Abstract
Objective
To systematically review the literature to examine whether there has been adequate assessment of the effects of dietary intervention on quality of life (QOL) independent of weight loss, assess which instruments are being used to measure nutrition-related QOL, identify gaps in the literature, and suggest future directions.
Design
Systematic review guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement.
Results
A total of 24 studies were eligible for inclusion. The Short Form–36 Health Survey was the most widely used instrument to assess QOL. Other disease-specific instruments were used. Several different dietary approaches (eg, low carbohydrate, low calorie, low fat, combinations) were recommended. Across studies, QOL generally improved after participating in behavioral weight loss interventions, but findings revealed a lack of evidence to definitively determine whether reported changes in QOL were a result of weight loss or independent of it.
Conclusions and Implications
It is important to consider how making broad dietary recommendations for all individuals might affect overall QOL in both positive and negative directions when considering factors other than weight loss and health improvement. If dietary interventions are adversely affecting QOL in other domains (eg, social, economic) and this relationship is not being detected or reported by current research practices, barriers for successful and sustainable dietary changes may not be fully understood.
Keywords: quality of life, diet, weight loss, review
INTRODUCTION
Behavioral lifestyle interventions that include recommendations for dietary changes are widely used to promote weight loss, which, for some individuals, results in decreased risk for several chronic diseases including type 2 diabetes,1 hypertension,2 and some cancers.3 These interventions include a range of dietary approaches (eg, low fat/low calorie, low carbohydrate, low energy density) for creating the energy deficit needed for weight loss. Indeed, the implementation of a variety of dietary interventions has produced at least modest weight loss for many and substantial weight loss for some. However, despite the apparent benefits of dietary interventions on weight and weight-related health outcomes, the independent effect of these various dietary interventions on quality of life (QOL) remains unclear.
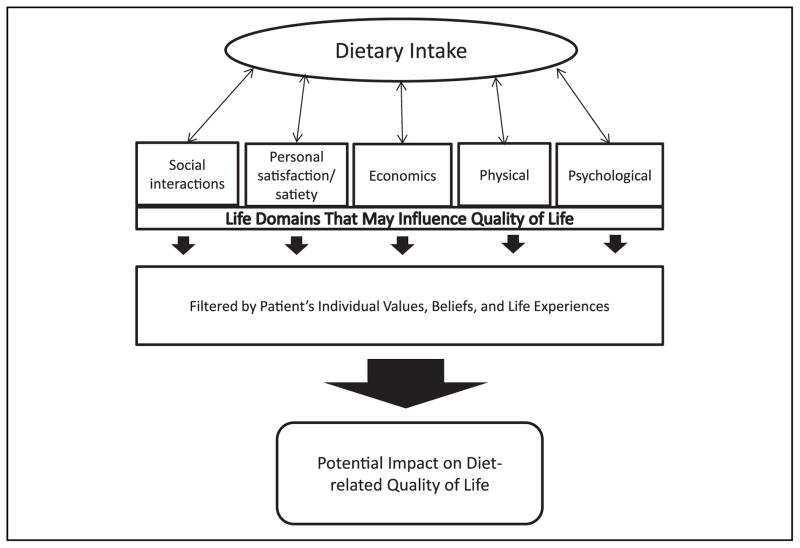
Broadly, QOL is a multidimensional concept that includes an individual’s subjective evaluation of both positive and negative aspects of life.4 Specific areas of study may explore QOL related to a particular discipline, such as a specific disease, overall health, or weight. Research examining the effect of weight loss on QOL is largely mixed depending on whether the QOL measure is obesity specific, and on the intervention modality.5,6 In addition, much of these data are limited to examining only changes in QOL related to weight loss and improvement in health conditions. This approach fails to consider an independent effect that implementing behavior change, altering dietary consumption, or simply participating in an intervention program may have on an individual independent of weight loss. Figure 1 proposes a conceptual model for the relationship between dietary intake and QOL. It illustrates the relationship between dietary intake and several life domains that may ultimately influence QOL. This figure highlights important areas to consider when examining how dietary changes may affect QOL in both positive and negative ways and regardless of whether weight loss occurs. For example, whereas weight loss that results from dietary change may improve some domains of QOL for some individuals, dietary change may also have negative effects on QOL by affecting that individual’s economic situation or social interactions, which are often food centered. Thus, if an individual’s QOL is diminished in some way as a result of dietary change, that individual may be less likely to continue to implement the change, which will ultimately limit successful weight loss and/or weight loss maintenance.
Figure 1.
Conceptual model of the potential impact of dietary intake on quality of life.
To date, the majority of nutrition-or weight-related QOL research has focused on the relationship between dietary intake and QOL by way of physical measures such as weight loss or risk factor reduction. However, it is plausible that making dietary changes can have a meaningful effect—positive or negative—on QOL through other avenues that are less well understood. Guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement,7 the purpose of this report was to systematically review the literature to examine whether there has been adequate assessment of the effects of dietary intervention on QOL independent of weight loss, to assess which instruments are currently being used to measure nutrition-related QOL, to identify gaps in the current literature, and to suggest future research directions.
METHODS
Published results of nutrition/dietary interventions intended to promote weight loss were reviewed. The primary outcome of interest was change in QOL. Secondary outcomes of interest were changes in weight and attrition.
With the assistance of a reference librarian, articles were retrieved using searches performed in PubMed, CINAHL, Psychinfo, Scopus, and the Cochrane Library. Searches for MeSH headings and key words were conducted to identify publications for inclusion, using the following limits: date, human studies, age and language. Searches were performed using combinations of the following terms: “quality of life,” “nutrition,” “diet,” “food,” “weight,” “weight loss,” and “intervention.”
Inclusion and Exclusion Criteria
All studies were evaluated according to the following inclusion criteria: (1) The study reported QOL as an outcome; (2) the study was a dietary intervention; (3) the study was intended to promote weight loss; (4) the intervention was at least 12 weeks in duration; (5) the study was a human study; (6) study participants were adults (age ≥ 19 years); (7) the publication was available in the English language; (8) the study was conducted in the United States; and (9) the publication date was between January 1, 1990 and August 31, 2012. Studies were excluded if the intervention provided food, surgery, or pharmaceutical means for weight loss, unless a dietary intervention arm that met the inclusion criteria was included in the trial as a comparison group. Studies were also excluded if they met none of the stated inclusion criteria (ie, studies were required to meet all inclusion criteria to be evaluated for this report).
Filtering Steps
All search results were first combined into a master reference database and duplicate references were deleted. Studies that clearly did not meet inclusion criteria based on reading the titles and abstracts were excluded. For all remaining papers, the full text of the paper was read to determine whether the study met inclusion criteria.
Methodological Quality Assessment
Each study was assessed for bias using the Methodological Index for Non-Randomized Studies, a tool for assessing risk of bias in both non-comparative and comparative studies.8 Two co-authors (B.H. and O.A.) independently rated each study (not reported = 0, reported but inadequate = 1, or reported and adequate = 2) for the following items: clearly stated aim, inclusion of consecutive patients, prospective data collection, appropriate end points, unbiased assessment of study end point, appropriate follow-up period, < 5% loss to follow-up, prospective calculation of the sample size. For comparative studies, items also included an adequate control group, contemporary groups, baseline equivalence of groups, and adequate statistical analyses. These ratings were used as the basis for the overall score of quality for each study, with the possibility of 24 points for comparative studies and 16 points for non-comparative studies. The 2 reviewers discussed the ratings and arrived at an agreement on the quality score in each study. When consensus could not be reached, a third co-author (T.L.C.) reviewed the study to adjudicate the quality score.
Data Extraction
Data were extracted by the co-authors individually using data extraction tables. Data extracted included study name and dates of study, intervention setting and duration, sample size, gender and race composition of sample, anthropometrics, QOL instrument used, and changes in QOL.
RESULTS
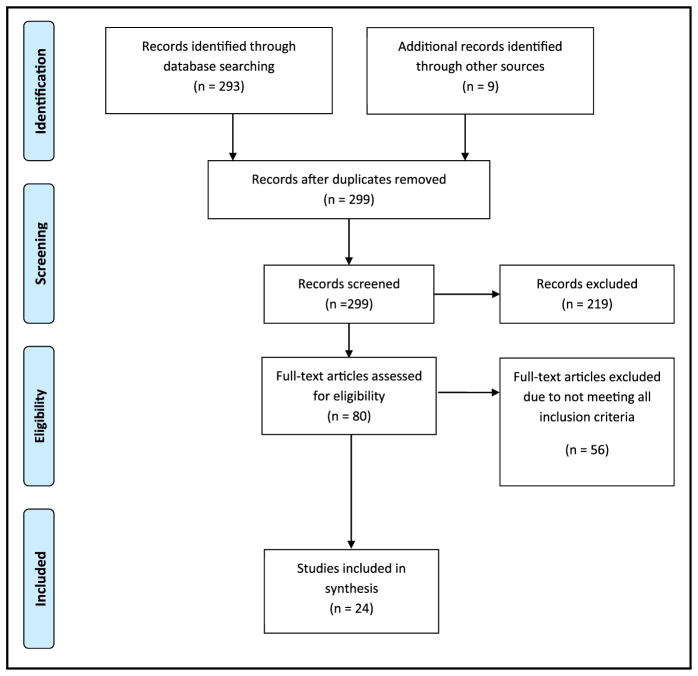
The initial search yielded 302 articles (Figure 2). After removal of 3 duplicates, title and abstract review of the remaining results led to exclusion of 219 articles. The primary reasons for exclusion at this point in the review were studies not meant to promote weight loss, studies conducted outside the United States, surgical interventions, or pediatric populations. Thus, 80 articles were deemed potentially eligible. Of the 80 potentially eligible articles, 56 were excluded because they did not meet all eligibility criteria. Descriptive characteristics of the 24 included studies are shown in Table 1. All but 39–11 of the included studies were randomized trials ranging in duration from 12 to 104 weeks in treatment and duration. Study samples included mostly women (50% to 100% of participants) for all included studies except Evangelista et al12 and Pope et al.13
Figure 2.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram detailing the review filtering process.
Table 1.
Characteristics of Included Studies (n = 24)
| Study | Design, Setting | Intervention Groups, Component Details | Treatment Duration, Follow-up Duration | Sample Characteristics (Group Size, n; Age, y [% Female Gender]) | Quality of Life Instrument Used |
|---|---|---|---|---|---|
| Ackerman et al14 | RCT, clinical | G1: placebo G2: metformin (increased to 1,700 mg/d) G3: lifestyle: goal-based diet and PA intervention; 150 min/wk moderate PA; 1,200–2,000 kcal/d based on baseline weight and dietary fat to < 25% of total calories |
104 wk, 104 wk | G1: n = 1,082; 50.6 (68%) G2: n = 1,073; 50.6 (68%) G3: n = 1,079; 50.6 (68%) |
SF-36 and QWB-SA |
| Barham et al29 | RCT, worksite | G1: wait-list control G2: lifestyle: goal-based diet and PA intervention; 150 min/wk moderate PA; 1,200–2,000 kcal/d based on baseline weight and dietary fat to < 25% of total calories |
52 wk, 52 wk | G1: n = 24; 51.2 (81%) G2: n = 21; 51.1 (81%) |
HRQOL SF-12, IWQOL, 3-Factor Eating Questionnaire |
| Blissmer et al9 | Cohort, clinical | G1: increased F/V and whole grains, set fat goals of 20%/25%/30% of total calories | 24 wk, 24 wk | G1: n = 144; 50.2 (78%) | SF-36 |
| Darga et al32 | RCT, community | G1: control: received National Cancer Institute’s Action Guide to Healthy Eating and Food Guide Pyramid pamphlets with no other instruction G2: Weight Watchers G3: One-on-one dietary counseling of calorie/fat restriction G4: combined Weight Watchers and individualized counseling group |
24 wk, 24 wk | Overall (not group specific): n = 39; 52.1 (100%) | FACT-An and FACT-G |
| Davis et al15 | RCT, clinic/university | G1: low-CHO diet; 2-wk phase of CHO restriction of 20–25 g daily with gradual 5-g increase G2: low-fat diet; 25% of energy needs based on baseline weight |
52 wk, 52 wk | G1: n = 55; 54 (81%) G2: n = 50; 53 (74%) |
SF-36 and IWQOL-Lite |
| Evangelista et al12 | RCT, clinical | G1: AHA recommended conventional diet G2: high protein; 40% CHO, 30% protein, 30% fat G3: standard protein, hypocaloric; 55% CHO, 15% protein, 30% fat |
12 wk, 12 wk | G1: n = 4; 62.2 (25%) G2: n = 5; 56.4 (20%) G3: n = 5; 58.6 (20%) |
Minnesota Living With Heart Failure questionnaire |
| Fontaine et al26 | RCT, community | G1: control: lifestyle PA to increase PA throughout day G2: diet + PA; traditional aerobics and reduced-calorie, reduced-fat diet; 1,000 kcal less than maintenance |
13 wk, 13 wk | G1: n = not stated; 37.3 (53.3%) G2: n = not stated; 36.4 (46.7%) |
SF-36, BDI |
| Heshka et al16 | RCT, clinic | G1: self-help program; 20-min counseling sessions with nutritionist and provision of self-help resources G2: commercial Weight Watchers weight loss program: food plan, activity plan, and cognitive restructuring behavior modification plan |
104 wk, 104 wk | G1: n = 211; 45 (82%) G2: n = 212; 44 (87%) |
SF-36 and IWQOL-Lite |
| Imayama et al27 | RCT, community and cancer research center | G1: wait-list control G2: lifestyle; goal-based diet and PA intervention; 150 min/wk moderate PA; 1,200–2,000 kcal/d based on baseline weight and dietary fat < 30% of total calories G3: exercise; 45 min/d MVPA, 5 d/wk G4: diet and exercise combined |
52 wk, 52 wk | G1: n = 118; 54.7 (100%) G2: n = 87; 57.4 (100%) G3: n = 117; 58.1 (100%) G4: n = 117; 58 (100%) |
SF-36 |
| Kennedy et al30 | RCT, community | G1: general nutrition education, group G2: general nutrition education, individual |
24 wk, 24 wk | G1: n = 20; 44 (NP) G2: n = 20; 44 (NP) |
IWQOL |
| Ladson et al33 | RCT, clinical | G1: metformin (increased to 2,000 mg/d) + caloric restriction; 500 kcal less than maintenance G2: lifestyle-alone caloric restriction; 500 kcal less than maintenance |
24 wk, 24 wk | G1: n = 55; 29 (100%) G2: n = 59; 28.8 (100%) |
PCOS HRQOL |
| Malone et al10 | Cohort, university | G1: general nutrition education, group | 20 wk, 20 wk | G1: n = 90; 48 (82%) | SF-36 |
| Melanson et al17 | RCT, community | G1: exercise only G2: lifestyle; 25% to 40% calories from meal replacements with F/V, whole grains, if dairy; 50% CHO, 25% protein, 25% fat |
12 wk, 24 wk | G1: n = 47; 42.3 (85.1%) G2: n = 43; 43.0 (86.0%) |
SF-36 |
| Pope et al13 | RCT, community | G1: standard cardiac rehabilitation + caloric restriction; 500 kcal less than maintenance G2: high-calorie expenditure + caloric restriction; 500 kcal less than maintenance |
20 wk, 20 wk | G1: n = 36; 63 (16.7%) G2: n = 38; 64 (21%) |
SF-36 |
| Rejeski et al18 | RCT, university | G1: control; group sessions of usual care G2: diet; emphasis on changing eating habits to lower caloric intake G3: exercise; 60 min, 3 d/wk G4: combined diet and exercise |
72 wk, 72 wk | G1: n = 68; 68.6 (66.7%) G2: n = 73; 68.1 (74.1%) G3: n = 69; 68.96 (73.8%) G4: n = 68; 68.5 (73.3%) |
SF-36 |
| Rippe et al19 | Randomized prospective trial, Weight Watchers International | G1: control; maintain regular lifestyle G2: diet and exercise; commercial Weight Watchers program G3: exercise; expenditure of 3,139.5 kJ in self-selected PA; gradual increase to maximum of 6,379 kJ |
12 wk, 12 wk | G1: n = 40; 35.6 (100%) G2: n = 30; 37.4 (100%) |
SF-36 |
| Ross et al11 | Cohort, community | G1: lifestyle; goal-based diet and PA intervention; 150 min/wk moderate PA; 1,200–2,000 kcal/d based on baseline weight and dietary fat to < 25% of total calories | 24 wk, 24 wk | G1: n = 274; 59.0 (100%) | SF-36 |
| Villareal et al20 | RCT, university | G1: control; maintain regular lifestyle G2: diet and exercise; caloric restriction of 750 kcal less than maintenance |
26 wk, 26 wk | G1: n = 10; 71.1 (60%) G2: n = 17; 69.4 (71%) |
SF-36 |
| Villareal et al21 | RCT, university | G1: control G2: diet; 500–750 kcal less than daily energy requirement G3: exercise; 90-min aerobic, strength, and flexibility exercises G4: diet and exercise combined |
52 wk, 52 wk | G1: n = 27; 69.0 (67%) G2: n = 26; 70.0 (65%) G3: n = 26; 70.0 (62%) G4: n = 28; 70.0 (57%) |
SF-36 |
| von Gruenighen et al34 | RCT, clinical/community | G1: control; usual care brochure G2: general nutrition/PA education |
24 wk, 52 wk | G1: n = 23; 55.5 (100%) G2: n = 22; 54.0 (100%) |
FACT-G |
| Williamson et al25 | RCT, multi-site clinical | G1: control; diabetes support and education G2: lifestyle; goal-based diet and PA intervention; 175 min/wk moderate PA; 1,200–2,000 kcal/d based on baseline weight and dietary fat to < 25% of total calories |
52 wk, up to 11.5 y | G1: n = 2,575; 58.8 (59.7%) G2: n = 2,570; 58.6 (59.4%) |
SF-36, BDI-II |
| Wolf et al22 | RCT, university | G1: control; usual care brochure G2: general nutrition/PA education |
52 wk, 52 wk | G1: n = 71; 53.4 (58%) G2: n = 73; 53.3 (62%) |
SF-36 |
| Womble et al23 | RCT, community | G1: weight loss manual; 1,200–1,500 kcal/d self-selected diet of conventional foods based on Food Guide Pyramid G2: commercial program; e-diets; conventional foods with caloric restriction based on body mass index |
52 wk, 52 wk | G1: n = 24; 43.3 (100%) G2: n = 23; 44.2 (100%) |
SF-36 |
| Yancy et al24 | RCT, Veterans Administration | G1: low-CHO/low-ketogenic diet; < 20 g/d G2: low-fat diet; < 30% of daily energy |
24 wk, 24 wk | G1: n = 59; 44.2 (75%) G2: n = 60; 45.6 (78%) |
SF-36 |
AHA indicates the American Heart Association; BDI, Beck Depression Inventory; CHO, carbohydrates; F/V, fruits and vegetables; FACT-G/An, Functional Assessment of Cancer Therapy–General/Anemia; G1, G2, G3, Group 1, 2, or 3; HRQOL, Health-Related Quality of Life; IWQOL, Impact of Weight on Quality of Life; MVPA, moderate and vigorous physical activity; NP, not provided; PA, physical activity; PCOS, polycystic ovarian syndrome; QWB-SA, Quality of Well-Being Scale–Self-administered; RCT, randomized controlled trial; SF-36, Short Form–36 Health Survey.
Methodological Quality Assessments
The mean quality score was 19.6 ± 2.6 (81.8%) for comparative studies and 8.7 ± 0.9 (54.2%) for non-comparative studies, respectively. Unbiased end point assessment (ie, blinding), sample size calculation, and loss to follow-up received the 3 lowest ratings (range, 0.71–1.29 out of 2) across all items irrespective of study design. These findings suggest that attention is needed to improve the methods and reporting of studies in the nutrition literature assessing QOL.
Quality of Life Measures
Eight different surveys were used to measure QOL in the included studies. The majority (71%) of the studies9–11,13–27 used the Short Form–36, a generic tool for assessing QOL using 36 items and including both a physical and mental component summary.28 Four studies15,16,29,30 used the Impact of Weight on Quality of Life–Lite, which was designed to specifically assess QOL related to weight.31 Other disease-specific instruments (eg, Minnesota Living With Heart Failure Questionnaire, Functional Assessment of Cancer Therapy–General, and Polycystic Ovarian Syndrome Health–Related Quality of Life) were employed in 4 of the included studies.12,32–34
Recommended dietary interventions included calorie restriction alone; fat restriction alone; calorie and fat restriction combined; low-carbohydrate, high-protein, low-sodium/high-potassium, commercial programs such as Weight Watchers; or a general “healthy diet” recommendation. Calorie restriction was the most frequently endorsed approach. With the exception of 3 studies,13,33,34 all treatment arms with any type of dietary intervention component reported a within-group improvement in QOL (Table 2). Similarly, all active treatment arms reported some weight loss, although the amount ranged from 0.8 to 10.0 kg (Table 2). Based on reported analyses, 4 studies clearly demonstrated that changes in QOL were independent of weight loss,9,15,18,24 whereas 11 studies indicated that changes were likely a result of weight loss (Table 2).11–14,16,19,25–27,32,34 Based on information provided, the role of weight loss in QOL change was unclear for the remaining 9 studies.10,17,20–23,29,30,33 Independent changes in QOL were noted, although not consistently observed, in studies of several different strategies for weight loss, including fat restriction,9 calorie restriction,18 and studies including low-carbohydrate recommendations (Table 3).15,24
Table 2.
Quality of Life and Weight Change Outcomes, by Study Group
| Study Authors | Attrition by Study Group (%) | Baseline Weight, kg | Weight Change by Study Group, kg | Within-Group QOL Improvement | Between-Group Differences in QOL Change | QOL Independent of Weight Change? (Yes/No/Cannot Determine) |
|---|---|---|---|---|---|---|
| Ackerman et al14 | Placebo: 0.0 Metformin: 0.0 kcal/fat restriction: 0.0 |
Placebo: 93.89 Metformin: 91.5 kcal/fat restriction: 87.2 |
Placebo: −0.4 Metformin: −2.7 kcal/fat restriction: −6.8 |
Placebo: no Metformin: no kcal/fat restriction: yes |
Yes | No |
| Barham et al29 | Control: NP kcal/fat restriction: NP |
Control: 96.5 kcal/fat restriction: 107.3 |
Control: +0.7 kcal/fat restriction: −2.3 |
Control: yes kcal/fat restriction: yes |
Yes | Cannot determine |
| Blissmer et al9 | Fat restriction: 24.0 | Fat restriction: 89.7 | Fat restriction: −5.6 | Fat restriction: yes | NA | Yes |
| Darga et al32,a | Control: 23.0 Commercial diet: 23.0 kcal/fat restriction: 23.0 Combination: 23.0 |
Control: 94.5 Commercial diet: 94.5 kcal/fat restriction: 94.5 Combination: 94.5 |
Control and commercial diet: −0.5 kcal/fat restriction and combination: −8.7 |
Control: yes Commercial diet: yes kcal/fat restriction: yes Combination: yes |
Unclear | No |
| Davis et al15 | Low-CHO: 19.0 Fat restriction: 19.0 |
Low-CHO: 93.6 Fat restriction: 101.0 |
Low-CHO: −3.1 Fat restriction: −3.1 |
Low-CHO: yes Fat restriction: yes |
No | Yes |
| Evangelista et al12 | Control: 0.0 High protein: 0.0 kcal restriction: 0.0 |
Control: 109.6 High protein: 110.8 kcal restriction: 99.5 |
Control: −1.5 High protein: −9.9 kcal restriction: −5.6 |
Control: yes High protein: yes kcal restriction: yes |
Yes | No |
| Fontaine et al26 | Control: NP kcal/fat restriction: NP |
Control: 85.2 kcal/fat restriction: 87.2 |
Control: −7.0 kcal/fat restriction: −8.7 |
Control: yes kcal/fat restriction: yes |
No | No |
| Heshka et al16 | Control: 25.0 Commercial: 28.0 |
Control: 93.1 Commercial: 94.2 |
Control: −0.1 Commercial: −3.0 |
Control: yes Commercial: yes |
No | No |
| Imayama et al27 | Control: 0.0 kcal/fat restriction: 0.0 Exercise: 1.0 Combination: 0.0 |
Control: 30.7 (BMI) kcal/fat restriction: 31.0 (BMI) Exercise: 30.7 (BMI) Combination: 31.0 (BMI) |
Control: not stated kcal/fat restriction: −7.2 Exercise: −2.0 kg Combination: −8.9 |
Control: yes kcal/fat restriction: yes Exercise: yes Combination: yes |
Yes | No |
| Kennedy et al30 | Healthy diet, group: 20.0 Healthy diet, individual: 0.0 |
Healthy diet, group: 103.7 Healthy diet, individual: 103.4 |
Healthy diet, group: −3.1 Healthy diet, individual: −3.4 |
Healthy diet, group: no Healthy diet, individual: no |
No | NA |
| Ladson et al33 | Drug + kcal restriction: 72.0 kcal restriction: 60.0 |
Drug + kcal restriction: 102.7 kcal restriction: 104 |
Drug + kcal restriction: −3.4 kcal restriction: −2.0 |
Drug + kcal restriction: no kcal restriction: yes |
No | Cannot determine |
| Malone et al10 | Healthy diet: 57.0 | Healthy diet: 100.9 | Healthy diet: −4.1 | Healthy diet: yes | No | Cannot determine |
| Melanson et al17 | Exercise: 59.6 kcal restriction: 49.8 |
Exercise: 84.3 kcal restriction: 88.8 |
Exercise: −0.4 kcal restriction: −7.1 |
Exercise: no kcal restriction: yes |
Not stated | Cannot determine |
| Pope et al13 | Standard rehabilitation + kcal restriction: 5.6 High activity + kcal restriction: 2.6 |
Standard rehabilitation + kcal restriction: 95.4 High activity + kcal restriction: 93.5 |
Standard rehabilitation + kcal restriction: −3.7 High activity + kcal restriction: −8.2 |
Standard rehabilitation + kcal restriction: no High activity + kcal restriction: yes |
Yes | No |
| Rejeski et al18 | Control: 22.0 kcal restriction: 20.0 Exercise: 18.0 Combination: 24.0 |
Control: 95.8 kcal restriction: 95.1 Exercise: 94.1 Combination: 91.9 |
control: −1.2 kcal restriction: −5.4 Exercise: −2.4 Combination: −4.0 |
Control: yes kcal restriction: yes Exercise: yes Combination: yes |
Yes | Yes |
| Rippe et al19 | Control: 35.0 Commercial: 25.0 |
Control: 82.1 Commercial: 81.2 |
Control: −1.3 Commercial: −6.1 |
Control: yes Commercial: yes |
Yes | No |
| Ross et al11 | kcal/fat restriction: 15.0 | kcal/fat restriction: 96.3 | kcal/fat restriction: −10 | kcal/fat restriction: yes | NA | No |
| Villareal et al20 | Control: 0.0 kcal restriction: 0.0 |
Control: 103.2 kcal restriction: 99.7 |
Control: +0.7 kcal restriction: −8.2 |
Control: yes kcal restriction: yes |
Yes | Cannot determine |
| Villareal et al21 | Control: 0.0 kcal restriction: 0.0 Exercise: 0.0 Combination: 0.0 |
Control: 101.0 kcal restriction: 104.1 Exercise: 99.2 Combination: 99.1 |
Control: −0.1 kcal restriction: −9.7 Exercise: −0.5 Combination: −8.6 |
Control: no kcal restriction: yes Exercise: yes Combination: yes |
Yes | Cannot determine |
| Von Gruenighen et al34 | Control: 10.0 Healthy diet, individual: 22.0 |
Control: 41.1 (BMI) Healthy diet, individual: 43.5 (BMI) |
Control: −1.4 Healthy diet, individual: −3.5 |
Control: no healthy diet, individual: no |
No | No |
| Williamson et al25 | Control: 4.3 kcal/fat restriction: 2.9 |
Control: 100.8 kcal/fat restriction: 100.5 |
Control: −0.9 kcal/fat restriction: −8.7 |
Control: no kcal/fat restriction: yes |
Yes | No |
| Wolf et al22 | Control: 14.0 Healthy diet, individual: 26.0 |
Control: 107.6 Healthy diet, individual: 107.1 |
Control: −0.6 Healthy diet, individual: −2.4 |
Control: yes Healthy diet, individual: yes |
Yes | Cannot determine |
| Womble et al23 | kcal restriction, manual: 33.3 kcal restriction, commercial: 34.5 |
kcal restriction, manual: 87.9 kcal restriction, commercial: 93.4 |
kcal restriction, manual: −3.3 kcal restriction, commercial: −0.8 |
kcal restriction, manual: yes kcal restriction, commercial: yes |
No | Cannot determine |
| Yancy et al24 | Low CHO: 25.0 Fat restriction: 55.0 |
Low CHO: 97.8 Fat restriction: 96.8 |
Low CHO: none stated Fat restriction: none stated |
Low CHO: yes Fat restriction: yes |
Yes | Yes |
BMI indicates body mass index; NA, not available; other abbreviations as in Table 1.
Attrition and baseline values for body weight were not provided by group. Thus, the overall sample average is reported for each group. Also, weight loss outcomes were combined as shown in the table.
Table 3.
Summary of Dietary Interventions Used in Included Studies and Their Effect on QOL
| Type of Dietary Intervention Recommendation | Summary of Intervention Effects on QOL and Supporting Quotations |
|---|---|
Caloric restriction alone
|
All study interventions of calorie restriction produced improved QOL. No studies clearly indicated whether QOL improvements were independent of weight loss. |
Fat restriction alone
|
All intervention arms endorsing a low-fat diet produced improved QOL. Most evidence suggested that QOL improvements were not completely attributable to weight loss. However, improvements in HRQOL did not appear to be dependent solely on weight loss.9 Our findings suggest QOL improvement are limited to the domains of sexual function and energy and mobility independent of the dietary approach used and independent of changes in weight and A1C.15 |
Low calorie, low-fat
|
All but 1 study intervention arm endorsing calorie restriction with emphasis on fat reduction produced improved QOL. Evidence suggested that QOL improvements were mostly, but not completely, attributable to weight loss. We also found significant associations between weight loss, increased aerobic fitness, and improvements in HRQOL and psychological factors, suggesting that these factors may explain, at least in part, the improved HRQOL observed in the diet and exercise interventions.27 Our findings demonstrate that improvements in HRQOL occurring across different diabetes prevention interventions in the DPP were mediated primarily by weight loss, and no significant improvement in global HRQOL occurred through intervention pathways independent of weight loss.14 |
Low carbohydrate
|
Both study intervention arms endorsing low-carbohydrate diets produced improved QOL. Evidence suggested that at least some aspect of QOL improvement was independent of weight loss. Our findings suggest QOL improvement are limited to the domains of sexual function and energy and mobility independent of the dietary approach used and independent of changes in weight and A1C.15 Compared with a low-fat diet, a low-carbohydrate diet led to similar improvements in the physical aspects of HRQOL and greater improvements in mental aspects of HRQOL as measured by the SF-36. The greater improvement in the mental aspects of HRQOL appeared to be related more to some aspect of the low-carbohydrate diet than to the greater weight loss that occurred on this diet.24 |
High protein
|
Evangelista et al12 reported that improvements in QOL for those consuming a high protein diet were associated with weight loss. The positive effects of short-term weight loss on QOL in overweight and obese individuals have been documented in the obesity literature and confirmed by data from the current study that showed improvements in overall and physical QOL at the end of the 12-week dietary intervention in which there was moderate weight loss.12 |
| Commercial diet | All interventions endorsing a commercial weight loss program such as Weight Watchers produced improved QOL and largely suggested that QOL improvements were related to weight losses. The current study’s investigators demonstrated that the beneficial effects of weight loss on physical and functional QOL extend to obese breast cancer survivors; however, whether that was a result of the weight loss or the exercise that was part of the weight loss program is difficult to determine.32 Weight strongly predicted total score and all subscale scores, with the strongest relationships for public distress, physical function, and total score.16 |
| General healthy diet | All study interventions of generally healthy diets produced improved QOL. No studies clearly indicated whether QOL improvements were independent of weight loss. |
BMI indicates body mass index; CHO, carbohydrates; HRQOL, Health-Related Quality of Life; QOL, quality of life; SF-36, Short Form–36 Health Survey.
DISCUSSION
A total of 24 studies were included in this systematic review, designed to assess whether dietary intervention alone affects QOL for individuals attempting weight loss. Across these studies, the Short Form–36, a general health QOL instrument, was the most widely used. Several studies also used a disease-specific survey (eg, Impact of Weight on Quality of Life–Lite, Functional Assessment of Cancer Therapy–General, Polycystic Ovarian Syndrome Health–Related Quality of Life, and Minnesota Living with Heart Failure) to measure QOL in various populations. This review revealed that the large majority (21 of 24; 88%) of studies reported improvement in QOL over time; however, for nearly half of the studies, it was unclear whether improvement in QOL was as a result of weight loss and/or risk factor reduction rather than actual implementation of dietary changes.
Based on the findings of this review, there is a lack of data to support whether implementing dietary change positively or negatively affects QOL independent of weight loss. Although it is widely accepted that there is no downside to encouraging generally healthy, overweight, and obese persons to eat more fruits and vegetables or eat less calories, the results of this review suggest that it remains unclear whether making dietary changes translates into improved QOL regardless of whether the individual actually loses weight. The only dietary recommendation consistently associated with improved QOL was the low-carbohydrate diet, but this was limited to only 2 studies15,24 Yancy et al24 suggested that the allowance of unlimited consumption of certain food groups while on a low-carbohydrate diet may improve QOL in contrast to diets focused solely on calorie restriction. Because diet is linked to a range of factors, as illustrated in Figure 1, it is important to consider how making broad dietary recommendations for all individuals might affect overall QOL in both positive and negative directions. For example, attempting to adopt standard dietary recommendations such as eating more fruits and vegetables or fewer calories may have social implications by making an individual feel isolated or disconnected from his or her social circles, which may not be attempting to adopt the same recommended eating patterns. These recommendations may also have economic implications that negatively affect the QOL for those with a limited income. In contrast, adoption of a healthier dietary pattern may lead to increased personal satisfaction associated with successful implementation of a behavior change, and thus improved QOL, regardless of whether weight loss occurs. Further exploration of mechanisms influencing QOL is warranted.
One apparent limitation for advancing research to examine the effect of diet on QOL is the lack of nutrition-specific tools for assessment. Initial work from Barr and Schumacher35,36 yielded the Nutrition Quality of Life questionnaire. However, reported use in the literature has been infrequent.37 Recently, another nutrition-specific QOL tool was developed by Schunemann and colleagues38 for Italian populations, but it has not been adapted or tested in other populations such as the United States. Nevertheless, statistical approaches can be employed to begin to disentangle the possible relationships between treatment effect, weight loss, and QOL in behavioral weight loss interventions, even in the absence of specific tools for measuring nutrition-related QOL.
This study was also limited. The range of dates for included studies may have affected the QOL surveys included. A start year of 1990 was selected to coincide with the onset of the obesity epidemic and the era in which there was a marked increase in the number of behavioral weight control studies. However, new QOL instruments have been developed over the past 20 years that were not available to be used in earlier studies.
Most studies indicated that participants in behavioral weight control studies report improved QOL after the intervention; however, there are limited published data to determine whether an independent effect of implementing dietary change on QOL exists. Evidence for the impact of diet on QOL would be strengthened by a nutrition-specific QOL tool. The effect of implementing recommended dietary changes for weight loss–seeking individuals may affect QOL through a range of domains other than weight loss or health improvement.
IMPLICATIONS FOR RESEARCH AND PRACTICE
The lack of evidence needed to fully understand the impact of dietary interventions on QOL has research and clinical implications that must be considered and addressed. If dietary interventions are adversely affecting QOL and this potential relationship is not being detected or reported by current research practices, barriers for successful dietary changes and maintenance of changes may not be fully understood. Statistical methodology (eg, modeling, mediation tests) can be used with current tools to begin to explore the effect of dietary changes alone on QOL. In addition, the optimal approach is to develop specific tools to accurately assess the effect of implementing dietary changes on the full spectrum of factors that influence QOL.
Acknowledgments
Dr. Hidalgo was funded by National Heart, Lung, and Blood Institute, University of Alabama Statistical Genetics Postdoctoral Training Program Grant 5T32HL072757-10.
References
- 1.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appel LJ, Champagne CM, Harsha DW, et al. Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA. 2003;289:2083–2093. doi: 10.1001/jama.289.16.2083. [DOI] [PubMed] [Google Scholar]
- 3.Chlebowski RT, Blackburn GL, Thomson CA, et al. Dietary fat reduction and breast cancer outcome: interim efficacy results from the Women’s Intervention Nutrition Study. J Natl Cancer Inst. 2006;98:1767–1776. doi: 10.1093/jnci/djj494. [DOI] [PubMed] [Google Scholar]
- 4.The WHOQOL Group. The World Health Organization Quality of Life Assessment. Development and psychometric properties. Soc Sci Med. 1998;46:1569–1585. doi: 10.1016/s0277-9536(98)00009-4. [DOI] [PubMed] [Google Scholar]
- 5.Maciejewski ML, Patrick DL, Williamson DF. A structured review of randomized controlled trials of weight loss showed little improvement in health-related quality of life. J Clin Epidemiol. 2005;58:568–578. doi: 10.1016/j.jclinepi.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 6.Fontaine KR, Barfosky I. Obesity and health-related quality of life. Obes Rev. 2001;2:173–182. doi: 10.1046/j.1467-789x.2001.00032.x. [DOI] [PubMed] [Google Scholar]
- 7.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA Statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 8.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 9.Blissmer B, Riebe D, Dye G, Ruggiero L, Greene G, Caldwell M. Health-related quality of life following a clinical weight loss intervention among overweight and obese adults: intervention and 24 month follow-up effects. Health Qual Life Outcomes. 2006;4:43. doi: 10.1186/1477-7525-4-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malone M, Alger-Mayer SA, Anderson DA. The lifestyle challenge program: a multidisciplinary approach to weight management. Ann Pharmacother. 2005;39:2015–2020. doi: 10.1345/aph.1G287. [DOI] [PubMed] [Google Scholar]
- 11.Ross KM, Milsom VA, Rickel KA, et al. The contributions of weight loss and increased physical fitness to improvements in health-related quality of life. Eat Behav. 2009;10:84–88. doi: 10.1016/j.eatbeh.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evangelista LS, Heber D, Li Z, Bowerman S, Hamilton MA, Fonarow GC. Reduced body weight and adiposity with a high-protein diet improves functional status, lipid profiles, glycemic control, and quality of life in patients with heart failure: a feasibility study. J Cardiovasc Nurs. 2009;24:207–215. doi: 10.1097/JCN.0b013e31819846b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pope L, Harvey-Berino J, Savage P, et al. The impact of high-calorie-expenditure exercise on quality of life in older adults with coronary heart disease. J Aging Phys Act. 2011;19:99–116. doi: 10.1123/japa.19.2.99. [DOI] [PubMed] [Google Scholar]
- 14.Ackermann RT, Edelstein SL, Narayan KM, et al. Changes in health state utilities with changes in body mass in the Diabetes Prevention Program. Obesity (Silver Spring) 2009;17:2176–2181. doi: 10.1038/oby.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis NJ, Tomuta N, Isasi CR, Leung V, Wylie-Rosett J. Diabetes-specific quality of life after a low-carbohydrate and low-fat dietary intervention. Diabetes Educ. 2012;38:250–255. doi: 10.1177/0145721711436132. [DOI] [PubMed] [Google Scholar]
- 16.Heshka S, Anderson JW, Atkinson RL, et al. Weight loss with self-help compared with a structured commercial program: a randomized trial. JAMA. 2003;289:1792–1798. doi: 10.1001/jama.289.14.1792. [DOI] [PubMed] [Google Scholar]
- 17.Melanson KJ, Dell’Olio J, Carpenter MR, Angelopoulos TJ. Changes in multiple health outcomes at 12 and 24 weeks resulting from 12 weeks of exercise counseling with or without dietary counseling in obese adults. Nutrition. 2004;20:849–856. doi: 10.1016/j.nut.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Rejeski WJ, Focht BC, Messier SP, Morgan T, Pahor M, Penninx B. Obese, older adults with knee osteoarthritis: weight loss, exercise, and quality of life. Health Psychol. 2002;21:419–426. doi: 10.1037//0278-6133.21.5.419. [DOI] [PubMed] [Google Scholar]
- 19.Rippe JM, Price JM, Hess SA, et al. Improved psychological well-being, quality of life, and health practices in moderately overweight women participating in a 12-week structured weight loss program. Obes Res. 1998;6:208–218. doi: 10.1002/j.1550-8528.1998.tb00339.x. [DOI] [PubMed] [Google Scholar]
- 20.Villareal DT, Banks M, Sinacore DR, Siener C, Klein S. Effect of weight loss and exercise on frailty in obese older adults. Arch Intern Med. 2006;166:860–866. doi: 10.1001/archinte.166.8.860. [DOI] [PubMed] [Google Scholar]
- 21.Villareal DT, Chode S, Parimi N, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011;364:1218–1229. doi: 10.1056/NEJMoa1008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolf AM, Conaway MR, Crowther JQ, et al. Translating lifestyle intervention to practice in obese patients with type 2 diabetes: Improving Control with Activity and Nutrition (ICAN) study. Diabetes Care. 2004;27:1570–1576. doi: 10.2337/diacare.27.7.1570. [DOI] [PubMed] [Google Scholar]
- 23.Womble LG, Wadden TA, McGuckin BG, Sargent SL, Rothman RA, Krauthamer-Ewing ES. A randomized controlled trial of a commercial internet weight loss program. Obes Res. 2004;12:1011–1018. doi: 10.1038/oby.2004.124. [DOI] [PubMed] [Google Scholar]
- 24.Yancy WS, Jr, Almirall D, Maciejewski ML, Kolotkin RL, McDuffie JR, Westman EC. Effects of two weight-loss diets on health-related quality of life. Qual Life Res. 2009;18:281–289. doi: 10.1007/s11136-009-9444-8. [DOI] [PubMed] [Google Scholar]
- 25.Williamson DA, Rejeski J, Lang W, et al. Impact of a weight management program on health-related quality of life in overweight adults with type 2 diabetes. Arch Intern Med. 2009;169:163–171. doi: 10.1001/archinternmed.2008.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fontaine KR, Barofsky I, Andersen RE, et al. Impact of weight loss on health-related quality of life. Qual Life Res. 1999;8:275–277. doi: 10.1023/a:1008835602894. [DOI] [PubMed] [Google Scholar]
- 27.Imayama I, Alfano CM, Kong A, et al. Dietary weight loss and exercise interventions effects on quality of life in overweight/obese postmenopausal women: a randomized controlled trial. Int J Behav Nutr Phys Act. 2011;8:118. doi: 10.1186/1479-5868-8-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ware JE, Kosinski M, Keller SD. SF-36 Physical and Mental Health Summary Scales: A User’s Manual. Boston, MA: New England Medical Center; 1994. [Google Scholar]
- 29.Barham K, West S, Trief P, Morrow C, Wade M, Weinstock RS. Diabetes prevention and control in the workplace: a pilot project for county employees. J Public Health Manag Pract. 2011;17:233–241. doi: 10.1097/PHH.0b013e3181fd4cf6. [DOI] [PubMed] [Google Scholar]
- 30.Kennedy BM, Paeratakul S, Champagne CM, et al. A pilot church-based weight loss program for African-American adults using church members as health educators: a comparison of individual and group intervention. Ethn Dis. 2005;15:373–378. [PubMed] [Google Scholar]
- 31.Kolotkin RL, Head S, Hamilton M, Tse CK. Assessing impact of weight on quality of life. Obes Res. 1995;3:49–56. doi: 10.1002/j.1550-8528.1995.tb00120.x. [DOI] [PubMed] [Google Scholar]
- 32.Darga LL, Magnan M, Mood D, Hryniuk WM, DiLaura NM, Djuric Z. Quality of life as a predictor of weight loss in obese, early-stage breast cancer survivors. Oncol Nurs Forum. 2007;34:86–92. doi: 10.1188/07.ONF.86-92. [DOI] [PubMed] [Google Scholar]
- 33.Ladson G, Dodson WC, Sweet SD, et al. The effects of metformin with lifestyle therapy in polycystic ovary syndrome: a randomized double-blind study. Fertil Steril. 2011;95:1059–1066. e1–7. doi: 10.1016/j.fertnstert.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von Gruenigen VE, Gibbons HE, Kavanagh MB, Janata JW, Lerner E, Courneya KS. A randomized trial of a lifestyle intervention in obese endometrial cancer survivors: quality of life outcomes and mediators of behavior change. Health Qual Life Outcomes. 2009;7:17. doi: 10.1186/1477-7525-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barr J, Schumacher G. Using focus groups to determine what constitutes quality of life in clients receiving medical nutrition therapy: first steps in the development of a nutrition quality-of-life survey. J Am Diet Assoc. 2003;103:844–851. doi: 10.1016/s0002-8223(03)00385-7. [DOI] [PubMed] [Google Scholar]
- 36.Barr JT, Schumacher GE. The need for a nutrition-related quality-of-life measure. J Am Diet Assoc. 2003;103:177–180. doi: 10.1053/jada.2003.50058. [DOI] [PubMed] [Google Scholar]
- 37.Lin LP, Elena W, Razif SM. Nutrition quality of life among female-majority Malay undergraduate students of health sciences. Malays J Med Sci. 2012;19:37–49. [PMC free article] [PubMed] [Google Scholar]
- 38.Schunemann HJ, Sperati F, Barba M, et al. An instrument to assess quality of life in relation to nutrition: item generation, item reduction and initial validation. Health Qual Life Outcomes. 2010;8:26. doi: 10.1186/1477-7525-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]