The brain has limited fuel reserves and is highly dependent on a constant supply of oxygen and energy substrates delivered through blood flow (Hossmann, 1994). Interruption of cerebral blood flow (CBF) leads to brain dysfunction and, if the interruption is prolonged, brain death. Cerebral blood vessels are endowed with powerful regulatory mechanisms that assure that the brain is perfused at all times. However, during cerebral ischemia these mechanisms become dysfunctional and fail to compensate for the reduction in CBF. Thus cerebral ischemia produces profound alterations of the major control mechanisms governing the cerebral circulation. Such dysregulation, termed vasomotor paralysis (Langfitt et al., 1964; Hoedt-Rasmussen et al., 1967; Paulson, 1971), occurs in the setting of both focal cerebral ischemia, in which CBF is reduced in a restricted brain region, and global cerebral ischemia, in which CBF is globally reduced throughout the brain. The cerebrovascular dysregulation undermines the ability of the brain to maintain CBF, aggravates the intensity of the ischemic insult, and amplifies the tissue damage. Therapies aimed at restoring cerebrovascular regulation after ischemia offer the opportunity to improve cerebral perfusion and limit ischemic injury. In this chapter, we will briefly review the effects of cerebral ischemia on the regulation of CBF, focusing on the potential mechanisms involved in the cerebrovascular dysfunction and on the implications for ischemic brain injury.
14.1. Effects of cerebral ischemia and reperfusion on cerebral blood flow
14.1.1. Cerebral blood flow after arterial occlusion
The introduction of techniques for measuring regional CBF provided new insights into the alterations of CBF distribution following focal or global cerebral ischemia. Occlusion of a major cerebral artery produces a severe reduction in CBF to the area of the brain supplied by that particular artery. Thus, the CBF reduction is greatest in the center of the ischemic territory. Surrounding this ‘core’ area of cerebral ischemia there is an area in which a less severe CBF reduction is found. This peripheral region of the ischemic territory is termed ischemic penumbra. Whereas brain tissue in the ischemic core is irreversibly damaged, the penumbra represents potentially salvageable brain tissue, in which neuronal activity is suppressed but the tissue is potentially viable (Astrup et al., 1981). The fate of the penumbra depends on the residual CBF and the duration of the flow reduction (Astrup et al., 1981; Heiss and Rosner, 1983). Numerous clinical studies have attempted to define the CBF values corresponding to the ischemic penumbra. Using different imaging techniques the range of CBF in the penumbra has been estimated to be between 12 and 22 ml/100 g per minute (Heiss et al., 2001). If CBF drops below this critical threshold for a sufficient period of time the tissue will be damaged.
14.1.2. Cerebral blood flow at reperfusion
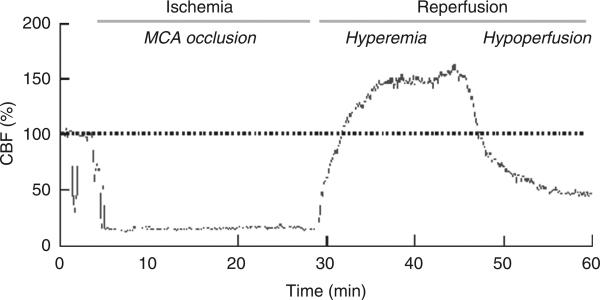
In human ischemic stroke, the arterial occlusion can be either permanent or transient. It has been shown that an early re-establishment of the CBF in ischemic areas (reperfusion) can be associated with a favorable functional outcome (Minematsu et al., 1992; Ringelstein et al., 1992). On the other hand, reperfusion can also exacerbate brain injury through the development of space-occupying hemorrhagic transformations or cerebral edema (Nakagawa et al., 1990; Wang and Lo, 2003). Therefore, CBF changes at reperfusion have important pathophysiological implications. Focal cerebral ischemia induced by an acute occlusion of a cerebral artery leads to a pronounced decrease in CBF in the dependent brain area. After reopening the vessel occlusion, reperfusion in the ischemic core typically shows a biphasic pattern: a transient increase in CBF (post-ischemic hyperperfusion) followed by a more sustained reduction in CBF (hypoperfusion; Fig. 14.1). Post-ischemic hyperperfusion, also known as ‘luxury perfusion’, has long been demonstrated in animal stroke models and human stroke as well (Lassen, 1966; Sundt and Waltz, 1971; Pulsinelli et al., 1982; Traupe et al., 1982; Todd et al., 1986; Heiss et al., 1997; Marchal et al., 1999). Because of a lack of pre-ischemic CBF values, post-ischemic hyperperfusion is usually termed ‘post-ischemic hyperemia’ (Hoedt-Rasmussen et al., 1967; Olsen et al., 1981; Baron et al., 1989). The length of post-ischemic hyperperfusion is directly proportional to the duration of the ischemic phase (Gourley and Heistad, 1984). The hyperemic phase is not mediated by an increase in oxygen or glucose utilization (Gourley and Heistad, 1984) and can be attributed to abnormal vasodilation in the ischemic territory (Marchal et al., 1996, 1999). Such abnormal vasodilation has multiple causes, including lactic acidosis secondary to ischemia-induced anaerobic glycolysis (Rehncrona et al., 1981) and/or release of vasoactive mediators from the ischemic brain, including ions, metabolites, and reactive oxygen species (Berne et al., 1974; Traystman et al., 1991; Nelson et al., 1992; Silver and Erecinska, 1992; Wei et al., 1996).
Fig. 14.1.
Recording of cerebral blood flow (CBF) measured by laser Doppler flowmetry in the center of the ischemic territory (ischemic core) before, during, and after occlusion of the middle cerebral artery using an intravascular filament in mice. CBF is markedly reduced during the arterial occlusion. At reperfusion, CBF first increases (post-ischemic hyperemia) and then drops below pre-ischemic levels (post-ischemic hypoperfusion).
The mechanisms leading to post-ischemic hypoperfusion are still unclear. Measurements of intravascular pressure and segmental vascular resistance in cats following global cerebral ischemia have shown that ischemia–reperfusion is followed by an increase in cerebrovascular resistance involving both extra- and intracranial arteries (Schmidt-Kastner et al., 1987). This increase in vascular resistance could be, in part, due to microvascular compression and vasospasm, as suggested by a scanning electron microscopy study of corrosion casts in rats following transient focal ischemia (Ohtake et al., 2004). In this study, microvessels appeared constricted, compressed, and narrowed, effects attributed to edema, hemorrhage, and vasospasm presumably induced by vasoconstrictors released from the ischemic brain (Ohtake et al., 2004). Additional causes of post-ischemic hypoperfusion may include reduced metabolic demand, reduced synthesis of parenchymal and endothelial vasoactive agents, and intravascular plugging by platelets and leukocytes (Hossmann, 1983; del Zoppo and Mabuchi, 2003). A deficit of the potent vasodilator nitric oxide (NO) does not seem to be involved because inhibition of NO synthesis reduces CBF further, suggesting that NO production helps to counteract the post-ischemic increase in vascular resistance (Clavier et al., 1994). Furthermore, administration of prostacyclin does not normalize post-ischemic flow, suggesting that the hypoperfusion is not due to a lack of endothelial-derived prostacyclin (van den Kerckhoff et al., 1983).
14.2. Cerebrovascular autoregulation
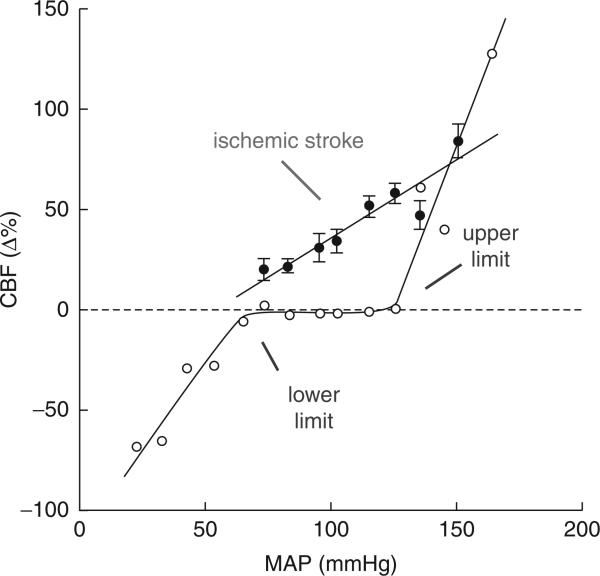
Cerebral blood flow is relatively independent of changes in mean arterial pressure with a certain range. The lower and upper limits of CBF autoregulation correspond to mean arterial pressures of approximately 50–60 and 150–160 mmHg respectively (Chillon and Baumbach, 2002; see Lassen, 1959; Heistad and Kontos, 1983; Paulson et al., 1990 for review) (Fig. 14.2). This property of the cerebral circulation, termed auto-regulation, is also a characteristic of the circulation of other organs (Paulson et al., 1990). The vascular adjustments underlying autoregulation consist of a constriction of cerebral resistance vessels when cerebral perfusion pressure (arterial pressure minus intracranial pressure) increases and a vasodilation of these vessels when perfusion pressure decreases (Kontos et al., 1978). Thus, the changes in cerebral perfusion pressure induced by changes in arterial pressure are counteracted by changes in cerebrovascular resistance, which tend to keep CBF constant. However, if the change in arterial pressure exceeds the capacity of the vessels to compensate, autoregulation is lost. Thus, increases in arterial pressure above the upper limit of autoregulation lead to increases in CBF, while decreases below the lower limit lead to reductions in CBF (Chillon and Baumbach, 2002). Furthermore, rapid variations in arterial pressure are not compensated for by autoregulation and produce changes in CBF (Aaslid et al., 1989; Florence and Seylaz, 1992). This is because the vascular adjustments initiated by arterial pressure are not instantaneous and take several seconds to take effect. The cellular basis of cerebrovascular autoregulation resides in the intrinsic property of vascular smooth muscles to react to changes in transmural pressure (myogenic tone; Bayliss, 1902; Harder et al., 1995; Wellman et al., 2002). Neural activity, hypoxia, hypo- and hypercapnia, or stimulation of cerebrovascular nerves can modulate the intrinsic vascular myogenic response and may influence the rapidity of the vascular adjustments, the slope of the pressure–flow relationship, and the range of pressures over which CBF is autoregulated (Heistad and Kontos, 1983; Busija and Heistad, 1984; Paulson et al., 1990).
Fig. 14.2.
Relationship between cerebral blood flow (CBF) and mean arterial pressure (MAP) in physiological conditions (gray) and following cerebral ischemia (black). In the normal state, CBF is kept constant over a relatively wide range of MAPs, a phenomenon termed cerebrovascular auto-regulation. Thus, cerebral resistance vessels dilate when MAP decreases and constrict when MAP increases and, as a result, CBF remains relatively constant. When the change in MAP exceeds this compensatory ability of cerebral vessels, CBF increases when MAP rises (upper limit) and decreases when MAP is reduced (lower limit). Following cerebral ischemia, cerebral resistance arteries are in a state of vasoparalysis and lose their ability to constrict or dilate in response to changes in MAP. Thus, autoregulation is lost and CBF follows MAP passively. Such disruption of autoregulation can amplify the damage by aggravating cerebral ischemia if MAP falls and by inducing blood-barrier damage and cerebral edema when MAP rises.
14.2.1. Effect of cerebral ischemia on autoregulation: animal studies
It is well established that cerebral ischemia impairs autoregulation. Whereas most experimental studies have investigated the effect of either hypertension or hypotension on CBF, a few studies have tested the full range of pressures over which CBF is autoregulated (Table 14.1). Experimental studies in different species have investigated cerebrovascular autoregulation during reperfusion following transient cerebral ischemia. One such study investigated CBF autoregulation before, during, and after transient bilateral carotid artery ligation in rats (Shiokawa et al., 1986). Resting CBF was markedly reduced 30 min after ligation and autoregulation during hypotension was severely impaired, resulting in almost zero flow. Such autoregulatory dysfunction persisted, even though less pronounced, in the acute phase after restoration of CBF to the ischemic lesion (Shiokawa et al., 1986). In the phase of hypoperfusion following transient global cerebral ischemia in dogs, autoregulation was present but attenuated (Christopherson et al., 1993). In that study, the CBF response to CO2 was also markedly attenuated. This finding is at variance with other investigations that demonstrated restored autoregulation during the phase of post-ischemic hypoperfusion while the CO2 response is still reduced (Hossmann et al., 1973; Nemoto et al., 1975). Perhaps, this discrepancy can be attributed to the different methods used for the induction of cerebral ischemia.
Table 14.1.
Cerebral ischemia and autoregulation—selected animal studies
| Ischemia model (duration) | Species | CBF measurement | Effect on autoregulation (upper–lower limit) | Reference |
|---|---|---|---|---|
| Global ischemia (30 and 60 min) | Cat | 133Xe-injection | Preserved (LL) | Hossmann et al., 1973 |
| 3 years after pMCAO | Baboon | H2 clearance | Abolished in infarct, impaired in peri-infarct area (LL) | Symon et al., 1975 |
| Global ischemia (15 min) | Dog | 133Xe-injection | Partially preserved (UL, LL) | Nemoto et al., 1975 |
| pMCAO | Cat | Heated thermocouple | Impaired (UL) | Shima et al., 1983 |
| Global ischemia (60 min) | Rat | H2 clearance | Abolished (LL) | Shiokawa et al., 1986 |
| pMCAO | Rat (SHR) | LDF | Impaired if ischemic CBF >30%, abolished if ischemic CBF <30% (UL, LL) | Dirnagl and Pulsinelli, 1990 |
| Global ischemia (12 and 18 min) | Dog | Sagittal sinus venous outflow | Impaired (UL, LL) | Christopherson et al., 1993 |
| pMCAO | Rat | LDF | Impaired, dependent on proximity to core (UL, LL) | Lauer et al., 1998 |
| tMCAO (endothelin-1 administration) | Rat | IAP, SPECT | Abolished in viable tissue with normal CBF (LL) | MacGregor et al., 2000 |
CBF, cerebral blood flow; IAP, C14-iodoantipyrine; LDF, laser-Doppler flowmetry; LL, lower limit; pMCAO, permanent middle cerebral artery occlusion; SHR, spontaneously hypertensive rats; SPECT, single photon emission computed tomography; tMCAO, transient middle cerebral artery occlusion; UL, upper limit.
The upper limit of autoregulation was impaired within the first hour after permanent middle cerebral artery occlusion in cats (Shima et al., 1983). However, 2 h after ischemia, autoregulation was partially restored (Shima et al., 1983). Measurement of pial artery pressure revealed that autoregulation in non-occluded collateral arteries supplying the ischemic territory is preserved (Shima et al., 1983). Therefore, collateral pathways could partially compensate for the CBF reduction following middle cerebral artery occlusion (Shima et al., 1983). Other studies found that autoregulation remains markedly impaired for several years after cerebral ischemia. For example, Symon and colleagues (1975) investigated autoregulation during hypotension in baboons 3 years after focal cerebral ischemia. They found that autoregulation was abolished in the infarct itself but it was present, albeit significantly impaired, in brain areas surrounding the infarcted tissue.
The degree of impairment of autoregulation is related to the magnitude of the CBF reduction. Dirnagl and Pulsinelli (1990) tested autoregulation during hypertension and hypotension in rats following permanent combined middle cerebral artery and common carotid artery occlusion. Autoregulation was attenuated in areas in which CBF fell to levels between 30% and 60% of preocclusion values and was lost in areas where CBF was below 30%. Similarly, in a study using intra-arterial injection of silicone for the occlusion of intracranial arteries, autoregulation was more or less impaired depending on the proximity of the region to the ischemic core, where the CBF reduction is most pronounced (Lauer et al., 1998). Another important finding that emerged from experimental studies is that in the ischemic brain autoregulation is also impaired in remote non-ischemic areas. For example, moderate hypotension 24 hours after transient focal cerebral ischemia in rats demonstrated that autoregulation is impaired in the normally perfused peri-infarct cortex and subcortical white matter, as well as in the subcortical white matter of the contralateral hemisphere (MacGregor et al., 2000). In another study, the lower limit of autoregulation was reduced in the rat cerebellum after transient bilateral carotid occlusion (Shiokawa et al., 1986). These remote effects are likely to be mechanistically related to the concept of diaschisis (see section 14.4.1).
14.2.2. Human studies
Alterations of cerebrovascular autoregulation following ischemic brain injury have also been investigated in humans (Table 14.2). The use of positron emission tomography (PET) provided valuable insights into the compensatory mechanisms that maintain cerebral perfusion in the initial stages of CBF reduction before brain injury occurs. Powers (1991) categorized these cerebrovascular adjustments into three stages: stage 0, when cerebral perfusion pressure is normal; stage 1, when cerebral perfusion pressure is reduced and autoregulation dilates cerebral vessels to maintain CBF; and stage 2, when the compensatory capacity for cerebral vasodilation is exceeded, CBF begins to decrease, and oxygen extraction fraction increases to maintain the cerebral metabolic rate of O2 (CMRO2) (Powers, 1991). In stage 2 cerebrovascular autoregulation is disrupted.
Table 14.2.
Cerebral ischemia and autoregulation—selected human studies
| Pathology | CBF measurement | Effect on autoregulation (upper–lower limit) | Reference |
|---|---|---|---|
| TIA (ICA territory) | 133Xe-injection | Impaired/abolished in 2 of 12 patients (UL, LL) | Skinhoj et al., 1970 |
| Stroke (ICA territory) | 133Xe-injection | Abolished, restored by hypocapnia (UL) | Paulson et al., 1972 |
| Stroke within 72 h (MCA territory) | 133Xe-injection | Impaired in 3 of 16 patients (UL) | Olsen et al., 1981 |
| Subacute stroke (MCA territory) | 133Xe-injection | Impaired in collaterally perfused areas (UL) | Olsen et al., 1983 |
| TIA (bilateral ICA stenosis/occlusion) | 133Xe-inhalation, TCD | Impaired (LL) | Tatemichi et al., 1990 |
| Stroke (ICA, MCA territory) | PET | Impaired (LL) | Ouchi et al., 2001 |
| Stroke within 72 h (ICA, VB territories) | TCD* | Impaired (spontaneous ABP fluctuations) | Eames et al., 2002 |
| ICA stenosis/occlusion | TCD* | Impaired (spontaneous ABP fluctuations) | Gooskens et al., 2003 |
| ICA stenosis/occlusion | TCD* | Impaired (spontaneous ABP fluctuations) | Reinhard et al., 2003 |
| MCA stroke, lacunar stroke (within 72 h) | TCD* | Impaired (spontaneous ABP fluctuations) | Immink et al., 2005 |
| MCA stenosis/occlusion | TCD* | Impaired, improvement after MCA stent angioplasty (thigh cuff) | Gong et al., 2006 |
| ICA stenosis (before/after angioplasty) | TCD* | Impaired (spontaneous ABP fluctuations), improvement after angioplasty | Haubrich et al., 2007 |
‘Dynamic autoregulation’ was estimated based on spontaneous ABP fluctuations except for Gong et al. (2006), who used the thigh cuff technique.
ABP, arterial blood pressure; CBF, cerebral blood flow; ICA, internal carotid artery; LL, lower limit; MCA, middle cerebral artery; PET, positron emission tomography; TCD, transcranial Doppler; TIA, transient ischemic attack; UL, upper limit; VB, vertebrobasilar artery.
In patients with acute stroke, all studies found an impaired autoregulation in the affected vascular territory, even though different techniques were used for CBF measurement and estimation of autoregulation (Olsen et al., 1981; Heiss et al., 1992; Eames et al., 2002; Immink et al., 2005). Immink et al. demonstrated, using measurement of dynamic cerebral autoregulation, that patients with acute unilateral lacunar strokes have bilaterally impaired autoregulation, whereas, in patients with an acute middle cerebral artery territory stroke, autoregulation is affected only in the ischemic hemisphere (Immink et al., 2005). These results are consistent with the assumption that patients with acute lacunar strokes suffer from bilateral small vessel disease and are therefore at increased risk of further ischemic events. In addition, autoregulation was transiently impaired in patients who had recently suffered a transient ischemic attack, and the autoregulatory impairment outlasted the clinical symptoms (Skinhoj et al., 1970). Moreover, Olsen et al. (1983) found an impairment of autoregulation in collateral arteries supplying the penumbra in patients less than 72 h after middle cerebral artery occlusion. Remote disturbances of autoregulation in the contralateral hemisphere of two patients with subacute (< 6 days) cerebral ischemia have also been described (Paulson et al., 1972). However, these patients had space-occupying lesions and the possibility that the remote effect was due to an increase of intracranial pressure could not be ruled out.
Autoregulation is also impaired by stenosis or occlusion of cerebral arteries. Two investigations using transcranial Doppler sonography found that cerebral autoregulation is impaired in patients with critical internal carotid artery stenoses or occlusions (Gooskens et al., 2003; Reinhard et al., 2003). Using two different paradigms to assess autoregulation, both groups found a correlation between degree of stenosis and loss of autoregulation. Furthermore, both studies concluded that the impairment in autoregulation is more severe in cases with bilateral internal carotid artery stenoses or occlusions, and that the alteration also depends on the collateral supply through the circle of Willis. Furthermore, Haubrich et al. (2007) demonstrated in a cohort of elderly patients that impaired cerebral auto-regulation may recover after carotid angioplasty. Gong et al. (2006) measured autoregulation in patients with middle cerebral artery stenosis and found impaired dynamic autoregulation, which correlated with the degree of stenosis, insufficient collateralization, and the severity of ischemic stroke. Furthermore, autoregulation improved after middle cerebral artery stentingangioplasty (Gong et al., 2006). Additional evidence of autoregulatory compromise in patients with internal carotid artery or middle cerebral artery occlusion was provided by Ouchi et al. (2001) using PET to measure CBF. A change in posture from supine to sitting was used as the autoregulatory challenge. Assuming that the sitting position was associated with a reduction in CBF and an increase in oxygen extraction fraction in regions ipsilateral to the stenotic vessels provided evidence for impaired autoregulation in post-stenotic resistance vessels. Similar results were described in a patient with recurrent transient ischemic attacks who had a critical ipsilateral internal carotid artery stenosis and a contralateral internal carotid artery occlusion and in whom CBF was measured during hypotension using xenon-133 inhalation (Tatemichi et al., 1990).
In summary, cerebral ischemia in animals, as in humans, impairs cerebrovascular autoregulation. The degree of impairment depends on the magnitude of the local CBF reduction but autoregulation is also impaired in remote non-ischemic regions. Although the temporal profile of the autoregulatory impairment following ischemic stroke suggests a trend towards improvement over time, severe disturbances in auto-regulation have also been observed in chronic stroke. In addition to acute cerebral ischemia, critical stenosis of cerebral arteries is also capable of compromising cerebrovascular autoregulation. Preliminary evidence suggests that stenting or angioplasty of diseased cerebral vessels may ameliorate the autoregulatory deficit.
14.3. Cerebrovascular reactivity to hypercapnia
Arterial partial pressure of carbon dioxide (PCO2) is a potent vasoactive stimulus for the cerebral circulation. Hypercapnia produces vasodilation whereas hypocapnia produces vasoconstriction. The cerebrovascular effects of hypercapnia are thought to be mediated by the changes in perivascular pH that accompany increases in PCO2 (Kontos et al., 1977). The mechanisms of the pH effect are multifactorial and involve direct effects on smooth muscle cells via adenosine triphosphate (ATP)-sensitive K+ channels (Faraci et al., 1994), prostanoids derived from cyclooxygenase-1 (Niwa et al., 2001), and NO (Iadecola, 1992; Faraci et al., 1994; Sandor et al., 1994; Thompson et al., 1996).
14.3.1. Effect of cerebral ischemia on cerebrovascular reactivity: animal studies
The cerebrovascular reactivity to changes in PCO2 is impaired following focal or global cerebral ischemia (Table 14.3). Most studies have tested CBF reactivity to hypercapnia, while a few also examined the effects of hypocapnia (Nemoto et al., 1975; Jones et al., 1989; Christopherson et al., 1993). With permanent ischemia several studies found that CO2 reactivity is abolished in the ischemic core but is still present but reduced in the brain tissue surrounding the core area (Symon et al., 1975; Jones et al., 1989; Dettmers et al., 1993; Harris et al., 2001). However, in transient ischemia, improvements in CO2 reactivity have been reported during reperfusion (Koch et al., 1984; Schmidt-Kastner et al., 1986; Ono et al., 1997; Olah et al., 2000). With reperfusion, CO2 reactivity recovered faster in penumbral regions whereas the ischemic core showed a lack of recovery (Olah et al., 2000). However, in the penumbra CO2 reactivity, while improved, remained below normal values (Olah et al., 2000). The impairment of CBF reactivity to CO2 is present despite full recovery of energy metabolism (Olah et al., 2000). During post-ischemic hypoperfusion, most investigators found an attenuated or even abolished response to CO2 (Nemoto et al., 1975; van den Kerckhoff et al., 1983; Schmidt-Kastner et al., 1987; Leffler et al., 1989; Christopherson et al., 1993). Most investigations suggest an association between persistently impaired CO2 reactivity and irreversible brain damage (Seki et al., 1984; Schmidt-Kastner et al., 1986).
Table 14.3.
Cerebral ischemia and cerebral blood flow reactivity to carbon dioxide—selected animal studies
| Ischemia model | Species | CBF measurement | Effect on CO2 reactivity | Reference |
|---|---|---|---|---|
| Global ischemia (30 and 60 min) | Cat | 133Xe-injection | Abolished | Hossmann et al., 1973 |
| Global ischemia (15 min) | Dog | 133Xe-injection | Abolished | Nemoto et al., 1975 |
| pMCAO | Baboon | H2 clearance | Impaired, steal phenomenon | Symon et al., 1974 |
| 3 years after pMCAO | Baboon | H2 clearance | Impaired | Symon et al., 1975 |
| pMCAO | Baboon | 133Xe-injection | Paradoxical CBF increase in ischemic area during hypocapnia | Ott et al., 1975 |
| pMCAO | Cat | Heated thermocouple | Abolished or steal phenomenon | Shima et al., 1983 |
| Global ischemia (60 min) | Cat | 133Xe-injection | Abolished during postischemic hypoperfusion | Van den Kerckhoff et al., 1983 |
| Global ischemia (12 min) | Dog | Microspheres | Abolished at 3 h, restored at 24 h after ischemia | Koch et al., 1984 |
| tMCAO (30 min, 1, 2, 6 h) | Dog | H2 clearance | Impaired, dependent on duration and severity of stroke | Seki et al., 1984 |
| Global ischemia (60 min) | Cat | 133Xe-injection | Prolonged impairment | Schmidt-Kastner et al., 1986 |
| Global ischemia (60 min) | Cat | 133Xe-injection | Abolished during postischemic hypoperfusion | Schmidt-Kastner et al., 1987 |
| pMCAO | Rat | IAP | Abolished in core | Jones et al., 1989 |
| Global ischemia (20 min) | Piglet | Pial diameter | Abolished | Leffler et al., 1989 |
| Global ischemia (12 and 18 min) | Dog | Sagittal venous outflow | Impaired | Christopherson et al., 1993 |
| pMCAO | Baboon | Microspheres | Core: abolished or steal phenomenon Penumbra: impaired |
Dettmers et al., 1993 |
| p or tMCAO (30 and 90 min) | Rat | MRI (T2*, DWI) | Abolished, recovery of CO2 reactivity in viable tissue | Ono et al., 1997 |
| tMCAO (60 min) | Rat | Perfusion MRI | Prolonged impairment | Olah et al., 2000 |
| pMCAO | Rat | BOLD imaging | Core: abolished/reduced Borderzone: normal |
Harris et al., 2001 |
BOLD, blood-oxygen-level-dependent; CBF, cerebral blood flow; DWI, diffusion-weighted imaging; IAP, C14-iodoantipyrine; MRI, magnetic resonance imaging; pMCAO, permanent middle cerebral artery occlusion; tMCAO, transient middle cerebral artery occlusion.
In some cases, hypercapnia produced a paradoxical reduction in CBF (Shima et al., 1983; Ono et al., 1997; Olah et al., 2000). Such reversal of hypercapnic vasodilation to vasoconstriction has been attributed to a ‘steal’ of blood flow into neighboring areas where CO2 reactivity is retained (Symon et al., 1974). Although this phenomenon has been confirmed in some animal studies (Waltz, 1970; Paulson, 1971; Symon et al., 1971; Ott et al., 1975; Olah et al., 2000), other studies could not find evidence for an intracerebral steal in hypercapnia (Olsen et al., 1983; Jones et al., 1989) or during administration of cerebrovasodilators (Date and Hossmann, 1984; Gogolak et al., 1985). Therefore, the existence of an intracerebral steal phenomenon during hypercapnia or vasodilator administration remains controversial. Like cerebrovascular autoregulation, CBF reactivity to CO2 is also altered in regions remote from the ischemic lesion. For example, in baboons it has been shown that the CO2 reactivity 6 h after permanent middle cerebral artery occlusion is also attenuated in non-infarcted, non-penumbral tissue supplied by the affected middle cerebral artery (Dettmers et al., 1993).
14.3.2. Human studies
Consistent with findings obtained in animal studies, investigations in patients with ischemic stroke have demonstrated alterations in the cerebrovascular reactivity to changes in PCO2 (Table 14.4). Olsen et al. (1981) examined cerebrovascular reactivity to hyper- and hypocapnia in hyperemic areas of nine patients with acute ischemic stroke and found an impaired CO2 response in four of these patients. Other studies have shown a more consistent attenuation in CO2 reactivity. For example, Novak and colleagues (2003) used transcranial Doppler sonography for the analysis of CO2 reactivity in 20 patients with minor stroke and found an impaired response in the ipsilateral hemisphere.
Table 14.4.
Cerebral ischemia and cerebral blood flow reactivity to carbon dioxide—selected human studies
| Pathology | CBF measurement | Effect on CO2 reactivity | Reference |
|---|---|---|---|
| Stroke (hemispheric, VB) | 133Xe-inhalation | Impaired | Yamamoto et al., 1980 |
| Stroke within 72 h (MCA territory) | 133Xe-injection | Impaired | Olsen et al., 1981 |
| TIA or stroke (MCA territory) | 133Xe-inhalation | Impaired | Norrving et al., 1982 |
| TIA, stroke in patients with ICA stenosis/occlusion | 133Xe-inhalation | Impaired | Bullock et al., 1985 |
| Uni-/bilateral ICA stenosis occlusion (TIA, stroke, no symptoms) | 133Xe-injection | Impaired | Brown et al., 1986 |
| Hemispherical stroke/TIA (ICA occlusion), follow-up after EC/IC bypass | 133Xe-inhalation | Acetazolamide reactivity impaired in 9 of 18 patients, two patients with steal, improvement in two patients after EC/IC bypass | Vorstrup et al., 1986 |
| Stroke/TIA, patients with unilateral ICA occlusion | 133Xe-inhalation | Impaired | Clifton et al., 1988 |
| ICA stenosis occlusion (TIA, stroke, no symptoms) | PET, 133Xe-injection | Impaired, correlates with CBF/CBV ratio | Herold et al., 1988 |
| ICA/MCA stenosis occlusion, moyamoya disease | PET | Impaired, correlates with OEF | Kanno et al., 1988 |
| TIA, stroke, ICA stenosis occlusion | 133Xe-inhalation | Impaired | Keyeux et al., 1988 |
| TIA, minor stroke (ICA territory) | PET | Impaired | Levine et al., 1988 |
| Uni-/bilateral ICA occlusion (stroke, TIA, no symptoms) | TCD | Impaired | Ringelstein et al., 1988 |
| Chronic capsular stroke | 133Xe-injection | Vasoconstriction to hypocapnia attenuated in regions with diaschisis | Takano et al., 1988 |
| TIA (ICA territory) | 133Xe-inhalation | Acetazolamide reactivity impaired in 14 of 15 patients | Chollet et al., 1989 |
| TIA, severe ICA disease | 133Xe-inhalation, TCD | Impaired | Tatemichi et al., 1990 |
| TIA (ICA territory), ICA stenosis occlusion | PET | Impaired | Levine et al., 1991 |
| TIA, stroke, ICA stenosis occlusion | TCD | Impaired | Miller et al., 1992 |
| ICA stenosis occlusion (TIA, stroke, no symptoms) | TCD | CO2 and acetazolamide reactivity impaired | Ringelstein et al., 1992 |
| Stroke (ICA territory) | TCD | Impaired | Maeda et al., 1993 |
| Uni-/bilateral ICA occlusion (stroke, TIA, no symptoms) | TCD | Impaired | Ringelstein et al., 1994 |
| TIA, stroke (ICA/MCA territory, moyamoya disease) | Stable Xe-CT, PET | Acetazolamide reactivity impaired | Nariai et al., 1995 |
| TIA, stroke (MCA territory) | 133Xe-inhalation | Preserved reactivity; CO2: 23/24pts, acetazolamide: 13/24pts | Kazumata et al., 1996 |
| Stroke, TIA (ICA/MCA stenosis occlusion) | Dynamic MRI | Acetazolamide reactivity impaired in 5/8 patients | Schreiber et al., 1998 |
| CADASIL | TCD | Impaired | Pfefferkorn et al., 2001 |
| TIA (ICA territory) | TCD | Impaired | Bakker et al., 2003 |
| Lacunar stroke (MCA territory, within 1 week) | TCD | Impaired | De Leeuw et al., 2003 |
| ICA stenosis/occlusion | TCD | CO2 reactivity correlates with degree of stenosis | Gooskens et al., 2003 |
| Minor stroke (MCA territory) | TCD | Impaired | Novak et al., 2003 |
| ICA stenosis/occlusion | TCD | Impaired | Reinhard et al., 2003 |
| ICA occlusion | PET | Correlation between reduced acetazolamide reactivity and OEF increase to acetazolamide | Nemoto et al., 2007 |
CADASIL, cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy; CBF, cerebral blood flow; CBV, cerebral blood volume; CT, computed tomography; EC/IC, extracranial/intracranial; ICA, internal carotid artery; MCA, middle cerebral artery; MRI, magnetic resonance imaging; OEF, oxygen extraction fraction; PET, positron emission tomography; TCD, transcranial Doppler; TIA, transient ischemic attack; Xe, Xenon; VB, vertebrobasilar.
Simultaneous measurements of cerebrovascular autoregulation and CO2 reactivity revealed that CO2 reactivity is better preserved than autoregulation in hemodynamically compromised brain areas (Paulson et al., 1972). Thus in this study, a state of dissociated vasoparalysis with impaired autoregulation and preserved CO2 reactivity was described. The authors stated that dissociated vasoparalysis might represent a stage in a gradual change between normal vasomotor function and complete vasoparalysis (Paulson et al., 1972).
As in animal studies, remote alterations in cerebrovascular CO2 reactivity have also been found in humans. In some cases, these remote alterations could be related to the phenomenon of diaschisis (see section 14.4.1). For example, in patients with capsular infarcts the CBF response to hypocapnia was reduced and the response to hypercapnia was enhanced in a restricted ipsilateral cortical area with reduced basal CBF, presumably because of diaschisis (Takano et al., 1988). More diffuse changes in CBF reactivity to CO2 have also been reported in patients with cerebrovascular diseases. Maeda and colleagues (1993) compared CO2 reactivity in patients with cortical or lacunar infarction with those of normal subjects. Patients with cortical infarctions exhibited reduced CO2 reactivity in the ischemic hemisphere compared to normal control subjects. In contrast, patients with subcortical infarctions exhibited a diffuse attenuation in CO2 responses in both hemispheres. These findings have been confirmed more recently (de Leeuw et al., 2003). Based on the finding of a reduced CO2 response in the non-ischemic hemisphere also, it was concluded that this impaired CO2 reactivity is a marker of generalized small-vessel disease. Another example of generalized impaired CO2 reactivity was provided by Yamamoto and colleagues (1980). In this study, the hemispheric response to hypercapnia was significantly reduced in patients with hemispheric infarction as well as in patients with vertebrobasilar ischemia. Similarly, Levine et al. (1988) demonstrated reduced CO2 reactivity in the ischemic hemisphere of patients with transient ischemic attack or minor ischemic stroke, as well as in the nonischemic hemisphere of stroke patients. The influence of generalized pathological alterations in the cerebral vasculature on the CBF reactivity to CO2 has also been demonstrated in patients with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) in whom a profound attenuation was reported (Pfefferkorn et al., 2001). These findings underline the importance of the functional state of cerebral blood vessels in the mechanisms of the vascular reactivity to CO2.
As noted for cerebrovascular autoregulation, CBF reactivity to CO2 is reduced in patients with stenosis or occlusion of one or more large cerebral arteries (Clifton et al., 1988; Keyeux et al., 1988; Tatemichi et al., 1990; Miller et al., 1992; Bakker et al., 2003). Several studies found a correlation between the degree of internal carotid artery stenosis, the number of affected arteries, and the impairment in CO2 reactivity (Brown et al., 1986; Levine et al., 1991; Gooskens et al., 2003; Reinhard et al., 2003). Furthermore, some studies considered the degree of collateral circulation to be an important factor for the preservation of CO2 reactivity. Accordingly, it was found that the CO2 reactivity is more impaired in patients with poor collateral blood supply (Norrving et al., 1982; Bullock et al., 1985; Ringelstein et al., 1988, 1994). Two PET studies correlated CO2 reactivity with CBF, cerebral blood volume (CBV) and oxygen extraction fraction in patients with occlusive carotid artery disease. In one study, a positive correlation between the CBF/CBV ratio and CO2 reactivity was found, while oxygen extraction fraction was negatively correlated with CO2 reactivity in most patients (Herold et al., 1988). In the other study, a negative correlation between oxygen extraction fraction and hypercapnic response was observed, a finding suggesting a relationship between reduced vasodilatory capacity of cerebral arteries and increase in oxygen extraction fraction. Using this correlation, Kanno et al. (1988) defined the oxygen extraction fraction value that corresponds to exhausted vasodilatatory capacity in cerebral arteries. Interestingly, this value coincided with the upper limit of the oxygen extraction fraction measured in normal tissue. The robust correlation between CBF response to CO2 and oxygen extraction fraction indicates that CO2 reactivity is a suitable method for assessing the cerebrovascular and metabolic reserves of the hemodynamically compromised brain (Herold et al., 1988; Kanno et al., 1988).
An alternative approach to assess cerebrovascular reactivity in humans is the intravenous injection of acetazolamide, a carbonic anhydrase inhibitor. Administration of acetazolamide produces a gradual decline in brain pH (Heuser et al., 1975). Acidosis is a potent stimulus for cerebral arteries to dilate. Considering that the cerebrovascular effects of arterial PCO2 are mediated by the changes in perivascular pH (Kontos et al., 1977), the acetazolamide test simulates the cerebrovascular vasodilation induced by hypercapnia. Unlike CO2, assessing cerebrovascular reactivity with acetazolamide does not require the assistance of the patient, which is an advantage in acutely ill stroke patients who might be unable to cooperate. In general, CO2 and acetazolamide responses show similar results in patients with cerebrovascular diseases (Ringelstein et al., 1992). The CBF reactivity to acetazolamide is impaired in patients with transient ischemic attack (Chollet et al., 1989), occlusive internal carotid artery disease (Schreiber et al., 1998), and acute and chronic cerebral ischemic disease (Vorstrup et al., 1986; Nariai et al., 1995). Nemoto et al. (2007) published a PET study in which the relationship between the cerebrovascular acetazolamide reserve and the oxygen extraction fraction reactivity to acetazolamide was analyzed in patients with symptomatic internal carotid artery occlusion. The authors found a close correlation between hemispheric cerebrovascular reserve and oxygen extraction fraction reactivity. They suggest that the oxygen extraction fraction reactivity to acetazolamide may be a valuable measure of hemodynamic compromise (Nemoto et al., 2007). However, by comparing acetazolamide and hypercapnic challenges, some studies found differences in the cerebrovascular reactivity to acetazolamide and hypercapnia in patients with cerebrovascular occlusive diseases (Kazumata et al., 1996). Therefore, the CBF reactivity to CO2 and acetazolamide cannot always be considered to be identical.
In summary, cerebral ischemia impairs the reactivity of CBF to CO2, and the degree of impairment is related to the magnitude of the local CBF reduction. CO2 reactivity recovers after reperfusion in areas that are not damaged. Following focal cerebral ischemia, CO2 reactivity is reduced in non-ischemic areas, suggesting a remote effect leading to impairment even in the normally perfused brain. Moreover, CO2 reactivity is globally impaired in patients with diffuse microvascular disease. Because of its close correlation with indices of brain oxygen metabolism, cerebrovascular CO2 reactivity may be a valuable tool to assess the vascular and metabolic reserves of the hemodynamically compromised brain.
14.4. Neurovascular coupling
There is substantial evidence that neural activity is a major factor controlling CBF. In the resting brain, there is a general correspondence between the flow of a given brain region and its rate of cerebral glucose utilization, a variable reflecting neural activity (Reivich, 1974; Sokoloff et al., 1977; Kuschinsky, 1987; Iadecola, 1993). Thus, regions with low glucose utilization, such as the white matter, have low flow, whereas regions with high glucose use, such as the auditory cortex, have high flow (Kuschinsky, 1987). In addition, when the activity of the brain is enhanced, either focally or globally, local blood flow increases in proportion to the intensity of the activation (Raichle et al., 1975; Greenberg et al., 1979; Tsubokawa et al., 1980; Iadecola et al., 1983; Fox and Raichle, 1984; Frostig et al., 1990). Conversely, if brain activity is decreased CBF decreases proportionally (Nilsson et al., 1978; Ueki et al., 1988). The close correspondence between CBF and neural activity reflects a homeostatic mechanism by which the delivery of substrates through blood flow, as well as the removal of metabolites produced by brain activity, are coupled to the energy demands of neurons and glia. Neuronal, glial, and vascular factors, acting in concert, mediate the increase in CBF produced by neural activity (Zonta et al., 2003; Cauli et al., 2004; Mulligan and MacVicar, 2004; Takano et al., 2006; Iadecola and Nedergaard, 2007). Neurons and astrocytes release vasoactive agents, such as ions (K+, H+), neurotransmitters (acetylcholine, vasoactive intestinal polypeptide, etc.), and neuromodulators (adenosine, nitric oxide, prostanoids, p450 metabolites) that act on local vessels to initiate the response (Iadecola, 1993; Iadecola and Nedergaard, 2007). Vascular cells, including endothelial cells, pericytes, and smooth muscle cells, transduce these neuronal and glial signals into changes in vascular resistance that ultimately mediate the increases in CBF (Iadecola and Nedergaard, 2007).
14.4.1. Effect of ischemia on neurovascular coupling: animal studies
Cerebral ischemia attenuates the CBF increase produced by functional activation (Table 14.5). Some studies have focused on the effect of focal or global ischemia on the coupling between CBF and cerebral glucose utilization in the resting state, while other studies have focused on the increase in CBF induced by brain activity. For example, Ginsberg et al. (1989) found reduced resting CBF and cerebral glucose utilization in the left barrel-field cortex and other left cortical regions in rats with a left frontal photothrombotic ischemia. Moreover, following contralateral vibrissa stimulation a marked suppression of the increase in CBF and cerebral glucose utilization produced by activation was observed throughout the barrel-field cortex (Ginsberg et al., 1989). In support of these findings, Kunz et al. (2007b) showed that the increase in CBF produced by whisker stimulation is markedly attenuated 1 h after transient focal cerebral ischemia in mice. Similarly, permanent middle cerebral artery occlusion attenuated the increase in CBF and estimated CMRO2 produced by somatosensory activation in rats, in which CBF was measured by magnetic resonance imaging (MRI) with arterial spin labeling (Shen et al., 2005). However, the increases in CBF and CMRO2 produced by somatosensory activation were not attenuated 30 min after middle cerebral artery occlusion lasting 15 min, an ischemic challenge that did not lead to brain injury (Shen et al., 2005). Therefore, the alterations in neurovascular coupling are graded according to the intensity of the ischemic insult.
Table 14.5.
Cerebral ischemia and neurovascular coupling—selected animal studies
| Ischemia model | Species | CBF measurement | Effect on CO2 reactivity | Reference |
|---|---|---|---|---|
| Global ischemia (10 min) | Rat | Mitochondrial redox state | Transient mitochondrial dysfunction | Duckrow et al., 1981 |
| Global ischemia (30 min) | Rat | 2-DG | Transient reduction of CGU | Dietrich et al., 1986 |
| Photothrombotic frontal lesion | Rat | IAP, 2-DG | 5 days post-lesion: activation of rCBF in barrel cortex suppressed | Ginsberg et al., 1989 |
| Global ischemia (30 min) | Rat | IAP, 2-DG | Impaired neurovascular coupling | Ueki et al., 1988 |
| Focal ischemia (30 min) | Mouse | LDF | Impaired neurovascular coupling | Kunz et al., 2007b |
2-DG, 2-deoxyglucose; CGU, cerebral glucose utilization; IAP, C14-iodoantipyrine; LDF, laser-Doppler flowmetry; rCBF, regional cerebral blood flow.
Duckrow et al. (1981) examined the effect of transient global ischemia on the activation-induced changes in the redox state of cytochrome a,a3 in the rat cerebral cortex. Before ischemia, focal electrical stimulation of the cerebral cortex increased the oxidation of cytochrome a,a3, and increased local blood volume, a parameter reflecting stimulus-induced hyperemia. After ischemia, the amplitude of the oxidative response and of the blood volume increase was reduced up to 30 min after ischemia. Also, the length of time required for the oxidative response to return to baseline was increased, despite full recovery of the mitochondrial baseline redox state. These observations provide evidence that increasing the work of the post-ischemic cortex unveils metabolic abnormalities that cannot be appreciated in the resting state. Another study investigated the correlation between hemodynamic parameters and field potentials evoked by somatosensory activation after transient global ischemia in the rat (Ueki et al., 1988). In the normal state, somatosensory stimulation increased CBF, cerebral glucose utilization, and local brain lactate concentration. By 3 h after global ischemia, the increases in CBF were suppressed while somatosensory evoked potentials had already reappeared (Ueki et al., 1988). No local changes in brain pH, lactate, glucose, and ATP were observed. Therefore, after global ischemia, both CBF and metabolic responses are depressed, despite recovery of somatosensory evoked potentials. The data suggest that, after ischemia, the metabolic workload of the cortex during functional activation is reduced and does not require increased delivery of substrates via an increase in CBF. The findings also indicate that ischemia disrupts the coupling between synaptic activity and CBF/cerebral glucose utilization, which may have long-term consequences for brain function.
Dietrich et al. (1986) investigated the time course of the ischemia-induced attenuation of the cerebral glucose utilization increase evoked by stimulation of the facial whiskers in the rat. They found that, 1 day after transient global ischemia, metabolic responses are severely depressed in the neocortex and thalamus but not in the trigeminal complex. Resting cerebral glucose utilization in the cortical barrel field and in the ventrobasal thalamus were also decreased. However, the activation-induced increase in cerebral glucose utilization returned to baseline 5 days after the ischemic insult. At this time, the spatial pattern of activation within the cortical barrel field was different from that of normal rats, suggesting that different local pathways were used. These observations support the idea that ischemia induces reorganization of cortical circuits in an attempt to compensate for the neuronal dysfunction and loss (Nudo, 2003). Alterations in regional blood flow and metabolism have also been described in brain areas remote from the ischemic lesion. These alterations are probably related to the concept of diaschisis (von Monakow, 1914), a term indicating brain dysfunction in areas remote from the ischemic regions, crossed cerebellar diaschisis being a typical example (Feeney and Baron, 1986). Gold and Lauritzen (2002) examined the neurophysiological basis of crossed cerebellar diaschisis in rat. Inactivation of the cerebral cortex by focal ischemia, spreading depression, or topical application of the Na+ channel blocker tetrodotoxin reduced contralateral Purkinje cells’ spiking activity and cerebellar blood flow (Gold and Lauritzen, 2002). These cerebellar alterations, which represent the neurophysiological basis of crossed cerebellar diaschisis, were not related to reductions in the excitability of Purkinje cells or cerebellar vascular reactivity. Thus, crossed cerebellar diaschisis is mediated by deactivation of the cerebellar cortex resulting from interruption of neocortical excitatory inputs (Gold and Lauritzen, 2002). Therefore, the remote changes in brain function produced by cerebral ischemia are likely to result from deafferentation rather than intrinsic changes in the neural circuitry or blood vessels of the remote region.
14.4.2. Human studies
Although several investigations have addressed the effects of ischemic stroke on neurovascular coupling, most of these studies have been performed in patients with chronic stroke (Table 14.6). Inao et al. (1998) used PET to examine patients with minor strokes and critical ipsilateral internal carotid–middle cerebral artery stenosis or occlusion. Despite an impaired ipsi-lateral acetazolamide response, the authors found a nearly normal CBF response to neural activation. Based on these results, it can be hypothesized that CBF response to neural activity is more robust than CBF reactivity to CO2. Unfortunately, the authors only tested the acetazolamide response and not the CO2 response or cerebrovascular autoregulation. Yamauchi et al. (2005) examined CBF responses in the primary visual cortex during visual stimulation in patients with internal carotid artery stenoses or occlusions using PET. Visual stimulation increased CBF in the primary visual cortex ipsilateral to the internal carotid artery lesion. However, the CBF increase in the surrounding regions was significantly reduced compared to the non-affected hemisphere. This finding raises the possibility that the local hemodynamic response evoked by visual stimulation might occur at the expense of perfusion of the surrounding region in brain areas with an already compromised vascular reactivity (Yamauchi et al., 2005).
Table 14.6.
Cerebral ischemia and functional activation—selected human studies
| Pathology | CBF measurement | Effect on functional activation | Reference |
|---|---|---|---|
| TIA, stroke (ICA territory) | PET | Motor activation: ipsilateral CBF increase Acetazolamide: no CBF increase |
Inao et al., 1998 |
| Stroke (MCA territory) | NIRS | Increased deoxyhemoglobin (five of ten patients) | Sakatani et al., 1998 |
| ICA stenosis/occlusion | BOLD-fMRI | Reduced BOLD response in auditory cortex if ischemic borderzone lesions present | Bilecen et al., 2002 |
| ICA/MCA stenosis/occlusion | BOLD-fMRI | Reduced BOLD response ipsilateral | Carusone et al., 2002 |
| TIA, stroke (within 3–12 months) | BOLD-fMRI, NIRS | Reduced BOLD response ipsilaterally, increased deoxyhemoglobin | Murata et al., 2002 |
| Lacunar stroke | BOLD-fMRI | Reduced BOLD response (ipsi-/contralateral) | Pineiro et al., 2002 |
| TIA (ICA territory), ICA occlusion | BOLD-fMRI | Negative BOLD response in a patient with impaired CO2 reactivity | Rother et al., 2002 |
| TIA (stenosis extra-/intracranial) | BOLD-fMRI | Reduced BOLD response in patients with impaired CO2 reactivity | Hamzei et al., 2003 |
| Stroke, TIA (ICA territory) | BOLD-fMRI, NIRS | BOLD: limited activation, NIRS: sustained increase in deoxyhemoglobin | Sakatani et al., 2003 |
| TIA, stroke (ICA territory) | MEG, evoked field BOLD-fMRI | VMR preserved, BOLD absent | Rossini et al., 2004 |
| Stroke (frontal lobe) | BOLD-fMRI | Reduced BOLD response on lesion side | Krainik et al., 2005 |
| ICA stenosis/occlusion (stroke, TIA, no symptoms) | PET | Increased CBF in primary visual cortex, decreased CBF in surrounding areas | Yamauchi et al., 2005 |
| TIA, stroke | BOLD-fMRI, NIRS | Reduced BOLD response and increased deoxyhemoglobin after severe ischemia | Murata et al., 2006 |
BOLD, blood-oxygen-level-dependent; CBF, cerebral blood flow; fMRI, functional magnetic resonance imaging; ICA, internal carotid artery; MCA, middle cerebral artery; MEG, magnetoencephalography; NIRS, near-infrared spectroscopy; PET, positron emission tomography; TIA, transitory ischemic attack; VMR, vasomotor reserve.
Blood-oxygen-level-dependent (BOLD) functional MRI (fMRI) is a powerful tool for studying functional activation in humans. BOLD-fMRI is based on the paramagnetic property of deoxyhemoglobin. During functional activation, a local decrease in the concentration of deoxyhemoglobin is observed (Ogawa et al., 1990). This decrease in deoxyhemoglobin gives rise to the BOLD signal, which reflects the interplay between changes in CBF, CBV, and CMRO2 during functional activation (Dirnagl et al., 2002). A few studies have examined functional activation using BOLD-fMRI in ischemic stroke patients. Krainik et al. (2005) investigated BOLD signal changes induced by manual tasks in patients who had fully recovered from stroke in the frontal lobe. The authors found that the BOLD signal changes in the sensorimotor cortex and supplementary motor area of the lesioned hemisphere was decreased despite the fact that these regions were anatomically intact. Interestingly, an impaired BOLD response to hypocapnia was a predictor of impaired BOLD response to functional activation as well. Based on these findings, the authors could provide a relationship between a reduced CO2 reactivity in brain areas surrounding the ischemic infarct and an impaired neurovascular coupling. Similar findings were described in a population of patients with a lacunar stroke (Pineiro et al., 2002). These patients exhibited a lower BOLD signal increase and a lower rate of rise of the BOLD signal during a sequential finger-tapping task performed contralaterally to the ischemic lesion. These changes were also found in the non-lesioned hemisphere for the same task performed with the corresponding hand. Unfortunately, CBF reactivity to CO2 was not investigated in these patients. Since CO2 reactivity is globally impaired in patients with lacunar stroke (Maeda et al., 1993; de Leeuw et al., 2003), it can be assumed that cerebral small-vessel disease is a major factor responsible for the impairment of both CO2 reactivity and neurovascular coupling. Also, Sakatani et al. (2003) described only limited activation areas with BOLD-fMRI in the sensorimotor cortex on the lesion side in ischemic stroke patients performing a motor task. In addition to BOLD-fMRI measurements, the authors performed near-infrared spectroscopy (NIRS) during the motor tasks, and they were able to measure the local changes in deoxyhemoglobin, oxyhemoglobin, and total hemoglobin. During the hand grasping task, increases in the focal concentrations of oxyand total hemoglobin were observed in the sensorimotor cortex, reflecting local increases in CBF. Also, a sustained increase in the concentration of deoxyhemoglobin was observed throughout the task in stroke patients, whereas a decrease was observed in control subjects. The increased deoxyhemoglobin signal measured by NIRS in stroke patients was accompanied by reduced BOLD signal activation in sensorimotor cortex (Sakatani et al., 2003; Murata et al., 2006). Similar changes in NIRS signals were also found in the left prefrontal cortex of aphasic stroke patients (Sakatani et al., 1998). The mechanism of the increase in deoxyhemoglobin in stroke patients remains unclear, although the possibility of a more pronounced increase in oxygen consumption by neuronal activity in patients with ischemic stroke has been considered (Sakatani et al., 2003).
The relationship between BOLD-fMRI and neural activity after stroke was examined more directly by Rossini et al. (2004). These investigators studied neural activity detected by magnetoencephalography (MEG) and BOLD-fMRI in patients with a history of stroke or transient ischemic attack, using median nerve stimulation to activate the somatosensory cortex. While MEG signals were detected in the affected and the unaffected hemispheres, the corresponding BOLD signal was not observed. Such uncoupling between neuro-physiological responses and BOLD signal was related to a reduced vasomotor reactivity as assessed by transcranial Doppler sonography during CO2 inhalation. Therefore, even though CO2 reactivity and functional hyperemia are impaired in stroke patients, the neural response evoked by the activation is still preserved (Rossini et al., 2004). Similar findings were described in a patient with bilateral internal carotid artery occlusions and unilateral vertebral artery occlusion (Rother et al., 2002).
In general, the results with BOLD-fMRI in patients with stroke differ from those with PET in that a reduction in the BOLD response to activation was usually found. Changes in regional CBF evoked by neural activation measured by PET are likely to reflect more precisely the hemodynamic changes. This is not surprising considering that the BOLD response is an indirect measure of CBF and also reflects CBV and CMRO2 (Dirnagl et al., 2002; Ugurbil et al., 2003). This is especially true after stroke, where the normal relationship between CBF, CBV, and CMRO2 may be altered by the disease process (Sakatani et al., 2003). Therefore, observations with fMRI need to be interpreted with caution because the BOLD signal may not reliably reflect CBF in the injured brain (Sakatani et al., 2003).
A few studies investigated BOLD responses in patients with stenosis or occlusion of major cerebral arteries. Bilecen et al. (2002) performed fMRI of the auditory cortex during auditory stimulation (1000 Hz, sine-tone) in acute stroke patients with unilateral internal carotid artery stenosis or occlusion and found a reduced BOLD response only in patients with ischemic lesions in the watershed areas between the anterior and middle or posterior and middle cerebral arteries. Patients without cerebral lesions or internal carotid artery disease had symmetric responses. The authors concluded that the presence of borderzone lesions in patients with reduced BOLD responses suggests that internal carotid artery stenosis is hemodynamically significant and contributes to the reduced BOLD response (Bilecen et al., 2002). Reduced or negative BOLD effects in patients with occlusive diseases of major cerebral arteries and without ischemic stroke were also described (Carusone et al., 2002; Hamzei et al., 2003). The negative BOLD effect is difficult to interpret and could reflect a more pronounced increase in CMRO2 compared to CBF, as suggested by the observation that deoxyhemoglobin is increased in patients with stroke during activation (Sakatani et al., 2003).
In summary, acute cerebral ischemia in animals results in an attenuation of the CBF and metabolic responses induced by neural activation. Most studies in humans have been performed in patients with chronic stroke. While in PET and NIRS studies the increases in local CBF and metabolism evoked by activation were found to not be impaired, in studies using BOLD-fMRI an impaired cerebrovascular response to neural activation was found, often coupled to a reduction in CO2 reactivity. In addition, alterations in neurovascular coupling are also present in patients with generalized impairment of cerebrovascular reactivity due to cerebral small-vessel disease or occlusive disease of major cerebral arteries. Although BOLD-fMRI seems to be more sensitive to the neurovascular disturbance induced by stroke, the basic mechanisms of the BOLD response in the ischemic brain have not been completely elucidated and BOLD-fMRI findings need to be interpreted with caution.
14.5. Role of reactive oxygen species in the cerebrovascular dysregulation following cerebral ischemia
There is accumulating evidence that reactive oxygen species are a major contributor to the disturbances in the regulation of the cerebral vasculature following ischemic brain injury. Reactive oxygen species are molecules that often have an unpaired electron in the outer orbital ring. Cerebral ischemia results in an overproduction of reactive oxygen species, which overwhelms endogenous antioxidant enzymes (Traystman et al., 1991; Chan, 2001). The production of reactive oxygen species following cerebral ischemia has been demonstrated in several animal studies (Armstead et al., 1988; Cao et al., 1988; Kontos et al., 1992; Zhang and Piantadosi, 1994; Globus et al., 1995; Kil et al., 1996; Piantadosi and Zhang, 1996; Chan et al., 1998; Murakami et al., 1998; Kawase et al., 1999; Kim et al., 2000, 2001, 2002; Kunz et al., 2007a). A peak in reactive oxygen species production occurs in the early reperfusion period after global ischemia in cats (Nelson et al., 1992), raising the possibility that reperfusion provides the oxygen needed for the formation of reactive oxygen species. Similarly, in transient focal cerebral ischemia in mice, reactive oxygen species production exhibits a peak at 2 h after reperfusion and at 72 h as well (Kunz et al., 2007a). The late post-ischemic increase in reactive oxygen species formation may reflect a radical produced by inflammatory cells infiltrating the ischemic brain through the enzyme NADPH oxidase (Kunz et al., 2007a). However, reactive oxygen species production has also been reported to occur before reperfusion (Liu et al., 2003), indicating that reestablishment of CBF is not needed to provide the oxygen for the formation of reactive oxygen species.
14.5.1. Reactive oxygen species and ischemic cerebrovascular dysregulation
Reactive oxygen species, including superoxide, hydrogen peroxide, and peroxynitrite, are potent dilators of cerebral arterioles and may play a role in normal cerebrovascular regulation (Kontos, 2001; Niwa et al., 2001). However, higher concentrations produce vasoconstriction and disrupt cerebrovascular regulation (Traystman et al., 1991; Kontos, 2001; Iadecola, 2004; Faraci, 2005). There is growing evidence that the cerebrovascular dysregulation associated with several brain diseases is mediated by reactive oxygen species (Iadecola, 2004; Faraci, 2005; Girouard and Iadecola, 2006). For example, the cerebrovascular dys-regulation that occurs in Alzheimer's disease, diabetes, hyperhomocystinemia, and angiotensin-II-induced hypertension is mediated by reactive oxygen species (Mayhan and Sharpe, 1998; Zhang et al., 1998; Iadecola et al., 1999; Didion and Faraci, 2003; Kazama et al., 2004; Park et al., 2004, 2005; Girouard et al., 2006).
The impaired cerebrovascular regulation observed after cerebral ischemia is also related to vascular oxidative stress. Cerebral ischemia induces markers of oxidative damage in cerebral blood vessels (Chan et al., 1998; Maneen et al., 2006). The cerebrovascular response to acetylcholine, which is mediated by endothelial NO, is abolished following transient global cerebral ischemia in cats (Nelson et al., 1992) and is prevented by the superoxide scavengers superoxide dismutase and catalase. Additionally, the alterations in CO2 reactivity are prevented by the administration of deferoxamine, an iron scavenger, suggesting a role also for the hydroxyl radical in cerebrovascular dysregulation after transient ischemia (Nelson et al., 1992). Free radical scavengers prevent the attenuation in the cerebrovascular response to NMDA induced by global ischemia in piglets (Bari et al., 1996). Furthermore, intravenous administration of polyethylene-glycol-conjugated superoxide dismutase improves post-ischemic hypercapnic CBF after global cerebral ischemia in piglets (Kirsch et al., 1993). In a global ischemia model in dogs, combined administration of superoxide dismutase and deferoxamine reduced post-ischemic hypoperfusion and enhanced recovery of evoked potentials (Cerchiari et al., 1987). In the isolated rat middle cerebral artery, studied after ischemia reperfusion, the attenuation in the smooth muscle relaxation produced by activation of Kir2.x, a barium-sensitive inward rectifier K+ channel, is counteracted by a free radical scavenger (Petrault et al., 2004). Interestingly, in this model, the ischemia-induced attenuation in endothelium-dependent vasodilation to acetylcholine was not normalized by the free radical scavenger (Petrault et al., 2004), suggesting that factors other than reactive oxygen species may also be involved. Similar results were obtained in rodents with topical cortical application of acetylcholine in situ (Rosenblum and Wormley, 1995; Rosenblum, 1997). Subsequent experiments demonstrated that the endothelium-dependent dysfunction, but not the Kir2.x dysfunction, was ameliorated by inducing neutropenia prior to ischemia, implicating neutrophils in mechanisms of the vascular dysregulation, possibly through metalloprotease-induced disruption of microvessels (Petrault et al., 2004). Supporting a role of neutrophils, activation of intravascular neutrophils induces endothelial cell dysfunction in rabbits (Akopov et al., 1994). Therefore, although reactive oxygen species are important pathogenic effectors in the cerebrovascular dysregulation induced by ischemia, loss of microvascular integrity through metalloprotease activation, disruption of the extracellular matrix, and loss of integrins is also likely to play a role (del Zoppo and Mabuchi, 2003).
14.5.1.1. NADPH oxidase as a potential source of reactive oxygen species mediating cerebrovascular dysfunction
There are several cellular and enzymatic sources of reactive oxygen species in the ischemic brain (Traystman et al., 1991; Chan, 2001). However, because reactive oxygen species diffuse poorly and are highly reactive, they tend to act at the site of formation (Stamler, 1996; Halliwell and Whiteman, 2004). It is therefore likely that the reactive oxygen species responsible for the cerebrovascular dysfunction are produced in cerebrovascular cells. The enzyme NADPH oxidase has recently emerged as a major source of reactive oxygen species in cerebral blood vessels (Cai et al., 2003). NADPH oxidase is a multi-unit enzyme initially described in phagocytic cells and responsible for reactive oxygen species production in leukocytes and macrophages (Babior, 2004; Lambeth, 2004). NADPH oxidase is composed of membrane-bound subunits (Nox and p22phox) and cytoplasmic subunits (p40phox, p47phox, p67phox, Rac1 and Rac2) (Lambeth, 2004). Activation stimuli lead to phosphorylation of p47phox, which results in the assembly of the enzyme and production of superoxide (Vignais, 2002). NADPH oxidase is involved in production of reactive oxygen species and cerebrovascular dysfunction in several brain conditions associated with vascular oxidative stress, including models of Alzheimer's disease (Park et al., 2005), and with the cerebrovascular complications of angiotensin II hypertension, diabetes, and hyperhomocystinemia (Cooper et al., 2002; Faraci and Lentz, 2004; Kazama et al., 2004; Girouard et al., 2006). Mice lacking the Nox2 subunit of NADPH oxidase have reduced ischemic brain injury (Walder et al., 1997) and reduced reactive oxygen species production 72 h after middle cerebral artery occlusion (Kunz et al., 2007a) but it has not been established whether vascular factors contribute to the protection. Therefore additional studies are needed to clarify the role of NADPH oxidase in the mechanisms of the cerebrovascular dysregulation associated with cerebral ischemia.
14.5.1.2. Mechanisms by which reactive oxygen species mediate cerebrovascular dysfunction
The mechanisms by which reactive oxygen species disrupt cerebrovascular regulation are diverse (Iadecola, 2004; Faraci, 2006). One mechanism is based the interaction of superoxide with NO. NO reacts with superoxide highly efficiently, forming peroxynitrite (Beckman et al., 1990; Pacher et al., 2007). Recent studies implicate peroxynitrite in the cerebrovascular dys-function induced by ischemia. Transient ischemia with reperfusion reduces the ability of the middle cerebral artery to constrict in response to increased intramural pressure (myogenic tone) (Cipolla et al., 1997). Such reperfusion-induced reduction in myogenic tone can account for the profound disruption of autoregulation observed after focal cerebral ischemia (see section 14.2). The effect was more pronounced for longer duration of reperfusion and was related to depolymerization of the vascular smooth muscle protein F-actin (Maneen et al., 2006). The loss of myogenic tone was associated with an increase in the peroxynitrite marker nitrotyrosine and could be reproduced by exogenous peroxynitrite (Maneen et al., 2006). In addition to its actions on myogenic tone, peroxynitrite has other deleterious effects on cerebrovascular regulation. First, peroxynitrite formation reduces the bioavailability of NO, resulting in an impairment of NO-dependent vasodilation (Adachi et al., 2004; Faraci, 2005). Peroxynitrite inhibits the enzyme prostacyclin synthase and reduces the ability of cerebral arteries to dilate through impaired synthesis of the potent vasodilator prostacyclin (Zou et al., 1999). Peroxynitrite also inhibits the mitochondrial isoform of superoxide dismutase, enhancing ischemia-induced oxidative stress (Guo et al., 2003). Furthermore, peroxynitrite induces DNA strand breaks, which activate the DNA repair enzyme poly-ADP-ribose polymerase and lead to endothelial cell dysfunction through energy depletion (Soriano et al., 2001). Reactive oxygen species and peroxynitrite oxidize tetrahydrobiopterin, a cofactor for the enzymatic activity of nitric oxide synthase. Deficiency of tetrahydrobiopterin results in uncoupling of nitric oxide synthase, a process by which this enzyme produces superoxide instead of NO and increases oxidative stress (Katusic, 2001). However, peroxynitrite is not uniformly deleterious and can also be beneficial in certain settings. For example, low levels of peroxynitrite are required for the neuroprotection induced by preconditioning with the proinflammatory mediator lipopolysaccharide (Kunz et al., 2007b). However, relatively large amounts of peroxynitrite produced during ischemia–reperfusion are likely to be damaging to the brain and blood vessels.
In summary, reactive oxygen species can alter post-ischemic cerebrovascular regulation through numerous factors, including depletion of vasodilators (NO, prostacyclin), enhanced oxidative stress and energy deplede tion. However, reactive oxygen species are not the only factor responsible for the cerebrovascular dys-function and loss of microvascular integrity through disruption of extracellular matrix and integrins is also likely to play a role.
14.6. Conclusions
Cerebral ischemia results in a profound disruption of cerebrovascular regulatory mechanisms that assure that the brain is adequately perfused. Cerebral ischemia impairs cerebrovascular autoregulation, attenuates functional hyperemia, and alters hypercapnic vasodilation, critical mechanisms by which active brain cells maintain the homeostasis of their microenvironment through delivery of nutrients and removal of waste. These alterations are in large part mediated by a surge of free radicals, which leads to neurovascular oxidative stress and impairs the function of neurons, glia, and vascular cells. These alterations in vascular regulation render the brain more vulnerable to the changes in perfusion pressure and to the metabolic dysfunction induced by ischemia, thereby increasing the susceptibility of the tissue to injury. Therapeutic strategies targeting the cerebrovascular dysfunction induced by ischemia offer the opportunity to preserve post-ischemic vascular regulation and improve the outcome of cerebral ischemia. Such a vasoprotective approach, in combination with cytoprotective therapies, may open new avenues for the treatment of ischemic stroke.
References
- Aaslid R, Lindegaard KF, Sorteberg W, et al. Cerebral autoregulation dynamics in humans. Stroke. 1989;20:45–52. doi: 10.1161/01.str.20.1.45. [DOI] [PubMed] [Google Scholar]
- Adachi T, Weisbrod RM, Pimentel DR, et al. S-glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat Med. 2004;10:1200–1207. doi: 10.1038/nm1119. [DOI] [PubMed] [Google Scholar]
- Akopov SE, Sercombe R, Seylaz J. Endothelial dysfunction in cerebral vessels following carotid artery infusion of phorbol ester in rabbits: the role of polymorphonuclear leukocytes. J Cereb Blood Flow Metab. 1994;14:1078–1087. doi: 10.1038/jcbfm.1994.141. [DOI] [PubMed] [Google Scholar]
- Armstead WM, Mirro R, Busija DW, et al. Post-ischemic generation of superoxide anion by newborn pig brain. Am J Physiol. 1988;255:H401–H403. doi: 10.1152/ajpheart.1988.255.2.H401. [DOI] [PubMed] [Google Scholar]
- Astrup J, Siesjo BK, Symon L. Thresholds in cerebral ischemia—the ischemic penumbra. Stroke. 1981;12:723–725. doi: 10.1161/01.str.12.6.723. [DOI] [PubMed] [Google Scholar]
- Babior BM. NADPH oxidase. Curr Opin Immunol. 2004;16:42–47. doi: 10.1016/j.coi.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Bakker FC, Klijn CJ, Jennekens-Schinkel A, et al. Cognitive impairment in patients with carotid artery occlusion and ipsilateral transient ischemic attacks. J Neurol. 2003;250:1340–1347. doi: 10.1007/s00415-003-0222-1. [DOI] [PubMed] [Google Scholar]
- Bari F, Errico RA, Louis TM, et al. Differential effects of short-term hypoxia and hypercapnia on N-methyl-D-aspartate-induced cerebral vasodilatation in pig-lets. Stroke. 1996;27:1634–1639. doi: 10.1161/01.str.27.9.1634. discussion 1639–1640. [DOI] [PubMed] [Google Scholar]
- Baron JC, Frackowiak RS, Herholz K, et al. Use of PET methods for measurement of cerebral energy metabolism and hemodynamics in cerebrovascular disease. J Cereb Blood Flow Metab. 1989;9:723–742. doi: 10.1038/jcbfm.1989.105. [DOI] [PubMed] [Google Scholar]
- Bayliss WM. On the local reactions of the arterial wall to changes of internal pressure. J Physiol. 1902;28:220–231. doi: 10.1113/jphysiol.1902.sp000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman JS, Beckman TW, Chen J, et al. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berne RM, Rubio R, Curnish RR. Release of adenosine from ischemic brain: effect on cerebral vascular resistance and incorporation into adenine nucleotides. Circ Res. 1974;35:262–271. [Google Scholar]
- Bilecen D, Radu EW, Schulte AC, et al. fMRI of the auditory cortex in patients with unilateral carotid artery steno-occlusive disease. J Magn Reson Imaging. 2002;15:621–627. doi: 10.1002/jmri.10117. [DOI] [PubMed] [Google Scholar]
- Brown MM, Wade JP, Bishop CC, et al. Reactivity of the cerebral circulation in patients with carotid occlusion. J Neurol Neurosurg Psychiatry. 1986;49:899–904. doi: 10.1136/jnnp.49.8.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock R, Mendelow AD, Bone I, et al. Cerebral blood flow and CO2 responsiveness as an indicator of collateral reserve capacity in patients with carotid arterial disease. Br J Surg. 1985;72:348–351. doi: 10.1002/bjs.1800720506. [DOI] [PubMed] [Google Scholar]
- Busija DW, Heistad DD. Factors involved in the physiological regulation of the cerebral circulation. Rev Physiol Biochem Pharmacol. 1984;101:161–211. doi: 10.1007/BFb0027696. [DOI] [PubMed] [Google Scholar]
- Cai H, Griendling KK, Harrison DG. The vascular NAD(P)H oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol Sci. 2003;24:471–478. doi: 10.1016/S0165-6147(03)00233-5. [DOI] [PubMed] [Google Scholar]
- Cao W, Carney JM, Duchon A, et al. Oxygen free radical involvement in ischemia and reperfusion injury to brain. Neurosci Lett. 1988;88:233–238. doi: 10.1016/0304-3940(88)90132-2. [DOI] [PubMed] [Google Scholar]
- Carusone LM, Srinivasan J, Gitelman DR, et al. Hemodynamic response changes in cerebrovascular disease: implications for functional MR imaging. AJNR Am J Neuroradiol. 2002;23:1222–1228. [PMC free article] [PubMed] [Google Scholar]
- Cauli B, Tong XK, Rancillac A, et al. Cortical GABA interneurons in neurovascular coupling: relays for subcortical vasoactive pathways. J Neurosci. 2004;24:8940–8949. doi: 10.1523/JNEUROSCI.3065-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerchiari EL, Hoel TM, Safar P, et al. Protective effects of combined superoxide dismutase and deferoxa-mine on recovery of cerebral blood flow and function after cardiac arrest in dogs. Stroke. 1987;18:869–878. doi: 10.1161/01.str.18.5.869. [DOI] [PubMed] [Google Scholar]
- Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001;21:2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- Chan PH, Kawase M, Murakami K, et al. Overexpression of SOD1 in transgenic rats protects vulnerable neurons against ischemic damage after global cerebral ischemia and reperfusion. J Neurosci. 1998;18:8292–8299. doi: 10.1523/JNEUROSCI.18-20-08292.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chillon JM, Baumbach GL. Autoregulation: arterial and intracranial pressure. In: Edvinsson L, Krause DN, editors. Cerebral Blood Flow and Metabolism. 2nd edn. Lippincott Williams & Wilkins; Philadelphia, PA: 2002. [Google Scholar]
- Chollet F, Celsis P, Clanet M, et al. SPECT study of cerebral blood flow reactivity after acetazolamide in patients with transient ischemic attacks. Stroke. 1989;20:458–464. doi: 10.1161/01.str.20.4.458. [DOI] [PubMed] [Google Scholar]
- Christopherson TJ, Milde JH, Michenfelder JD. Cerebral vascular autoregulation and CO2 reactivity following onset of the delayed postischemic hypoperfusion state in dogs. J Cereb Blood Flow Metab. 1993;13:260–268. doi: 10.1038/jcbfm.1993.32. [DOI] [PubMed] [Google Scholar]
- Cipolla MJ, McCall AL, Lessov N, et al. Reperfusion decreases myogenic reactivity and alters middle cerebral artery function after focal cerebral ischemia in rats. Stroke. 1997;28:176–180. doi: 10.1161/01.str.28.1.176. [DOI] [PubMed] [Google Scholar]
- Clavier N, Kirsch JR, Hurn PD, et al. Effect of post-ischemic hypoperfusion on vasodilatory mechanisms in cats. Am J Physiol. 1994;267:H2012–H2018. doi: 10.1152/ajpheart.1994.267.5.H2012. [DOI] [PubMed] [Google Scholar]
- Clifton GL, Haden HT, Taylor JR, et al. Cerebrovascular CO2 reactivity after carotid artery occlusion. J Neurosurg. 1988;69:24–28. doi: 10.3171/jns.1988.69.1.0024. [DOI] [PubMed] [Google Scholar]
- Cooper D, Stokes KY, Tailor A, et al. Oxidative stress promotes blood cell–endothelial cell interactions in the microcirculation. Cardiovasc Toxicol. 2002;2:165–180. doi: 10.1007/s12012-002-0002-7. [DOI] [PubMed] [Google Scholar]
- Date H, Hossmann KA. Effect of vasodilating drugs on intracortical and extracortical vascular resistance following middle cerebral artery occlusion in cats. Ann Neurol. 1984;16:330–336. doi: 10.1002/ana.410160309. [DOI] [PubMed] [Google Scholar]
- De Leeuw FE, Van Huffelen A, Kappelle J. Cerebrovascular reactivity in patients with a recent lacunar infarction. J Neurol. 2003;250:232–233. doi: 10.1007/s00415-003-0949-8. [DOI] [PubMed] [Google Scholar]
- Del Zoppo GJ, Mabuchi T. Cerebral microvessel responses to focal ischemia. J Cereb Blood Flow Metab. 2003;23:879–894. doi: 10.1097/01.WCB.0000078322.96027.78. [DOI] [PubMed] [Google Scholar]
- Dettmers C, Young A, Rommel T, et al. CO2 reactivity in the ischaemic core, penumbra, and normal tissue 6 hours after acute MCA-occlusion in primates. Acta Neurochir (Wien) 1993;125:150–155. doi: 10.1007/BF01401843. [DOI] [PubMed] [Google Scholar]
- Didion SP, Faraci FM. Angiotensin II produces superoxide-mediated impairment of endothelial function in cerebral arterioles. Stroke. 2003;34:2038–2042. doi: 10.1161/01.STR.0000081225.46324.AA. [DOI] [PubMed] [Google Scholar]
- Dietrich WD, Ginsberg MD, Busto R. Effect of transient cerebral ischemia on metabolic activation of a somatosensory circuit. J Cereb Blood Flow Metab. 1986;6:405–413. doi: 10.1038/jcbfm.1986.73. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Pulsinelli W. Autoregulation of cerebral blood flow in experimental focal brain ischemia. J Cereb Blood Flow Metab. 1990;10:327–336. doi: 10.1038/jcbfm.1990.61. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Edvinsson L, Villringer A. Measuring cerebral blood flow and metabolism. In: dvinsson LE, Krause DN, editors. Cerebral Blood Flow and Metabolism. 2nd edn. Lippincott Williams & Wilkins; Philadelphia, PA: 2002. [Google Scholar]
- Duckrow RB, Lamanna JS, Rosenthal M. Disparate recovery of resting and stimulated oxidative metabolism following transient ischemia. Stroke. 1981;12:677–686. doi: 10.1161/01.str.12.5.677. [DOI] [PubMed] [Google Scholar]
- Eames PJ, Blake MJ, Dawson SL, et al. Dynamic cerebral autoregulation and beat to beat blood pressure control are impaired in acute ischaemic stroke. J Neurol Neurosurg Psychiatry. 2002;72:467–472. doi: 10.1136/jnnp.72.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraci FM. Oxidative stress: the curse that underlies cerebral vascular dysfunction? Stroke. 2005;36:186–188. doi: 10.1161/01.STR.0000153067.27288.8b. [DOI] [PubMed] [Google Scholar]
- Faraci FM. Reactive oxygen species: influence on cerebral vascular tone. J Appl Physiol. 2006;100:739–743. doi: 10.1152/japplphysiol.01044.2005. [DOI] [PubMed] [Google Scholar]
- Faraci FM, Lentz SR. Hyperhomocysteinemia, oxidative stress, and cerebral vascular dysfunction. Stroke. 2004;35:345–347. doi: 10.1161/01.STR.0000115161.10646.67. [DOI] [PubMed] [Google Scholar]
- Faraci FM, Breese KR, Heistad DD. Cerebral vasodilation during hypercapnia. Role of glibenclamide-sensitive potassium channels and nitric oxide. Stroke. 1994;25:1679–1683. doi: 10.1161/01.str.25.8.1679. [DOI] [PubMed] [Google Scholar]
- Feeney DM, Baron JC. Diaschisis. Stroke. 1986;17:817–830. doi: 10.1161/01.str.17.5.817. [DOI] [PubMed] [Google Scholar]
- Florence G, Seylaz J. Rapid autoregulation of cerebral blood flow: a laser-Doppler flowmetry study. J Cereb Blood Flow Metab. 1992;12:674–680. doi: 10.1038/jcbfm.1992.92. [DOI] [PubMed] [Google Scholar]
- Fox PT, Raichle ME. Stimulus rate dependence of regional cerebral blood flow in human striate cortex, demonstrated by positron emission tomography. J Neurophysiol. 1984;51:1109–1120. doi: 10.1152/jn.1984.51.5.1109. [DOI] [PubMed] [Google Scholar]
- Frostig RD, Lieke EE, Ts'o DY, et al. Cortical functional architecture and local coupling between neuronal activity and the microcirculation revealed by in vivo high-resolution optical imaging of intrinsic signals. Proc Natl Acad Sci U S A. 1990;87:6082–6086. doi: 10.1073/pnas.87.16.6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg MD, Castella Y, Dietrich WD, et al. Acute thrombotic infarction suppresses metabolic activation of ipsilateral somatosensory cortex: evidence for functional diaschisis. J Cereb Blood Flow Metab. 1989;9:329–341. doi: 10.1038/jcbfm.1989.51. [DOI] [PubMed] [Google Scholar]
- Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol. 2006;100:328–335. doi: 10.1152/japplphysiol.00966.2005. [DOI] [PubMed] [Google Scholar]
- Girouard H, Park L, Anrather J, et al. Angiotensin II attenuates endothelium-dependent responses in the cerebral microcirculation through nox-2-derived radicals. Arterioscler Thromb Vasc Biol. 2006;26:826–832. doi: 10.1161/01.ATV.0000205849.22807.6e. [DOI] [PubMed] [Google Scholar]
- Globus MY, Busto R, Lin B, et al. Detection of free radical activity during transient global ischemia and recirculation: effects of intraischemic brain temperature modulation. J Neurochem. 1995;65:1250–1256. doi: 10.1046/j.1471-4159.1995.65031250.x. [DOI] [PubMed] [Google Scholar]
- Gogolak I, Gotoh F, Tomita M, et al. No intracerebral steal phenomenon in the ischemic brain following papaverine administration. Stroke. 1985;16:114–117. doi: 10.1161/01.str.16.1.114. [DOI] [PubMed] [Google Scholar]
- Gold L, Lauritzen M. Neuronal deactivation explains decreased cerebellar blood flow in response to focal cerebral ischemia or suppressed neocortical function. Proc Natl Acad Sci U S A. 2002;99:7699–7704. doi: 10.1073/pnas.112012499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong XP, Li Y, Jiang WJ, et al. Impaired dynamic cerebral autoregulation in middle cerebral artery stenosis. Neurol Res. 2006;28:76–81. doi: 10.1179/016164106X91915. [DOI] [PubMed] [Google Scholar]
- Gooskens I, Schmidt EA, Czosnyka M, et al. Pressure-autoregulation, CO2 reactivity and asymmetry of haemodynamic parameters in patients with carotid artery stenotic disease. A clinical appraisal. Acta Neurochir (Wien) 2003;145:527–532. doi: 10.1007/s00701-003-0045-y. [DOI] [PubMed] [Google Scholar]
- Gourley JK, Heistad DD. Characteristics of reactive hyperemia in the cerebral circulation. Am J Physiol. 1984;246:H52–H58. doi: 10.1152/ajpheart.1984.246.1.H52. [DOI] [PubMed] [Google Scholar]
- Greenberg J, Hand P, Sylvestro A, et al. Localized metabolic-flow couple during functional activity. Acta Neurol Scand. 1979;72:12–13. [Google Scholar]
- Guo W, Adachi T, Matsui R, et al. Quantitative assessment of tyrosine nitration of manganese superoxide dismutase in angiotensin II-infused rat kidney. Am J Physiol Heart Circ Physiol. 2003;285:H1396–H1403. doi: 10.1152/ajpheart.00096.2003. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol. 2004;142:231–255. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamzei F, Knab R, Weiller C, et al. The influence of extra- and intracranial artery disease on the BOLD signal in FMRI. Neuroimage. 2003;20:1393–1399. doi: 10.1016/S1053-8119(03)00384-7. [DOI] [PubMed] [Google Scholar]
- Harder DR, Narayanan J, Gebremedhin D, et al. Transduction of physical forces by the vascular wall: role of phospholipase C and cytochrome P450 metabolites of arachidonic acid. Trends Cardiovasc Med. 1995;5:7–14. doi: 10.1016/1050-1738(94)00026-R. [DOI] [PubMed] [Google Scholar]
- Harris NG, Lythgoe MF, Thomas DL, et al. Cerebrovascular reactivity following focal brain ischemia in the rat: a functional magnetic resonance imaging study. Neuroimage. 2001;13:339–350. doi: 10.1006/nimg.2000.0689. [DOI] [PubMed] [Google Scholar]
- Haubrich C, Kruska W, Diehl RR, et al. Recovery of the blood pressure—cerebral flow relation after carotid stenting in elderly patients. Acta Neurochir (Wien) 2007;149:131–136. doi: 10.1007/s00701-006-0888-0. discussion 137. [DOI] [PubMed] [Google Scholar]
- Heiss WD, Rosner G. Functional recovery of cortical neurons as related to degree and duration of ischemia. Ann Neurol. 1983;14:294–301. doi: 10.1002/ana.410140307. [DOI] [PubMed] [Google Scholar]
- Heiss WD, Huber M, Fink GR, et al. Progressive derangement of periinfarct viable tissue in ischemic stroke. J Cereb Blood Flow Metab. 1992;12:193–203. doi: 10.1038/jcbfm.1992.29. [DOI] [PubMed] [Google Scholar]
- Heiss WD, Graf R, Lottgen J, et al. Repeat positron emission tomographic studies in transient middle cerebral artery occlusion in cats: residual perfusion and efficacy of postischemic reperfusion. J Cereb Blood Flow Metab. 1997;17:388–400. doi: 10.1097/00004647-199704000-00004. [DOI] [PubMed] [Google Scholar]
- Heiss WD, Forsting M, Diener HC. Imaging in cerebrovascular disease. Curr Opin Neurol. 2001;14:67–75. doi: 10.1097/00019052-200102000-00011. [DOI] [PubMed] [Google Scholar]
- Heistad DD, Kontos H. Cerebral circulation. In: Abboud F, Shephard J, editors. Handbook of Physiology: The Cardiovascular System. American Physiological Society; Bethesda, MA: 1983. [Google Scholar]
- Herold S, Brown MM, Frackowiak RS, et al. Assessment of cerebral haemodynamic reserve: correlation between PET parameters and CO2 reactivity measured by the intravenous 133 xenon injection technique. J Neurol Neurosurg Psychiatry. 1988;51:1045–1050. doi: 10.1136/jnnp.51.8.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser D, Astrup J, Lassen NA, et al. Brain carbonic acid acidosis after acetazolamide. Acta Physiol Scand. 1975;93:385–390. doi: 10.1111/j.1748-1716.1975.tb05827.x. [DOI] [PubMed] [Google Scholar]
- Hoedt-Rasmussen K, Skinhoj E, Paulson O, et al. Regional cerebral blood flow in acute apoplexy. The ‘luxury perfusion syndrome’ of brain tissue. Arch Neurol. 1967;17:271–281. doi: 10.1001/archneur.1967.00470270049007. [DOI] [PubMed] [Google Scholar]
- Hossmann KA. Hemodynamics of postischemic reperfusion of the brain. In: Weinstein PR, Faden AI, editors. Protection of the Brain from Ischemia. Williams & Wilkins; Baltimore, MD.: 1983. [Google Scholar]
- Hossmann KA. Viability thresholds and the penumbra of focal ischemia. Ann Neurol. 1994;36:557–565. doi: 10.1002/ana.410360404. [DOI] [PubMed] [Google Scholar]
- Hossmann KA, Lechtape-Gruter H, Hossmann V. The role of cerebral blood flow for the recovery of the brain after prolonged ischemia. Z Neurol. 1973;204:281–299. doi: 10.1007/BF00316009. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Does nitric oxide mediate the increases in cerebral blood flow elicited by hypercapnia? Proc Natl Acad Sci U S A. 1992;89:3913–3916. doi: 10.1073/pnas.89.9.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C. Regulation of the cerebral microcirculation during neural activity: is nitric oxide the missing link? Trends Neurosci. 1993;16:206–214. doi: 10.1016/0166-2236(93)90156-g. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Nakai M, Mraovitch S, et al. Global increase in cerebral metabolism and blood flow produced by focal electrical stimulation of dorsal medullary reticular formation in rat. Brain Res. 1983;272:101–114. doi: 10.1016/0006-8993(83)90367-0. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Zhang F, Niwa K, et al. SOD1 rescues cerebral endothelial dysfunction in mice overexpressing amyloid precursor protein. Nat Neurosci. 1999;2:157–161. doi: 10.1038/5715. [DOI] [PubMed] [Google Scholar]
- Immink RV, Van Montfrans GA, Stam J, et al. Dynamic cerebral autoregulation in acute lacunar and middle cerebral artery territory ischemic stroke. Stroke. 2005;36:2595–2600. doi: 10.1161/01.STR.0000189624.06836.03. [DOI] [PubMed] [Google Scholar]
- Inao S, Tadokoro M, Nishino M, et al. Neural activation of the brain with hemodynamic insufficiency. J Cereb Blood Flow Metab. 1998;18:960–967. doi: 10.1097/00004647-199809000-00005. [DOI] [PubMed] [Google Scholar]
- Jones SC, Bose B, Furlan AJ, et al. CO2 reactivity and heterogeneity of cerebral blood flow in ischemic, border zone, and normal cortex. Am J Physiol. 1989;257:H473–H482. doi: 10.1152/ajpheart.1989.257.2.H473. [DOI] [PubMed] [Google Scholar]
- Kanno I, Uemura K, Higano S, et al. Oxygen extraction fraction at maximally vasodilated tissue in the ischemic brain estimated from the regional CO2 responsiveness measured by positron emission tomography. J Cereb Blood Flow Metab. 1988;8:227–235. doi: 10.1038/jcbfm.1988.53. [DOI] [PubMed] [Google Scholar]
- Katusic ZS. Vascular endothelial dysfunction: does tetrahydrobiopterin play a role? Am J Physiol Heart Circ Physiol. 2001;281:H981–H986. doi: 10.1152/ajpheart.2001.281.3.H981. [DOI] [PubMed] [Google Scholar]
- Kawase M, Murakami K, Fujimura M, et al. Exacerbation of delayed cell injury after transient global ischemia in mutant mice with CuZn superoxide dismutase deficiency. Stroke. 1999;30:1962–1968. doi: 10.1161/01.str.30.9.1962. [DOI] [PubMed] [Google Scholar]
- Kazama K, Anrather J, Zhou P, et al. Angiotensin II impairs neurovascular coupling in neocortex through NADPH oxidase-derived radicals. Circ Res. 2004;95:1019–1026. doi: 10.1161/01.RES.0000148637.85595.c5. [DOI] [PubMed] [Google Scholar]
- Kazumata K, Tanaka N, Ishikawa T, et al. Dissociation of vasoreactivity to acetazolamide and hypercapnia. Comparative study in patients with chronic occlusive major cerebral artery disease. Stroke. 1996;27:2052–2058. doi: 10.1161/01.str.27.11.2052. [DOI] [PubMed] [Google Scholar]
- Keyeux A, Laterre C, Beckers C. Resting and hyper-capnic rCBF in patients with unilateral occlusive disease of the internal carotid artery. J Nucl Med. 1988;29:311–319. [PubMed] [Google Scholar]
- Kil HY, Zhang J, Piantadosi CA. Brain temperature alters hydroxyl radical production during cerebral ischemia/reperfusion in rats. J Cereb Blood Flow Metab. 1996;16:100–106. doi: 10.1097/00004647-199601000-00012. [DOI] [PubMed] [Google Scholar]
- Kim GW, Sugawara T, Chan PH. Involvement of oxidative stress and caspase-3 in cortical infarction after photothrombotic ischemia in mice. J Cereb Blood Flow Metab. 2000;20:1690–1701. doi: 10.1097/00004647-200012000-00008. [DOI] [PubMed] [Google Scholar]
- Kim GW, Lewen A, Copin J, et al. The cytosolic antioxidant, copper/zinc superoxide dismutase, attenuates blood–brain barrier disruption and oxidative cellular injury after photothrombotic cortical ischemia in mice. Neuroscience. 2001;105:1007–1018. doi: 10.1016/s0306-4522(01)00237-8. [DOI] [PubMed] [Google Scholar]
- Kim GW, Kondo T, Noshita N, et al. Manganese superoxide dismutase deficiency exacerbates cerebral infarction after focal cerebral ischemia/reperfusion in mice: implications for the production and role of superoxide radicals. Stroke. 2002;33:809–815. doi: 10.1161/hs0302.103745. [DOI] [PubMed] [Google Scholar]
- Kirsch JR, Helfaer MA, Haun SE, et al. Polyethylene glycol-conjugated superoxide dismutase improves recovery of postischemic hypercapnic cerebral blood flow in piglets. Pediatr Res. 1993;34:530–537. doi: 10.1203/00006450-199310000-00030. [DOI] [PubMed] [Google Scholar]
- Koch KA, Jackson DL, Schmiedl M, et al. Total cerebral ischemia: effect of alterations in arterial PCO2 on cerebral microcirculation. J Cereb Blood Flow Metab. 1984;4:343–349. doi: 10.1038/jcbfm.1984.51. [DOI] [PubMed] [Google Scholar]
- Kontos HA. Oxygen radicals in cerebral ischemia: the 2001 Willis lecture. Stroke. 2001;32:2712–2716. doi: 10.1161/hs1101.098653. [DOI] [PubMed] [Google Scholar]
- Kontos CD, Wei EP, Williams JI, et al. Cytochemical detection of superoxide in cerebral inflammation and ischemia in vivo. Am J Physiol. 1992;263:H1234–H1242. doi: 10.1152/ajpheart.1992.263.4.H1234. [DOI] [PubMed] [Google Scholar]
- Kontos HA, Raper AJ, Patterson JL. Analysis of vasoactivity of local pH, PCO2 and bicarbonate on pial vessels. Stroke. 1977;8:358–360. doi: 10.1161/01.str.8.3.358. [DOI] [PubMed] [Google Scholar]
- Kontos HA, Wei EP, Navari RM, et al. Responses of cerebral arteries and arterioles to acute hypotension and hypertension. Am J Physiol. 1978;234:H371–H383. doi: 10.1152/ajpheart.1978.234.4.H371. [DOI] [PubMed] [Google Scholar]
- Krainik A, Hund-Georgiadis M, Zysset S, et al. Regional impairment of cerebrovascular reactivity and BOLD signal in adults after stroke. Stroke. 2005;36:1146–1152. doi: 10.1161/01.STR.0000166178.40973.a7. [DOI] [PubMed] [Google Scholar]
- Kunz A, Anrather J, Zhou P, et al. Cyclooxygenase-2 does not contribute to postischemic production of reactive oxygen species. J Cereb Blood Flow Metab. 2007a;27:545–551. doi: 10.1038/sj.jcbfm.9600369. [DOI] [PubMed] [Google Scholar]
- Kunz A, Park L, Abe T, et al. Neurovascular protection by ischemic tolerance: role of nitric oxide and reactive oxygen species. J Neurosci. 2007b;27:7083–7093. doi: 10.1523/JNEUROSCI.1645-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuschinsky W. Coupling of function metabolism and blood flow in the brain. News Physiol Sci. 1987;2:217–220. doi: 10.1007/BF00310651. [DOI] [PubMed] [Google Scholar]
- Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- Langfitt TW, Weinstein JD, Kassell NF. Cerebral vasomotor paralysis as a cause of brain swelling. Trans Am Neurol Assoc. 1964;89:214–215. [PubMed] [Google Scholar]
- Lassen NA. Cerebral blood flow and oxygen consumption in man. Physiol Rev. 1959;39:183–238. doi: 10.1152/physrev.1959.39.2.183. [DOI] [PubMed] [Google Scholar]
- Lassen NA. The luxury-perfusion syndrome and its possible relation to acute metabolic acidosis localised within the brain. Lancet. 1966;2:1113–1115. doi: 10.1016/s0140-6736(66)92199-4. [DOI] [PubMed] [Google Scholar]
- Lauer KK, Shen H, Hudetz AG, et al. Regional cerebral blood flow autoregulation in a silicone embolus model of focal brain ischemia as assessed by multichannel laser Doppler flowmetry. In: Hudetz AG, Bruley DF, editors. Oxygen Transport to Tissue XX. Plenum Press; New York: 1998. [DOI] [PubMed] [Google Scholar]
- Leffler CW, Busija DW, Mirro R, et al. Effects of ischemia on brain blood flow and oxygen consumption of newborn pigs. Am J Physiol. 1989;257:H1917–H1926. doi: 10.1152/ajpheart.1989.257.6.H1917. [DOI] [PubMed] [Google Scholar]
- Levine RL, Sunderland JJ, Lagreze HL, et al. Cerebral perfusion reserve indexes determined by fluoro-methane positron emission scanning. Stroke. 1988;19:19–27. doi: 10.1161/01.str.19.1.19. [DOI] [PubMed] [Google Scholar]
- Levine RL, Dobkin JA, Rozental JM, et al. Blood flow reactivity to hypercapnia in strictly unilateral carotid disease: preliminary results. J Neurol Neurosurg Psychiatry. 1991;54:204–209. doi: 10.1136/jnnp.54.3.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Liu M, Peterson S, et al. Hydroxyl radical formation is greater in striatal core than in penumbra in a rat model of ischemic stroke. J Neurosci Res. 2003;71:882–888. doi: 10.1002/jnr.10534. [DOI] [PubMed] [Google Scholar]
- MacGregor DG, Carswell HV, Graham DI, et al. Impaired cerebral autoregulation 24 h after induction of transient unilateral focal ischaemia in the rat. Eur J Neurosci. 2000;12:58–66. doi: 10.1046/j.1460-9568.2000.00880.x. [DOI] [PubMed] [Google Scholar]
- Maeda H, Matsumoto M, Handa N, et al. Reactivity of cerebral blood flow to carbon dioxide in various types of ischemic cerebrovascular disease: evaluation by the transcranial Doppler method. Stroke. 1993;24:670–675. doi: 10.1161/01.str.24.5.670. [DOI] [PubMed] [Google Scholar]
- Maneen MJ, Hannah R, Vitullo L, et al. Peroxynitrite diminishes myogenic activity and is associated with decreased vascular smooth muscle F-actin in rat posterior cerebral arteries. Stroke. 2006;37:894–899. doi: 10.1161/01.STR.0000204043.18592.0d. [DOI] [PubMed] [Google Scholar]
- Marchal G, Furlan M, Beaudouin V, et al. Early spontaneous hyperperfusion after stroke. A marker of favourable tissue outcome? Brain. 1996;119:409–419. doi: 10.1093/brain/119.2.409. [DOI] [PubMed] [Google Scholar]
- Marchal G, Young AR, Baron JC. Early postischemic hyperperfusion: pathophysiologic insights from positron emission tomography. J Cereb Blood Flow Metab. 1999;19:467–482. doi: 10.1097/00004647-199905000-00001. [DOI] [PubMed] [Google Scholar]
- Mayhan WG, Sharpe GM. Superoxide dismutase restores impaired histamine-induced increases in venular macromolecular efflux during diabetes mellitus. Microcirculation. 1998;5:211–218. [PubMed] [Google Scholar]
- Miller JD, Smith RR, Holaday HR. Carbon dioxide reactivity in the evaluation of cerebral ischemia. Neuro-surgery. 1992;30:518–521. doi: 10.1227/00006123-199204000-00008. [DOI] [PubMed] [Google Scholar]
- Minematsu K, Yamaguchi T, Omae T. ‘Spectacular shrinking deficit’: rapid recovery from a major hemispheric syndrome by migration of an embolus. Neurology. 1992;42:157–162. doi: 10.1212/wnl.42.1.157. [DOI] [PubMed] [Google Scholar]
- Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431:195–199. doi: 10.1038/nature02827. [DOI] [PubMed] [Google Scholar]
- Murakami K, Kondo T, Kawase M, et al. Mitochondrial susceptibility to oxidative stress exacerbates cerebral infarction that follows permanent focal cerebral ischemia in mutant mice with manganese superoxide dismutase deficiency. J Neurosci. 1998;18:205–213. doi: 10.1523/JNEUROSCI.18-01-00205.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y, Sakatani K, Katayama Y, et al. Increase in focal concentration of deoxyhaemoglobin during neuronal activity in cerebral ischaemic patients. J Neurol Neurosurg Psychiatry. 2002;73:182–184. doi: 10.1136/jnnp.73.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y, Sakatani K, Hoshino T, et al. Effects of cerebral ischemia on evoked cerebral blood oxygenation responses and BOLD contrast functional MRI in stroke patients. Stroke. 2006;37:2514–2520. doi: 10.1161/01.STR.0000239698.50656.3b. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Fujimoto N, Matsumoto K, et al. Morphological changes in acute cerebral ischemia after occlusion and reperfusion in the rat. Adv Neurol. 1990;52:21–27. [PubMed] [Google Scholar]
- Nariai T, Suzuki R, Hirakawa K, et al. Vascular reserve in chronic cerebral ischemia measured by the acetazolamide challenge test: comparison with positron emission tomography. AJNR Am J Neuroradiol. 1995;16:563–570. [PMC free article] [PubMed] [Google Scholar]
- Nelson CW, Wei EP, Povlishock JT, et al. Oxygen radicals in cerebral ischemia. Am J Physiol. 1992;263:H1356–H1362. doi: 10.1152/ajpheart.1992.263.5.H1356. [DOI] [PubMed] [Google Scholar]
- Nemoto EM, Snyder JV, Carroll RG, et al. Global ischemia in dogs: cerebrovascular CO2 reactivity and autoregulation. Stroke. 1975;6:425–431. doi: 10.1161/01.str.6.4.425. [DOI] [PubMed] [Google Scholar]
- Nemoto EM, Yonas H, Pindzola RR, et al. PET OEF reactivity for hemodynamic compromise in occlusive vascular disease. J Neuroimaging. 2007;17:54–60. doi: 10.1111/j.1552-6569.2006.00080.x. [DOI] [PubMed] [Google Scholar]
- Nilsson B, Rehncrona S, Siesjo BK. Ciba Foundation Symposium 56 (New series) Elsevier North-Holland; Amsterdam: 1978. Coupling of cerebral metabolism and blood flow in epileptic seizures, hypoxia and hypoglycemia. In: Cerebral Vascular Smooth Muscle and Its Control. [DOI] [PubMed] [Google Scholar]
- Niwa K, Haensel C, Ross ME, et al. Cyclooxygenase-1 participates in selected vasodilator responses of the cerebral circulation. Circ Res. 2001;88:600–608. doi: 10.1161/01.res.88.6.600. [DOI] [PubMed] [Google Scholar]
- Norrving B, Nilsson B, Risberg J. rCBF in patients with carotid occlusion. Resting and hypercapnic flow related to collateral pattern. Stroke. 1982;13:155–162. doi: 10.1161/01.str.13.2.155. [DOI] [PubMed] [Google Scholar]
- Novak V, Chowdhary A, Farrar B, et al. Altered cerebral vasoregulation in hypertension and stroke. Neurology. 2003;60:1657–1663. doi: 10.1212/01.wnl.0000068023.14587.06. [DOI] [PubMed] [Google Scholar]
- Nudo RJ. Functional and structural plasticity in motor cortex: implications for stroke recovery. Phys Med Rehabil Clin North Am. 2003;14:S57–S76. doi: 10.1016/s1047-9651(02)00054-2. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Kay AR, et al. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtake M, Morino S, Kaidoh T, et al. Three-dimensional structural changes in cerebral microvessels after transient focal cerebral ischemia in rats: scanning electron microscopic study of corrosion casts. Neuropathology. 2004;24:219–227. doi: 10.1111/j.1440-1789.2004.00560.x. [DOI] [PubMed] [Google Scholar]
- Olah L, Franke C, Schwindt W, et al. CO2 reactivity measured by perfusion MRI during transient focal cerebral ischemia in rats. Stroke. 2000;31:2236–2244. doi: 10.1161/01.str.31.9.2236. [DOI] [PubMed] [Google Scholar]
- Olsen TS, Larsen B, Skriver EB, et al. Focal cerebral hyperemia in acute stroke. Incidence, pathophysiology and clinical significance. Stroke. 1981;12:598–607. doi: 10.1161/01.str.12.5.598. [DOI] [PubMed] [Google Scholar]
- Olsen TS, Larsen B, Herning M, et al. Blood flow and vascular reactivity in collaterally perfused brain tissue. Evidence of an ischemic penumbra in patients with acute stroke. Stroke. 1983;14:332–341. doi: 10.1161/01.str.14.3.332. [DOI] [PubMed] [Google Scholar]
- Ono Y, Morikawa S, Inubushi T, et al. T2*-weighted magnetic resonance imaging of cerebrovascular reactivity in rat reversible focal cerebral ischemia. Brain Res. 1997;744:207–215. doi: 10.1016/S0006-8993(96)01079-7. [DOI] [PubMed] [Google Scholar]
- Ott EO, Abraham J, Meyer JS, et al. Regional cerebral blood flow measured by the gamma camera after direct injection of 133Xe into the distal stump of the occluded middle cerebral artery. Stroke. 1975;6:376–381. doi: 10.1161/01.str.6.4.376. [DOI] [PubMed] [Google Scholar]
- Ouchi Y, Nobezawa S, Yoshikawa E, et al. Postural effects on brain hemodynamics in unilateral cerebral artery occlusive disease: a positron emission tomography study. J Cereb Blood Flow Metab. 2001;21:1058–1066. doi: 10.1097/00004647-200109000-00003. [DOI] [PubMed] [Google Scholar]
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L, Anrather J, Forster C, et al. Ab-induced vascular oxidative stress and attenuation of functional hyper-emia in mouse somatosensory cortex. J Cereb Blood Flow Metab. 2004;24:334–342. doi: 10.1097/01.WCB.0000105800.49957.1E. [DOI] [PubMed] [Google Scholar]
- Park L, Anrather J, Zhou P, et al. NADPH-oxidase-derived reactive oxygen species mediate the cerebrovascular dysfunction induced by the amyloid beta peptide. J Neurosci. 2005;25:1769–1777. doi: 10.1523/JNEUROSCI.5207-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson OB. Cerebral apoplexy (stroke): pathogenesis, pathophysiology and therapy as illustrated by regional blood flow measurements in the brain. Stroke. 1971;2:327–360. doi: 10.1161/01.str.2.4.327. [DOI] [PubMed] [Google Scholar]
- Paulson OB, Olesen J, Christensen MS. Restoration of autoregulation of cerebral blood flow by hypocapnia. Neurology. 1972;22:286–293. doi: 10.1212/wnl.22.3.286. [DOI] [PubMed] [Google Scholar]
- Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev. 1990;2:161–192. [PubMed] [Google Scholar]
- Petrault O, Bastide M, Cotelle N, et al. The neuro-protective effect of the antioxidant flavonoid derivate ditert-butylhydroxyphenyl is parallel to the preventive effect on post-ischemic Kir2.x impairment but not to post-ischemic endothelial dysfunction. Naunyn Schmiedebergs Arch Pharmacol. 2004;370:395–403. doi: 10.1007/s00210-004-0966-x. [DOI] [PubMed] [Google Scholar]
- Pfefferkorn T, Von Stuckrad-Barre S, Herzog J, et al. Reduced cerebrovascular CO2 reactivity in CADASIL: a transcranial Doppler sonography study. Stroke. 2001;32:17–21. doi: 10.1161/01.str.32.1.17. [DOI] [PubMed] [Google Scholar]
- Piantadosi CA, Zhang J. Mitochondrial generation of reactive oxygen species after brain ischemia in the rat. Stroke. 1996;27:327–331. doi: 10.1161/01.str.27.2.327. discussion 332. [DOI] [PubMed] [Google Scholar]
- Pineiro R, Pendlebury S, Johansen-Berg H, et al. Altered hemodynamic responses in patients after subcortical stroke measured by functional MRI. Stroke. 2002;33:103–109. doi: 10.1161/hs0102.100482. [DOI] [PubMed] [Google Scholar]
- Powers WJ. Cerebral hemodynamics in ischemic cerebrovascular disease. Ann Neurol. 1991;29:231–240. doi: 10.1002/ana.410290302. [DOI] [PubMed] [Google Scholar]
- Pulsinelli WA, Levy DE, Duffy TE. Regional cerebral blood flow and glucose metabolism following transient forebrain ischemia. Ann Neurol. 1982;11:499–502. doi: 10.1002/ana.410110510. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Gado MH, Grubb RL, Jr, et al. In vivo correlation between regional cerebral blood flow and oxygen utilization in man. Trans Am Neurol Assoc. 1975;100:68–71. [PubMed] [Google Scholar]
- Rehncrona S, Rosen I, Siesjo BK. Brain lactic acidosis and ischemic cell damage: 1. Biochemistry and neuro-physiology. J Cereb Blood Flow Metab. 1981;1:297–311. doi: 10.1038/jcbfm.1981.34. [DOI] [PubMed] [Google Scholar]
- Reinhard M, Muller T, Roth M, et al. Bilateral severe carotid artery stenosis or occlusion—cerebral autoregulation dynamics and collateral flow patterns. Acta Neurochir (Wien) 2003;145:1053–1059. doi: 10.1007/s00701-003-0137-8. [DOI] [PubMed] [Google Scholar]
- Reivich M. Blood flow metabolism couple in brain. Res Publ Assoc Res Nerv Ment Dis. 1974;53:125–140. [PubMed] [Google Scholar]
- Ringelstein EB, Sievers C, Ecker S, et al. Noninvasive assessment of CO2-induced cerebral vasomotor response in normal individuals and patients with internal carotid artery occlusions. Stroke. 1988;19:963–969. doi: 10.1161/01.str.19.8.963. [DOI] [PubMed] [Google Scholar]
- Ringelstein EB, Biniek R, Weiller C, et al. Type and extent of hemispheric brain infarctions and clinical outcome in early and delayed middle cerebral artery recanalization. Neurology. 1992;42:289–298. doi: 10.1212/wnl.42.2.289. [DOI] [PubMed] [Google Scholar]
- Ringelstein EB, Weiller C, Weckesser M, et al. Cerebral vasomotor reactivity is significantly reduced in low-flow as compared to thromboembolic infarctions: the key role of the circle of Willis. J Neurol Sci. 1994;121:103–109. doi: 10.1016/0022-510x(94)90163-5. [DOI] [PubMed] [Google Scholar]
- Rosenblum WI. Selective impairment of response to acetylcholine after ischemia/reperfusion in mice. Stroke. 1997;28:448–451. doi: 10.1161/01.str.28.2.448. discussion 451–452. [DOI] [PubMed] [Google Scholar]
- Rosenblum WI, Wormley B. Selective depression of endothelium-dependent dilations during cerebral ischemia. Stroke. 1995;26:1877–1881. doi: 10.1161/01.str.26.10.1877. discussion 1882. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Altamura C, Ferretti A, et al. Does cerebrovascular disease affect the coupling between neuronal activity and local haemodynamics? Brain. 2004;127:99–110. doi: 10.1093/brain/awh012. [DOI] [PubMed] [Google Scholar]
- Rother J, Knab R, Hamzei F, et al. Negative dip in BOLD fMRI is caused by blood flow–oxygen consumption uncoupling in humans. Neuroimage. 2002;15:98–102. doi: 10.1006/nimg.2001.0965. [DOI] [PubMed] [Google Scholar]
- Sakatani K, Xie Y, Lichty W, et al. Language-activated cerebral blood oxygenation and hemodynamic changes of the left prefrontal cortex in poststroke aphasic patients: a near-infrared spectroscopy study. Stroke. 1998;29:1299–1304. doi: 10.1161/01.str.29.7.1299. [DOI] [PubMed] [Google Scholar]
- Sakatani K, Murata Y, Fukaya C, et al. BOLD functional MRI may overlook activation areas in the damaged brain. Acta Neurochir Suppl. 2003;87:59–62. doi: 10.1007/978-3-7091-6081-7_13. [DOI] [PubMed] [Google Scholar]
- Sandor P, Komjati K, Reivich M, et al. Major role of nitric oxide in the mediation of regional CO2 responsiveness of the cerebral and spinal cord vessels of the cat. J Cereb Blood Flow Metab. 1994;14:49–58. doi: 10.1038/jcbfm.1994.8. [DOI] [PubMed] [Google Scholar]
- Schmidt-Kastner R, Ophoff BG, Hossmann KA. Delayed recovery of CO2 reactivity after one hour's complete ischaemia of cat brain. J Neurol. 1986;233:367–369. doi: 10.1007/BF00313924. [DOI] [PubMed] [Google Scholar]
- Schmidt-Kastner R, Hossmann KA, Ophoff BG. Pial artery pressure after one hour of global ischemia. J Cereb Blood Flow Metab. 1987;7:109–117. doi: 10.1038/jcbfm.1987.16. [DOI] [PubMed] [Google Scholar]
- Schreiber WG, Guckel F, Stritzke P, et al. Cerebral blood flow and cerebrovascular reserve capacity: estimation by dynamic magnetic resonance imaging. J Cereb Blood Flow Metab. 1998;18:1143–1156. doi: 10.1097/00004647-199810000-00011. [DOI] [PubMed] [Google Scholar]
- Seki H, Yoshimoto T, Ogawa A, et al. The CO2 response in focal cerebral ischemia—sequential changes following recirculation. Stroke. 1984;15:699–704. doi: 10.1161/01.str.15.4.699. [DOI] [PubMed] [Google Scholar]
- Shen Q, Ren H, Cheng H, et al. Functional, perfusion and diffusion MRI of acute focal ischemic brain injury. J Cereb Blood Flow Metab. 2005;25:1265–1279. doi: 10.1038/sj.jcbfm.9600132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima T, Hossmann KA, Date H. Pial arterial pressure in cats following middle cerebral artery occlusion. 1. Relationship to blood flow, regulation of blood flow and electrophysiological function. Stroke. 1983;14:713–719. doi: 10.1161/01.str.14.5.713. [DOI] [PubMed] [Google Scholar]
- Shiokawa O, Sadoshima S, Kusuda K, et al. Cerebral and cerebellar blood flow autoregulation in acutely induced cerebral ischemia in spontaneously hypertensive rats—transtentorial remote effect. Stroke. 1986;17:1309–1313. doi: 10.1161/01.str.17.6.1309. [DOI] [PubMed] [Google Scholar]
- Silver IA, Erecinska M. Ion homeostasis in rat brain in vivo: intra- and extracellular [Ca2+] and [H+] in the hippocampus during recovery from short-term, transient ischemia. J Cereb Blood Flow Metab. 1992;12:759–772. doi: 10.1038/jcbfm.1992.107. [DOI] [PubMed] [Google Scholar]
- Skinhoj E, Hoedt-Rasmussen K, Paulson OB, et al. Regional cerebral blood flow and its autoregulation in patients with transient focal cerebral ischemic attacks. Neurology. 1970;20:485–493. doi: 10.1212/wnl.20.5.485. [DOI] [PubMed] [Google Scholar]
- Sokoloff L, Reivich M, Kennedy C, et al. The [14C] deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977;28:897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- Soriano FG, Virag L, Szabo C. Diabetic endothelial dysfunction: role of reactive oxygen and nitrogen species production and poly(ADP-ribose) polymerase activation. J Mol Med. 2001;79:437–448. doi: 10.1007/s001090100236. [DOI] [PubMed] [Google Scholar]
- Stamler JS. Alzheimer's disease. A radical vascular connection. Nature. 1996;380:108–111. [PubMed] [Google Scholar]
- Sundt TM, Jr, Waltz AG. Cerebral ischemia and reactive hyperemia. Studies of cortical blood flow and micro-circulation before, during, and after temporary occlusion of middle cerebral artery of squirrel monkeys. Circ Res. 1971;28:426–433. doi: 10.1161/01.res.28.4.426. [DOI] [PubMed] [Google Scholar]
- Symon L, Khodadad G, Montoya G. Effect of carbon dioxide inhalation on the pattern of gaseous metabolism in ischaemic zones of the primate cortex. An experimental study of the ‘intracerebral steal’ phenomenon in baboons. J Neurol Neurosurg Psychiatry. 1971;34:481–486. doi: 10.1136/jnnp.34.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symon L, Pasztor E, Branston NM. The distribution and density of reduced cerebral blood flow following acute middle cerebral artery occlusion: an experimental study by the technique of hydrogen clearance in baboons. Stroke. 1974;5:355–364. doi: 10.1161/01.str.5.3.355. [DOI] [PubMed] [Google Scholar]
- Symon L, Crockard HA, Dorsch NW, et al. Local cerebral blood flow and vascular reactivity in a chronic stable stroke in baboons. Stroke. 1975;6:482–492. doi: 10.1161/01.str.6.5.482. [DOI] [PubMed] [Google Scholar]
- Takano T, Nagatsuka K, Ohnishi Y, et al. Vascular response to carbon dioxide in areas with and without diaschisis in patients with small, deep hemispheric infarction. Stroke. 1988;19:840–845. doi: 10.1161/01.str.19.7.840. [DOI] [PubMed] [Google Scholar]
- Takano T, Tian GF, Peng W, et al. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci. 2006;9:260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- Tatemichi TK, Young WL, Prohovnik I, et al. Perfusion insufficiency in limb-shaking transient ischemic attacks. Stroke. 1990;21:341–347. doi: 10.1161/01.str.21.2.341. [DOI] [PubMed] [Google Scholar]
- Thompson BG, Pluta RM, Girton ME, et al. Nitric oxide mediation of chemoregulation but not autoregulation of cerebral blood flow in primates. J Neurosurg. 1996;84:71–78. doi: 10.3171/jns.1996.84.1.0071. [DOI] [PubMed] [Google Scholar]
- Todd NV, Picozzi P, Crockard HA, et al. Reperfusion after cerebral ischemia: influence of duration of ischemia. Stroke. 1986;17:460–466. doi: 10.1161/01.str.17.3.460. [DOI] [PubMed] [Google Scholar]
- Traupe H, Kruse E, Heiss WD. Reperfusion of focal ischemia of varying duration: postischemic hyper- and hypo-perfusion. Stroke. 1982;13:615–622. doi: 10.1161/01.str.13.5.615. [DOI] [PubMed] [Google Scholar]
- Traystman RJ, Kirsch JR, Koehler RC. Oxygen radical mechanisms of brain injury following ischemia and reperfusion. J Appl Physiol. 1991;71:1185–1195. doi: 10.1152/jappl.1991.71.4.1185. [DOI] [PubMed] [Google Scholar]
- Tsubokawa T, Katayama Y, Kondo T, et al. Changes in local cerebral blood flow and neuronal activity during sensory stimulation in normal and sympathectomized cats. Brain Res. 1980;190:51–64. doi: 10.1016/0006-8993(80)91159-2. [DOI] [PubMed] [Google Scholar]
- Ueki M, Linn F, Hossmann KA. Functional activation of cerebral blood flow and metabolism before and after global ischemia of rat brain. J Cereb Blood Flow Metab. 1988;8:486–494. doi: 10.1038/jcbfm.1988.89. [DOI] [PubMed] [Google Scholar]
- Ugurbil K, Toth L, Kim DS. How accurate is magnetic resonance imaging of brain function? Trends Neurosci. 2003;26:108–114. doi: 10.1016/S0166-2236(02)00039-5. [DOI] [PubMed] [Google Scholar]
- Van den Kerckhoff W, Hossmann KA, Hossmann V. No effect of prostacyclin on blood flow, regulation of blood flow and blood coagulation following global cerebral ischemia. Stroke. 1983;14:724–730. doi: 10.1161/01.str.14.5.724. [DOI] [PubMed] [Google Scholar]
- Vignais PV. The superoxide-generating NADPH oxidase: structural aspects and activation mechanism. Cell Mol Life Sci. 2002;59:1428–1459. doi: 10.1007/s00018-002-8520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Monakow C. Die Lokalisation im Grosshirn und der Abbau der Funktion durch kortikale Herde. JF Berg-mann; Wiesbaden: 1914. [Google Scholar]
- Vorstrup S, Brun B, Lassen NA. Evaluation of the cerebral vasodilatory capacity by the acetazolamide test before EC-IC bypass surgery in patients with occlusion of the internal carotid artery. Stroke. 1986;17:1291–1298. doi: 10.1161/01.str.17.6.1291. [DOI] [PubMed] [Google Scholar]
- Walder CE, Green SP, Darbonne WC, et al. Ischemic stroke injury is reduced in mice lacking a functional NADPH oxidase. Stroke. 1997;28:2252–2258. doi: 10.1161/01.str.28.11.2252. [DOI] [PubMed] [Google Scholar]
- Waltz AG. Effect of PaCO2 on blood flow and micro-vasculature of ischemic and nonischemic cerebral cortex. Stroke. 1970;1:27–37. doi: 10.1161/01.str.1.1.27. [DOI] [PubMed] [Google Scholar]
- Wang X, Lo EH. Triggers and mediators of hemorrhagic transformation in cerebral ischemia. Mol Neurobiol. 2003;28:229–244. doi: 10.1385/MN:28:3:229. [DOI] [PubMed] [Google Scholar]
- Wei EP, Kontos HA, Beckman JS. Mechanisms of cerebral vasodilation by superoxide, hydrogen peroxide, and peroxynitrite. Am J Physiol. 1996;271:H1262–H1266. doi: 10.1152/ajpheart.1996.271.3.H1262. [DOI] [PubMed] [Google Scholar]
- Wellman GC, Nathan DJ, Saundry CM, et al. Ca2+ sparks and their function in human cerebral arteries. Stroke. 2002;33:802–808. doi: 10.1161/hs0302.104089. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Meyer JS, Sakai F, et al. Aging and cerebral vasodilator responses to hypercarbia: responses in normal aging and in persons with risk factors for stroke. Arch Neurol. 1980;37:489–496. doi: 10.1001/archneur.1980.00500570037005. [DOI] [PubMed] [Google Scholar]
- Yamauchi H, Kudoh T, Sugimoto K, et al. Altered patterns of blood flow response during visual stimulation in carotid artery occlusive disease. Neuroimage. 2005;25:554–560. doi: 10.1016/j.neuroimage.2004.11.047. [DOI] [PubMed] [Google Scholar]
- Zhang F, Slungaard A, Vercellotti GM, et al. Super-oxide-dependent cerebrovascular effects of homocysteine. Am J Physiol. 1998;274:R1704–R1711. doi: 10.1152/ajpregu.1998.274.6.R1704. [DOI] [PubMed] [Google Scholar]
- Zhang J, Piantadosi CA. Prolonged production of hydroxyl radical in rat hippocampus after brain ischemiareperfusion is decreased by 21-aminosteroids. Neurosci Lett. 1994;177:127–130. doi: 10.1016/0304-3940(94)90061-2. [DOI] [PubMed] [Google Scholar]
- Zonta M, Angulo MC, Gobbo S, et al. Neuron-toastrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
- Zou MH, Leist M, Ullrich V. Selective nitration of prostacyclin synthase and defective vasorelaxation in atherosclerotic bovine coronary arteries. Am J Pathol. 1999;154:1359–1365. doi: 10.1016/S0002-9440(10)65390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]