Abstract
Background. Epidemiologic evidence suggests that nonsteroidal anti-inflammatory drugs (NSAIDs) contribute to more severe group A streptococcal (GAS) infections, yet a beneficial role for NSAIDs has been demonstrated in other experimental bacterial infections.
Methods. Nonselective (ketorolac tromethamine, ibuprofen, indomethacin), COX-1–selective (SC-560), or COX-2–selective (SC-236) NSAIDs ± antibiotics (penicillin, clindamycin) were given to mice challenged intramuscularly with M-type 3 GAS and disease course was followed for 14 days.
Results. All nonselective NSAIDs significantly accelerated mortality and reduced antibiotic efficacy; COX-selective NSAIDs had no significant effects.
Conclusions. Use of nonselective NSAIDs, either alone or as adjuncts to antibiotic therapy, for GAS soft tissue infection may contribute to worse outcomes.
Keywords: Streptococcus pyogenes, myonecrosis, NSAIDs
The annual incidence of invasive group A streptococcal (GAS) infections in the United States remains at 3.5/100 000 population per year, and mortality ranges between 30% and 60% (reviewed in [1]). In the approximately 50% of patients lacking an obvious portal of bacterial entry, deep-seeded necrotizing fasciitis/myonecrosis often develops at the site of minor, nonpenetrating trauma or muscle strain, which progresses rapidly to involve an entire extremity. Diagnosis of these “cryptic” infections is often delayed until after shock and organ failure are manifest, causing mortality in this group to approach 85% (reviewed in [1]).
To alleviate pain after muscle injury, nonsteroidal anti-inflammatory drugs (NSAIDs) are frequently self-prescribed or given in emergency departments. Based on evidence that NSAIDs dysregulate production of the cytokines mediating septic shock, Stevens proposed that NSAIDs may predispose individuals to more severe GAS infection [2]. Since then, multiple retrospective and prospective clinical studies have sought to confirm or refute the NSAID/GAS association but have yielded conflicting results [3–6]. Our recent experimental studies have shown that the non-COX-selective NSAID ketorolac tromethamine (trade name, Toradol) greatly augments early GAS infection of injured muscle. Because NSAIDs exert their anti-inflammatory activity largely through their ability to inhibit 1 or both isoforms of cyclooxygenase (ie, COX-1 and -2) and because these enzymes are important in early skeletal muscle regeneration after injury [7] and during resolution of infection [7, 8], the present study elucidated the effects of selective and nonselective NSAIDs, alone and in combination with antibiotic therapy, on outcome of experimental GAS myonecrosis.
MATERIALS AND METHODS
Streptococcus pyogenes Strain
GAS strain ATCC 12384 (M-type 3) was used in this study as we have previously described [9] and is sensitive to both penicillin and clindamycin. GAS was routinely cultured in Todd-Hewitt broth (THB). For use in animals, an overnight culture was diluted 1:10 in fresh THB and grown to mid–log phase (4.5 hours) at 37°C, 5% CO2 with shaking (100 rpm). Bacteria were collected by centrifugation, washed, and diluted in sterile saline to a final concentration of 0.175–1.4 × 109 colony-forming units (CFU)/mL.
Experimental Infection and Treatment Regimens
Female Swiss Webster mice (n = 10/group) were infected intramuscularly in the right thigh muscle with 100 µL of prepared GAS. One hour after infection, animals were treated intraperitoneally with one of the NSAIDs listed in Table 1. Ketorolac tromethamine, SC-236, and SC560 were given every 8 hours for 72 hours; because of longer half-lives, ibuprofen and indomethacin were given every 24 hours for 72 hours. Control animals received NSAID vehicle (4% dimethyl sulfoxide in phosphate-buffered saline) on the same schedule.
Table 1.
Characteristics of Nonsteroidal Anti-inflammatory Drugs Used in This Study
| NSAID Class | NSAID Name | COX-1/COX-2 IC50 Ratio [12] | Subclass | Dual Acting NSAID? | Inhibits NFkB Activation? | Activates PPAR? | Dose Used in This Study |
|---|---|---|---|---|---|---|---|
| Highly Selective COX-1 Inhibitor | SC-560 | 0.001 | Coxib | No | Unknown | Unknown | 6 mg/kg |
| Nonselective COX Inhibitors | Ketorolac tromethamine | 0.35 | Propionic Acid | No | No | No | 7.5 mg/kg |
| Ibuprofen | 0.5 | Propionic Acid | No | Yes | Yes | 30 mg/kg | |
| Indomethacin | 1.9 | Acetic Acid | Yes | No | Yes | 5 mg/kg | |
| Highly Selective COX-2 Inhibitor | SC-236 | 1780 | Coxib | No | Unknown | Yes [29] | 2 mg/kg |
Abbreviations: IC50, inhibitory concentration-50; NSAID, nonsteroidal anti-inflammatory drug; PPAR, peroxisome proliferator-activated receptor.
Intraperitoneal antibiotic treatments began 8 hours after infection and continued every 8 hours for 72 hours and included either (1) antibiotic vehicle (sterile saline); (2) penicillin (98 mg/kg; Sigma); (3) clindamycin phosphate (1.7 mg/kg; a 50% effective dose [ED50, see below], Hospira) or (4) a combination of penicillin plus clindamycin. These antibiotics constitute the treatments of choice for severe GAS infections [10]. The doses used here were based on our previous studies [9, 11]. Specifically, we have shown that penicillin (at 98 mg/kg) delays mortality, but ultimately fails to protect animals from lethal infection, even when treatment begins immediately after challenge. Thus, any ability of NSAIDs to augment penicillin efficacy should be observable at this antibiotic dose. In contrast, we have shown that clindamycin (at 86 mg/kg) is 80%–100% protective even when treatment is delayed up to 16 hours after challenge. Thus, to demonstrate possible additive or synergistic effects of NSAIDs on clindamycin efficacy, an ED50 (1.7 mg/kg), established in pilot studies, was used. This dose of clindamycin is approximately 40-fold lower than that used therapeutically in our previous studies.
Studies involving animal subjects were approved by the Institutional Animal Care and Use Committee, US Department of Veterans Affairs Medical Center, Boise, Idaho.
Statistical Analyses
Statistical differences in survival times and rates were performed using a log-rank test and Dunnett method. A P value of <.05 was considered statistically significant. All statistical computations were made in SAS version 9.2.
RESULTS
Mortality Rate in Experimental GAS Myonecrosis is Dose Dependent
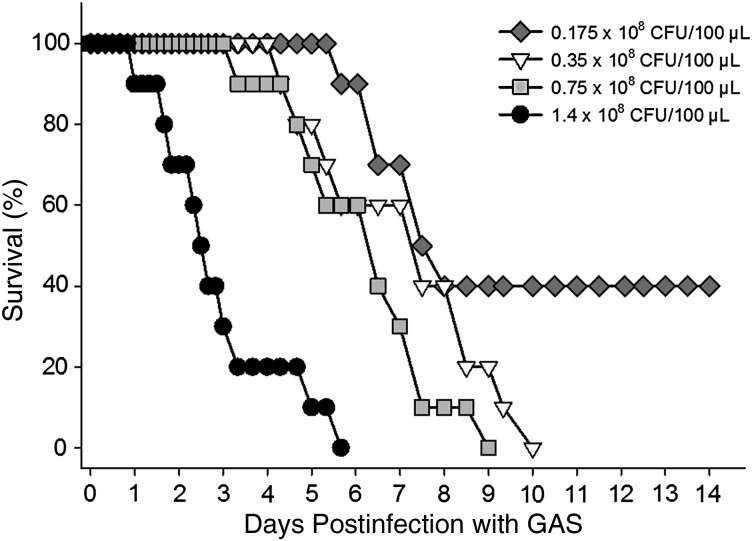
The times to 50% and 100% mortality of untreated animals challenged intramuscularly with M-type 3 GAS was clearly inoculum size dependent (Figure 1). The development and severity of localized swelling and necrosis of the fascia, muscle, and foot paralleled the mortality rates (data not shown). At the highest inoculum tested (1.4 × 108 CFU), 100% of animals died by day 6. Reducing the inoculum by 2- and 4-fold delayed the time to 100% mortality by 3 and 4 days, respectively. Further reductions in the inoculum size did not result in uniformly fatal infection. These results are in agreement with our previous publications using this model [9, 11]. To increase the likelihood of observing any NSAID effects on the disease course, whether beneficial or detrimental, animals in subsequent treatment experiments were challenged with either 1.4 × 108 CFU GAS (100% lethal dose-high [LD100-high]) or 0.35 × 108 CFU (LD100-low). These inoculum sizes routinely resulted in 100% mortality in 6–8 and 9–10 days, respectively.
Figure 1.
Mortality rate in experimental group A streptococcal (GAS) myonecrosis is inoculum size dependent. Swiss Webster mice (n = 10/group) were challenged intramuscularly with 2-fold decreasing doses of washed, log-phase M-type 3 GAS in the right thigh muscle. Mortality was recorded over a 14-day observation period. Abbreviation: CFU, colony-forming units.
Nonselective NSAIDs Increase the Severity of Experimental GAS Infection
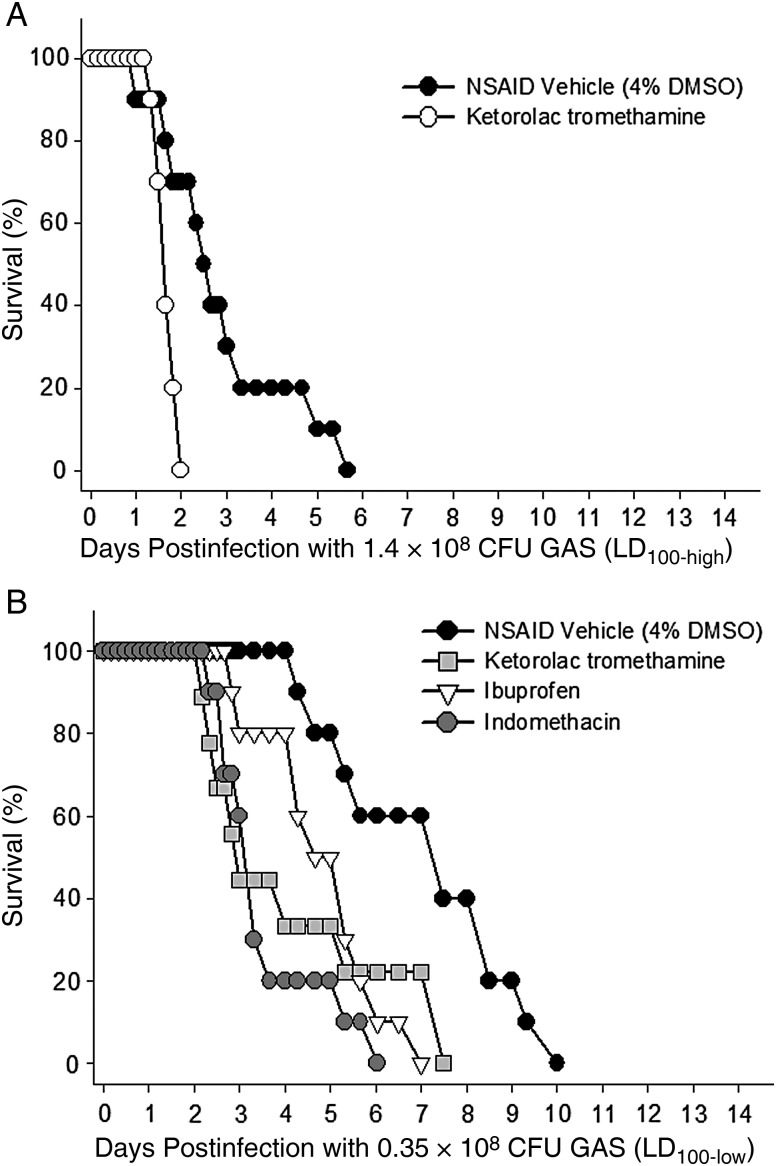
Initial experiments examined the effects of ketorolac tromethamine on survival in animals challenged with LD100-high GAS. As expected, this dose of GAS resulted in fulminant infection in which untreated animals died by day 6 (Figure 2A). However, ketorolac tromethamine–treated animals developed a significantly more severe and rapidly progressive disease course resulting in 100% mortality by day 2. Separate studies demonstrated no mortality or overt signs of toxicity in noninfected animals treated with an identical ketorolac tromethamine regimen (data not shown).
Figure 2.
Nonselective nonsteroidal anti-inflammatory drugs (NSAIDs) increase severity of group A streptococcal (GAS) myonecrosis. Mice were infected intramuscularly with either (A) 1.4 × 108 (LD100-high) or (B) 0.35 × 108 (LD100-low) washed, log-phase M-type 3 GAS. One hour after infection, animals were treated intraperitoneally with one of the indicated nonselective NSAIDs. NSAID administration continued every 8 hours (for ketorolac tromethamine, 7.5 mg/kg) or every 24 hours (for ibuprofen and indomethacin, 30 and 5 mg/kg, respectively) for 72 hours. Control animals received NSAID vehicle (4% dimethyl sulfoxide in phosphate-buffered saline) on the every 8 hours schedule. Abbreviation: DMSO, dimethyl sulfoxide.
To ensure that the results obtained above were independent of both the inoculum size used and the nonselective NSAID chosen, the experiment was repeated using 4-fold less GAS (ie, the LD100-low dose) and 2 additional nonselective NSAIDs, ibuprofen and indomethacin, one of which—indomethacin—is in a different NSAID chemical subclass (Table 1) and is considered “dual acting” (ie, also inhibits 5-lipoxygenase) [12]. Results demonstrated that all 3 nonselective COX inhibitors greatly accelerated the disease progression and significantly shortened the times to 50% and 100% mortality by as much as 4 days (P < .05; Figure 2B). Interestingly, development of signs and symptoms of systemic illness (scruffy fur, loss of activity) were markedly delayed in indomethacin-treated animals (data not shown); however, upon autopsy all such animals had widespread infection over the thigh, flank, and abdomen, comparable to that observed in animals in the untreated and other nonselective NSAID treatment groups.
Nonselective NSAIDs Reduce Antibiotic Efficacy
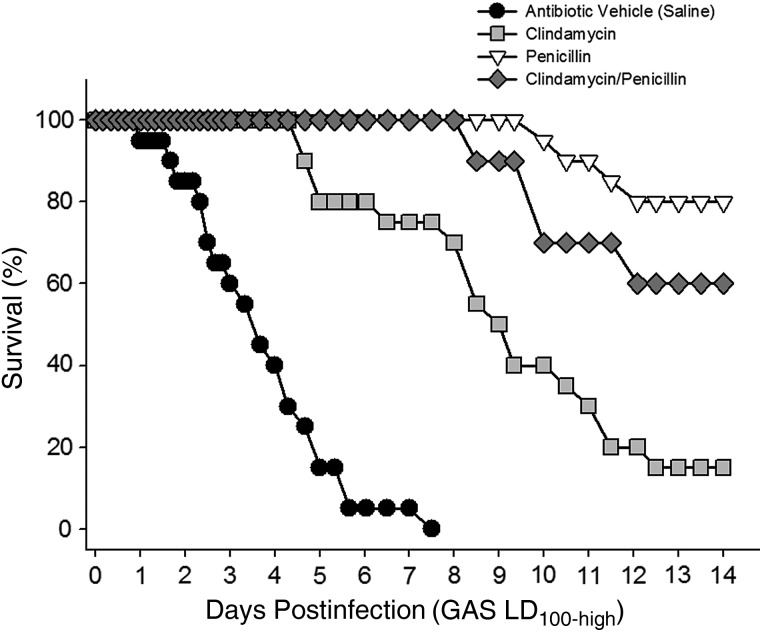
Penicillin, clindamycin, or a combination of penicillin and clindamycin were initiated 8 hours after infection with LD100-high GAS and continued every 8 hours for 72 hours. As expected, all antibiotic treatment regimens demonstrated significant, but transient, therapeutic benefits (Figure 3). Specifically, clindamycin, given at an ED50, significantly delayed mortality compared to untreated control animals (P < .001), and 20% of the clindamycin-treated animals survived for the full 14-day observation period. Similarly, mortality in the penicillin treatment group was significantly delayed. The combination of penicillin plus clindamycin was also therapeutic, but the disease course and overall mortality were not significantly different than penicillin treatment alone.
Figure 3.
Antibiotics delay mortality in experimental group A streptococcal (GAS) myonecrosis. Mice were infected intramuscularly with LD100-high GAS as described in Figure 2. Intraperitoneal antibiotic treatments began 8 hours after infection and continued every 8 hours for 72 hours. Antibiotics were (1) penicillin (98 mg/kg; Sigma); (2) clindamycin phosphate (1.7 mg/kg; Hospira); or (3) a combination of penicillin plus clindamycin. Control animals received antibiotic vehicle (sterile saline) on the same schedule. Mortality was recorded over 14 days. Data are pooled from 2 independent experiments with n = 10 mice per treatment group.
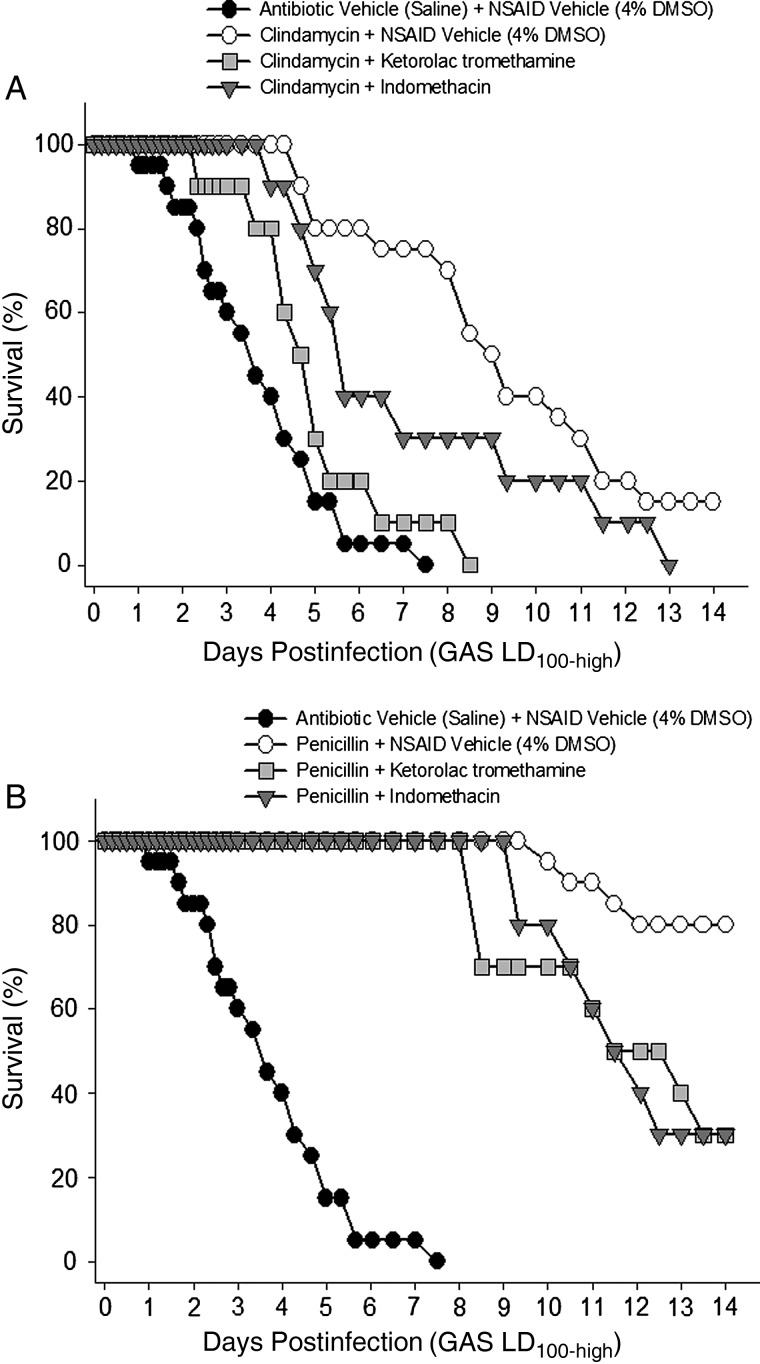
Addition of ketorolac tromethamine to the clindamycin regimen shortened the time to 50% mortality by more than 3 days (P < .05), and 100% of animals receiving this combination died before day 9 (Figure 4A). Similarly, indomethacin shortened the time to 50% mortality in clindamycin-treated animals by approximately 4 days (P < .05), and 100% of animals receiving this combination died by day 13 (Figure 4A).
Figure 4.
Nonselective nonsteroidal anti-inflammatory drugs (NSAIDs) reduce antibiotic efficacy. Animals infected with lethal dose-high (LD100-high) group A streptococci were treated with the indicated nonselective NSAIDs (or NSAID vehicle) in combination with (A) clindamycin or (B) penicillin. NSAIDs were begun 1 hour after infection and continued every 8 hours (for ketorolac tromethamine) or every 24 hours (for indomethacin) for 72 hours. Antibiotics were initiated 8 hours after infection and continued every 8 hours for 72 hours. Mortality was recorded over 14 days. Abbreviation: DMSO, dimethyl sulfoxide.
Addition of ketorolac tromethamine or indomethacin to the penicillin regimen (Figure 4B) also resulted in a more rapidly progressive infection with statistically greater mortality at day 14 compared to antibiotic treatment alone (P < .05). These NSAIDs also reduced the efficacy of combined clindamycin/penicillin treatment (data not shown).
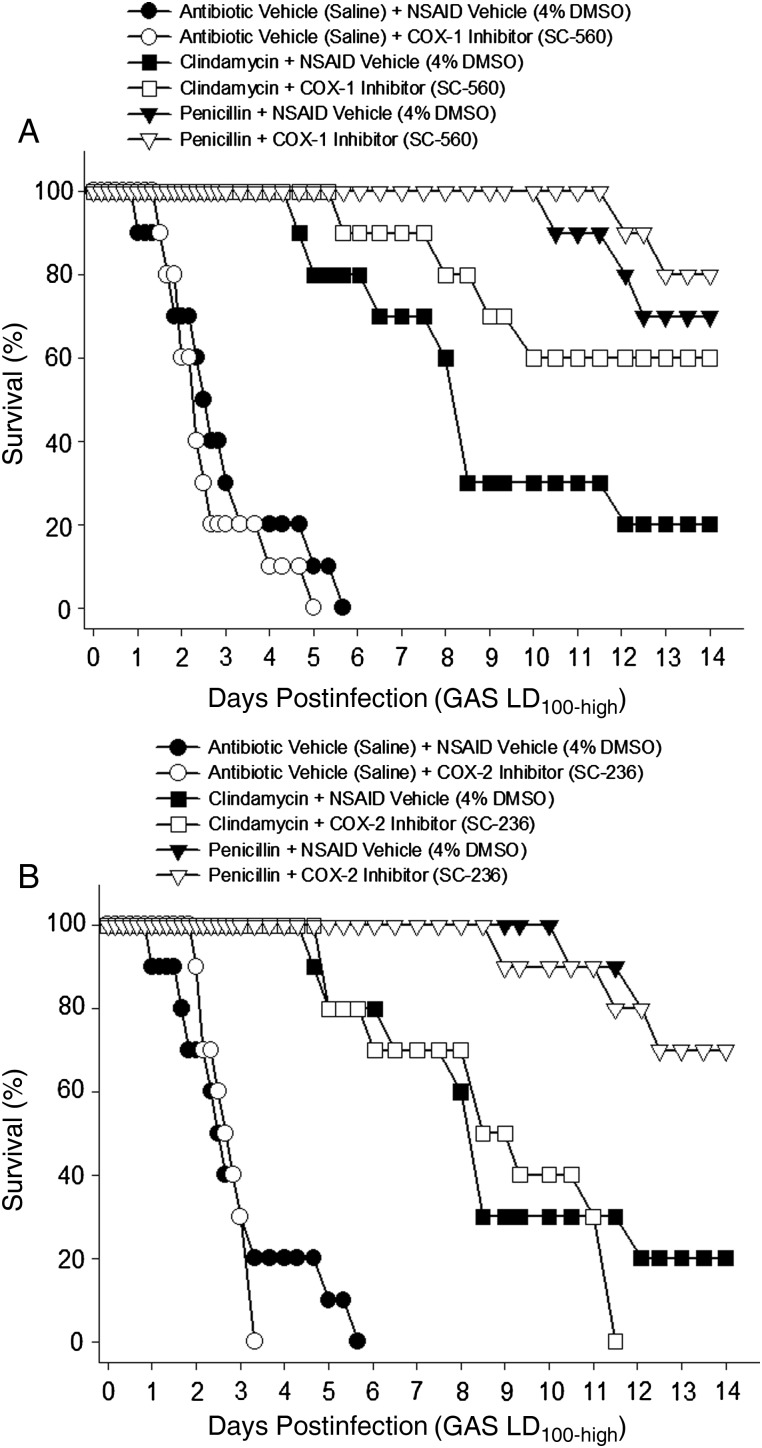
Effects of COX-Selective NSAIDs on Severity of GAS Infection
The COX-selective NSAIDs had differing effects on outcome when administered alone or in combination with antibiotics (Figure 5). Specifically, when given alone, the COX-1 selective NSAID did not significantly alter the disease process in animals challenged with either the LD100-high (Figure 5A) or LD100-low (not shown) dose of GAS. In animals challenged with the LD100-high dose of GAS, the COX-2 selective NSAID did not alter the time to 50% mortality, but significantly shortened the time to 100% mortality compared to untreated controls (3.3 vs 5.7 days, respectively; P < .05; Figure 5B). The COX-2–selective NSAID was not tested in animals challenged with the LD100-low dose of GAS.
Figure 5.
COX-selective, nonsteroidal anti-inflammatory drugs (NSAIDs) do not increase severity of experimental group A streptococcal (GAS) myonecrosis or reduce antibiotic efficacy. Mice were infected with lethal dose-high (LD100-high) GAS as described in Figure 2. A, COX-1 selective (SC-560, 6 mg/kg) or B, COX-2 selective (SC-236, 2 mg/kg) NSAIDs (or NSAID vehicle) were administered intraperitoneally 1 hour after infection and continued every 12 hours for 72 hours. Penicillin or clindamycin (or antibiotic vehicle) were begun 8 hours after infection and continued every 8 hours for 72 hours. Mortality was monitored for 14 days. Abbreviation: DMSO, dimethyl sulfoxide.
Differences were also noted when the COX-selective NSAIDs were given in combination with antibiotics. The 14-day survival rates of animals receiving the COX-1–selective NSAID plus clindamycin was 60% compared to 20% in animals receiving clindamycin treatment alone (Figure 5A). This COX-1–induced improvement in survival of the clindamycin-treated animals approached, but did not attain, statistical significance (P = .085). Additional experiments performed to increase treatment group size did not improve the significance level. When combined with penicillin, the COX-1–selective NSAID also caused a slight, nonsignificant, improvement in survival (Figure 5A). Addition of the COX-2–selective NSAID to the antibiotic treatment regimens did not significantly alter the disease progression or outcome compared to antibiotic treatment alone (Figure 5B).
DISCUSSION
Based on their ability to modify the host immune response, a direct role for NSAIDs in development of severe GAS infection was proposed by Stevens in 1995 [2]. This hypothesis was based on the ability of these agents to interrupt the negative feedback loop that limits production of tumor necrosis factor (TNF) α–a key mediator of septic shock. Others argue that NSAIDs merely mask the signs and symptoms of developing infection, such that diagnosis and antibiotic treatment are delayed. Investigators worldwide have performed retrospective and prospective studies to confirm or refute the NSAID/GAS association, yet controversy remains. For example, in one report, 92% of patients with streptococcal toxic shock syndrome (TSS) reported antecedent NSAID use [3]. Similarly, in a case-control study of children with varicella, 42% of patients who developed life-threatening GAS necrotizing fasciitis used NSAIDs compared to only 15% of patients who developed other, nonnecrotizing soft tissue infections [4]. Yet a different prospective study in children with primary varicella demonstrated only a slightly increased risk of GAS infection in those receiving both acetaminophen and ibuprofen vs controls [5], causing the authors to doubt the NSAID/GAS association. A 2003 literature review by Aronoff and Bloch concluded that prospective studies do not support a risk of developing GAS necrotizing fasciitis as a result of NSAID therapy, or a worsening of established streptococcal infection [6]. However, a more recent prospective epidemiologic study in the United Kingdom demonstrated that use of NSAIDs was independently associated with increased risk for streptococcal TSS [13]. Several case reports and larger case-control studies have demonstrated that NSAIDs contribute to severe infections caused by pathogens other than GAS [14–16]. For example, a recent retrospective study found a strong association between NSAID use and severe necrotizing soft tissue infection in which 47% were caused by GAS, 23% were caused by staphylococci, and the remaining cases were caused by various gram-negative species [14].
Thus, a preponderance of clinical evidence suggests that NSAIDs may contribute to worse outcomes in bacterial infections, including necrotizing infections caused by GAS. Despite this evidence, there is an increasing trend in clinical practice, supported by some experimental data, to include NSAIDs as treatment adjuncts for bacterial skin and soft tissue infections.
The present study investigated the effects of selective and nonselective NSAIDs in a well-established murine model of GAS myonecrosis [9, 17] in which NSAIDs were initiated 1 hour after bacterial challenge and 7 hours prior to antibiotic treatment. This design mimics the human scenario in which NSAID administration (usually nonselective NSAIDs) often precedes antibiotic therapy. This is because physicians frequently do not suspect an existing deep necrotizing soft tissue infection when the patient initially presents, especially in the approximately 50% of cases that lack an obvious portal of bacterial entry [18]. In these cryptogenic GAS infections, patients are prescribed NSAIDs for the associated pain and sent home. Only after the patient returns to the emergency department 24–28 hours later in shock and multiorgan failure are antibiotics initiated.
Our current findings, and those from our prior work [19], clearly demonstrate that nonselective NSAIDs augment both the initiation of this infection and directly worsen the disease process once infection is established. This conclusion is supported by findings of Weng et al [20] in which the nonselective NSAID ibuprofen significantly increased mortality in animals challenged intramuscularly with a sublethal dose of GAS. Furthermore, our results demonstrate that the addition of commonly used nonselective NSAIDs to different therapeutic antibiotic regimens significantly reduces antibiotic efficacy. Together, these findings strongly support the notion that in GAS soft tissue infections, nonselective NSAID administration shifts the host/pathogen interaction in favor of the pathogen, and that once infection is established, even a brief exposure to such NSAIDs limits antibiotic efficacy and contributes to worse outcome.
We further show that early administration of a COX-2–selective NSAID has little beneficial effect on the disease course or outcome of infection. These results contrast with other experimental murine infection studies in which strategies targeting COX-2 provided protection against group B streptococcus peritonitis [21], Pseudomonas aeruginosa pneumonia [22], and of particular interest, GAS bacteremia [23]. In the latter study, Goldmann et al [23] found that the COX-2 expression levels in C3H/HeN mice with GAS bacteremia (of unspecified M-type) strongly correlated with the severity of infection, and that genetic ablation of COX-2 or treatment with a COX-2–selective NSAID significantly delayed, but did not ultimately prevent, mortality [23]. In each of these studies, the authors concluded that COX-2–selective NSAIDs hold potential as an adjunct to antibiotic therapy for treatment of severe infections, especially those caused by antibiotic-resistant organisms. In contrast to the present work, these studies administered the COX-2–selective NSAID prior to bacterial challenge. The various routes of bacterial administration may also account for the differences in outcomes with COX-2 inhibitors.
Our present data also demonstrate a trend toward improved outcome in animals receiving antibiotics plus the COX-1–selective NSAID. This suggests there may be potential benefit from addition of COX-1–selective NSAIDs to antibiotic regimens for the treatment of soft tissue infection caused by GAS. This notion is supported by studies demonstrating that COX-1 is the predominant prostaglandin-generating cyclooxygenase isoform that is active during infection [24], and that pharmacologic blockade of COX-1 improves neutrophil-mediated clearance of pathogens, especially when used in combination with antibiotics [21]. However, the precise role of COX-1 in severe GAS soft tissue infection requires further study.
The question remains as to how nonselective, but not COX-selective, NSAIDs augment the initial GAS infection of injured muscles [19], and increase mortality and reduce antibiotic efficacy in experimental GAS myonecrosis (this study). Nonselective NSAIDs inhibit both isoforms of cyclooxygenase, and recent evidence suggests that COX-1 and COX-2 each contribute in unique and in overlapping ways to the acute and resolution phases of the inflammatory response (reviewed in [25]). Some NSAIDs have pharmacologic effects other than the inhibition of COX activity—including the abilities to inhibit the transcription factor NFkB and to activate the nuclear peroxisome proliferator-activated receptors (PPAR) α and γ. When activated on macrophages, PPAR-γ downregulates proinflammatory cytokine production and increases nonphlogistic (proresolution) phagocytosis. Whether our results with nonselective NSAIDs are mediated by these activities remains to be determined.
Differences between our results and those of Goldmann et al [23] could also be attributed to the mouse species chosen for study. As in humans, different mouse species vary in their susceptibility to GAS [26, 27]. This variation is marked among inbred mouse strains and appears related to a genetically programmed immune bias toward either T-helper (Th) 1– or Th2-type responses, resulting in high levels of interferon-γ/interleukin [IL]-2/TNF-α vs IL-4/IL-10/IL-13, respectively. Such an immune bias is also manifested as a predisposition toward 1 of 2 macrophage phenotypes (proinflammatory M1 and anti-inflammatory M2). For example, C57BL6 mice are resistant to GAS bacteremia [26, 27] and display a Th1/M1 bias [28], whereas DBA/2 mice are highly GAS susceptible [26, 27] and are Th2/M2-biased [28]. In addition to the Th1/M1 cytokine bias, GAS-resistant mice also demonstrate a relative downregulation of both prostaglandin E synthase genes (ie, cyclooxygenases 1 and 2) postchallenge compared to susceptible strains [27]. Weng et al [20] used Balb/c mice (Th2/M2 bias) and showed that NSAIDs worsened GAS mortality, whereas Goldmann et al [23] used C3H/HeN and wild-type C57BL/6 mice (each with Th1/M1 bias) and saw protective NSAID effects. The present study used Swiss Webster outbred mice that are presumably unbiased. However, experimental injury–associated cryptic GAS infection in these mice results in a high level of IL-4 gene expression in infected muscles, suggestive of an M2 bias (author's unpublished observations). Thus, the apparent dichotomous ability of NSAIDs to either worsen or improve GAS infection in mice may depend, at least in part, on the host's genetically predetermined response to infectious stimuli.
In summary, NSAIDs are important anti-inflammatory and analgesic drugs; however, the marked differences in outcomes of experimental infection suggest that the host's genotype, the etiologic agent, the site and/or stage of infection, the NSAID chosen, and its timing of administration relative to the onset of infection may each influence the end result, suggesting that more study is required before the general use of NSAIDs to treat human bacterial infections can be advocated.
Specifically with respect to GAS soft tissue infection, our data suggest that nonselective NSAIDs, given early in the course, do more than simply mask the signs and symptoms of developing infection. Instead, these agents enhance GAS infection of injured tissues [19] and dramatically accelerate GAS disease progression in established soft tissue infection. Even short-term exposure to such NSAIDs has lasting and profound deleterious effects on antibiotic efficacy. Although additional studies are required to elucidate the mechanisms responsible, physicians should be alert to possible complications in patients with suspected or established GAS soft tissue infection who take nonselective NSAIDs, even in conjunction with appropriate antibiotics.
Notes
Acknowledgments. We offer special thanks to the members of the Infectious Diseases research group for their technical assistance and to Laura Bond (Boise State University, Boise, Idaho) for statistical analyses. This material is based upon work supported by the U.S. Department of Veterans Affairs, Office of Research & Development, Biomedical Laboratory Research Program (A.E.B. and D.L.S.).
This material is based upon work supported by the U.S. Department of Veterans Affairs, Office of Research & Development, Biomedical Laboratory Research Program (A.E.B. and D.L.S.).
Financial support. This work was supported by the Office of Research and Development, Medical Research Service, US Department of Veterans Affairs (to A. E. B. and D. L. S.), and the National Institutes of Health (grant number P20 RR0116454/GM103408).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Bisno AL, Stevens DL. In: Streptococcus pyogenes. 6th ed. Mandell GL, Bennett JE, Dolin R, editors. Philadelphia, PA: Elsevier Churchill Livingstone; 2005. pp. 2362–79. Principles and practice of infectious diseases. [Google Scholar]
- 2.Stevens DL. Could nonsteroidal anti-inflammatory drugs (NSAIDs) enhance the progression of bacterial infections to toxic shock syndrome? Clin Infect Dis. 1995;21:977–80. doi: 10.1093/clinids/21.4.977. [DOI] [PubMed] [Google Scholar]
- 3.Barnham MR, Weightman NC, Anderson AW, et al. Streptococcal toxic shock syndrome: a description of 14 cases from North Yorkshire, UK. Clin Microbiol Infect. 2002;8:174–81. doi: 10.1046/j.1469-0691.2002.00396.x. [DOI] [PubMed] [Google Scholar]
- 4.Zerr DM, Alexander ER, Duchin JS, et al. A case-control study of necrotizing fasciitis during primary varicella. Pediatrics. 1999;103:783–90. doi: 10.1542/peds.103.4.783. [DOI] [PubMed] [Google Scholar]
- 5.Lesko SM, O'Brien KL, Schwartz B, et al. Invasive group A streptococcal infection and nonsteroidal antiinflammatory drug use among children with primary varicella. Pediatrics. 2001;107:1108–15. doi: 10.1542/peds.107.5.1108. [DOI] [PubMed] [Google Scholar]
- 6.Aronoff DM, Bloch KC. Assessing the relationship between the use of nonsteroidal antiinflammatory drugs and necrotizing fasciitis caused by group A streptococcus. Medicine (Baltimore) 2003;82:225–35. doi: 10.1097/01.md.0000085060.63483.bb. [DOI] [PubMed] [Google Scholar]
- 7.Bondesen BA, Mills ST, Kegley KM, et al. The COX-2 pathway is essential during early stages of skeletal muscle regeneration. Am J Physiol Cell Physiol. 2004;287:C475–83. doi: 10.1152/ajpcell.00088.2004. [DOI] [PubMed] [Google Scholar]
- 8.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–61. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stevens DL, Bryant-Gibbons AE, Bergstrom R, et al. The Eagle effect revisited: efficacy of clindamycin, erythromycin, and penicillin in the treatment of streptococcal myositis. J Infect Dis. 1988;158:23–8. doi: 10.1093/infdis/158.1.23. [DOI] [PubMed] [Google Scholar]
- 10.Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41:1373–406. doi: 10.1086/497143. [DOI] [PubMed] [Google Scholar]
- 11.Stevens DL, Yan S, Bryant AE. Penicillin binding protein expression at different growth stages determines penicillin efficacy in vitro and in vivo: an explanation for the inoculum effect. J Infect Dis. 1993;167:1401–5. doi: 10.1093/infdis/167.6.1401. [DOI] [PubMed] [Google Scholar]
- 12.Brooks PM, Day RO. Nonsteroidal antiinflammatory drugs—differences and similarities. N Engl J Med. 1991;324:1716–25. doi: 10.1056/NEJM199106133242407. [DOI] [PubMed] [Google Scholar]
- 13.Lamagni TL, Neal S, Keshishian C, et al. Severe Streptococcus pyogenes Infections, United Kingdom, 2003–2004. Emerg Infect Dis. 2008;14:201–9. doi: 10.3201/eid1402.070888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Souyri C, Olivier P, Grolleau S, et al. Severe necrotizing soft-tissue infections and nonsteroidal anti-inflammatory drugs. Clin Exp Dermatol. 2008;33:249–55. doi: 10.1111/j.1365-2230.2007.02652.x. [DOI] [PubMed] [Google Scholar]
- 15.Dial S, Delaney JA, Barkun AN, et al. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA. 2005;294:2989–95. doi: 10.1001/jama.294.23.2989. [DOI] [PubMed] [Google Scholar]
- 16.Ott E, Nussmeier NA, Duke PC, et al. Efficacy and safety of the cyclooxygenase 2 inhibitors parecoxib and valdecoxib in patients undergoing coronary artery bypass surgery. J Thorac Cardiovasc Surg. 2003;125:1481–92. doi: 10.1016/s0022-5223(03)00125-9. [DOI] [PubMed] [Google Scholar]
- 17.Yan S, Bohach GA, Stevens DL. Persistent acylation of high-molecular weight penicillin binding proteins by penicillin induces the post antibiotic effect in Streptococcus pyogenes. J Infect Dis. 1994;170:609–14. doi: 10.1093/infdis/170.3.609. [DOI] [PubMed] [Google Scholar]
- 18.Stevens DL, Tanner MH, Winship J, et al. Reappearance of scarlet fever toxin A among streptococci in the Rocky Mountain West: severe group A streptococcal infections associated with a toxic shock-like syndrome. N Eng J Med. 1989;321:1–7. doi: 10.1056/NEJM198907063210101. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton SM, Bayer CR, Stevens DL, et al. Muscle injury, vimentin expression, and nonsteroidal anti-inflammatory drugs predispose to cryptic group A streptococcal necrotizing infection. J Infect Dis. 2008;198:1692–8. doi: 10.1086/593016. [DOI] [PubMed] [Google Scholar]
- 20.Weng TC, Chen CC, Toh HS, et al. Ibuprofen worsens Streptococcus pyogenes soft tissue infections in mice. J Microbiol Immunol Infect. 2011;44:418–23. doi: 10.1016/j.jmii.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 21.Stables MJ, Newson J, Ayoub SS, et al. Priming innate immune responses to infection by cyclooxygenase inhibition kills antibiotic-susceptible and -resistant bacteria. Blood. 2010;116:2950–9. doi: 10.1182/blood-2010-05-284844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sadikot RT, Zeng H, Azim AC, et al. Bacterial clearance of Pseudomonas aeruginosa is enhanced by the inhibition of COX-2. Eur J Immunol. 2007;37:1001–9. doi: 10.1002/eji.200636636. [DOI] [PubMed] [Google Scholar]
- 23.Goldmann O, Hertzen E, Hecht A, et al. Inducible cyclooxygenase released prostaglandin E2 modulates the severity of infection caused by Streptococcus pyogenes. J Immunol. 2010;185:2372–81. doi: 10.4049/jimmunol.1000838. [DOI] [PubMed] [Google Scholar]
- 24.Wallace JL, Bak A, McKnight W, et al. Cyclooxygenase 1 contributes to inflammatory responses in rats and mice: implications for gastrointestinal toxicity. Gastroenterology. 1998;115:101–9. doi: 10.1016/s0016-5085(98)70370-1. [DOI] [PubMed] [Google Scholar]
- 25.Langenbach R, Loftin C, Lee C, et al. Cyclooxygenase knockout mice: models for elucidating isoform-specific functions. Biochem Pharmacol. 1999;58:1237–46. doi: 10.1016/s0006-2952(99)00158-6. [DOI] [PubMed] [Google Scholar]
- 26.Aziz RK, Kansal R, Abdeltawab NF, et al. Susceptibility to severe Streptococcal sepsis: use of a large set of isogenic mouse lines to study genetic and environmental factors. Genes Immun. 2007;8:404–15. doi: 10.1038/sj.gene.6364402. [DOI] [PubMed] [Google Scholar]
- 27.Abdeltawab NF, Aziz RK, Kansal R, et al. An unbiased systems genetics approach to mapping genetic loci modulating susceptibility to severe streptococcal sepsis. PLoS Pathog. 2008;4:e1000042. doi: 10.1371/journal.ppat.1000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butler NS, Monick MM, Yarovinsky TO, et al. Altered IL-4 mRNA stability correlates with Th1 and Th2 bias and susceptibility to hypersensitivity pneumonitis in two inbred strains of mice. J Immunol. 2002;169:3700–9. doi: 10.4049/jimmunol.169.7.3700. [DOI] [PubMed] [Google Scholar]
- 29.Planaguma A, Claria J, Miquel R, et al. The selective cyclooxygenase-2 inhibitor SC-236 reduces liver fibrosis by mechanisms involving non-parenchymal cell apoptosis and PPARgamma activation. FASEB J. 2005;19:1120–2. doi: 10.1096/fj.04-2753fje. [DOI] [PubMed] [Google Scholar]