGF-α in double transgenic mice [25]. In our study, HGF was synthesized in the surrounding non-cancerous liver tissue. Therefore, the reduction of its gene expression might be involved in HCC in dogs.
In conclusion, higher levels of PDGF-B expression were observed in canine HCC, suggesting that PDGF-B has the potential to become a valuable ancillary target for the medical treatment. Further investigations on the association between the molecular changes and the proliferation of canine HCC are needed for the establishment of targeted therapy in dogs with HCC.
REFERENCES
- 1.Abramsson A., Lindblom P., Betsholtz C.2003. Endothelial and nonendothelial sources of PDGF-B regulate pericyte recruitment and influence vascular pattern formation in tumors. J. Clin. Invest. 112: 1142–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altimari A., Fiorentino M., Gabusi E., Gruppioni E., Corti B., D’Errico A., Grigioni W. F.2003. Investigation of ErbB1 and ErbB2 expression for therapeutic targeting in primary liver tumours. Dig. Liver Dis. 35: 332–338. doi: 10.1016/S1590-8658(03)00077-X [DOI] [PubMed] [Google Scholar]
- 3.Bartlett D. L., DiBisceglie A. M., Dawson L. A.2011. Cancer of the liver. pp. 997–1018. In: Cancer: Principles and Practice of Oncology, 9th ed. (De-Vita, V. T., Lawrence, T. S. and Rosenberg, S. A. eds.), Lippincott Williams & Wilkins, Philadelphia. [Google Scholar]
- 4.Cervello M., McCubrey J. A., Cusimano A., Lampiasi N., Azzolina A., Montalto G.2012. Targeted therapy for hepatocellular carcinoma: novel agents on the horizon. Oncotarget 3: 236–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng A. L., Kang Y. K., Chen Z., Tsao C. J., Qin S., Kim J. S., Luo R., Feng J., Ye S., Yang T. S., Xu J., Sun Y., Liang H., Liu J., Wang J., Tak W. Y., Pan H., Burock K., Zou J., Voliotis D., Guan Z.2009. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 10: 25–34. doi: 10.1016/S1470-2045(08)70285-7 [DOI] [PubMed] [Google Scholar]
- 6.Ciardiello F., Tortora G.2008. EGFR antagonists in cancer treatment. N. Engl. J. Med. 358: 1160–1174. doi: 10.1056/NEJMra0707704 [DOI] [PubMed] [Google Scholar]
- 7.Fernández M., Semela D., Bruix J., Colle I., Pinzani M., Bosch J.2009. Angiogenesis in liver disease. J. Hepatol. 50: 604–620. doi: 10.1016/j.jhep.2008.12.011 [DOI] [PubMed] [Google Scholar]
- 8.Hahn K. A., Ogilvie G., Rusk T., Devauchelle P., Leblanc A., Legendre A., Powers B., Leventhal P. S., Kinet J. P., Palmerini F., Dubreuil P., Moussy A., Hermine O.2008. Masitinib is safe and effective for the treatment of canine mast cell tumors. J. Vet. Intern. Med. 22: 1301–1309. doi: 10.1111/j.1939-1676.2008.0190.x [DOI] [PubMed] [Google Scholar]
- 9.Hamazaki K., Yunoki Y., Tagashira H., Mimura T., Mori M., Orita K.1997. Epidermal growth factor receptor in human hepatocellular carcinoma. Cancer Detect. Prev. 21: 355–360 [PubMed] [Google Scholar]
- 10.Harada K., Shiota G., Kawasaki H.1999. Transforming growth factor-alpha and epidermal growth factor receptor in chronic liver disease and hepatocellular carcinoma. Liver 19: 318–325. doi: 10.1111/j.1478-3231.1999.tb00056.x [DOI] [PubMed] [Google Scholar]
- 11.Hsu C., Huang C. L., Hsu H. C., Lee P. H., Wang S. J., Cheng A. L.2002. HER-2/neu overexpression is rare in hepatocellular carcinoma and not predictive of anti-HER-2/neu regulation of cell growth and chemosensitivity. Cancer 94: 415–420. doi: 10.1002/cncr.10180 [DOI] [PubMed] [Google Scholar]
- 12.Isotani M., Ishida N., Tominaga M., Tamura K., Yagihara H., Ochi S., Kato R., Kobayashi T., Fujita M., Fujino Y., Setoguchi A., Ono K., Washizu T., Bonkobara M.2008. Effect of tyrosine kinase inhibition by imatinib mesylate on mast cell tumors in dogs. J. Vet. Intern. Med. 22: 985–988. doi: 10.1111/j.1939-1676.2008.00132.x [DOI] [PubMed] [Google Scholar]
- 13.Jhappan C., Stahle C., Harkins R. N., Fausto N., Smith G. H., Merlino G. T.1990. TGF alpha overexpression in transgenic mice induces liver neoplasia and abnormal development of the mammary gland and pancreas. Cell 61: 1137–1146. doi: 10.1016/0092-8674(90)90076-Q [DOI] [PubMed] [Google Scholar]
- 14.Kira S., Nakanishi T., Suemori S., Kitamoto M., Watanabe Y., Kajiyama G.1997. Expression of transforming growth factor alpha and epidermal growth factor receptor in human hepatocellular carcinoma. Liver 17: 177–182. doi: 10.1111/j.1600-0676.1997.tb00803.x [DOI] [PubMed] [Google Scholar]
- 15.Kiss A., Wang N. J., Xie J. P., Thorgeirsson S. S.1997. Analysis of transforming growth factor (TGF)-alpha/epidermal growth factor receptor, hepatocyte growth Factor/c-met,TGF-beta receptor type II, and p53 expression in human hepatocellular carcinomas. Clin. Cancer Res. 3: 1059–1066 [PubMed] [Google Scholar]
- 16.Letard S., Yang Y., Hanssens K., Palmérini F., Leventhal P. S., Guéry S., Moussy A., Kinet J. P., Hermine O., Dubreuil P.2008. Gain-of-function mutations in the extracellular domain of KIT are common in canine mast cell tumors. Mol. Cancer Res. 6: 1137–1145. doi: 10.1158/1541-7786.MCR-08-0067 [DOI] [PubMed] [Google Scholar]
- 17.Lindahl P., Boström H., Karlsson L., Hellström M., Kalén M., Betsholtz C.1999. Role of platelet-derived growth factors in angiogenesis and alveogenesis. Curr. Top. Pathol. 93: 27–33. doi: 10.1007/978-3-642-58456-5_4 [DOI] [PubMed] [Google Scholar]
- 18.Llovet J. M., Ricci S., Mazzaferro V., Hilgard P., Gane E., Blanc J., De Oliveira A. C., Santoro A., Raoul J., Forner A., Schwartz M., Porta C., Zeuzem S., Bolondi L., Greten T. F., Galle P. R., Seitz J., Borbath I., Häussinger D., Giannaris T., Shan M., Moscovici M., Voliotis D., Bruix J.2008. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 359: 378–390. doi: 10.1056/NEJMoa0708857 [DOI] [PubMed] [Google Scholar]
- 19.Maisonpierre P. C., Suri C., Jones P. F., Bartunkova S., Wiegand S. J., Radziejewski C., Compton D., McClain J., Aldrich T. H., Papadopoulos N., Daly T. J., Davis S., Sato T. N., Yancopoulos G. D.1997. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 277: 55–60. doi: 10.1126/science.277.5322.55 [DOI] [PubMed] [Google Scholar]
- 20.Massagué J.1990. Transforming growth factor-alpha. A model for membrane-anchored growth factors. J. Biol. Chem. 265: 21393–21396 [PubMed] [Google Scholar]
- 21.Nakamura T., Nishizawa T., Hagiya M., Seki T., Shimonishi M., Sugimura A., Tashiro K., Shimizu S.1989. Molecular cloning and expression of human hepatocyte growth factor. Nature 342: 440–443. doi: 10.1038/342440a0 [DOI] [PubMed] [Google Scholar]
- 22.Oliner J., Min H., Leal J., Yu D., Rao S., You E., Tang X., Kim H., Meyer S., Han S. J., Hawkins N., Rosenfeld R., Davy E., Graham K., Jacobsen F., Stevenson S., Ho J., Chen Q., Hartmann T., Michaels M., Kelley M., Li L., Sitney K., Martin F., Sun J. R., Zhang N., Lu J., Estrada J., Kumar R., Coxon A., Kaufman S., Pretorius J., Scully S., Cattley R., Payton M., Coats S., Nguyen L., Desilva B., Ndifor A., Hayward I., Radinsky R., Boone T., Kendall R.2004. Suppression of angiogenesis and tumor growth by selective inhibition of angiopoietin-2. Cancer Cell 6: 507–516. doi: 10.1016/j.ccr.2004.09.030 [DOI] [PubMed] [Google Scholar]
- 23.Sandgren E. P., Luetteke N. C., Qiu T. H., Palmiter R. D., Brinster R. L., Lee D. C.1993. Transforming growth factor alpha dramatically enhances oncogene-induced carcinogenesis in transgenic mouse pancreas and liver. Mol. Cell. Biol. 13: 320–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen Y. C., Hsu C., Cheng A. L.2010. Molecular targeted therapy for advanced hepatocellular carcinoma: current status and future perspectives. J. Gastroenterol. 45: 794–807. doi: 10.1007/s00535-010-0270-0 [DOI] [PubMed] [Google Scholar]
- 25.Shiota G., Kawasaki H., Nakamura T., Schmidt E. V.1995. Characterization of double transgenic mice expressing hepatocye growth factor and transforming growth factor alpha. Res. Commun. Mol. Pathol. Pharmacol. 90: 17–24 [PubMed] [Google Scholar]
- 26.Shiota G., Rhoads D. B., Wang T. C., Nakamura T., Schmidt E. V.1992. Hepatocyte growth factor inhibits growth of hepatocellular carcinoma cells. Proc. Natl. Acad. Sci. U.S.A. 89: 373–377. doi: 10.1073/pnas.89.1.373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song S., Ewald A. J., Stallcup W., Werb Z., Bergers G.2005. PDGFRbeta+ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nat. Cell Biol. 7: 870–879. doi: 10.1038/ncb1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su M. C., Lien H. C., Jeng Y. M.2005. Absence of epidermal growth factor receptor exon 18–21 mutation in hepatocellular carcinoma. Cancer Lett. 224: 117–121. doi: 10.1016/j.canlet.2004.10.010 [DOI] [PubMed] [Google Scholar]
- 29.Takehara T., Matsumoto K., Nakamura T.1992. Cell density-dependent regulation of albumin synthesis and DNA synthesis in rat hepatocytes by hepatocyte growth factor. J. Biochem. 112: 330–334 [DOI] [PubMed] [Google Scholar]
- 30.Tanaka S., Mori M., Sakamoto Y., Makuuchi M., Sugimachi K., Wands J. R.1999. Biologic significance of angiopoietin-2 expression in human hepatocellular carcinoma. J. Clin. Invest. 103: 341–345. doi: 10.1172/JCI4891 [DOI] [PMC free article] [PubMed] [Google Scholar]