Abstract
Lysins are bacteriophage-derived enzymes that degrade bacterial peptidoglycans. Lysin CF-301 is being developed to treat Staphylococcus aureus because of its potent, specific, and rapid bacteriolytic effects. It also demonstrates activity on drug-resistant strains, has a low resistance profile, eradicates biofilms, and acts synergistically with antibiotics. CF-301 was bacteriolytic against 250 S. aureus strains tested including 120 methicillin-resistant S. aureus (MRSA) isolates. In time-kill studies with 62 strains, CF-301 reduced S. aureus by 3-log10 within 30 minutes compared to 6–12 hours required by antibiotics. In bacteremia, CF-301 increased survival by reducing blood MRSA 100-fold within 1 hour. Combinations of CF-301 with vancomycin or daptomycin synergized in vitro and increased survival significantly in staphylococcal-induced bacteremia compared to treatment with antibiotics alone (P < .0001). Superiority of CF-301 combinations with antibiotics was confirmed in 26 independent bacteremia studies. Combinations including CF-301 and antibiotics represent an attractive alternative to antibiotic monotherapies currently used to treat S. aureus bacteremia.
Keywords: Staphylococcus aureus, MRSA, lysin, daptomycin, vancomycin, bacteremia
Methicillin-resistant Staphylococcus aureus (MRSA) infections occur in both hospital and community settings, and in the United States approximately 100 000 patients are hospitalized annually with invasive MRSA infections resulting in >18 000 deaths [1]. Among invasive infections, the annual incidence rate of S. aureus bacteremia varies from 3.6 to 6.0 per 100 000 person-years [2]. Of further concern, MRSA strains are now evolving additional resistances against standard-of-care (SOC) antibiotics [3]. This high unmet clinical need and associated healthcare costs [4] underscore the need for new therapeutic alternatives to treat MRSA infections.
Emerging options for treating gram-positive bacterial infections are lysins [5]. In the natural setting, these bacteriophage-encoded hydrolytic enzymes liberate progeny phage from infected bacteria by degrading peptidoglycan from inside the cell, causing lysis of the host bacterium. Therapeutically, lysins are being developed as antimicrobial agents to lyse pathogenic bacteria by attacking peptidoglycan from outside the cell [6, 7]. The efficacy of lysin therapy has been demonstrated in rodents with experimental pharyngitis [7], pneumonia [8], otitis media [9], abscesses [10], bacteremia [11], endocarditis [12], and meningitis [13]. In addition, lysins are generally highly specific for bacterial species and rarely lyse nontarget organisms, including commensal gut bacteria, which may be beneficial in maintaining gastrointestinal homeostasis [14, 15].
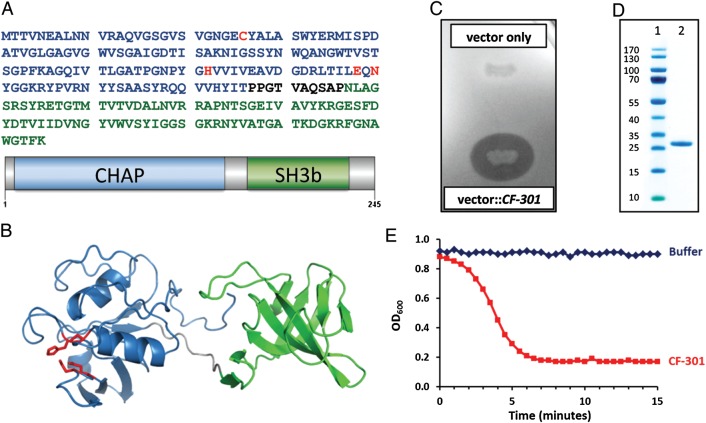
CF-301, also referred to as PlySs2, was identified as an antistaphylococcal lysin encoded within a prophage of the Streptococcus suis genome [16]. It has a domain arrangement characteristic of most bacteriophage lysins, defined by a catalytic N-terminal domain linked to a cell wall–binding C-terminal domain [5] (Figure 1A). The N-terminal domain belongs to the cysteine-histidine–dependent amidohydrolases/peptidases (CHAP) family [20], common among lysins and other bacterial cell wall–modifying enzymes. The C-terminal domain belongs to the SH3b family [21] that often forms the cell wall–binding element of lysins. Unlike most recombinant lysins that digest S. aureus, which are often poorly expressed and display low solubilities [22–24], recombinant CF-301 is highly expressed in E. coli within the soluble fraction [16].
Figure 1.
General features of CF-301. A, Protein sequence and diagrammatic representation of CF-301 depicted with the N-terminal cysteine-histidine–dependent amidohydrolases/peptidases (CHAP) domain in blue and the C-terminal SH3b domain in green. The CHAP domain active-site residues (Cys26, His102, Glu118, and Asn120) identified by homology to Protein Data Bank (PDB) entry 2K3A [17] are highlighted in red. B, Ribbon representation of a CF-301 model built with the I-TASSER server [18] using PDBs 2K3A and 1R77 as templates for the N-terminal and C-terminal domains, respectively, with the side chains of the active site residues depicted. The coloring scheme is the same as in panel A. The model was rendered in PyMOL [19]. C, The lytic activity of Escherichia coli expressing CF-301 (compared to a vector control) shown on a lawn of Staphylococcus aureus MW2. D, Sodium dodecyl sulfate polyacrylamide gel electrophoresis analysis of CF-301 (lane 2). Molecular weight markers (PAGE Ruler, Thermo Scientific; lane 1); CF-301 (lane 2). E, Time course of CF-301 lytic activity against S. aureus MW2. Exponential phase cells were treated with either CF-301 (128 µg/mL) or buffer. The OD600 values were recorded every 30 seconds for 15 minutes. A decrease in optical density was associated with bacteriolysis.
In the present study we show that CF-301 differs from SOC antibiotics by its potency, speed, specificity, and activity against antibiotic-resistant strains. We also demonstrate a low resistance profile, anti-biofilm activity, and synergy with antibiotics in vitro. Furthermore, we show that CF-301 significantly enhances SOC antibiotic activities against staphylococcal-induced murine bacteremia under challenging conditions where high doses of single antibiotics fail, underscoring the effectiveness of the combination strategy for treating bacteremia.
METHODS
Bacterial Strains and Growth Conditions
Bacterial strains were maintained on BBL Trypticase Soy Agar II with 5% sheep blood (Becton, Dickinson and Company) at 37°C in ambient air. Liquid growth media was Mueller-Hinton Broth (Becton, Dickinson and Company) or Mueller Hinton II Broth (Becton, Dickinson and Company) and supplemented as indicated with DL-dithiothreitol (DTT; Sigma-Aldrich), CaCl2 (Sigma-Aldrich), or NaCl (Sigma-Aldrich). Contemporary clinical S. aureus isolates were obtained from JMI Laboratories. Additional strains were obtained from the Network on Antimicrobial Resistance in Staphylococcus aureus (NRS strain designations), American Type Culture Collection (ATCC strains), the Centers for Disease Control and Prevention, and The Lancefield Collection of Streptococcus Strains (The Rockefeller University). The S. aureus strain CFS 955 is a daptomycin-resistant (minimum inhibitory concentration [MIC], 16 µg/mL) derivative of strain MW2 (NRS 123). Strains CFS738, CFS832 and CFS544 correspond to MRSA strains NRS192, NRS61 and ATCC 25923 respectively.
MIC Determinations
MIC values for CF-301 and comparator antibiotics were determined in triplicate by broth microdilution [25]. Bacteria were suspended in growth media (5 × 105 colony-forming units [CFU]/mL and exposed to CF-301 supplemented with 1 mM DTT or antibiotics in a series of 2-fold serial dilutions in 96-well polypropylene microtiter plates (Becton, Dickinson, and Company). After 24 hours of incubation at 35°C in ambient air, MIC values were recorded as the most dilute drug concentration that inhibited bacterial growth. The addition of DTT was restricted to MIC, in vitro resistance, and combination MIC experiments involving CF-301. For strain MW2, the MIC values for CF-301 were 8 and 32 µg/mL in the presence and absence of DTT, respectively.
Murine Bacteremia Model
Female BALB/c mice, 5–7 weeks of age, with 16–19.5 g body weight (Jackson Laboratories) were housed and provided chow and water. Exponential phase bacterial inocula were generated by growing cells to an optical density at 600 nm (OD600) of 0.5 harvested, washed, and concentrated to 1.5–2 × 109 CFU/mL. The bacterial pellet was suspended in a volume of 5% (w/v) mucin (Sigma; lot number 081M1507V or SLBD5666V) and placed on ice. Five hundred microliters (7.5 × 106 to 1 × 109 CFU) was injected intraperitoneally into mice. Drug doses were weight-adjusted with an injected volume between 160 and 200 µL. Postinfection survival was assessed every 3 or 6 hours for the first 24 hours, then at 48 and 72 hours. Experiments were repeated 2–3 times with each treatment group containing between 10 and 20 mice. Due to lot-to-lot variability among commercial mucins, prescreening was performed to discard lots that did not support robust infections and treatments (lot variability is expected as commercial mucins are sourced from various providers and are crude preparations obtained by peptic digests of hog stomach, followed by precipitation and other steps). Blood was collected in K2-EDTA tubes (Terumo Medical Corporation), vortexed, serially diluted in phosphate-buffered saline, and spotted onto blood agar plates. Following overnight incubation at 37°C, CFUs were quantified. All in vivo studies were performed under the guidelines and protocols of the Institutional Animal Care and Use Committee of ContraFect Corporation.
Statistical Methods
Statistical analysis was performed using the GraphPad Prism 5 software package. Time-kill experiments were analyzed by 2-tailed unpaired Student t test. Results are presented as mean ± SEM. Actuarial mouse survival was analyzed using the log-rank (Mantel–Cox) test. P < .05 was considered statistically significant.
RESULTS
Modeling and Preparation of Lysin CF-301
A CF-301 homology model was built in silico and Figure 1B indicates the location of the CHAP domain active site residues [17]. Escherichia coli clones expressing recombinant CF-301 Yeiled a pronounced lytic zone on agar plates overlaid with live S. aureus cells (Figure 1C). The CF-301 protein is 26 kDa with an isoelectric point of 9.15. Conditions to efficiently produce and purify CF-301 at high Yieled (>9 g/L) were developed (Supplementary Methods). The purified lysin (Figure 1D) was bacteriolytic against S. aureus cells in a lytic assay in vitro (Figure 1E).
CF-301 Is Active Against a Narrow Range of Bacterial Species Including Multidrug-Resistant Strains of S. aureus
CF-301 demonstrated potent inhibitory activity on 250 S. aureus strains tested, including 103 methicillin-sensitive S. aureus (MSSA) and 120 MRSA (Table 1). It was also active against 54 Streptococcus pyogenes, 51 Streptococcus agalactiae, and 10 Staphylococcus lugdunensis strains (Supplementary Table 1). Little or no activity was observed against the other gram-positive or gram-negative bacteria tested.
Table 1.
In Vitro Activity of CF-301 and Standard-of-Care Antibiotics Against Staphylococcus aureus
| Strains (N = 250) | CF-301 (mw = 26 000 Da) |
Daptomycin (mw = 1620 Da) |
Vancomycin (mw = 1486 Da) |
Linezolid (mw = 337 Da) |
||||
|---|---|---|---|---|---|---|---|---|
| µg/mL | µM | µg/mL | µM | µg/mL | µM | µg/mL | µM | |
| MSSA (n = 103) | 8 | 0.31 | 1 | 0.62 | 1 | 0.67 | 1 | 3.0 |
| MRSA (n = 120) | 8 | 0.31 | 1 | 0.62 | 1 | 0.67 | 2 | 5.9 |
| DRSA (n = 8) | 4 | 0.15 | 16 | 9.88 | 1 | 0.67 | 2 | 5.9 |
| VRSA (n = 14) | 4 | 0.15 | 1 | 0.62 | >16 | >10.8 | 2 | 5.9 |
| LRSA (n = 5) | 2 | 0.08 | 1 | 0.62 | 1 | 0.67 | >64 | >190 |
The antibiotic resistance profile of each Staphylococcus aureus group and the molecular weight of each drug are shown. The minimum inhibitory concentrations at which 90% of the isolates (MIC90) were inhibited are shown in µg/mL. The correspond μM values are shown as well.
Abbreviations: mw, molecular weight; DRSA, daptomycin-resistant S. aureus; LRSA, linezolid-resistant S. aureus; MRSA, methicillin-resistant S. aureus; MSSA, methicillin-sensitive S. aureus; mw, ; VRSA, vancomycin-resistant S. aureus.
CF-301 inhibited growth of MRSA strains additionally resistant to vancomycin, daptomycin, or linezolid. Shown in Table 1, CF-301 inhibited the growth of 27 multidrug-resistant strains tested. On a molar basis, CF-301 was 2- to 40-fold more potent than the SOC antibiotics tested.
CF-301 Kills S. aureus Rapidly In Vitro
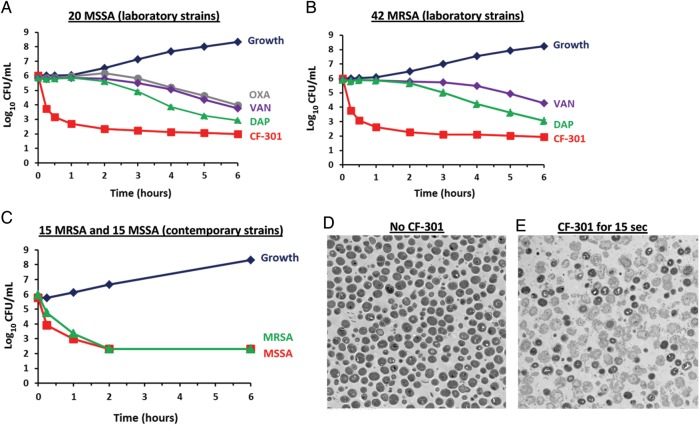
Time-kill assays [26] were used to test the rate of antimicrobial activity in vitro. CF-301 and SOC antibiotics at their minimum inhibitory concentrations (1× MIC) were tested against 20 MSSA and 42 MRSA laboratory strains. CF-301 reached bactericidal levels (≥3-log10 CFU reduction [26]) within 30 minutes against both MSSA and MRSA (Figure 2A and 2B). In contrast, daptomycin required 6 hours to reach similar levels against MSSA and MRSA whereas vancomycin achieved only a 2-log10 kill in the same time frame. Oxacillin, when tested on MSSA, also demonstrated a 2-log10 kill requiring 6 hours.
Figure 2.
CF-301 rapidly kills Staphylococcus aureus in vitro. A and B, Composite time-kill curves of CF-301 compared to control buffer (growth), oxacillin (OXA), vancomycin (VAN), and daptomycin (DAP). Each kill curve is a composite result from 20 methicillin-sensitive S. aureus (MSSA) laboratory strains or 42 methicillin-resistant S. aureus (MRSA) laboratory strains. In individual analyses, drug concentrations correspond to strain-specific 1× minimum inhibitory concentration (MIC) values. Mean values (±SEM) are shown for each timepoint. C, Composite time-kill curves of CF-301 against clinical MSSA and MRSA strains. Each kill curve is a composite result from either 15 MRSA or 15 MSSA contemporary strains tested against strain-specific 1× MIC values. Mean values (±SEM) are shown for each timepoint. A, B, and C, A 6-hour time-course is shown to focus on the speed of the antibacterial effect. D and E, Transmission electron micrographs (×3300 magnification) of S. aureus MW2 before and after treatment for 15 seconds with 8 µg/mL CF-301. Scale bars are 2 µm. Lysis results in the loss of darkly stained cytoplasmic components.
Time-kill experiments were also performed with CF-301 against contemporary strains of 15 MSSA and 15 MRSA (Figure 2C), demonstrating rapid bactericidal activity of CF-301 on recent clinical isolates. Potent activity of CF-301 was further illustrated by electron microscopy, where bacteriolysis was observed within 15 seconds of treatment (Figure 2D and 2E). The speed of the CF-301 lytic activity is consistent with a bactericidal effect that occurs upon contact.
CF-301 Kills S. aureus Rapidly In Vivo
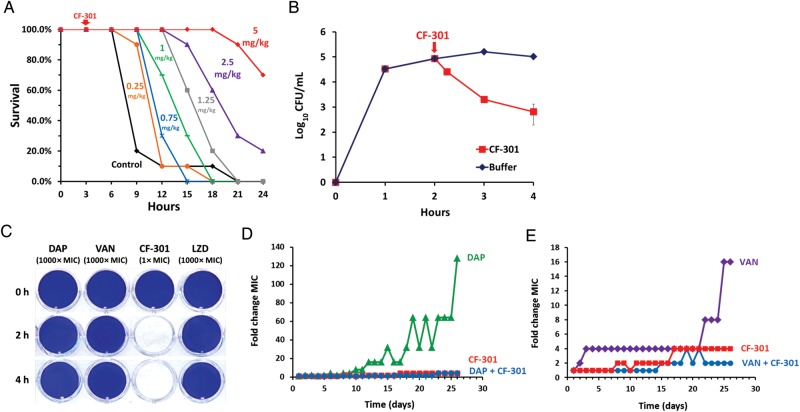
CF-301 exhibited a dose-response curve in treating murine MRSA-induced bacteremia. Measurable enhancement of survival occurred with 0.25 mg/kg CF-301, whereas a high level of protection (70%) was observed with 5 mg/kg (Figure 3A).
Figure 3.
In vitro and in vivo activities of CF-301. A, Dose response in murine bacteremia model. Mice were challenged with 108 colony-forming units (CFU) (methicillin-resistant Staphylococcus aureus [MRSA] MW2)/mouse intraperitoneally, treated with various amounts of CF-301 ranging from 0.25 to 5 mg/kg intraperitoneally (or buffer control with no CF-301) at 3 hours postinoculum, and survival was assessed over 24 hours. Dose groups were 10 mice each. B, CF-301-mediated in vivo killing in blood. Mice were challenged with 109 CFU (MRSA MW2)/mouse, and blood samples (n = 3 per timepoint) were drawn at various times after CF-301 (or buffer control) treatment and plated for CFU measurements. C, The activity of CF-301 and antibiotics against S. aureus biofilms. D, In vitro selection for decreased susceptibility to CF-301 and/or daptomycin. Over the 26-day passage, the CF-301 minimum inhibitory concentration (MIC) increased from 8 to 32 µg/mL (a 4-fold change) whereas the daptomycin MIC increased from 0.5 to 64 µg/mL (a 128-fold change) without CF-301 and from 0.25 to 1 µg/mL (a 4-fold change) with 1 µg/mL CF-301. E, In vitro selection for decreased susceptibility to CF-301 and/or vancomycin. Over the 26-day passage, the CF-301 MIC increased from 8 to 32 µg/mL (a 4-fold change) whereas the vancomycin MIC increased from 1 to 16 µg/mL (a 16-fold change) without CF-301 and from 0.5 to 2 µg/mL (a 4-fold change) with 2 µg/mL CF-301. Abbreviations: DAP, daptomycin; LZD, linezolid; VAN, vancomycin.
Rapid bactericidal activity of CF-301 was also demonstrated in the bloodstream. MRSA CFU were measured in the blood of infected mice before and after treatment with CF-301 (Figure 3B). Treatment with CF-301 at 2 hours postinfection produced a decrease of 0.5-log10 CFU within 15 minutes and a 2.0-log10 decrease in 60 minutes.
CF-301 Is Active Against S. aureus Biofilms
CF-301 was compared to SOC antibiotics for the ability to eradicate S. aureus biofilms. MRSA ATCC BAA-42 biofilms were grown for 24 hours in polystyrene dishes and subsequently treated with CF-301 (1× MIC) or with daptomycin, vancomycin, or linezolid (1000× MIC each). Following treatments for 2 or 4 hours, residual biofilms were visualized by staining with crystal violet. As shown in Figure 3C, CF-301 at 1× MIC removed all visual biomass by 2 hours, whereas the SOC antibiotics at 1000× MIC failed to remove biomass after 4 hours of treatment.
Resistance of S. aureus to CF-301 Is Low Relative to Resistance to Antibiotics
MRSA MW2 was serially passaged in the presence of increasing amounts of CF-301. After 26 days of passage, resistance to CF-301 increased no more than 4 times the initial MIC (red-colored datasets, Figure 3D and 3E). Similar methods were used to detect resistance to SOC antibiotics. Following 26 days of passage in increasing amounts of antibiotics, resistances of 128 and 16 times the initial MIC were observed for daptomycin and vancomycin, respectively (Figure 3D and 3E).
Antibiotic Resistance Is Suppressed by Growth in Combination With CF-301
MRSA MW2 was treated for 26 days with increasing concentrations of daptomycin or vancomycin in the presence of sub-MIC CF-301 (blue-colored datasets, Figure 3D and 3E). In contrast to the relatively high resistance levels observed when these SOC antibiotics were tested as single agents, growth of bacteria in antibiotics in the presence of CF-301 resulted in only a 4-fold increase in their resistances, demonstrating that growth of MRSA with CF-301 suppressed formation of antibiotic resistances to both vancomycin and daptomycin.
CF-301 Synergizes With Antibiotics In Vitro
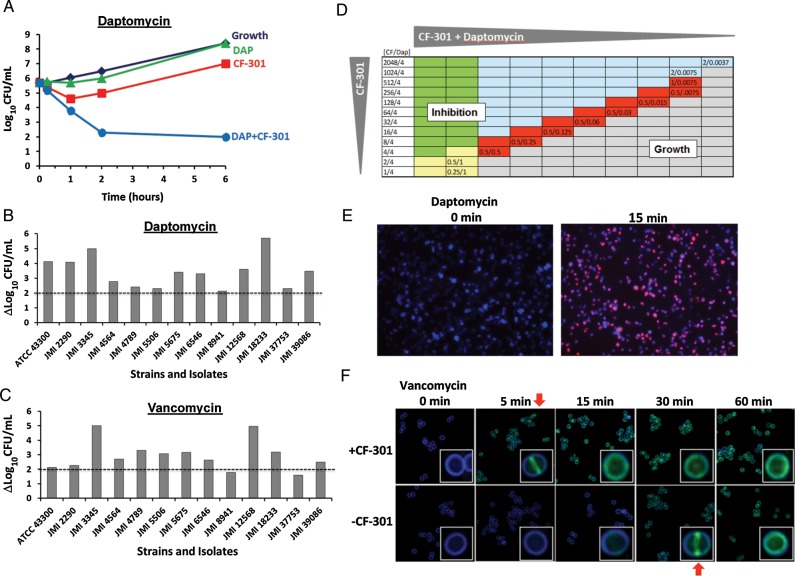
Synergy between CF-301 and SOC antibiotics was assessed using 3 different methods. The first method was the time-kill assay, a preferred technique for examining synergistic antimicrobial activity in vitro [26, 27]. Sub-MIC amounts of daptomycin and CF-301 were tested individually and found to have minimal kill against MRSA JMI 3345 (Figure 4A). However, when the same doses of daptomycin and CF-301 were combined, substantially more effective killing was observed than for either of the individual drugs alone, indicating synergy.
Figure 4.
CF-301 synergizes with antibiotics in vitro. A, Time-kill curves of methicillin-resistant Staphylococcus aureus (MRSA) strain JMI 3345 treated with buffer control or sub–minimum inhibitory concentration (MIC) amounts of either CF-301 (1 µg/mL), daptomycin (0.25 µg/mL), or combinations of CF-301 and daptomycin (DAP). B and C, Log changes in colony-forming units (CFU)/mL between combination and single-drug-treated cultures over 6 hours are shown for 13 MRSA strains. Horizontal dotted lines indicate a >2-log10 difference in viability (the cutoff value to score for synergy) between the combination and the most active single drug. D, Combination MIC assays for CF-301 and daptomycin. Combinations of CF-301 and daptomycin in micrograms per milliliter were serially diluted 2-fold across each row before adding MRSA ATCC BAA-1720. The most dilute combination to inhibit growth in each row is indicated. Wells are colored-coded to indicate the following conditions: (1) daptomycin at or above its MIC value (yellow); (2) CF-301 at or above its MIC value (blue); (3) daptomycin and CF-301 above their MIC values (green); and (4) daptomycin and CF-301 below their MIC values and acting synergistically (red). Wells in which cell growth occurred are gray. E, S. aureus strain CFS 955 labeled with BODIPY-FL-daptomycin (1/4× MIC) in the presence and absence of sub-MIC CF-301 (1/32× MIC). Cell walls are counterstained with Marina blue–labeled wheat germ agglutinin. Red fluorescence indicates daptomycin staining. F, Time course of S. aureus staining by BODIPY-FL-vancomycin (1/4× MIC) before and after adding sub-MIC CF-301 (1/32× MIC). Green fluorescence indicates daptomycin staining. Red arrows indicate times of binding equivalence.
Using the same methodology (Figure 4B and 4C), 12 additional MRSA strains were tested with sub-MIC daptomycin and sub-MIC vancomycin alone, as well as in combination with sub-MIC CF-301. Synergy was defined as a ≥2-log10 decrease in CFU/mL [26] at the 6-hour timepoint. Sub-MIC amounts of CF-301 demonstrated synergy when tested in combination with sub-MIC amounts of daptomycin (13/13 strains) and vancomycin (11/13 strains).
A second method to confirm synergy was the checkerboard assay [28, 29]. Checkerboards were generated using combinations of sub-MIC CF-301 with either sub-MIC daptomycin or vancomycin against 26 MRSA and 29 MSSA strains. Synergy was defined as inhibitory activity greater than what would be predicted by adding the 2 drugs together (ie, minimum fractional inhibitory concentration [FICmin] ≤ 0.5) [28]. With MRSA strains, CF-301 was synergistic with daptomycin (23/26 strains) and vancomycin (18/26 strains). For MSSA, CF-301 was synergistic with daptomycin (23/29 strains) and vancomycin (25/29 strains).
In the third method, the extent of synergy between CF-301 and daptomycin was tested in a combination MIC assay by diluting the 2 drugs together to determine the lowest concentration of the drug combinations required to inhibit growth (Figure 4D). Daptomycin alone inhibited growth of MRSA ATCC BAA-1720 at 1.0 µg/mL. In combination with sub-MIC CF-301, the amount of daptomycin required for inhibition decreased to 0.0075 µg/mL. This demonstrated synergy between CF-301 and daptomycin, with a 128-fold decrease in the amount of daptomycin required to inhibit growth. In subsequent studies using the identical format, mixtures of CF-301 and daptomycin were tested on 11 additional MRSA strains, with similar daptomycin susceptibility enhancements observed, ranging from 64- to 256-fold (data not shown). These results confirmed the findings in Figure 4D, underscoring the consistency of the synergistic effect.
CF-301 Accelerates Antibiotic Binding to the Bacterial Cell Wall and Membrane
Antibiotic binding to bacteria was performed with BODIPY-FL–labeled SOC antibiotics in the presence or absence of sub-MIC levels of CF-301. In the presence of CF-301, BODIPY-FL–labeled daptomycin stained S. aureus within 15 minutes, whereas without CF-301, no staining was observed (Figure 4E).
In a time-course analysis, BODIPY-FL–labeled vancomycin stained S. aureus within 5 minutes in the presence of sub-MIC CF-301 (Figure 4F). In contrast, staining required 30 minutes in the absence of CF-301. The confocal microscopic analysis demonstrated that labeled antibiotic was first observed at the bacterial division plane.
Accelerated staining with antibiotic is further illustrated in a video (Supplementary Video 1), where in the course of a 2-hour period, BODIPY-FL–labeled daptomycin bound much more rapidly to S. aureus in the presence of sub-MIC CF-301 compared to the rate of binding in its absence.
Combinations of CF-301 With Antibiotics Are More Efficacious in Treating S. aureus Bacteremia Than Treating With Antibiotics Alone
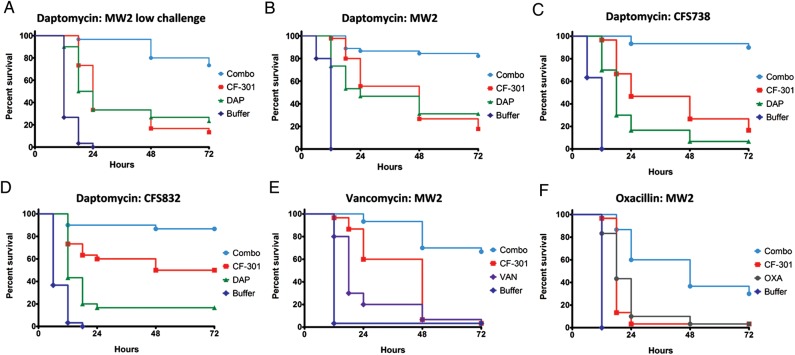
Mice with staphylococcal-induced bacteremia were treated with CF-301 and SOC antibiotics. In a low-challenge model, mice were inoculated intraperitoneally with 7.5 × 106 CFU of MRSA MW2 and treated 4 hours later with CF-301, daptomycin, or a combination of both (Figure 5A). At 72 hours, treatment with CF-301 yielded 13% survival, whereas treatment with daptomycin yielded 23% survival. Combination of CF-301 with daptomycin resulted in 73% survival, demonstrating that combination therapy was superior to either drug alone (P < .0001).
Figure 5.
Combination therapy is superior to monotherapy in murine models of Staphylococcus aureus bacteremia. At time 0 mice were challenged intraperitoneally with either 7.5 × 106 colony-forming units (CFU)/mouse (low-challenge model, A) or 1.0 × 109 CFU/mouse (high-challenge model, B–F) in mucin and were subsequently treated at various times with either antibiotic, CF-301, CF-301 combined with antibiotic, or buffer control. Single-bolus doses were used, with the exception of vancomycin (VAN; twice daily, E) and oxacillin (OXA; 4 times daily, F), which were administered as multiple doses over the first 24-hour period. Different routes of administration were used for CF-301 (intraperitoneal), daptomycin and vancomycin (subcutaneous), and oxacillin (intramuscular). P values were calculated for combinations vs antibiotic alone. Each dataset is a compilation of several individual studies (n = 10–20 per study) performed under identical conditions. A, Low-challenge model MRSA MW2 with daptomycin (DAP) at 2 mg/kg and CF-301 at 1.25 mg/kg. Dosing at 4 hours postinoculation (n = 30), P < .0001. B, High-challenge model with MW2 with daptomycin at 50 mg/kg and CF-301 at 5.25 mg/kg. Dosing at 2 hours postinoculation (n = 45), P < .0001. C, Same as (B), with MRSA CFS738 (n = 30), P < .0001. D, Same as (B), with MRSA CFS832 (n = 30), P < .0001. E, High-challenge model with MRSA MW2 with vancomycin at 110 mg/kg twice daily and CF-301 at 5.25 mg/kg. Dosing initiated at 2 hours postinoculation (n = 30), P < .0001. F, High-challenge model with methicillin-sensitive S. aureus CFS544 with oxacillin at 200 mg/kg 4 times daily and CF-301 at 5.25 mg/kg. Dosing initiated at 2 hours postinoculation (n = 30), P < .0001.
To test the robustness of combination therapy, the bacterial inoculum was increased to a point where high doses of SOC antibiotics were minimally efficacious. In this high-challenge model, mice were inoculated intraperitoneally with 1 × 109 CFU of MRSA MW2, MRSA CFS738, or MRSA CFS832 and treated 2 hours later with CF-301, daptomycin, or a combination of both (Figure 5B, 5C, and 5D). At 72 hours, treatment with CF-301 alone resulted in 17%–50% survival, whereas treatment with daptomycin alone resulted in 7%–31% survival. In contrast to these single-drug treatments, the combination of CF-301 with daptomycin improved survival to 82%–90% (P < .0001).
In another high-challenge study, mice were inoculated with MRSA MW2 and treated 2 hours later with CF-301, vancomycin, or a combination of CF-301 with vancomycin (Figure 5E). At 72 hours, treatment with either CF-301 or vancomycin alone resulted in 3% survival. Combination of CF-301 with vancomycin improved survival to 67% (P < .0001).
A high-challenge study was also performed with MSSA CFS544 (Figure 5F). Mice were inoculated intraperitoneally with 109 CFU and treated 2 hours later with CF-301, oxacillin, or a combination of CF-301 with oxacillin. At 72 hours, treatment with either CF-301 or oxacillin alone resulted in 3% survival. In contrast, treatment with the combination of CF-301 and oxacillin improved survival to 30% (P < .0001).
Similar enhanced activities of combinations were observed in 10 additional MRSA-induced bacteremia experiments (Supplementary Table 2). Together with the 16 individual experiments used to compile the graphs shown in Figure 5, superiority of combination over single-antibiotic treatments was confirmed in 26 independent studies.
DISCUSSION
Antimicrobial drugs used in combination should have complementary and synergistic activities that do not reinforce the liabilities of either drug [30–32]. Lysins are well suited for use in combinations as their properties uniquely complement those of SOC antibiotics. As illustrated with CF-301, beneficial properties include potent and rapid bacteriolytic effects, activity against drug-resistant strains, narrow specificity, eradication of biofilms, low levels of resistance, and synergy with antibiotics.
Unlike most antibiotics, lysins do not require bacterial metabolism or growth for activity and are bacteriolytic on contact [33]. The rapid CF-301–mediated killing effect was demonstrated in vitro by a >3-log10 reduction in bacterial viability within 30 minutes of treatment. The rapid-kill property of lysins makes them well suited to quickly reduce the bacterial burden in infected hosts. Although this property can be exploited to rapidly decolonize animals of resident pathogens [7, 10], the current study with CF-301 focused on systemic treatment. The rapid killing effect was directly demonstrated in vivo by the 2-log10 drop in pathogen CFU within 1 hour of dosing.
The breadth and potency of CF-301 was demonstrated by its bacteriolytic effect on all 250 S. aureus strains tested, including 120 MRSA and 27 multidrug-resistant strains, and its molar activity being 2- to 40-fold higher than SOC antibiotics tested.
Another beneficial property of lysins is their narrow spectrum of action [5]. Unlike broad-spectrum antibiotics, lysins do not kill beneficial organisms, which should reduce unwanted clinical complications such as Clostridium difficile–associated diarrhea [14, 15]. Whereas combinations of 2 standard broad-spectrum antibiotics could be highly disruptive to commensal flora, combinations incorporating a lysin should be preferred owing to their narrow species specificity.
Within infected tissues (eg, heart valves in endocarditis) or prosthetic implants, S. aureus forms biofilms that provide favorable environments for growth and persistence, protecting the pathogen from antibiotics and the immune system [34, 35]. CF-301 has potent activity against S. aureus biofilms and was able to remove biomass at 1× MIC, compared to the activity of SOC antibiotics, which failed to remove comparable biomass at 1000× MIC. Potent antibiofilm activity may be a general feature of the lysin family [36, 37], and this feature uniquely complements the action of antibiotics by enabling their access to bacteria in disrupted biofilms. We anticipate that combinations incorporating CF-301 may prove efficacious in treating endocarditis and infected implants.
Because of its microbial origin, CF-301 is immunogenic in mammals. However, anti–CF-301 antibodies are nonneutralizing because affinity-purified rabbit anti-CF-301 antisera did not inhibit in vitro lytic activity of CF-301 (data not shown), in agreement with previous studies on antipneumococcal lysin Cpl-1 [11]. Further, immunized mice with anti–CF-301 titers >1:100 000 remained responsive to CF-301 treatment in the murine bacteremia model, indicating that systemic CF-301 treatments may not be appreciably hindered by antibody-mediated neutralization or clearance.
Beyond the direct bacteriolytic activity provided by lysins as a single agent, CF-301 also exhibited potent synergy in vitro and significantly improved efficacy in vivo when combined with SOC antibiotics. This agrees with other studies where enhanced activities of lysin/antibiotic combinations have been reported in both in vitro assays [23, 38] and in vivo models [39, 40]. While in vitro synergy between antibiotics and CF-301 was demonstrated here in time-kill and checkerboard assays, it was best illustrated in MIC assays with combined drugs (Figure 4D). For example, in the presence of low levels of CF-301, the MIC of daptomycin decreased from 1 µg/mL to 0.0075 µg/mL, a 128-fold increase in potency. This powerful synergistic effect was additionally observed with 11 other MRSA strains where CF-301 increased daptomycin potency by 64- to 256-fold.
Fluorescent daptomycin and vancomycin derivatives displayed accelerated binding kinetics to the bacterial envelope in the presence of sub-MIC CF-301. Using similar methodologies, enhanced daptomycin binding to staphylococci has been observed in the presence of nafcillin and attributed to a reduction in net positive cell-wall charge mediated by the β-lactam [41]. In the present study, we propose a different hypothesis to account for the synergy between lysin and antibiotics, whereby at sub-MIC doses of CF-301, a sufficient number of bonds in the peptidoglycan are cleaved, resulting in a more permeable structure, enabling enhanced antibiotic penetration. This hypothesis is supported by preferential antibiotic staining at division septa, where newly formed peptidoglycan is dynamically shaped [42] and appears to be more sensitive to lysin action [33, 43]. Regardless of the mode of action, the in vitro synergy observed is likely to be a key reason for the enhanced efficacy of combinations observed in the animal studies.
Across 26 independent murine staphylococcal-bacteremia studies, multiple variables were examined, including different S. aureus strains (MRSA and MSSA), different bacterial inoculum amounts, and various SOC antibiotics. Invariably, combination treatments of CF-301 and SOC antibiotics significantly outperformed treatments by SOC antibiotics alone.
Although the strength of the bacterial inocula used in the high-challenge models were sufficient to cause failure of high-dose treatments of vancomycin or daptomycin (considered to be human-simulated doses [44, 45]), in all cases the combination treatments were superior. In other MRSA-induced bacteremia experiments, bolus dosing was consistently found to be more efficacious than multiple dosing of the same total amount of CF-301 (ie, twice- or thrice-daily dosing), presumably due to rapid reduction of the bacterial population early in the time-course of infection. Overall, the in vivo results demonstrate that CF-301 combined with SOC antibiotics is more efficacious than single-drug regimens for treating bacteremia, and that statistically significant results occurred with SOC antibiotics and all S. aureus strains tested.
These results have clinical implications for designing new therapeutic regimens employing combinations of lysins with antibiotics. We envisage an S. aureus bacteremia treatment based on administering a combination of CF-301 and SOC antibiotics. During each round of dosing, the fast-acting CF-301 would rapidly reduce the pathogen burden while the antibiotic would continue to act on residual bacteria. Additionally, synergy between the 2 antimicrobial agents would extend potency and duration of bactericidal action. Advantages of this approach would include limitation of unwanted killing of commensal bacteria due to the narrow-spectrum antimicrobial activity of lysins, and increased exposure and killing of pathogens by antibiotics due to the biofilm-disrupting capacity of lysins. Compared to currently available single-drug options with SOC antibiotics, therapies using lysins in combination with antibiotics should provide unique and efficacious alternatives for treating bacteremia caused by drug-resistant S. aureus.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We acknowledge Berice Ruisi for manuscript preparations; Alfredo Barea, Michael Kreiness, Benjamin Winer, Chad Euler (The Rockefeller University), Adam Vigil, and Robert Durso for technical assistance; Nadine Soplop and Kunihiro Uryo (Electron Microscopy Resource Center at The Rockefeller University) for electron microscopy studies; Alexander Epstein and Anna Blech for assistance with serial passage resistance assays; and the Process Development and Manufacturing team at Fujifilm Diosynth Biologics UK Ltd for CF-301 purification process development. We thank Alison North (Bio-Imaging Resource Center at The Rockefeller University) for support with DeltaVision microscopy; Torin Weisbrod and Travis Hartman (Howard Hughes Medical Institute at Albert Einstein College of Medicine) for assistance with video fluorescence microscopy; Keegan DeLisio for video editing; and Bernard Beall (Centers for Disease Control and Prevention) and Mary Windels (The Rockefeller University) for providing streptococcal strains.
Financial support. This work was supported by ContraFect Corporation. The work of V. A. F. and A. R. was supported by the National Institutes of Health (grant number AI075472 to V. A. F.).
Potential conflicts of interest. R. S., H. M. L., B. C. S., K. L. S., C. L., B. K. K., J. A. R., D. E. C., D. B. H., R. C. N., and M. W. are employees of ContraFect Corporation (Yonkers, New York), and they and V. A. F and Y. H. hold stock options in ContraFect Corporation. A. R. reports no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Klevens RM, Morrison MA, Nadle J, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–71. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 2.Landrum ML, Neumann C, Cook C, et al. Epidemiology of Staphylococcus aureus blood and skin and soft tissue infections in the US military health system, 2005–2010. JAMA. 2012;308:50–9. doi: 10.1001/jama.2012.7139. [DOI] [PubMed] [Google Scholar]
- 3.Brink AJ. Does resistance in severe infections caused by methicillin-resistant Staphylococcus aureus give you the 'creeps’? Cur Opin Crit Care. 2012;18:451–9. doi: 10.1097/MCC.0b013e3283578968. [DOI] [PubMed] [Google Scholar]
- 4.Ben-David D, Novikov I, Mermel LA. Are there differences in hospital cost between patients with nosocomial methicillin-resistant Staphylococcus aureus bloodstream infection and those with methicillin-susceptible S. aureus bloodstream infection? Infect Control Hosp Epidemiol. 2009;30:453–60. doi: 10.1086/596731. [DOI] [PubMed] [Google Scholar]
- 5.Fischetti VA. Bacteriophage lysins as effective antibacterials. Curr Opin Microbiol. 2008;11:393–400. doi: 10.1016/j.mib.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fenton M, Ross P, McAuliffe O, O'Mahony J, Coffey A. Recombinant bacteriophage lysins as antibacterials. Bioeng Bugs. 2010;1:9–16. doi: 10.4161/bbug.1.1.9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nelson D, Loomis L, Fischetti VA. Prevention and elimination of upper respiratory colonization of mice by group A streptococci by using a bacteriophage lytic enzyme. Proc Natl Acad Sci U S A. 2001;98:4107–12. doi: 10.1073/pnas.061038398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Witzenrath M, Schmeck B, Doehn JM, et al. Systemic use of the endolysin Cpl-1 rescues mice with fatal pneumococcal pneumonia. Crit Care Med. 2009;37:642–9. doi: 10.1097/CCM.0b013e31819586a6. [DOI] [PubMed] [Google Scholar]
- 9.McCullers JA, Karlstrom A, Iverson AR, Loeffler JM, Fischetti VA. Novel strategy to prevent otitis media cauesed by colonizing Streptococcus pneumoniae. PLoS Pathog. 2007;3:0001–3. doi: 10.1371/journal.ppat.0030028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pastagia M, Euler C, Chahales P, Fuentes-Duculan J, Krueger JG, Fischetti VA. A novel chimeric lysin shows superiority to mupirocin for skin decolonization of methicillin-resistant and -sensitive Staphylococcus aureus strains. Antimicrob Agents Chemother. 2011;55:738–44. doi: 10.1128/AAC.00890-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loeffler JM, Djurkovic S, Fischetti VA. Phage lytic enzyme Cpl-1 as a novel antimicrobial for pneumococcal bacteremia. Infect Immun. 2003;71:6199–204. doi: 10.1128/IAI.71.11.6199-6204.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Entenza JM, Loeffler JM, Grandgirard D, Fischetti VA, Moreillon P. Therapeutic effects of bacteriophage Cpl-1 lysin against Streptococcus pneumoniae endocarditis in rats. Antimicrob Agents Chemother. 2005;49:4789–92. doi: 10.1128/AAC.49.11.4789-4792.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grandgirard D, Loeffler JM, Fischetti VA, Leib SL. Phage lytic enzyme Cpl-1 for antibacterial therapy in experimental pneumococcal meningitis. J Infect Dis. 2008;197:1519–22. doi: 10.1086/587942. [DOI] [PubMed] [Google Scholar]
- 14.Blaser M. Stop killing beneficial bacteria. Nature. 2011;476:393–4. doi: 10.1038/476393a. [DOI] [PubMed] [Google Scholar]
- 15.Willing BP, Russell SL, Finlay BB. Shifting the balance: antibiotic effects on host-microbiota mutualism. Nature Rev Microbiol. 2011;9:233–43. doi: 10.1038/nrmicro2536. [DOI] [PubMed] [Google Scholar]
- 16.Gilmer DB, Schmitz JE, Euler CW, Fischetti VA. Novel bacteriophage lysin with broad lytic activity protects against mixed infection by Streptococcus pyogenes and methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2013;57:2743–50. doi: 10.1128/AAC.02526-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rossi P, Aramini JM, Xiao R, et al. Structural elucidation of the Cys-His-Glu-Asn proteolytic relay in the secreted CHAP domain enzyme from the human pathogen Staphylococcus saprophyticus. Proteins. 2009;74:515–9. doi: 10.1002/prot.22267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y. I-TASSER server for protein 3D structure prediction. BMC Bioinformatics. 2008;9:40. doi: 10.1186/1471-2105-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schrodinger, LLC. Portland, OR USA. The PyMOL molecular graphics system, Version 1.6.0.0. 2013. [Google Scholar]
- 20.Bateman A, Rawlings ND. The CHAP domain: a large family of amidases including GSP amidase and peptidoglycan hydrolases. Trends Biochem Sci. 2003;28:234–7. doi: 10.1016/S0968-0004(03)00061-6. [DOI] [PubMed] [Google Scholar]
- 21.Whisstock JC, Lesk AM. SH3 domains in prokaryotes. Trends Biochem Sci. 1999;24:132–3. doi: 10.1016/s0968-0004(99)01366-3. [DOI] [PubMed] [Google Scholar]
- 22.Fenton M, Casey PG, Hill C, et al. The truncated phage lysin CHAP(k) eliminates Staphylococcus aureus in the nares of mice. Bioeng Bugs. 2010;1:404–7. doi: 10.4161/bbug.1.6.13422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manoharadas S, Witte A, Blasi U. Antimicrobial activity of a chimeric enzybiotic towards Staphylococcus aureus. J Biotechnol. 2009;139:118–23. doi: 10.1016/j.jbiotec.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 24.O'Flaherty S, Coffey A, Meaney W, Fitzgerald GF, Ross RP. The recombinant phage lysin LysK has a broad spectrum of lytic activity against clinically relevant staphylococci, including methicillin-resistant Staphylococcus aureus. J Bacteriol. 2005;187:7161–4. doi: 10.1128/JB.187.20.7161-7164.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clinical and Laboratory Standards Institute. M07-A9. 8th ed. Wayne, PA: CLSI; 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard. [Google Scholar]
- 26.Clinical and Laboratory Standards Institute. M26-A. Wayne, PA: CLSI; 1999. Methods for determining bactericidal activity of antimicrobial agents; approved guideline. [Google Scholar]
- 27.Mueller M, de la Pena A, Derendorf H. Issues in pharmacokinetics and pharmacodynamics of anti-infective agents: kill curves versus MIC. Antimicrob Agents Chemother. 2004;48:369–77. doi: 10.1128/AAC.48.2.369-377.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moody J. Synergy testing: broth microdilution checkerboard and broth macrodilution methods. In: Garcia LS, editor. Clinical microbiology procedures handbook; Vol. 2. Washington, DC: ASM Press; 2010. pp. 5.12.1–23. [Google Scholar]
- 29.Tallarida RJ. Revisiting the isobole and related quantitative methods for assessing drug synergism. J Pharmacol Exp Ther. 2012;342:2–8. doi: 10.1124/jpet.112.193474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abad CL, Kumar A, Safdar N. Antimicrobial therapy of sepsis and septic shock—when are two drugs better than one? Crit Care Clin. 2011;27:e1–27. doi: 10.1016/j.ccc.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 31.Cottarel G, Wierzbowski J. Combination drugs, an emerging option for antibacterial therapy. Trends Biotechnol. 2007;25:547–55. doi: 10.1016/j.tibtech.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 32.Fischbach MA. Combination therapies for combating antimicrobial resistance. Curr Opin Microbiol. 2011;14:519–23. doi: 10.1016/j.mib.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loeffler JM, Nelson D, Fischetti VA. Rapid killing of Streptococcus pneumoniae with a bacteriophage cell wall hydrolase. Science. 2001;294:2170–2. doi: 10.1126/science.1066869. [DOI] [PubMed] [Google Scholar]
- 34.Costerton JW. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–22. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 35.Kiedrowski MR, Horswill AR. New approaches for treating staphylococcal biofilm infections. Ann NY Acad Sci. 2011;1241:104–21. doi: 10.1111/j.1749-6632.2011.06281.x. [DOI] [PubMed] [Google Scholar]
- 36.Domenech M, Garcia E, Moscoso M. In vitro destruction of Streptococcus pneumoniae biofilms with bacterial and phage peptidoglycan hydrolases. Antimicrob Agents Chemother. 2011;55:4144–8. doi: 10.1128/AAC.00492-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meng X, Shi Y, Ji W, et al. Application of a bacteriophage lysin to disrupt biofilms formed by the animal pathogen Streptococcus suis. Appl Environ Microb. 2011;77:8272–9. doi: 10.1128/AEM.05151-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rashel M, Uchiyama J, Ujihara T, et al. Efficient elimination of multidrug-resistant Staphylococcus aureus by cloned lysin derived from bacteriophage phi MR11. J Infect Dis. 2007;196:1237–47. doi: 10.1086/521305. [DOI] [PubMed] [Google Scholar]
- 39.Daniel A, Euler C, Collin M, Chahales P, Gorelick KJ, Fischetti VA. Synergism between a novel chimeric lysin and oxacillin protects against infection by methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2010;54:1603–12. doi: 10.1128/AAC.01625-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kokai-Kun JF, Chanturiya T, Mond JJ. Lysostaphin as a treatment for systemic Staphylococcus aureus infection in a mouse model. J Antimicrob Chemother. 2007;60:1051–9. doi: 10.1093/jac/dkm347. [DOI] [PubMed] [Google Scholar]
- 41.Dhand A, Bayer AS, Pogliano J, et al. Use of antistaphylococcal beta-lactams to increase daptomycin activity in eradicating persistent bacteremia due to methicillin-resistant Staphylococcus aureus: role of enhanced daptomycin binding. Clin Infect Dis. 2011;53:158–63. doi: 10.1093/cid/cir340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matias VR, Beveridge TJ. Cryo-electron microscopy of cell division in Staphylococcus aureus reveals a mid-zone between nascent cross walls. Mol Microbiol. 2007;64:195–206. doi: 10.1111/j.1365-2958.2007.05634.x. [DOI] [PubMed] [Google Scholar]
- 43.Schuch R, Nelson D, Fischetti V. A bacteriolytic agent that detects and kills Bacillus anthracis. Nature. 2002;418:884–9. doi: 10.1038/nature01026. [DOI] [PubMed] [Google Scholar]
- 44.Crandon JL, Kuti JL, Nicolau DP. Comparative efficacies of human simulated exposures of telavancin and vancomycin against methicillin-resistant Staphylococcus aureus with a range of vancomycin MICs in a murine pneumonia model. Antimicrob Agents Chemother. 2010;54:5115–9. doi: 10.1128/AAC.00062-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.LaPlante KL, Leonard SN, Andes DR, Craig WA, Rybak MJ. Activities of clindamycin, daptomycin, doxycycline, linezolid, trimethoprim-sulfamethoxazole, and vancomycin against community-associated methicillin-resistant Staphylococcus aureus with inducible clindamycin resistance in murine thigh infection and in vitro pharmacodynamic models. Antimicrob Agents Chemother. 2008;52:2156–62. doi: 10.1128/AAC.01046-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.