Abstract
The HPV16 E7 oncoprotein and 17β-estradiol are important factors for the induction of premalignant lesions and cervical cancer. The study of these factors is crucial for a better understanding of cervical tumorigenesis. Here, we assessed the global gene expression profiles induced by the HPV16 E7 oncoprotein and/or 17β-estradiol in cervical tissue of FvB and K14E7 transgenic mice. We found that the most dramatic changes in gene expression occurred in K14E7 and FvB groups treated with 17β-estradiol. A large number of differentially expressed genes involved in the immune response were observed in 17β-estradiol treated groups. The E7 oncoprotein mainly affected the expression of genes involved in cellular metabolism. Our microarray data also identified differentially expressed genes that have not previously been reported in cervical cancer. The identification of genes regulated by E7 and 17β-estradiol, provides the basis for further studies on their role in cervical carcinogenesis.
Keywords: HPV16 E7, 17β-estradiol, Microarrays, Cervix, Carcinogenesis, K14E7
Introduction
Cervical cancer (CC), a worldwide health problem, has been directly linked to genital infection by high-risk human papilloma-viruses (HR-HPVs) (Munger et al., 2004). The HPV16 is the most prevalent high-risk genotype in cervical carcinomas; it contains three viral oncoproteins, E5, E6 and E7, which are involved in the induction and maintenance of the transformed phenotype (Munger et al., 2004). The HPV16 E7 is considered the major transforming protein given its ability, in cell culture, to immortalize human epithelial cells (Halbert et al., 1991), and, in the context of genetically engineered mice expressing E7, to induce cervical tumors [in cooperation with 17β-estradiol (E2)] as well as to maintain precancerous lesions and cancers once they have arisen (Arbeit et al., 1996; Jabbar et al., 2009; Riley et al., 2003). E7's oncogenic potential is only partially attributed to its inactivation of the retinoblastoma tumor suppressor protein (pRb) (Darnell et al., 2007) given the observation that inactivation of pRb is not sufficient to explain all the oncogenic effects caused by E7 (Balsitis et al., 2006). It is well documented that the transforming activities of the E7 protein are related to the binding of a large number of cellular targets (McLaughlin-Drubin and Munger, 2009). In addition to the HR-HPV E7 oncoprotein, other factors are important in cervical carcinogenesis.
Hormones, like E2, are confirmed cofactors for HPV-related CC (Gariglio et al., 2009). Important epidemiologic studies have indicated the association between oral contraceptive intake and the risk of developing both adenocarcinomas and squamous cell carcinomas (Brisson et al., 1994; Moreno et al., 2002; Salazar et al., 2001). Riley et al. have examined the relationship between E2, E6 or E7 oncoproteins and CC using 7-month-old transgenic mice expressing either E6 or E7 from HPV16 under the control of the human keratin 14 promoter (K14E6 or K14E7 mice, respectively). In this study they treated mice with E2 for six months and then scored the severity of cervical neoplasia. They found that E7 cooperates with E2 to induce high-grade dysplasias, in situ carcinomas and microinvasive cancer, whereas E6 had little effect in the development of CC (Riley et al., 2003). This finding demonstrated that E7 is the most potent oncogene in driving cervical carcinogenesis together with estrogen, but does not discount a role of E6 in cervical cancer per se, as E6 does cooperate with E2 to induce cervical cancer when K14E6 mice are treated with estrogen for longer periods (Shai et al., 2007). Additionally, it has been shown that E2 contributes not only to the onset, but also to the persistence and malignant progression of CC in K14E7 transgenic mice (Brake and Lambert, 2005) and interestingly, E2 fails to promote either, dysplasia or CC in K14E7 mice lacking estrogen receptor (Chung et al., 2008). These studies suggest that E2 has a prominent effect in cell transformation and tumor development, and requires the cooperation of the E7 protein during cervical carcinogenesis.
To identify genes regulated by HPV16 E7 and E2 that may play a role in cervical carcinogenesis, the current study examines the global changes in the pattern of gene expression from cervical tissue of 4- month-old K14E7 transgenic and FvB mice treated with E2, as well as untreated mice. Some of the genes identified in this study were previously reported to be up- or down-regulated in CC, which validate our transgenic model of carcinogenesis; however, the differential expression of many other genes represents novel findings.
We found that immune response and metabolism are among the biological processes most affected in the mouse cervix by E2 and E7. These pathways likely represent early steps in E7 plus E2-induced carcinogenesis. Their identification as such provides important new insights into the molecular mechanisms by which HPV16 E7 and E2 contribute to the development of cervical cancer, and open the possibility for new diagnostic markers of HPV-associated carcinogenesis.
Results
Histological characterization of cervical premalignant lesions
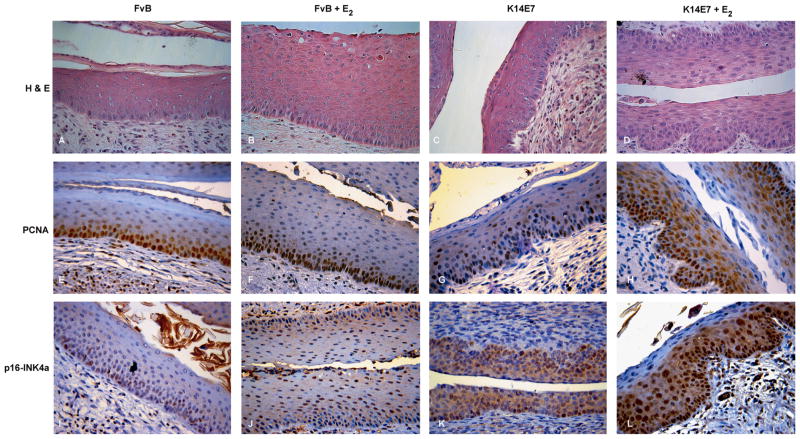
To establish the dysplastic degree or cancer in the mouse cervix, a “histopathological grading system for transgenic mouse cervical squamous carcinogenesis” developed by Riley et al. (2003) was performed (see “Materials and methods” section). Using cervical slides from 4-month-old mice we observed squamous epithelial hyperplasia, with an increase in the number of squamous epithelial cell layers, occasional basal cell mitotic figures and preservation of differentiated suprabasal keratinocytes in FvB+E2 mice (Fig. 1B). In stark contrast, squamous cervical tissue from K14E7+E2 mice contained cells with increased nuclear size, high degree of anaplasia, augmented frequency and distribution of dysplastic cells in the suprabasal layers of the squamous epithelium, and the basal aspect is projected into the cervical stroma (Fig. 1D); all above was previously reported by Elson et al. (2000) and Arbeit et al. (1996) as equivalent to human CIN2 lesions. Moreover, none of untreated mice developed hyperplasia or neoplasia (Fig. 1A and C).
Fig. 1.
Histopathology of mouse cervical tissue and biomarkers expression (PCNA and p16-INK4a). The images correspond to cross-sections of endocervical epithelium from 4-month-old nontransgenic (FvB) and HPV-transgenic mice (K14E7) untreated or treated with 17β-estradiol (E2) for 3 months. The sections were stained with H&E ((A)–(D)) and immunostained for PCNA ((E)–(H)) or p16-INK4a ((I)–(L)), as described under Materials and Methods. The brown signal represents positive cells for PCNA or p16-INK4a. Visual field at 40 × magnification.
To compare the histopathological diagnosis and the expression of established biomarkers for human cervical cancer (Branca et al., 2007; Herbsleb et al., 2001; Middleton et al., 2003), immunohistochemistry assays for PCNA and p16-INK4a were performed. In K14E7+E2 mice, PCNA and p16-INK4a staining was mainly nuclear, augmented and uniformly distributed through the full thickness of the epithelium (Fig. 1H and L). This indicates a generalized high-proliferative activity, similar to the staining pattern that originates in the basal epithelial cells and extends to the upper layers, usually observed in human CIN2 lesions (del Pino et al., 2009; Dray et al., 2005; Kurshumliu et al., 2009; Missaoui et al., 2010). On the contrary, in untreated K14E7 mice, PCNA staining was restricted in its expression to basal and parabasal compartments of the hyperproliferative stratified squamous epithelium (Fig. 1G). Moreover, in the K14E7 mice p16-INK4a staining was diffuse (Fig. 1K); however, the staining intensity was lower than that observed in the K14E7+E2 mice (Fig. 1L). In FvB+E2 mice, the expression of PCNA was only observed in the basal layer (Fig. 1F); and p16-INK4a was expressed in basal and suprabasal compartments (Fig. 1J). In FvB control mice the expression of PCNA and p16-INK4a was restricted to the basal compartment of the stratified squamous epithelium (Fig. 1E and I).
Effect of the E7 oncoprotein and 17β-estradiol on the global gene expression
In order to evaluate the global gene expression profile in cervical tissue induced by a combination of the E7 oncoprotein plus E2 or HPV16-E7 oncoprotein and E2 alone, we used FvB and K14E7 female mice treated with E2 pellets during three months and untreated K14E7 mice, all of them compared with untreated FvB mice (control). After the total RNA from cervix was extracted and converted to cDNA, the groups were examined with Whole Mice Genome Oligo Microarrays (See list of the differentially expressed genes in GEO ID: GSE46890). Using ANOVA, a fold change criteria of ≥2 and ≤−2, and a p-value <0.05, we determined that the most dramatic changes in gene expression occur in the groups treated with E2, independently if the strain corresponds to non-transgenic or K14E7 (Fig. 2A). When comparing gene expression profile among K14E7+E2, K14E7 and FvB+E2 groups, we noted that several genes were overlapped (Supplementary Table 1); therefore, a Venn diagram (Fig. 2B) was constructed to identify common as well as exclusively modulated genes by E7 oncoprotein and/or E2. The comparison between K14E7+E2 and FvB+E2 yielded 70 overlap-ping genes, 9 overlapping genes were found between K14E7+E2 and K14E7 and only 2 genes (Krtap3-3 and Serpinb3b) were overlapped for all groups. Interestingly, 134 (K14E7+E2), 198 (FvB+E2) and 30 (K14E7) genes were exclusively regulated in each group (Supplementary Tables 2–4) as compared to the control mice.
Fig. 2.
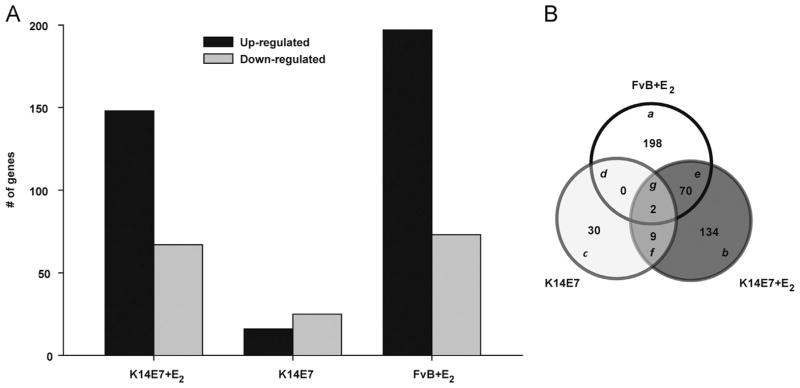
Global expression profile of K14E7+E2, K14E7 and FvB +E2 mice vs untreated FvB mice, respectively. To determine the differentially expressed genes, a fold change of ≥2 and ≤−2, and a p-value cutoff of <0.05 were used. (A) 148, 16 and 197 up-regulated genes and 67, 25 and 73 down-regulated genes in K14E7+E2, K14E7 and FvB +E2 mice vs untreated FvB mice were detected, respectively. (B) The Venn diagram shows unique ((a)–(c)) and common ((d)–(g)) differentially expressed genes between the K14E7+E2, K14E7 and FvB +E2 groups.
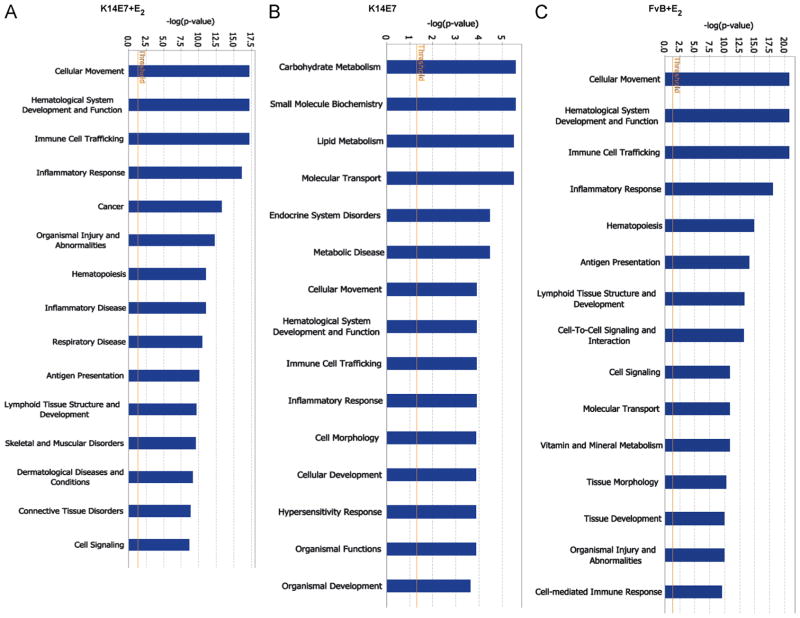
To classify differentially expressed genes involved in cellular processes, we performed a Gene Ontology analysis using Ingenuity Pathway (IPA) software. We observed that several processes associated to immunity (cellular movement, hematological system development, immune cell traffic and inflammatory response) were mainly modified in K14E7+E2 as well as in FvB +E2 mice groups (Fig. 3); whereas carbohydrate metabolism, small molecule biochemistry, lipid metabolism and molecular transport were the most modified processes in the K14E7 mice.
Fig. 3.
Gene ontology-based biological process pathways. For each experimental mice group, the genes were categorized into functional groups by the Ingenuity Pathway Advanced (IPA) software. Closed bars indicate—log (p-value), which was calculated by the IPA software showing the levels of relatedness. The left Y-axis shows the—log (p-value), and the X-axis indicates the name of functions.
Validation of the microarray data
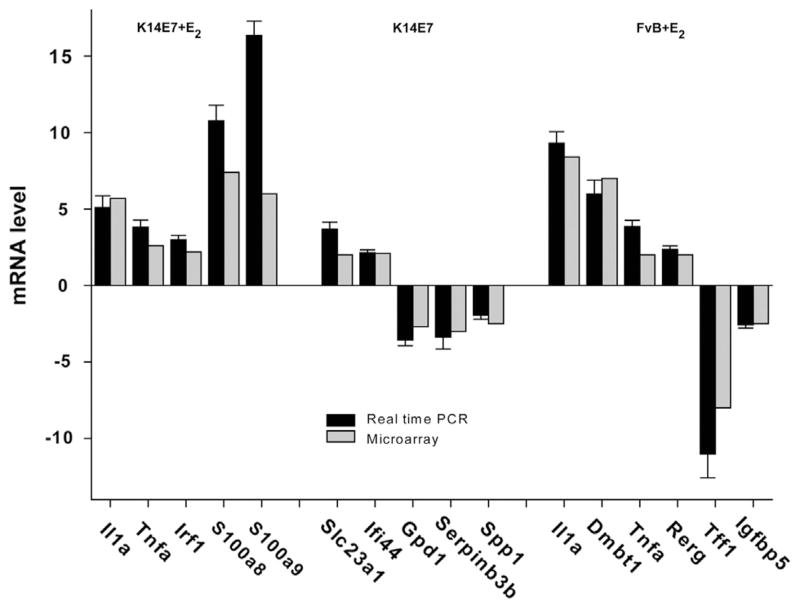
To validate the microarray data, we selected genes involved in different processes and in a variety of cancers. Five specific genes that showed altered expression in K14E7+E2 (Il1a, Tnfa, Irf1, S100a8, and S100a9), five from K14E7 (Slc23a1, Ifi44, Gpd1, Serpinb3b, and Spp1) and six genes from FvB+E2 (Il1a, Dmbt1, Tnfa, Rerg, Tff1, and Igfbp5) were validated by real-time PCR analysis. As shown in Fig. 4, the results for all the analyzed genes were consistent with the microarray data. Among validated genes, S100a8 and S100a9, members of the S100 protein family, were the most up-regulated genes in K14E7+E2 group, whereas that Slc23a1 was the most up-regulated and Gpd1 and Serpinb3b were the most down-regulated in K14E7 group. Otherwise, in the FvB+E2 animals an increased expression of Il1a and Dmbt1 genes and decreased expression of the Tff1 were observed.
Fig. 4.
Validation of microarray data by RT-qPCR. Comparison of the expression levels of selected genes determined by microarray analysis and real time PCR. All qPCR data were normalized with Hprt as a housekeeping gene. The relative expression was determined by 2−Δ ΔCt equation and the untreated FvB mice were used as a calibrator. The RT-qPCR data are expressed as the means±SD of three independent analyses. Black bars represent RT-qPCR values and gray bars represent microarray values.
Estradiol enhances the S100a9 expression
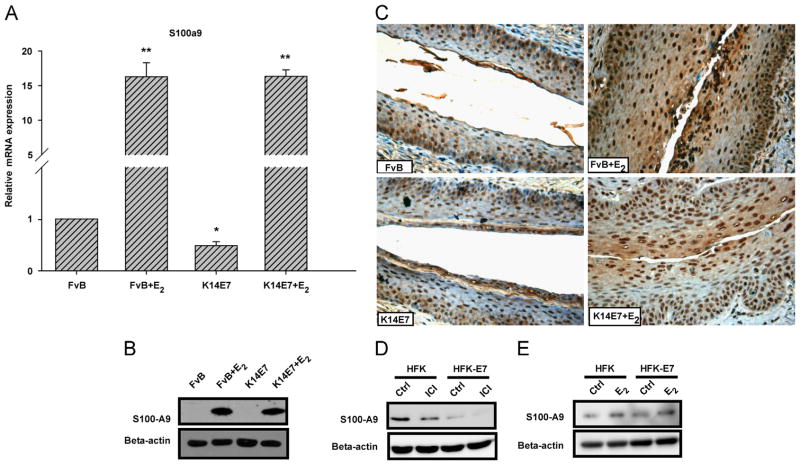
It has been determined that the level of human protein S100-A9 is increased in many cancers and plays a key role in inflammation-associated cancer (Markowitz and Carson, 2013) Since, in the K14E7+E2 the S100a9 gene was one of the most up-regulated genes, as determined by both microarrays and real time PCR, we decided to determine mRNA and protein expression level in endo-cervical epithelium from all experimental groups. Real time PCR and Western blot analysis revealed extensive mRNA and protein expression of S100a9, respectively in FvB+E2 and K14E7+E2 groups (Fig. 5A and B). It is interesting to note that in untreated mice (K14E7 and FvB) the protein was not detected by Western blot. We also evaluated the distribution and expression of protein S100-A9 in cervical squamous tissue.
Fig. 5.
The 17β-estradiol enhances the expression of S100-A9. Quantitative real-time PCR analysis showed a higher mRNA expression of S100a9 inK14E7+E2 and FvB +E2 mice compared to untreated mice (FvB or K14E7) (A). Bars (relative expression) represent the mean±SD of three independent experiments (*p<0.05, **p<0.01, Student's t-test) normalized to Hprt mRNA and compared with the control (untreated FvB mice). Western blot analysis (B) and immunohistochemistry (C) also revealed a marked up-regulated S100-A9 expression in K14E7+E2 and FvB +E2 mice. S100-A9 positive cells in immunohistochemistry show brown staining in the nucleus and cytoplasm. The results show a representative of three experiments, nuclei were counterstained with hematoxylin and visual field at 40 × magnification. Western blot analysis of Human primary and HPV16 E7 transformed keratinocytes treated with vehicle or ICI 182,780 (50 nM for 48 h) (D) and vehicle or E2 (10 nM) (E) for 24 h after 48 h starvation in the absence of any growth factor. Cell fractionation was conducted, and lysates were subjected to SDS-PAGE followed by Western blot with anti-S100-A9 and anti-beta-actin (control) antibodies. All gene and protein symbols were written according to the HUGO Gene Nomenclature Committee (HGNC) and UniProtKB, respectively.
Immunohistochemical staining of cervical tissue sections from E2 treated FvB and K14E7 mice revealed augmented expression of the protein S100-A9; its expression was evident in the cytoplasm and more intense in the nuclei of epithelial cells (Fig. 5C). In untreated K14E7 mice, the expression and localization of this protein was very similar to control FvB mice.
To corroborate the effect of HPV16 E7 oncoprotein and E2 on human S100A9 gene expression, we utilized in vitro assays. Human primary (HFK) and HPV16 E7 transformed keratinocytes (HFK-E7) were cultured in keratinocyte serum free medium and then treated with E2 or an estrogen antagonist (ICI 182,780). We found that protein S100-A9 was drastically down-regulated when the cells were treated with ICI 182,780 in comparison with control cells (Fig. 5D). In contrast, the cells that were treated with E2 showed a slight increase in the expression of protein S100-A9 (Fig. 5E). Additionally, the mRNA levels for S100A9 were measured, but no significant changes were observed between treated and control cells (Data not shown). These results suggest that E2 induces the protein S100-A9 expression in vivo and in vitro.
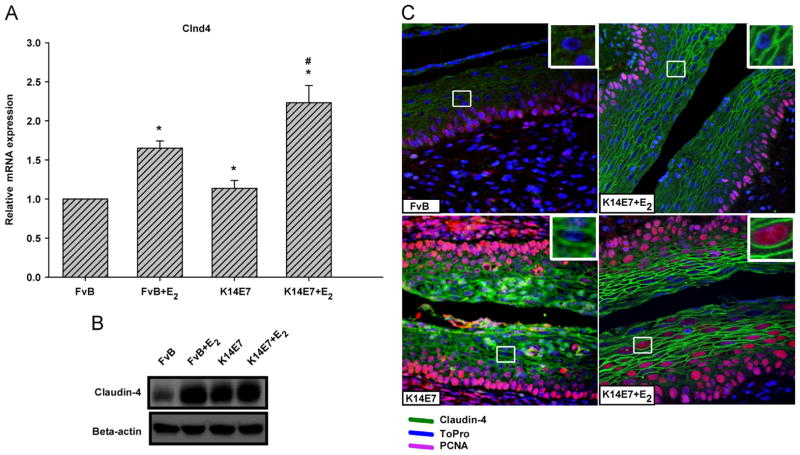
Estradiol and the HPV16 E7 oncoprotein have a significant effect on Claudin 4 expression
Given that the deregulation of the Cldn4 gene product has recently been associated with epithelial cancers and the microarray data of K14E7+E2 mice demonstrated that this gene was up-regulated, we decided to determine the effect of the E7 oncoprotein and/or E2 on Cldn4 gene expression in mouse cervical squamous tissue. We noted that mRNA expression was higher in K14E7+E2 and FvB+E2 mice than in FvB control mice (Fig. 6A). Moreover, in the K14E7 mice, the Cldn4 mRNA expression was very similar to that of FvB control mice. To assess whether the changes seen in mRNA expression of Cldn4 are also observed at the protein level, Western blotting was carried out. The data show that the Claudin-4 protein was increased by E7 and estradiol influence. It is noteworthy that the mRNA and protein expression in FvB+E2 and K14E7+E2 are correlated, but this was not observed for K14E7 mice in which only a high level of Claudin-4 protein was detected (Fig. 6B). The expression and distribution of Claudin-4 was also detected by immunofluorescence. Strikingly increased expression of Claudin-4, delineating the cell borders in the suprabasal layer, was detected in the cervical squamous tissue of the K14E7+E2 group (Fig. 6C). In FvB+E2 the honeycomb pattern was similar to that seen in FvB control; however, expression of Claudin-4 was significantly greater with E2 treatment. The Claudin-4 expression was also enhanced in K14E7 mice, but the honeycomb pattern, observed in the suprabasal layer, was diffuse. Interestingly, in the basal and parabasal layers of FvB control and experimental groups Claudin-4 was absent. These results suggest that the E7 oncoprotein alone or in conjunction with E2 enhance the expression of Claudin-4 only in suprabasal layers of cervical epithelium.
Fig. 6.
Effect of HPV16-E7 and/or 17β-estradiol on the expression of Cldn4. Real time PCR (A) and Western blotting (B) analysis showed an increased expression of the Cldn4 gene in treated mice (K14E7+E2 and FvB +E2), as well as in K14E7 mice compared with the untreated control (FvB). Immunofluorescence with an antibody against Claudin-4 (C) also showed a higher level of Claudin-4 protein in K14E7+E2 and FvB +E2 mice vs untreated FvB mice; in untreated K14E7 mice Claudin-4 was distributed diffusely throughout the plasma membrane (green). Proliferative and non-proliferative cells are shown as pink and blue signal, respectively. For RT-qPCR and Western blot, bars represent the mean±SD of three independent experiments (*p<0.05, **p<0.01, Student's t-test). Data were normalized to Hprt and compared with the control. Visual field at 40 × magnification.
Discussion
The molecular mechanisms by which the HPV16 E7 oncoprotein and the E2 contribute to the early stages of cervical carcino- genesis remain unclear. We took a global approach to identify potential mechanisms, by performing gene expression profiling of cervical tissues from HPV16 E7 transgenic and nontransgenic mice that had or had not been treated with estrogen. We found the largest number of differentially expressed genes when comparing E2-treated mice (K14E7 +E2 or FvB +E2) to untreated mice (K14E7 and FvB). This could be partially explained because the estrogens regulate the transcription of many genes through various mechanisms, such as (1) nuclear activation, including the classical interaction of the activated nuclear receptor with estrogen responsive elements on the DNA; (2) effects through protein–protein interactions with the Sp1, AP1, and NF-kB proteins (Nilsson et al., 2001), and (3) non-nuclear effects through cell surface receptors linked to the mitogen-activated protein kinase pathway (Gruber et al., 2002). The E7 oncoprotein lacks intrinsic enzymatic and specific DNA binding activities (Moody and Laimins, 2010), therefore its ability to alter cellular gene expression must derive from its ability to associate with and subvert the normal activities of cellular regulatory complexes (Ghittoni et al., 2010; McLaughlin-Drubin and Munger, 2009).
Due to the number of pathways affected by E7 and/or E2, it is difficult to discuss each in detail; therefore, we have chosen to discuss the most altered processes in this K14E7 cervical cancer model.
Many of the differentially expressed transcripts encode elements of the immune response, and many of such genes [Il1b, Il1a, Tnfa, Il8rb, Tnfaip3, Cd74, Cd274, S100a8, S100a9, Ccl3, Cd14, Cd3g, Cxcl13, H2-Q6, Iigp1, Inhba, Irg1, Tgtp, and Trem1 (see Supplementary Table 1)] are expressed in common and with similar fold change in both, K14E7 and FvB mice treated with E2. Previous reports have demonstrated that some genes like Il1b (Polan et al., 1988; Ruh et al., 1998), Tnfa, Tnfaip3 (Vendrell et al., 2007), Il8rb (Cxcr2) (Lei et al., 2003) and S100a9 (Stygar et al., 2007) are modulated by estrogen and its expression and secretion appears to depend on the cell types, milieu conditions, and the estrogen concentrations. Therefore, it is likely that in K14E7 mice treated with E2, the expression of genes encoding components of the immune response is primarily being modified by E2 rather than by the E7 oncoprotein.
It is also interesting to note that some genes up-regulated by E2 mentioned above (Il1b, Tnfa, Cd74, Cd274, S100a8 and S100a9) have been reported to be overexpressed in cervical cancer (Al-Tahhan et al., 2011; Cheng et al., 2011a; Choi et al., 2007; Karim et al., 2009; Manavi et al., 2007; Zhu et al., 2013). In these studies, however, the influence of estrogens was not evaluated. Some of the up-regulated genes that belong to the immune response such as Ccl3, Cxcl13, Il8rb (Cxcr2) (expressed by both the K14E7 and the FvB treated mice) have not been reported in cervical cancer, but these genes have been reported to be up-regulated in other cancers in which one finds inflammation. For example, Ccl3, Cxcl13, Il8rb (Cxcr2) and Inhba over-expression has been detected in oral squamous cell carcinoma (Silva et al., 2007), breast epithelial tumor cells (Panse et al., 2008), gastric cancer (Cheng et al., 2011b; Wang et al., 2012) and lung adenocarcinoma (Seder et al., 2009), respectively. These results raise the possibility that E2 may contribute to the differential expression of these genes and therefore contribute to modulation of the inflammatory process during cervical carcinogenesis in the K14E7+E2 mouse model.
Given the potential importance of immune responses and inflammation in cancer, we decided to validate the expression of one of the inflammation-associated genes, S100-A9, a calcium-and zinc-binding protein that belongs to the S100/calgranulins family and is found mainly in a variety of immune cells as well as in squamous epithelium. Human protein S100-A9 has antimicrobial, cytostatic, antiproliferative, apoptosis-inducing and chemo-tactic properties (Srikrishna, 2012). In the present study, we found overexpression of S100a9 gene in the E2-treated mice groups at both the mRNA and the protein level. In HFK cells, the treatment with an estrogen antagonist decreased drastically the level of S100-A9 protein, in agreement with our results in the mouse model, suggesting that E2 could be involved in the up-regulation of S100a9 in vivo.
This gene may be responding indirectly to E2, through inflammatory stimuli such as Interleukin-1 beta (Bando et al., 2007) or tumor necrosis factor (Hiratsuka et al., 2006) which promote the expression and the formation of protease-resistant S100-A9 homodimers (Riva et al., 2013). In this context, it is interesting to note that Il1b and Tnfa genes were found up-regulated in the E2 treated groups; therefore, these cytokines may be directly regulating the expression of S100a9 in the cervix.
Recently, protein S100-A9 over-expression has been reported in CIN 1, CIN 2, CIN 3 and squamous cervical cancer (Zhu et al., 2013, 2009). In other tumors it has been associated with development, invasion or metastasis (Ichikawa et al., 2011; Kallberg et al., 2012; Li et al., 2012). There are also reports that suggest that the complex S100A8/A9 induces apoptosis and inhibits metastasis in the CaSki cell line, and also reduces cell growth in other cell lines derived from cervical cancer (Qin et al., 2010; Tugizov et al., 2005); however, these effects have not yet been established in vivo.
A second set of genes we found altered in their expression are involved in metabolism, such as Ucp1, Cox7a1 and Cox8b, which were down-regulated in K14E7 and K14E7+E2 mice. The Ucp1 protein product, which is located in the mitochondria of brown adipocytes, acts as a proton carrier activated by free fatty acids and enhances respiration and cellular heat production (Mailloux and Harper, 2011). Over-expression of Ucp1 results in an increased oxygen consumption and decreased tumor growth (Chen et al., 2009; Lee et al., 2012). Ucp3 (a homologous to Ucp1), also increases the mitochondrial respiration and is associated with resistance to chemically-mediated multistage skin carcinogenesis (Lago et al., 2012). Other genes reduced in their expression in E7 transgenic mice that also participate in the respiratory chain are Cox7a1 and Cox8b, subunits of cytochrome C oxidase (COX). Suppression of mitochondrial respiration and COX activity (by inhibiting the expression of COX6C, COX7A, and COX7C subunits), as well as the induction of the glycolytic switch are mediated by induction of the Wnt pathway (Lee et al., 2012). These findings suggest that in our mice models (K14E7+E2 and K14E7), the down-regulation of Ucp1, Cox7a1 and Cox8b might decrease mitochondrial respiration, promote the glycolytic activity and increase tumor growth.
It is known that HPV16 E7 perturbs the keratinocytes differentiation program and allow DNA synthesis to occur in a subset of suprabasal cells of the cervical epithelial tissue (Flores et al., 2000). In agreement with this issue, we noted that PCNA, a marker of non-differentiated proliferative cells, is markedly increased by the E7 oncoprotein (K14E7+E2 and K14E7 mice) in the basal and suprabasal compartments of the cervical tissue; besides, it is interesting to note that a cluster of keratinocyte differentiation-related genes (late cornified envelope genes, precursors of the cornified envelope of the stratum corneum) were significantly down-regulated in K14E7 and K14E7+E2 mice. These findings confirm and extend the previous observations that genes involved in epithelial differentiation are down-regulated by HPV16 E7 expression, uncoupling the proliferation and differentiation processes (Gyongyosi et al., 2012; Santin et al., 2005).
We cannot rule out the possibility that changes in gene expression do not represent direct effects of E7 and/or estrogen, but rather indirect effects that reflect expansion of cells populations that remain proliferatively active and/or resistant to terminal differentiation. For example, HPV16 E7 and estradiol stimulate the production of cytokines like Il1a (previously mentioned) which in turn induces expression of polypeptide growth factors that stimulate proliferation of normal keratinocytes and carcinoma cells through autocrine and paracrine pathways (Bando et al., 2007; Castrilli et al., 1997). Also, it is not known that the E7 oncoprotein and estradiol regulate directly the expression of late cornified envelope genes, precursors of the cornified envelope of the stratum corneum (discussed above). These changes may reflect secondary effects of these factors.
Another up-regulated gene in K14E7+E2 mice was Cldn4. Claudin-4 is a component of tight junctions, which plays an important role in tumor cell invasion and metastasis (Shang et al., 2012). The tight junction proteins have been shown to be deregulated in a number of epithelial cancers. Whereas the expression of Claudin proteins is up-regulated in certain cancers including prostate cancer (Long et al., 2001) and pancreatic cancer (Nichols et al., 2004), Claudin proteins can also be down-regulated in other cancers such as head and neck cancer (Al Moustafa et al., 2002) and breast cancer (Kominsky et al., 2003). Moreover, over-expression of Claudin 3 and Claudin 4 both at the mRNA and protein levels has been observed in ovarian cancer (Agarwal et al., 2005). Forced expression of Claudin-4, in cells that do not normally express Claudin-4, increased cell motility. Recently, it has been shown that Claudin-4 can also promote motility in cells that normally express Claudin-4 and that both normal cells and different types of tumor cells exhibit the same phenomenon (Webb et al., 2013). These studies have led to the suggestion that Claudin proteins, either alone or in combination with other proteins, may represent useful biomarkers for the detection and diagnosis of certain cancers (Agarwal et al., 2005). In the present study we learned that HPV16 E7 and/or E2 increased Claudin-4 expression. Interestingly, we observed that this overexpression is limited to the suprabasal cells of stratified epithelium (see Fig. 6C). These results are consistent with another report that found the expression of Claudin-4 was exclusively in suprabasal layers of CIN lesions (Sobel et al., 2005). These data denoted a disturbance in cell proliferation and differentiation, so the Claudin-4 overexpres-sion could be an important marker of the interruption of normal differentiation and an early marker in cervical cancer.
We recognize that we are unable to strictly associate the observed changes in gene expression to a particular cell type. However, we found that data obtained from certain genes, such as Claudins (e.g. Cldn4) (Morin, 2005; Tsukita and Furuse, 2002) will likely agree with those of microdissected epithelium due to their preferential expression in epithelial cells. As a support of this observation, many genes analyzed in our work (see Supplementary Table 5) have been reported with similar expression profile in microdissected high-grade squamous intraepithelial lesions and cervical squamous cell carcinomas (Gius et al., 2007; Zhai et al., 2007). Only two genes differentially expressed in stromal tissue (Arhgdib, Igfbp5) were matched in our work (Gius et al., 2007). Therefore, the study of HPV16 E7 oncoprotein, estradiol, or a combination of both factors in the entire cervix might provide information similar to those of microdissected tissue, which may cooperate during cervical carcinogenesis. In this regard, it is relevant to note that we recently determined that the effects of estrogen on cervical carcinogenesis are mediated at least in part by estrogen/estrogen receptor α-mediated signaling within the cervical stroma (Chung et al., 2013).
In summary, the results presented here suggest that the HPV16 E7 oncoprotein mainly contribute to cervical carcinogenesis by editing the metabolism and E2 mainly modified processes associated to immunity. It is important to note that we also identified a number of aberrantly regulated genes previously reported to be involved in the pathogenesis of cervical cancer. In addition, we identified new genes which could play a role in the cervical tumorigenesis and might offer the potential of developing new diagnostic markers and therapeutic targets.
Materials and methods
Mouse models and hormone treatment
The K14E7 and FvB mice have been described previously (Riley et al., 2003). All the mice were housed and treated according to the American Association of Laboratory Animal Care (AALAC), and all experiments and procedures were approved by the Research Unit for Laboratory Animal Care Committee (UPEAL-CINVESTAV-IPN, Mexico; NOM-062-ZOO-1999). Hormone treatment was carried out for three months with E2 pellets, one-month virgin female transgenic and nontransgenic mice were implanted in the dorsal skin with continuous release pellets delivering 0.05 mg of E2 over 60 days (Innovative Research of America). To complete the treatment two pellets were used and then the mice were sacridiced to 4-month-old. We also used K14E7 and FvB (control) untreated mice of the same age.
Tissue procurement and histopathology
K14E7 hemizygote and nontransgenic FvB control virgin female untreated and treated mice were sacrificed by cervical dislocation. All specimens were formalin-fixed and paraffin embedded. Sections were deparaffinized and rehydrated as described previously (Ibarra Sierra et al., 2012). Serial sections were cut (5 μm thick) and stained with H&E (haematoxylin and eosin).
A histopathological grading system for transgenic mouse cervical squamous carcinogenesis developed by Riley et al. was used to classify histological samples. Using this mouse cervical neoplasia grading system, CIN1 consists of a 2-fold increase in the basal/ basaloid cell layers of cervical and vaginal squamous epithelia of transgenic compared with estrogen-treated nontransgenic mice. CIN2 lesions contain cells with additional increases in nuclear size, degree of anaplasia, frequency, and distribution of dysplastic cells in the suprabasal layers of the squamous epithelium. Moreover, the basal aspect of the squamous epithelium is projected into papillary folds projecting into the underlying vaginal or cervical stroma. CIN3 lesions contain abundant anaplastic cells, some with pronounced increases in nuclear size. CIS demonstrates a pronounced degree of remodeling and undulation of the epithelial–stromal border and most of the cellular features of well-differentiated squamous carcinoma, with retention of an intact basement membrane without evidence of microinvasion on serial sections.
Microarray sample processing
K14E7 hemizygote and nontransgenic FvB control virgin female untreated and treated mice were sacrificed by cervical dislocation. Cervical biopsies were immediately stored in RNA later Solution (Ambion) at 4 °C overnight. Tissue was recovered from RNA later solution with sterile forceps, quickly blotted to remove excess RNA later and immediately snap frozen in liquid nitrogen. Total RNA was extracted from snap frozen tissue using standard procedures (TRIzol reagent, Ambion). Total RNA collected from nine female mice of each group was extracted. RNA quantity and quality were assessed on an Agilent 2100 Bioanalyzer (Agilent Technologies). Only RNA samples with a RNA Integrity Number greater than 8.0 were further processed for microarray analysis. cDNA synthesis, ampliflcation, and gene expression profiling were performed according to the manufacturer's instructions (Affymetrix WT Sense Target labeling assay Manual). The RNA from three different mice was pooled and three independents pools were used by each group. Each micro-array experiment was performed as biological triplicates for each group using GeneChip Mouse Gene 1.0 ST Array.
Analysis array data
Signal intensities from each array were analyzed using Partek Genomic Suite version 6.4 (Partek). Raw intensity probe values were normalized using robust multiarray analysis background correction (RMA). A two way ANOVA was performed to identify differentially expressed genes. Only genes with statistically significant differences in expression levels (p-value <0.05) and a fold change criteria of ≥2 and ≤ −2 were included in the final set of differentially expressed genes. The microarray data were deposited to the NCBIGEO database [GEOID: GSE46890]. To identify those biological processes altered by E7 and/or E2, we used Ingenuity Pathway software (Ingenuity Systems), a bioinformatic tool for visualizing expression data in the context of KEGG-defined biological pathways. All human and mouse gene and protein symbols were written according the HUGO Gene Nomenclature Committee (HGNC) and UniProtKB, respectively.
Relative mRNA quantification by real-time quantitative PCR and data analysis using the 2−Δ ΔCt method
Isolated RNA from six mice from each group (K14E7, FvB, K14E7+E2 and FvB +E2) was purified and its quality determined by electrophoresis on a 2% agarose gel and visualization of ribosomal RNA by ethidium bromide staining. RNA was quantified by spectrophotometric analysis at 260 nm and 280 nm. cDNA synthesis was done as described according to manufacturer's instructions (Invitrogen). Quantitative real-time PCR (RT-qPCR) was carried out using a LightCycler 2.0 apparatus (Roche) and a DNA Master SYBR Green I kit (Roche). The templates were amplified in 45 cycles of a 3-step PCR process, which included 30 s of denaturation step at 95 °C, a 30-s primer-dependent annealing phase (60 °C), and a 30-s template-dependent elongation at 72 °C. Each gene-specific RNA was quantified in triplicate by real time PCR and mRNA ratios relative to the house-keeping gene HPRT were calculated for standardization of gene expression levels across samples using the Ct method. All primer sequences and product size are described in Table 1
Table 1.
List of primer sequences for real time PCR used to validate the microarrays.
| * Gene symbol | Forward | Reverse | Amplicon size (pb) |
|---|---|---|---|
| Il1a | TCACCTTCAAGGAGAGCCG | ATCTGGGTTGGATGGTCTCTT | 150 |
| Tnfa | CATCTTCTCAAAATTCGAGTGACAA | TGGGAGTAGACAAGGTACAACCC | 175 |
| Irf1 | AGCATAGTCCCACTGCAAACAG | GCCTCTGCCTTACACCTCAGA | 150 |
| S100a8 | AAATCACCATGCCCTCTACAAG | CCCACTTTTATCACCATCGCAA | 165 |
| S100a9 | ATACTCTAGGAAGGAAGGACACC | TCCATGATGTCATTTATGAGGGC | 129 |
| Slc23a1 | AAAGCAGCATGAGGTCGTGG | ACTGAAGCACGTCAGGTAATG | 163 |
| I344 | AACTGACTGCTCGCAATAATGT | GTAACACAGCAATGCCTCTTGT | 102 |
| Gpd1 | ATGGCTGGCAAGAAAGTCTG | CGTGCTGAGTGTTTGATGATCT | 175 |
| Serpinb3b | CATGCAACAGAAGAGAGCGAA | GCTACTGCTTAGGCTCCCAC | 102 |
| Spp1 | AGCAAGAAACTCTTCCAAGCAA | GTGAGATTCGTCAGATTCATCCG | 134 |
| Dmbt1 | ACCTTCAGTCCATGGGCTATTC | TCTCGTTGTCAGCCTGTTTGA | 150 |
| Rerg | GCAAGTCAGCGATTGTAGTGA | CTTCATCGTCTATGGTTGCCTG | 105 |
| Tff1 | TTCCCGTGAAGCTGCCATGG | TGTTTCTTCCTGGGCCTGGG | 115 |
| Igfbp5 | GGTGTGTGGACAAGTACGGAATGA | ACGTTACTGCTGTCGAAGGCGT | 88 |
| Hprt | GTAATGATCAGTCAACGGGG | CCAGCAAGCTTGCAACCTTAAC | 177 |
Official gene symbol by HUGO Gene Nomenclature Committee (HGNC).
Immunochemistry and immunofluorescence procedures
The cervical sections were processed for immunohistochemical staining. The protein detection for immunohistochemistry was conducted using the Mouse/Rabbit PolyDetector HRP/DAB Detection System (Bio SB) according to manufacturer's recommendations and HPR conjugated anti-rat antibody. The samples were incubated overnight with primary antibodies against PCNA, p16-INK4a, (Santa Cruz Biotechnology) Claudin-4 (Invitrogen) or protein S100-A9 (Abcam). Anti-rat HPR conjugated was used as secondary antibody for detection of protein S100-A9 antibody. Following the immunohistochemical procedures, the tissues were counterstained with hematoxylin and mounted in GVA-mount reagent (Zymed). For the immunofluorescence procedures, the cervical sections were rinsed in 1 × PBS and blocked for 2 h at 4 °C with 1 × PBS that was supplemented with 0.3% Triton X-100 and 1% bovine serum albumin; they were washed three times with 1 × PBS and incubated for 1 h at 37 °C with an anti-Claudin 4 antibody (Santa Cruz Biotechnology). The sections were then incubated with a fluorescein isothiocyanate (FITC)-labeled secondary antibody (Zymed) for 30 min at room temperature; they were rinsed above, counterstained with propidium iodide, and mounted in Vectashield (Vector). The preparations were examined by confocal microscopy using an SP2 (Leica Microsystems). Captured images were imported into Adobe Photoshop CS6 (Adobe Systems) to produce maximum projections.
Cell culture
Human foreskin primary and HPV16 E7 transformed keratino-cytes were cultured in keratinocyte serum free medium containing recombinant epidermal growth factor and bovine pituitary extract as supplements (Gibco, Life Technologies).
Western blot
The whole cervices were subjected to total protein extraction and 20 μg were used to SDS-PAGE and transferred to an Immobilon-P membrane (Millipore). The membrane was blocked in 5% nonfat dry milk in 1 × TBS (20 mmol/L Tris–HCl, pH 7.5, 150 mmol/L NaCl, and 0.5% Tween-20) for 1 h, blotted with an anti-S100-A9 (1:2000) or anti-Claudin-4 (1:1000) primary antibodies overnight, and then incubated with a horseradish peroxidase (HRP)-linked anti-rat or anti-rabbit secondary antibody (GE Healthcare) for 1 h. The membranes were developed using the Millipore Immobilon Western Chemiluminescent HRP Substrate according to the manufacturer's instructions. Chemiluminescence was detected using a FujiFilm LAS-3000 imaging system.
Human foreskin primary and HPV16 E7 transformed keratinocytes were treated with vehicle (0.5% ethanol) or 50 nM ICI 182,780 (Tocris Bioscience) for 48 h. ICI 182,780 stock solution was prepared by dissolving in ethanol at a 10 μM concentration. For β-estradiol treatment, cells were first serum starved for 48 h, then treated with β-estradiol (10 nM) for 24 h. Water-soluble 17-β estradiol (Sigma-Aldrich) was prepared fresh by dissolving in keratinocyte serum free medium as a 10 mM stock solution (106 × fold). Western blot analysis was performed under conventional conditions. Cells grown to 80% confluency were lysed with cell lysis buffer (1% Triton X-100, 100 mM NaCl, 50 mM HEPES, pH 7.9, 10 mM EDTA, 4 mM NaPP, 10 mM NaF, 2 mM vanadate, 1 mM PMSF, 2 μg/mL aprotinin and 2 μg/mL leupeptin). Total protein (50 μg) was loaded into each well, resolved by 12% SDS-polyacrylamide gel electrophoresis (PAGE), and then electro-blotted onto Immobilon-P membrane (Millipore). Anti-hS100A9 (B-5) (Santa Cruz Biotechnology) antibody (1:300) was added to the membrane in 5% nonfat dry milk in 1 × TTBS for 2 h. The membrane was then washed three times in 1 × TTBS and HRP-linked anti-mouse secondary antibody (GE Healthcare) was added for 1 h. Actin HRP-linked primary antibody (Santa Cruz Biotech-nology) was utilized as a control. Blots were washed three times in 1 × TTBS and then developed using Millipore Immobilon Western Chemiluminescent HRP Substrate per the manufacturer's instructions (Millipore). Chemiluminescence was detected using a Fuji-film LAS-3000 imaging system.
Statistical analysis
For all data comparison, the Student's t-test was performed using Microsoft Excel. A p value of <0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
The authors would like to thank to Raúl Mojica (INMEGEN), Laura Uribe Figueroa (INMEGEN), Enrique García Villa, Elizabeth Álvarez Ríos, Rodolfo Ocádiz Delgado and Lauro Macias Gonzales for technical support. P.G. was supported by grants from the ICYT326/11 to perform this work. This research was supported in part by the Intramural Research Program of the NIH, NCI and CONACYT (D.M.V.). P.F.L. was supported by grants from the NIH: R01CA120847 and P01CA022443. This study was performed in partial fulfillment of the requirement for the doctoral degree of E.M.C.M. in Biomedical Sciences at Universidad Nacional Autonóma de México.
Appendix A. Supplementary material
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.virol.2013.08.036.
Contributor Information
Enoc M. Cortés-Malagón, Email: enoc.cortes@salud.gob.mx.
José Bonilla-Delgado, Email: jose.bonilla@salud.gob.mx.
José Díaz-Chávez, Email: josediaz030178@hotmail.com.
Alfredo Hidalgo-Miranda, Email: ahidalgo@inmegen.gob.mx.
Sandra Romero-Cordoba, Email: sromero_cordoba@hotmail.com.
Aykut Üren, Email: au26@georgetown.edu.
Haydar Çelik, Email: hc547@georgetown.edu.
Matthew McCormick, Email: mjm444@georgetown.edu.
José A. Munguía-Moreno, Email: antoniomgeminis@yahoo.com.mx.
Eloisa Ibarra-Sierra, Email: eibarra@cinvestav.mx.
Jaime Escobar-Herrera, Email: moemras@yahoo.com.
Paul F. Lambert, Email: plambert@wisc.edu.
Daniel Mendoza-Villanueva, Email: daniel.mendozavillanueva@nih.gov.
Rosa M. Bermudez-Cruz, Email: roberm@cinvestav.mx.
Patricio Gariglio, Email: vidal@cinvestav.mx.
References
- Agarwal R, D'Souza T, Morin PJ. Claudin-3 and claudin-4 expression in ovarian epithelial cells enhances invasion and is associated with increased matrix metalloproteinase-2 activity. Cancer Res. 2005;65 (16):7378–7385. doi: 10.1158/0008-5472.CAN-05-1036. [DOI] [PubMed] [Google Scholar]
- Al-Tahhan MA, Etewa RL, El Behery MM. Association between circulating interleukin-1 beta (IL-1beta) levels and IL-1beta C-511T polymorphism with cervical cancer risk in Egyptian women. Mol Cell Biochem. 2011;353 (1–2):159–165. doi: 10.1007/s11010-011-0782-9. [DOI] [PubMed] [Google Scholar]
- Al Moustafa AE, Alaoui-Jamali MA, Batist G, Hernandez-Perez M, Serruya C, Alpert L, Black MJ, Sladek R, Foulkes WD. Identification of genes associated with head and neck carcinogenesis by cDNA microarray comparison between matched primary normal epithelial and squamous carcinoma cells. Oncogene. 2002;21 (17):2634–2640. doi: 10.1038/sj.onc.1205351. [DOI] [PubMed] [Google Scholar]
- Arbeit JM, Howley PM, Hanahan D. Chronic estrogen-induced cervical and vaginal squamous carcinogenesis in human papillomavirus type 16 transgenic mice. Proc Nat Acad Sci USA. 1996;93 (7):2930–2935. doi: 10.1073/pnas.93.7.2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsitis S, Dick F, Dyson N, Lambert PF. Critical roles for non-pRb targets of human papillomavirus type 16 E7 in cervical carcinogenesis. Cancer Res. 2006;66 (19):9393–9400. doi: 10.1158/0008-5472.CAN-06-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bando M, Hiroshima Y, Kataoka M, Shinohara Y, Herzberg MC, Ross KF, Nagata T, Kido J. Interleukin-1alpha regulates antimicrobial peptide expression in human keratinocytes. Immunol Cell Biol. 2007;85 (7):532–537. doi: 10.1038/sj.icb.7100078. [DOI] [PubMed] [Google Scholar]
- Brake T, Lambert PF. Estrogen contributes to the onset, persistence, and malignant progression of cervical cancer in a human papillomavirus-transgenic mouse model. Proc Nat Acad Sci USA. 2005;102 (7):2490–2495. doi: 10.1073/pnas.0409883102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branca M, Ciotti M, Giorgi C, Santini D, Di Bonito L, Costa S, Benedetto A, Bonifacio D, Di Bonito P, Paba P, Accardi L, Syrjanen S, Favalli C, Syrjanen K. Up-regulation of proliferating cell nuclear antigen (PCNA) is closely associated with high-risk human papillomavirus (HPV) and progression of cervical intraepithelial neoplasia (CIN), but does not predict disease outcome in cervical cancer. Eur J Obstet Gynaecol Reprod Biol. 2007;130 (2):223–231. doi: 10.1016/j.ejogrb.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Brisson J, Morin C, Fortier M, Roy M, Bouchard C, Leclerc J, Christen A, Guimont C, Penault F, Meisels A. Risk factors for cervical intraepithelial neoplasia: differences between low- and high-grade lesions. Am J Epidemiol. 1994;140 (8):700–710. doi: 10.1093/oxfordjournals.aje.a117318. [DOI] [PubMed] [Google Scholar]
- Castrilli G, Tatone D, Diodoro MG, Rosini S, Piantelli M, Musiani P. Interleukin 1alpha and interleukin 6 promote the in vitro growth of both normal and neoplastic human cervical epithelial cells. Br J Cancer. 1997;75 (6):855–859. doi: 10.1038/bjc.1997.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Cairns R, Papandreou I, Koong A, Denko NC. Oxygen consumption can regulate the growth of tumors, a new perspective on the Warburg effect. PLoS One. 2009;4 (9):e7033. doi: 10.1371/journal.pone.0007033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng RJ, Deng WG, Niu CB, Li YY, Fu Y. Expression of macrophage migration inhibitory factor and CD74 in cervical squamous cell carcinoma. Int J Gynecol Cancer. 2011a;21 (6):1004–1012. doi: 10.1097/IGC.0b013e31821c45b7. [DOI] [PubMed] [Google Scholar]
- Cheng WL, Wang CS, Huang YH, Tsai MM, Liang Y, Lin KH. Overexpression of CXCL1 and its receptor CXCR2 promote tumor invasion in gastric cancer. Ann Oncol. 2011b;22 (10):2267–2276. doi: 10.1093/annonc/mdq739. [DOI] [PubMed] [Google Scholar]
- Choi YW, Kim YW, Bae SM, Kwak SY, Chun HJ, Tong SY, Lee HN, Shin JC, Kim KT, Kim YJ, Ahn WS. Identification of differentially expressed genes using annealing control primer-based GeneFishing in human squamous cell cervical carcinoma. Clin Oncol (R Coll Radiol) 2007;19 (5):308–318. doi: 10.1016/j.clon.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Chung SH, Shin MK, Korach KS, Lambert PF. Requirement for stromal estrogen receptor alpha in cervical neoplasia. Horm Cancer. 2013;4 (1):50–59. doi: 10.1007/s12672-012-0125-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SH, Wiedmeyer K, Shai A, Korach KS, Lambert PF. Requirement for estrogen receptor alpha in a mouse model for human papillomavirus-associated cervical cancer. Cancer Res. 2008;68 (23):9928–9934. doi: 10.1158/0008-5472.CAN-08-2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell GA, Schroder WA, Antalis TM, Lambley E, Major L, Gardner J, Birrell G, Cid-Arregui A, Suhrbier A. Human papillomavirus E7 requires the protease calpain to degrade the retinoblastoma protein. J Biol Chem. 2007;282 (52):37492–37500. doi: 10.1074/jbc.M706860200. [DOI] [PubMed] [Google Scholar]
- del Pino M, Garcia S, Fuste V, Alonso I, Fuste P, Torne A, Ordi J. Value of p16(INK4a) as a marker of progression/regression in cervical intraepithelial neoplasia grade 1. Am J Obstet Gynecol. 2009;201(5):488, e1–7. doi: 10.1016/j.ajog.2009.05.046. [DOI] [PubMed] [Google Scholar]
- Dray M, Russell P, Dalrymple C, Wallman N, Angus G, Leong A, Carter J, Cheerala B. p16(INK4a) as a complementary marker of high-grade intraepithelial lesions of the uterine cervix. I: Experience with squamous lesions in 189 consecutive cervical biopsies. Pathology. 2005;37 (2):112–124. doi: 10.1080/00313020500058607. [DOI] [PubMed] [Google Scholar]
- Elson DA, Riley RR, Lacey A, Thordarson G, Talamantes FJ, Arbeit JM. Sensitivity of the cervical transformation zone to estrogen-induced squamous carcinogenesis. Cancer Res. 2000;60 (5):1267–1275. [PubMed] [Google Scholar]
- Flores ER, Allen-Hoffmann BL, Lee D, Lambert PF. The human papillo-mavirus type 16 E7 oncogene is required for the productive stage of the viral life cycle. J Virol. 2000;74 (14):6622–6631. doi: 10.1128/jvi.74.14.6622-6631.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariglio P, Gutierrez J, Cortes E, Vazquez J. The role of retinoid deficiency and estrogens as cofactors in cervical cancer. Arch Med Res. 2009;40 (6):449–465. doi: 10.1016/j.arcmed.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Ghittoni R, Accardi R, Hasan U, Gheit T, Sylla B, Tommasino M. The biological properties of E6 and E7 oncoproteins from human papillomaviruses. Virus Genes. 2010;40 (1):1–13. doi: 10.1007/s11262-009-0412-8. [DOI] [PubMed] [Google Scholar]
- Gius D, Funk MC, Chuang EY, Feng S, Huettner PC, Nguyen L, Bradbury CM, Mishra M, Gao S, Buttin BM, Cohn DE, Powell MA, Horowitz NS, Whitcomb BP, Rader JS. Profiling microdissected epithelium and stroma to model genomic signatures for cervical carcinogenesis accommodating for covariates. Cancer Res. 2007;67 (15):7113–7123. doi: 10.1158/0008-5472.CAN-07-0260. [DOI] [PubMed] [Google Scholar]
- Gruber CJ, Tschugguel W, Schneeberger C, Huber JC. Production and actions of estrogens. N Engl J Med. 2002;346 (5):340–352. doi: 10.1056/NEJMra000471. [DOI] [PubMed] [Google Scholar]
- Gyongyosi E, Szalmas A, Ferenczi A, Konya J, Gergely L, Veress G. Effects of human papillomavirus (HPV) type 16 oncoproteins on the expression of involucrin in human keratinocytes. Virol J. 2012;9:36. doi: 10.1186/1743-422X-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbert CL, Demers GW, Galloway DA. The E7 gene of human papillo-mavirus type 16 is sufficient for immortalization of human epithelial cells. J Virol. 1991;65 (1):473–478. doi: 10.1128/jvi.65.1.473-478.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbsleb M, Knudsen UB, Orntoft TF, Bichel P, Norrild B, Knudsen A, Mogensen O. Telomerase activity, MIB-1, PCNA, HPV 16 and p53 as diagnostic markers for cervical intraepithelial neoplasia. APMIS: Acta Pathol Microbiol Immunol Scand. 2001;109:607–617. doi: 10.1034/j.1600-0463.2001.d01-182.x. [DOI] [PubMed] [Google Scholar]
- Hiratsuka S, Watanabe A, Aburatani H, Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol. 2006;8 (12):1369–1375. doi: 10.1038/ncb1507. [DOI] [PubMed] [Google Scholar]
- Ibarra Sierra E, Diaz Chavez J, Cortes-Malagon EM, Uribe-Figueroa L, Hidalgo-Miranda A, Lambert PF, Gariglio P. Differential gene expression between skin and cervix induced by the E7 oncoprotein in a transgenic mouse model. Virology. 2012;433 (2):337–345. doi: 10.1016/j.virol.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa M, Williams R, Wang L, Vogl T, Srikrishna G. S100A8/A9 activate key genes and pathways in colon tumor progression. Mol Cancer Res. 2011;9 (2):133–148. doi: 10.1158/1541-7786.MCR-10-0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbar SF, Abrams L, Glick A, Lambert PF. Persistence of high-grade cervical dysplasia and cervical cancer requires the continuous expression of the human papillomavirus type 16 E7 oncogene. Cancer Res. 2009;69 (10):4407–4414. doi: 10.1158/0008-5472.CAN-09-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallberg E, Vogl T, Liberg D, Olsson A, Bjork P, Wikstrom P, Bergh A, Roth J, Ivars F, Leanderson T. S100A9 interaction with TLR4 promotes tumor growth. PLoS One. 2012;7 (3):e34207. doi: 10.1371/journal.pone.0034207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim R, Jordanova ES, Piersma SJ, Kenter GG, Chen L, Boer JM, Melief CJ, van der Burg SH. Tumor-expressed B7-H1 and B7-DC in relation to PD-1+ T-cell infiltration and survival of patients with cervical carcinoma. Clin Cancer Res. 2009;15 (20):6341–6347. doi: 10.1158/1078-0432.CCR-09-1652. [DOI] [PubMed] [Google Scholar]
- Kominsky SL, Argani P, Korz D, Evron E, Raman V, Garrett E, Rein A, Sauter G, Kallioniemi OP, Sukumar S. Loss of the tight junction protein claudin-7 correlates with histological grade in both ductal carcinoma in situ and invasive ductal carcinoma of the breast. Oncogene. 2003;22 (13):2021–2033. doi: 10.1038/sj.onc.1206199. [DOI] [PubMed] [Google Scholar]
- Kurshumliu F, Thorns C, Gashi-Luci L. p16INK4A in routine practice as a marker of cervical epithelial neoplasia. Gynecol Oncol. 2009;115 (1):127–131. doi: 10.1016/j.ygyno.2009.06.020. [DOI] [PubMed] [Google Scholar]
- Lago CU, Nowinski SM, Rundhaug JE, Pfeiffer ME, Kiguchi K, Hirasaka K, Yang X, Abramson EM, Bratton SB, Rho O, Colavitti R, Kenaston MA, Nikawa T, Trempus C, Digiovanni J, Fischer SM, Mills EM. Mito-chondrial respiratory uncoupling promotes keratinocyte differentiation and blocks skin carcinogenesis. Oncogene. 2012;31 (44):4725–4731. doi: 10.1038/onc.2011.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Jeon HM, Ju MK, Kim CH, Yoon G, Han SI, Park HG, Kang HS. Wnt/Snail signaling regulates cytochrome C oxidase and glucose metabolism. Cancer Res. 2012;72 (14):3607–3617. doi: 10.1158/0008-5472.CAN-12-0006. [DOI] [PubMed] [Google Scholar]
- Lei ZB, Fu XJ, Lu ZT, Wang BC, Liu XL, You NZ. Effect of estradiol on chemokine receptor CXCR2 expression in rats: implications for atherosclerosis. Acta Pharmacol Sin. 2003;24 (7):670–674. [PubMed] [Google Scholar]
- Li C, Li S, Jia C, Yang L, Song Z, Wang Y. Low concentration of S100A8/9 promotes angiogenesis-related activity of vascular endothelial cells: bridges among inflammation, angiogenesis, and tumorigenesis? Mediators Inflamm. 2012;2012:248574. doi: 10.1155/2012/248574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long H, Crean CD, Lee WH, Cummings OW, Gabig TG. Expression of Clostridium perfringens enterotoxin receptors claudin-3 and claudin-4 in prostate cancer epithelium. Cancer Res. 2001;61 (21):7878–7881. [PubMed] [Google Scholar]
- Mailloux RJ, Harper ME. Uncoupling proteins and the control of mitochon-drial reactive oxygen species production. Free Radical Biol Med. 2011;51 (6):1106–1115. doi: 10.1016/j.freeradbiomed.2011.06.022. [DOI] [PubMed] [Google Scholar]
- Manavi M, Hudelist G, Fink-Retter A, Gschwandtler-Kaulich D, Pischinger K, Czerwenka K. Gene profiling in Pap-cell smears of high-risk human papillomavirus-positive squamous cervical carcinoma. Gynecol Oncol. 2007;105 (2):418–426. doi: 10.1016/j.ygyno.2006.12.032. [DOI] [PubMed] [Google Scholar]
- Markowitz J, Carson WE., 3rd Review of S100A9 biology and its role in cancer. Biochim Biophys Acta. 2013;1835 (1):100–109. doi: 10.1016/j.bbcan.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin-Drubin ME, Munger K. The human papillomavirus E7 onco-protein. Virology. 2009;384 (2):335–344. doi: 10.1016/j.virol.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton K, Peh W, Southern S, Griffin H, Sotlar K, Nakahara T, El-Sherif A, Morris L, Seth R, Hibma M, Jenkins D, Lambert P, Coleman N, Doorbar J. Organization of human papillomavirus productive cycle during neoplastic progression provides a basis for selection of diagnostic markers. J Virol. 2003;77 (19):10186–10201. doi: 10.1128/JVI.77.19.10186-10201.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missaoui N, Trabelsi A, Hmissa S, Fontaniere B, Yacoubi MT, Mokni M, Korbi S, Frappart L. p16INK4A overexpression in precancerous and cancerous lesions of the uterine cervix in Tunisian women. Pathol Res Pract. 2010;206 (8):550–555. doi: 10.1016/j.prp.2010.02.014. [DOI] [PubMed] [Google Scholar]
- Moody CA, Laimins LA. Human papillomavirus oncoproteins: pathways to transformation. Nat Rev Cancer. 2010;10 (8):550–560. doi: 10.1038/nrc2886. [DOI] [PubMed] [Google Scholar]
- Moreno V, Bosch FX, Munoz N, Meijer CJ, Shah KV, Walboomers JM, Herrero R, Franceschi S. Effect of oral contraceptives on risk of cervical cancer in women with human papillomavirus infection: the IARC multicentric case-control study. Lancet. 2002;359 (9312):1085–1092. doi: 10.1016/S0140-6736(02)08150-3. [DOI] [PubMed] [Google Scholar]
- Morin PJ. Claudin proteins in human cancer: promising new targets for diagnosis and therapy. Cancer Res. 2005;65 (21):9603–9606. doi: 10.1158/0008-5472.CAN-05-2782. [DOI] [PubMed] [Google Scholar]
- Munger K, Baldwin A, Edwards KM, Hayakawa H, Nguyen CL, Owens M, Grace M, Huh K. Mechanisms of human papillomavirus-induced oncogenesis. J Virol. 2004;78 (21):11451–11460. doi: 10.1128/JVI.78.21.11451-11460.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols LS, Ashfaq R, Iacobuzio-Donahue CA. Claudin 4 protein expression in primary and metastatic pancreatic cancer: support for use as a therapeutic target. Am J Clin Pathol. 2004;121 (2):226–230. doi: 10.1309/K144-PHVD-DUPD-D401. [DOI] [PubMed] [Google Scholar]
- Nilsson S, Makela S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson JA. Mechanisms of estrogen action. Physiol Rev. 2001;81 (4):1535–1565. doi: 10.1152/physrev.2001.81.4.1535. [DOI] [PubMed] [Google Scholar]
- Panse J, Friedrichs K, Marx A, Hildebrandt Y, Luetkens T, Barrels K, Horn C, Stahl T, Cao Y, Milde-Langosch K, Niendorf A, Kroger N, Wenzel S, Leuwer R, Bokemeyer C, Hegewisch-Becker S, Atanackovic D. Chemokine CXCL13 is overexpressed in the tumour tissue and in the peripheral blood of breast cancer patients. Br J Cancer. 2008;99 (6):930–938. doi: 10.1038/sj.bjc.6604621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polan ML, Daniele A, Kuo A. Gonadal steroids modulate human monocyte interleukin-1 (IL-1) activity. Fertil Steril. 1988;49 (6):964–968. [PubMed] [Google Scholar]
- Qin F, Song Y, Li Z, Zhao L, Zhang Y, Geng L. S100A8/A9 induces apoptosis and inhibits metastasis of CasKi human cervical cancer cells. Pathol Oncol Res. 2010;16 (3):353–360. doi: 10.1007/s12253-009-9225-2. [DOI] [PubMed] [Google Scholar]
- Riley RR, Duensing S, Brake T, Munger K, Lambert PF, Arbeit JM. Dissection of human papillomavirus E6 and E7 function in transgenic mouse models of cervical carcinogenesis. Cancer Res. 2003;63 (16):4862–4871. [PubMed] [Google Scholar]
- Riva M, He Z, Kallberg E, Ivars F, Leanderson T. Human S100A9 protein is stabilized by inflammatory stimuli via the formation of proteolytically-resistant homodimers. PLoS One. 2013;8 (4):e61832. doi: 10.1371/journal.pone.0061832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruh MF, Bi Y, D'Alonzo R, Bellone CJ. Effect of estrogens on IL-1beta promoter activity. J Steroid Biochem Mol Biol. 1998;66 (4):203–210. doi: 10.1016/s0960-0760(98)00042-9. [DOI] [PubMed] [Google Scholar]
- Salazar EL, Sojo-Aranda I, Lopez R, Salcedo M. The evidence for an etiological relationship between oral contraceptive use and dysplastic change in cervical tissue. Gynecol Endocrinol: Off J Int Soc Gynecol Endocrinol. 2001;15 (1):23–28. [PubMed] [Google Scholar]
- Santin AD, Zhan F, Bignotti E, Siegel ER, Cane S, Bellone S, Palmieri M, Anfossi S, Thomas M, Burnett A, Kay HH, Roman JJ, O'Brien TJ, Tian E, Cannon MJ, Shaughnessy J, Jr, Pecorelli S. Gene expression profiles of primary HPV16- and HPV18-infected early stage cervical cancers and normal cervical epithelium: identification of novel candidate molecular markers for cervical cancer diagnosis and therapy. Virology. 2005;331 (2):269–291. doi: 10.1016/j.virol.2004.09.045. [DOI] [PubMed] [Google Scholar]
- Seder CW, Hartojo W, Lin L, Silvers AL, Wang Z, Thomas DG, Giordano TJ, Chen G, Chang AC, Orringer MB, Beer DG. Upregulated INHBA expression may promote cell proliferation and is associated with poor survival in lung adenocarcinoma. Neoplasia. 2009;11 (4):388–396. doi: 10.1593/neo.81582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shai A, Brake T, Somoza C, Lambert PF. The human papillomavirus E6 oncogene dysregulates the cell cycle and contributes to cervical carcinogenesis through two independent activities. Cancer Res. 2007;67 (4):1626–1635. doi: 10.1158/0008-5472.CAN-06-3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang X, Lin X, Alvarez E, Manorek G, Howell SB. Tight junction proteins claudin-3 and claudin-4 control tumor growth and metastases. Neoplasia. 2012;14 (10):974–985. doi: 10.1593/neo.12942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva TA, Ribeiro FL, Oliveira-Neto HH, Watanabe S, Alencar Rde C, Fukada SY, Cunha FQ, Leles CR, Mendonca EF, Batista AC. Dual role of CCL3/CCR1 in oral squamous cell carcinoma: implications in tumor metastasis and local host defense. Oncol Rep. 2007;18 (5):1107–1113. [PubMed] [Google Scholar]
- Sobel G, Paska C, Szabo I, Kiss A, Kadar A, Schaff Z. Increased expression of claudins in cervical squamous intraepithelial neoplasia and invasive carcinoma. Hum Pathol. 2005;36 (2):162–169. doi: 10.1016/j.humpath.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Srikrishna G. S100A8 and S100A9: new insights into their roles in malignancy. J Innate Immun. 2012;4 (1):31–40. doi: 10.1159/000330095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stygar D, Masironi B, Eriksson H, Sahlin L. Studies on estrogen receptor (ER) alpha and beta responses on gene regulation in peripheral blood leukocytes in vivo using selective ER agonists. J Endocrinol. 2007;194 (1):101–119. doi: 10.1677/JOE-06-0060. [DOI] [PubMed] [Google Scholar]
- Tsukita S, Furuse M. Claudin-based barrier in simple and stratified cellular sheets. Curr Opin Cell Biol. 2002;14 (5):531–536. doi: 10.1016/s0955-0674(02)00362-9. [DOI] [PubMed] [Google Scholar]
- Tugizov S, Berline J, Herrera R, Penaranda ME, Nakagawa M, Palefsky J. Inhibition of human papillomavirus type 16 E7 phosphorylation by the S100 MRP-8/14 protein complex. J Virol. 2005;79 (2):1099–1112. doi: 10.1128/JVI.79.2.1099-1112.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendrell JA, Ghayad S, Ben-Larbi S, Dumontet C, Mechti N, Cohen PA. A20/TNFAIP3, a new estrogen-regulated gene that confers tamoxifen resistance in breast cancer cells. Oncogene. 2007;26 (32):4656–4667. doi: 10.1038/sj.onc.1210269. [DOI] [PubMed] [Google Scholar]
- Wang Q, Wen YG, Li DP, Xia J, Zhou CZ, Yan DW, Tang HM, Peng ZH. Upregulated INHBA expression is associated with poor survival in gastric cancer. Med Oncol. 2012;29 (1):77–83. doi: 10.1007/s12032-010-9766-y. [DOI] [PubMed] [Google Scholar]
- Webb PG, Spillman MA, Baumgartner HK. Claudins play a role in normal and tumor cell motility. BMC Cell Biol. 2013;14:19. doi: 10.1186/1471-2121-14-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Y, Kuick R, Nan B, Ota I, Weiss SJ, Trimble CL, Fearon ER, Cho KR. Gene expression analysis of preinvasive and invasive cervical squamous cell carcinomas identifies HOXC10 as a key mediator of invasion. Cancer Res. 2007;67 (21):10163–10172. doi: 10.1158/0008-5472.CAN-07-2056. [DOI] [PubMed] [Google Scholar]
- Zhu X, Jin L, Zou S, Shen Q, Jiang W, Lin W. Immunohistochemical expression of RAGE and its ligand (S100A9) in cervical lesions. Cell Biochem Biophys. 2013 doi: 10.1007/s12013-013-9515-x. [DOI] [PubMed] [Google Scholar]
- Zhu X, Lv J, Yu L, Wu J, Zou S, Jiang S. Proteomic identification of differentially-expressed proteins in squamous cervical cancer. Gynecol Oncol. 2009;112 (1):248–256. doi: 10.1016/j.ygyno.2008.09.045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.