INTRODUCTION
Acute decompensated heart failure (ADHF) represents the primary admitting diagnoses for more than 1 million patients per year. As the most common cause for admission in patients older than 65 years, visits because of ADHF have tripled in the last 3 decades.1,2 Given an increasing aged population and improved outcomes in sudden cardiac death and acute myocardial infarction, the trajectory of increasing ADHF presentations to acute-care institutions will undoubtedly continue.3 The in-hospital mortality of these patients is 5% and has decreased 40% over the past decade. Mean length of stay in hospital is 5 to 6 days and has decreased over the same period. However, re-admission rates remain unchanged at 25% within 30 days and 50% within 6 to 12 months. Mortality has persistently been 5% to 10% at 30 days after hospital discharge and 20% to 40% 6 to 12 months after hospital discharge.4–6
Given the staggering disease burden and recidivism, the cost of ADHF is substantial. Direct and inherent costs for treating ADHF were expected to total 34.8 billion dollars in 2008, with 75% of ADHF-related costs being incurred in the first 48 hours after presentation.7 Because more than 80% of patients with ADHF present to the emergency department (ED), significant pressures exist to manage these patients efficiently in the acute-care environment.8 Selected patients may be eligible to receive care for ADHF in an observation unit (OU), which may provide a safe and effective means to lower costs by providing an alternative to an inpatient stay.9 Previous studies have suggested that more than 50% of such patients are appropriate for a brief period of observation and treatment aimed at avoiding inpatient admission. However, the limited data in those discharged directly from the ED suggest that they have a high rate of adverse events.10,11 Thus, the decision to admit an ED patient with ADHF is often not based on acute severity of disease. Instead, it is largely a function of medical comorbidities and the uncertainty regarding near-term events. Thus, a safe alternative to hospital admission is a critical unmet need.
Although the hemodynamically unstable patient with ADHF poses significant challenges to the cardiologist and emergency physician, these presentations account for less than 5% of the total population with ADHF.4,12 Most are symptomatic yet hemodynamically stable, at least initially. The management of this patient population requires a balance between supporting hemodynamic stability, improving signs and symptoms, and decision making to minimize morbidity and mortality. Although it is easy for the emergency medicine (EM) physician to identify those patients who are hemodynamically unstable or critically ill, it is sometimes difficult to identify those patients with ADHF who require hospitalization versus those who can be discharged after a brief period of observation. Because there are few data to aid EM physicians when trying to identify low-risk patients who are safe for ED discharge, risk stratification in this vulnerable patient population is the subject of ongoing study.13–16
The initial evaluation of patients who present with signs and symptoms of ADHF focuses on increasing the degree of diagnostic certainty that ADHF is present rather than the myriad of other causes of acute dyspnea. Symptom relief is the therapeutic focus after initial stabilization. Risk stratification occurs concurrently with diagnosis and treatment and can guide the decision of where to best triage patients, such as the intensive care unit (ICU), telemetry, or observation. In this review, diagnostic and treatment modalities, clinical considerations, risk stratification tools, and disposition options in patients presenting to the ED with ADHF are discussed.
DIAGNOSIS
History
Patients with ADHF often present with dyspnea. Dyspnea on exertion, paroxysmal nocturnal dyspnea (PND), and orthopnea, although common, are not highly predictive of ADHF. In a large meta-analysis examining the predictors of ADHF,17 PND and orthopnea have intermediate likelihood ratios (LRs) of 2.6 and 2.2, respectively, for a diagnosis of ADHF. Historical features such as lower extremity edema, fatigue, previous episodes of ADHF, recent medication changes, dietary indiscretion, or modifications are often routinely asked about. Duration of symptoms often gives subtle cues to the phenotype of the heart failure (HF). Signs of volume overload and peripheral edema with an onset over days to weeks are often suggestive of gradual worsening of HF with reduced ejection fraction (HFREF).18 Poorly controlled blood pressure (BP), arrhythmias, or acute coronary syndrome (ACS) may cause more rapid decompensation.
Physical Examination
Vital signs can be helpful in determining the cause of ADHF as well as guiding initial management and therapy. Patients with diastolic dysfunction and HF with preserved ejection fraction (HFPEF) often present with high BP.19 However, both those with systolic and diastolic dysfunction can present with increased BP.20 Bradycardia might implicate heart block, drug toxicity (specifically digoxin), and electrolyte abnormalities as precipitants of the ADHF presentation. The physical examination findings most suggestive of ADHF include a third heart sound (S3), and jugular venous distention (JVD). Although bibasilar rales and peripheral edema are helpful, they are less specific for ADHF. An S3 gallop and JVD have an LR of 11 and 5.1, respectively, for ADHF, whereas rales and peripheral edema have an LR of 2.8 and 2.3, respectively.17
Chest Radiography
Cardiomegaly, cephalization, interstitial edema, alveolar edema, or pleural effusions are all found in ADHF. Interstitial edema is well correlated with ADHF, with an LR of 12.0.17 Cardiomegaly and pleural effusions, with LRs of 3.3 and 3.2, may be findings that are more common in chronic HF than AHDF. ED patients with ADHF have no radiographic findings of congestion in 13% to 15% of cases.21
Natriuretic Peptides
Natriuretic peptides (NP), released from myocytes secondary to myocardial stretch and increasing end diastolic pressures, have been found to be useful as a diagnostic tool in patients in whom there is still uncertainty after traditional testing.22,23 Increased NP levels are indicators of both the presence and severity of illness. The Breathing Not Properly trial suggested that brain NP (BNP) levels can be used to increase diagnostic accuracy when added to clinical judgment. The dyspneic patient with a plasma BNP level of less than 100 pg/dL or N-terminal (NT)-proBNP of less than 300 pg/dL is unlikely to have ADHF. BNP levels of greater than 500 pg/dL have 90% specificity in the prediction of ADHF as the cause of dyspnea. Of the 26.4% of patients with gray zone BNP levels between 100 and 500 pg/dL, two-thirds (16.5%) may still have HF as the cause of dyspnea.23 NT-proBNP has also shown a similar diagnostic usefulness.24 In certain patient populations, the NPs can be falsely high or low. Both are falsely increased in renal insufficiency, NT-proBNP more than BNP.25 Obese patients tend to have falsely lower BNP levels because of BNP metabolism in adipose tissue.18
Necrosis Markers
Serial monitoring of cardiac necrosis biomarkers, especially cardiac troponin (cTn), is recommended for diagnostic and prognostic purposes. Patients with ADHF with elevation of cTn have higher mortality than those with nondetectable cTn. The magnitude of the increase is not specified. Results from data from a large ADHF registry using troponin I levels of greater than 1 μg/L and troponin T levels of 0.1 μg/L found an independent association with in-hospital mortality.26 Outcome studies of lower-level troponin increases using ultrasensitive methods are ongoing. In HFPEF with ADHF, minor myocardial damage, defined as increases of cTn of more than 0.02 ng/mL, were found in 44% of patients.27 Patients with an increased Tn were also found to have higher markers of disease severity, such as lower ejection fraction (EF), higher serum creatinine level, higher NP level, and higher 6-month event rates.27
Electrocardiography
A 12-lead electrocardiogram (ECG) is important to identify treatable conditions such as cardiac ischemia and arrhythmias. Atrial fibrillation in the setting of dyspnea has been the most studied arrhythmia in patients with ADHF.17 However, new T-wave changes or any abnormal ECG can also be associated with ADHF. A normal ECG decreases the likelihood of ADHF.17
Evolving Diagnostic Strategies
Echocardiography was once considered a test that was obtained during inpatient admission. However, with the introduction of portable high-resolution ultrasound devices and advanced training for EM physicians, bedside echocardiography in the ED continues to evolve. After initial management and stabilization of the patient with suspected ADHF, limited bedside echocardiography can give the clinician information about cardiovascular pathophysiology. The use of mitral valve E point septal separation (EPSS) in M-mode ultrasonography has been established as an option to assess for reduced EF. A sensitivity of 87% for detecting an abnormal EF at a cutoff of greater than 7 mm has been reported.28 EM residents (PGY3/4) were able to obtain EPSS measurements that closely correlate with the visual estimates of EF made by experienced sonographers.29
Rapid assessment for increased central venous pressure (CVP) as a marker of right heart congestion can also be assessed. An inferior vena cava greater than 2 cm or collapsibility index of less than 50% indicates increased CVP. In the absence of significant pulmonary disease, this factor has been found to be highly correlated with pulmonary capillary wedge pressure.30 Measuring diastolic parameters can identify decreased left ventricular compliance and diastolic dysfunction. An early/late ratio greater than 2 indicates decreased left ventricular compliance and has been shown to be 100% sensitive and specific in predicting a left ventricular end diastolic pressure greater than 20 mm Hg.31
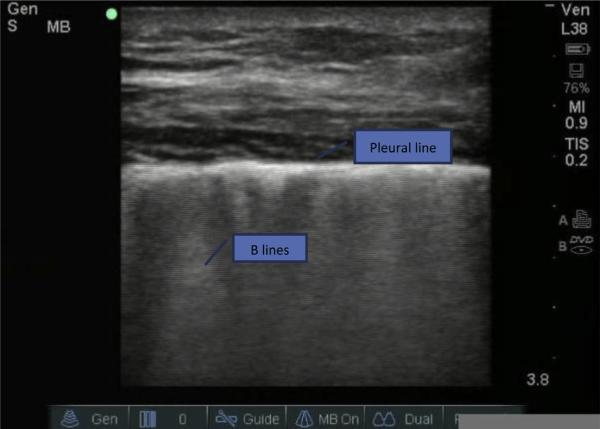
Comet tail artifacts, ultrasonographic evidence of B-lines, are additional diagnostic findings readily assessed by the EM physician using bedside ultrasonography (Fig. 1). B-lines arise from water-thickened interlobular septa at the pleural line. Assessment of B-lines can help differentiate between cardiogenic and noncardiogenic causes of dyspnea. The technique involves assessment of the anterior and lateral chest wall from the second to fifth intercostal spaces.32 Three or more B-lines in 1 viewing field are considered a positive finding for pulmonary edema. In one study33 evaluating 149 patients who presented with acute dyspnea, ultrasonographic B-lines were found in 93 of the 122 patients who were found to have a cardiogenic cause of dyspnea. Whereas the negative predictive value was superior for NT-proBNP (100%) over B-lines (45%), the positive predictive value for B-lines (97%) was marginally better than NT-proBNP (92%).
Fig. 1.
Ultrasound B-lines (comet tails). (Courtesy of Laura Kress, RDCS, RVT, Cincinnati, OH.)
THERAPY
General Approach to Therapy
ADHF therapy as applied to the ED setting can be conceptualized as a 2-fold approach: symptom relief and initiation of inpatient therapy. Although most patients known to have HF have been prescribed approved medications as outpatients, ED care pathways most often involve restarting the medications that have been missed or adjusting dosages on medications that the patient is are already taking. Symptom relief is often tailored to treat the presenting clinical profile.
Symptom relief
Non–potassium-sparing diuretics (ie, loop diuretics) are the mainstay of symptom therapy in ADHF. Although the goal of fluid removal remains primary in initial inpatient therapy, electrolyte imbalances, renal dysfunction, and alteration of neurohormonal balance can develop with high furosemide doses. The DOSE (Diuretic Optimization Strategies Evaluation) study34 randomized patients to low-dose (same as oral daily dose) or high-dose (2.5 times oral daily dose) parenteral furosemide therapy. Using a factorial design, patients were also randomized to bolus (twice daily) or continuous administration. Results show a trend toward greater symptom relief in the high-dose group, with secondary improvement in volume loss and decreased weight. The median change of serum creatinine level was 0.06 mg/dL and 0.01 mg/dL for the high-dose and low-dose protocols, respectively. Unlike previous studies, continuous infusion was not superior to intermittent bolus therapy. Patients were enrolled in this study long after ED presentation, making extrapolation of these data to the ED patient difficult. Despite the obvious clinical usefulness of diuretic use, clinicians should be aware of the possible association of diuretic use as a marker of increased risk.4 The use of ultrafiltration has not been adequately studied in the ED setting to advocate routine use. Thus far, trials of vasopressin antagonists have failed to show significant improvement in ADHF symptoms.35
Nitroglycerin is a potent arteriole and venous vasodilator and reduces both afterload and pre-load rapidly. Because it can be delivered by several routes, such as intravenous, sublingual, topical, spray, and tablet, nitroglycerin remains a popular first-line therapy for symptom relief in patients without low BP.21 The intravenous form can be titrated based on BP and symptom response, but often mandates admission to a critical care bed. Morphine has historically been used in ADHF because of its mild venodilator activity, preload reduction, and anxiolysis. The data supporting its use have been anecdotal and were contradicted by reports describing increased rates of endotracheal intubation, ICU admission, and prolonged hospital stay. Recent data from the ADHERE registry (Acute Decompensated Heart Failure National Registry) found an association between morphine use and increased in-hospital mortality.36 Given these reports, if morphine is used, it should be in a judicious manner.
Nesiritide was approved for the treatment of ADHF in the United States in 2001. Although it improves hemodynamics and dyspnea,37,38 pooled data raised concerns over worsening renal function and increased mortality.39 The safety and efficacy of nesiritide was studied in the ASCEND-HF (Acute Study of Clinical Effectiveness of Nesiritide in De-compensated Heart Failure) trial,40 a double-blind, placebo-controlled, multinational trial. Results suggest that it is safe, but did not meet the prespecified end point for improvement of dyspnea. The use of nesiritide in OUs has been evaluated by the PROACTION (Prospective Randomized Outcomes study of Acutely decompensated CHF Treated Initially as Outpatients with Nesiritide) trial. There were no differences in adverse outcomes between standard care and nesiritide. The nesiritide group was shown to have fewer hospital days (2.5 days vs 6.5 days; P = .03) in the month after administration.41,42
A recent randomized study of relaxin in ADHF suggests this therapy may safely improve dyspnea, minimize ongoing myocardial and renal injury, and perhaps improve long-term events.43 This study enrolled patients earlier than previous ADHF studies, with a minimum systolic BP of 125 mm Hg, which may be an explanation for the promising results. Early enrollment suggests that this therapy may have a role in ED patients with ADHF, but further study is necessary.44
Although the use of angiotensin-converting enzyme (ACE) inhibitors in chronic HF management is well supported, their role in the acute setting is less clear.45 The use of enalaprilat, an intravenous ACE inhibitor, in ADHF is a level C recommendation by the American College of Emergency Physicians, and is not recommended by the European Society of Cardiology.46 It has been shown to improve hemodynamics in small studies,47 but has not been definitively evaluated in a large-scale trial. Nitroprusside is a potent systemic and pulmonary vasodilator, which requires invasive monitoring and frequent titration, and is not well suited to an ED environment. Positive inotropes with properties favorable for patients with ADHF with low output states, like dobutamine, milrinone, and levosimendan (available in Europe) are useful in only a few patients who present with ADHF and signs of impaired perfusion.
Patients who present with ADHF may require oxygen supplementation. The amount of oxygen that must be administered after initial therapy is often a key triage data point. After initial steps at symptom relief, patients can be titrated down to nasal cannula administration that can be easily managed in a non-ICU environment. Ventilatory support through endotracheal intubation or noninvasive ventilation (NIV) mandates ICU admission. NIV support either through continuous positive airway pressure support or bilevel positive airway pressure may reduce the need for intubation, shorten ICU stay, and reduce cost but likely has no impact on mortality.48,49 If patients can be weaned off NIV in the ED, transferring their care to an OU or telemetry floor can be considered if other parameters are met. Hyperoxia should be avoided.50
Implementation of outpatient therapies
Depending on specific ADHF pathways, ED management of this patient population may include initiation of inpatient therapies in collaboration with the inpatient team of cardiologists or hospitalists. β-Blockers improve survival in HF and are a mainstay in outpatient treatment. There was controversy as to whether these agents should be withheld during acute exacerbations. Most recent guidelines suggest that patients taking long-term evidenced-based β-blockers (carvedilol, metoprolol CR/XL, or bisoprolol) should continue them even in ADHF unless hypoperfusion is present. Dosages can be down titrated or held if hypotension or bradycardia arises.51,52 ACE inhibitors and angiotensin receptor blockers (ARBs) are standard therapy in chronic HF management. They improve symptoms, decrease morbidity and mortality, and slow disease progression in patients with reduced left ventricular EF (EF <40%).46 They should be continued in those patients who are taking them chronically. Because they are contraindicated in patients with hyperkalemia (K >5.5 mmol/L), as well as pregnancy, symptomatic hypotension, bilateral renal artery stenosis, or angioedema, they should be given after potassium levels and renal function have been checked. ADHF protocols should have a standardized approach to β-blocker and ACE inhibitor/ARB delivery, with careful documentation of contraindications or intolerances. Oral digoxin has been historically used to improve hemodynamics and symptoms with chronic use, and when added to diuretic and ACE inhibitor, can decrease hospitalization.53 Intravenous digoxin use in ADHF is less well studied.
Tailored Therapy
The clinical profile is initially described based on the presenting hemodynamics; hypertensive, normotensive, and hypotensive. Patients who present with increased systolic BP (>140 mm Hg) have a predominance of pulmonary congestion, clinically or radiographically, with milder signs of systemic congestion. The onset of symptoms is often over 24 to 48 hours, and more than 50% of patients present with systolic BP greater than 140 mm Hg.21,54 These patients often have HFPEF and may present with acute, severe dyspnea and diaphoresis. Although the treating clinician may entertain delivering ventilatory support until other treatment modalities take effect, these patients often respond dramatically with aggressive, timely intervention. The treatment focuses on reducing afterload with vasodilators such as nitrates (sublingual, intravenous, or topical), hydralazine (intravenous), and ACE inhibitors. Although loop diuretics are often used, symptoms in these patients are often caused by rapid fluid shifts into the pulmonary vascular bed and are not necessarily caused by increased whole-body edema.55,56
By contrast, patients with HFREF may present with normal or moderately increased BP, with systemic congestion developing over days to weeks, with few signs or symptoms of pulmonary congestion. These patients often respond to more aggressive diuresis and may not require vasodilators acutely. The optimal diuretic regimen is the subject of recent investigation.34 A few (<8%) who present with low BP (<90 mm Hg) as a result of very low cardiac output, superimposed infection, or hypovolemia and decreased renal perfusion, or in cardiogenic shock, are unique therapeutic challenges.57 These patients should be triaged as quickly as possible to the ICU for advanced medical and mechanical therapies.
Overall, the management of the patient with ADHF in the ED is based on the physician's ability to identify critical elements of the patient's history and presentation to administer appropriate treatment and determine disposition. Table 1 shows the balance of considerations that the ED physician must consider to appropriately manage the patient with ADHF and also highlights the complexity and heterogeneity of ADHF presentations.21,58
Table 1.
Clinical considerations
| 1. BP Hypotensive (<5%) Normotensive Hypertensive (~50%) |
Associated Findings and Strategies Fluids Inotropes ICU admit Insidious onset (days, weeks) Diuretics ± vasodilators ED observation vs telemetry floor Acute onset (24–72 h) Vasodilator, diuretics ED observation vs telemetry floor |
| 2. Comorbidities Chronic renal insufficiency Obstructive pulmonary disorder Obesity |
Associated Findings Chronically increased BNP, Difficult diuresis Wheezing on physical examination Low BNP, Difficult physical examination |
| 3. Clinical Severity Present in extremis with pulmonary edema Insidious presentation | |
| 4. Heart Rate and Rhythm Rapid atrial fibrillation/flutter: consider β-blocker vs calcium channel blocker Bradycardia: consider heart block, drug toxicity, electrolyte abnormality | |
| 5. Precipitants Diet Medicine ACS, arrhythmia | |
| 6. De novo vs acute exacerbation of chronic HF De novo: broad differential, consider ACS/arrhythmia Chronic HF: consider precipitants, current medications | |
RISK STRATIFICATION
The patient who presents in cardiogenic shock caused by ADHF is at substantial risk of morbidity and mortality. However, most patients who present to the ED with dyspnea caused by ADHF and gradual worsening of chronic HF are not critically ill. This group also has substantial risk of immediate and short-term adverse events. One prospective cohort study59 showed that triage physicians overestimate the probability of severe complications in the patient with ADHF and as a result tend to overuse critical care resources for admission. Although the predominant consideration in risk stratification as it applies to the emergency care of the patient with ADHF is short-term event rates, no gold standard time frame exists. The events rates are often conceptualized by emergency physicians as immediate adverse events (those within 7 days or within the index hospitalization) and short-term (those events occurring within 30 days of ED presentation). Several studies have identified markers of short-term risk. They include increased blood urea nitrogen (BUN) or creatinine levels, hyponatremia, ECG evidence of myocardial ischemia, increased BNP, increased cTn, or low BP.54,60,61 However, because 80% of all patients with ADHF are admitted to the hospital and often to a monitored bed, the identification of the high-risk patient has less impact on emergency decision making than identification of the low-risk patient. The lack of high-risk features does not identify a patient who is low risk.
Given the reluctance of US clinicians to discharge patients directly from the ED, is there evidence supporting the identification of a low-risk cohort? Auble and colleagues62 aimed to derive a clinical prediction rule to identify patients with ADHF who are at low risk of death or serious medical complication based on readily available patient data in the ED. They used administrative data and a complex classification algorithm to retrospectively derive a tool that uses 21 variables to describe patients at low risk for poor outcome. This model was validated by applying this clinical prediction rule to a retrospective cohort of 8384 in-patients with a primary diagnosis of ADHF. Of this cohort, 1609 (19.2%) were identified as low risk. Of those identified as low risk, 12 (0.7%) died as inpatients, 28 (1.7%) survived after a serious hospital complication, and 47 (2.9%) died within 30 days of discharge.62,63 This tool has yet to be studied prospectively to determine how it would augment physician decision making. A recent Canadian study also explored the use of a risk scoring system to identify high-risk patients. Using variables readily available to the emergency physicians and a novel 3-minute walk test, Stiell and colleagues16 developed a 15-point, 10-variable scoring model. Using the serious adverse events definition of 30-day all-cause mortality, 14-day ADHF readmission, myocardial infarction, mechanical ventilation, percutaneous coronary intervention, coronary artery bypass graft, or renal replacement, the investigators describe low-risk score as follows: (score [risk%]): 0 (2.8%), 1 (5.1%), 2 (9.2%). This study is confounded by the significant practice variation in Canada, where less than 50% of patients with ADHF are admitted. Less than 10% of the cohort had a risk score of 0, categorized as low risk. This study highlights the difficulty of identifying a low-risk population and confirms the commonly accepted high-risk variables. The results of a recently completed study in a US cohort sponsored by the National Institutes of Health is forthcoming.13
DISPOSITION
Because clinical decision-making tools to aid in the risk stratification of the patient with ADHF are lacking, the disposition in the United States is often admission to the hospital. Given the uncertain reimbursement landscape, there are several unanswered questions in ADHF related to disposition decision making: is there a subset of patients who can be discharged safely from the ED with close follow-up? Who warrants 23-hour observation in an ED-based OU? Alternatives to admission are dependent on individual patient factors and the infrastructure of the health care system where care is being provided. Both hospital resources and outpatient resources are required for comprehensive treatment of the ED patient with ADHF. One ED disposition option is stabilization, medication adjustment, and discharge home after arranging close outpatient follow-up within the next 72 hours. One study examined the trends of early outpatient follow-up on 30-day readmission rates and found that patients discharged from an inpatient admission in hospitals with higher early follow-up rates have a lower risk of 30-day read-mission.64 This finding is specific to stabilization after an inpatient stay but may suggest similar outcomes if close follow-up were established after discharge from the ED or OU. One of the crucial factors to such a process is ensuring availability of close follow-up with either a cardiologist or primary care physician. Many patients do not have a primary care physician and rely on EDs for medication adjustment and acute treatment. This subset of patients would need to have an appointment scheduled with a new provider, possibly hindering their ability to be discharged directly from the ED or after OU management. Hospital resources dedicated to finding providers for these patients would be crucial for success.
Another alternative to ED discharge is the ED-based OU. Emergency physicians are skilled and well equipped to provide acute therapy for the first 24 to 48 hours for the patient with ADHF, thus making ED-based observation a logical and economical means to care immediately for this patient population.8,65 OUs were identified by the Institute of Medicine as central to improving resource use and patient flow. A recent study66 suggested that increased use of the OU has the potential to save $3.1 billion and avoid 2.4 million inpatient admissions. Appropriate OU use in ADHF management may contribute to this cost saving. The impact of hospitalization on postdischarge events has not been well elucidated.64,67,68 For the potential 50% of patients who present with ADHF and have no high-risk features, an ED-based OU may be a safe and appropriate alternative to admission to manage these patients and facilitate early discharge.69,70 An ED OU has the potential to provide the resources necessary to monitor BP, heart rate, urine output, and weight during a 23-hour observation period, which is also adequate time for many patients to have near-complete resolution of symptoms with standard therapy.71 Additional diagnostic testing can also be easily arranged in on OU setting, including formal echocardiography, electrolytes, and cardiac biomarkers.
SUMMARY
The initial evaluation of patients who present to the ED with ADHF remains challenging. Because more than 80% of patients with ADHF present to the ED, significant pressures exist to manage these patients efficiently in the acute-care environment. Although most patients present with worsening of chronic HF, some may present with undifferentiated dyspnea and new-onset HF. Others have significant comorbidities that complicate both the diagnosis and treatment. Although physical examination, ECG, chest radiography, NP, and necrosis markers remain as the cornerstone of diagnosis, the role of bedside ultrasonography will continue to expand. The treatment of patients with ADHF is prioritized based on vital signs and presenting phenotype. Although vasodilators and loop diuretics remain the mainstay in initial therapy, newer vasoactive compounds may be available soon. The risk stratification of patients, particularly those who may show low-risk features, is the subject of ongoing evaluation. The disposition of patients to areas other than a monitored inpatient bed, such as an ED-based OU, may prove effective in the ever-changing health care climate.
KEY POINTS.
More than 80% of patients with acute decompensated heart failure (ADHF) present to the emergency department (ED), and significant pressures exist to manage these patients efficiently in the acute-care environment.
Most patients with ADHF present to the ED with worsening of chronic heart failure (HF); some may present with undifferentiated dyspnea and new-onset HF, whereas others have significant comorbidities that complicate diagnosis and treatment.
Although physical examination, electrocardiography, chest radiography, natriuretic peptides, and necrosis markers remain the cornerstones of diagnosis, the role of bedside ultrasonography will continue to expand.
Treatment of patients with ADHF is prioritized based on vital signs and presenting phenotype.
Vasodilators and loop diuretics remain the mainstay in initial therapy, although newer vasoactive compounds may be available soon.
Risk stratification of patients, particularly those who may show low-risk features, is the subject of ongoing evaluation.
Disposition of patients to areas other than a monitored inpatient bed, such as an ED-based observation unit, may prove effective in the ever-changing health care climate.
ACKNOWLEDGMENTS
The authors wish to thank Danielle Cornwall for her assistance with the preparation of this article.
Disclosures: Dr Collins has received research support from NIH/NHLBI K23HL085387, Medtronic, Radiometer, and Cardiorentis. He has also been a consultant for Novartis, Trevena, The Medicines Company, and BRAHMS. Dr Fermann has received research support from Medtronic, Radiometer, Cardiorentis, Nanodetection Technologies, and Cubist Pharmaceuticals. He is also a Consultant to Pfizer.
REFERENCES
- 1.Ahmed A, Allman RM, Fonarow GC, et al. Incident heart failure hospitalization and subsequent mortality in chronic heart failure: a propensity-matched study. J Card Fail. 2008;14:211. doi: 10.1016/j.cardfail.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics–2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2–220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abraham WT, Fonarow GC, Albert NM, et al. Predictors of in-hospital mortality in patients hospitalized for heart failure: insights from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF). J Am Coll Cardiol. 2008;52:347. doi: 10.1016/j.jacc.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 4.Fonarow GC, Abraham WT, Albert NM, et al. Association between performance measures and clinical outcomes for patients hospitalized with heart failure. JAMA. 2007;297(1):61–70. doi: 10.1001/jama.297.1.61. [DOI] [PubMed] [Google Scholar]
- 5.Fonarow GC, Abraham WT, Albert NM, et al. Factors identified as precipitating hospital admissions for heart failure and clinical outcomes: findings from OPTIMIZE-HF. Arch Intern Med. 2008;168:847. doi: 10.1001/archinte.168.8.847. [DOI] [PubMed] [Google Scholar]
- 6.O'Connor CM, Abraham WT, Albert NM, et al. Predictors of mortality after discharge in patients hospitalized with heart failure: an analysis from the Organized Program to Initiate Lifesaving treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF). Am Heart J. 2008;156:662. doi: 10.1016/j.ahj.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 7.Fonarow GC. Epidemiology and risk stratification in acute heart failure. Am Heart J. 2008;155:200. doi: 10.1016/j.ahj.2006.10.043. [DOI] [PubMed] [Google Scholar]
- 8.Fermann GJ, Collins SP. Observation units in the management of acute heart failure syndromes. Curr Heart Fail Rep. 2010;7(3):125–33. doi: 10.1007/s11897-010-0020-x. [DOI] [PubMed] [Google Scholar]
- 9.Collins SP, Schauer DP, Gupta A, et al. Cost-effectiveness analysis of ED decision making in patients with non-high-risk heart failure. Am J Emerg Med. 2009;27(3):293. doi: 10.1016/j.ajem.2008.02.025. [DOI] [PubMed] [Google Scholar]
- 10.Lee DS, Schull MJ, Alter DA, et al. Early deaths in patients with heart failure discharged from the emergency department: a population-based analysis. Circ Heart Fail. 2010;3(2):228–35. doi: 10.1161/CIRCHEARTFAILURE.109.885285. [DOI] [PubMed] [Google Scholar]
- 11.Rame JE, Sheffield MA, Dries DL, et al. Outcomes after emergency department discharge with a primary diagnosis of heart failure. Am Heart J. 2001;142(4):714–9. doi: 10.1067/mhj.2001.118473. [DOI] [PubMed] [Google Scholar]
- 12.Fonarow GC, Abraham WT, Albert NM, et al. Day of admission and clinical outcomes for patients hospitalized for heart failure: findings from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure (OPTIMIZE-HF). Circ Heart Fail. 2008;1(1):50–7. doi: 10.1161/CIRCHEARTFAILURE.107.748376. [DOI] [PubMed] [Google Scholar]
- 13.Collins SP, Lindsell CJ, Jenkins CA, et al. Risk stratification in acute heart failure: rationale and design of the STRATIFY and DECIDE studies. Am Heart J. 2012;164(6):825–34. doi: 10.1016/j.ahj.2012.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins SP, Lindsell CJ, Pang PS, et al. Bayesian adaptive trial design in acute heart failure syndromes: moving beyond the mega trial. Am Heart J. 2012;164(2):138–45. doi: 10.1016/j.ahj.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pang PS, Jesse R, Collins SP, et al. Patients with acute heart failure in the emergency department: do they all need to be admitted? J Card Fail. 2012;18(12):900–3. doi: 10.1016/j.cardfail.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 16.Stiell IG, Clement CM, Brison RJ, et al. A risk scoring system to identify emergency department patients with heart failure at high risk for serious adverse events. Acad Emerg Med. 2013;20(1):17–26. doi: 10.1111/acem.12056. [DOI] [PubMed] [Google Scholar]
- 17.Wang CS, FitzGerald JM, Schulzer M, et al. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA. 2005;294(15):1944–56. doi: 10.1001/jama.294.15.1944. [DOI] [PubMed] [Google Scholar]
- 18.Weintraub NL, Collins SP, Pang PS, et al. Acute heart failure syndromes: emergency department presentation, treatment, and disposition: current approaches and future aims: a scientific statement from the American Heart Association. Circulation. 2010;122(19):1975–96. doi: 10.1161/CIR.0b013e3181f9a223. [DOI] [PubMed] [Google Scholar]
- 19.Slama M, Susic D, Varagic J, et al. Diastolic dysfunction in hypertension. Curr Opin Cardiol. 2002;17(4):368–73. doi: 10.1097/00001573-200207000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Chatterjee K, Massie B. Systolic and diastolic heart failure: differences and similarities. J Card Fail. 2007;13(7):569–76. doi: 10.1016/j.cardfail.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Collins S, Storrow AB, Kirk JD, et al. Beyond pulmonary edema: diagnostic, risk stratification, and treatment challenges of acute heart failure management in the emergency department. Ann Emerg Med. 2008;51(1):45–57. doi: 10.1016/j.annemergmed.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Maisel AS, Krishnaswamy P, Nowak RM, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347(3):161–7. doi: 10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]
- 23.McCullough PA, Nowak RM, McCord J, et al. B-type natriuretic peptide and clinical judgment in emergency diagnosis of heart failure: analysis from Breathing Not Properly (BNP) Multinational Study. Circulation. 2002;106(4):416–22. doi: 10.1161/01.cir.0000025242.79963.4c. [DOI] [PubMed] [Google Scholar]
- 24.Januzzi JL, Jr, Camargo CA, Anwaruddin S, et al. The N-terminal Pro-BNP investigation of dyspnea in the emergency department (PRIDE) study. Am J Cardiol. 2005;95(8):948–54. doi: 10.1016/j.amjcard.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 25.Lamb EJ, Vickery S, Price CP. Amino-terminal pro-brain natriuretic peptide to diagnose congestive heart failure in patients with impaired kidney function. J Am Coll Cardiol. 2006;48(5):1060–1. doi: 10.1016/j.jacc.2006.06.019. author reply: 1061. [DOI] [PubMed] [Google Scholar]
- 26.Peacock WF IV, De Marco T, Fonarow GC, et al. Cardiac troponin and outcome in acute heart failure. N Engl J Med. 2008;358:2117. doi: 10.1056/NEJMoa0706824. [DOI] [PubMed] [Google Scholar]
- 27.Perna ER, Aspromonte N, Cimbaro Canella JP, et al. Minor myocardial damage is a prevalent condition in patients with acute heart failure syndromes and preserved systolic function with long-term prognostic implications: a report from the CIASTHF (Collaborative Italo-Argentinean Study on Cardiac Troponin T in Heart Failure) study. J Card Fail. 2012;18(11):822–30. doi: 10.1016/j.cardfail.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Ahmadpour H, Shah AA, Allen JW, et al. Mitral E point septal separation: a reliable index of left ventricular performance in coronary artery disease. Am Heart J. 1983;106(1 Pt 1):21–8. doi: 10.1016/0002-8703(83)90433-7. [DOI] [PubMed] [Google Scholar]
- 29.Secko MA, Lazar JM, Salciccioli LA, et al. Can junior emergency physicians use E-point septal separation to accurately estimate left ventricular function in acutely dyspneic patients? Acad Emerg Med. 2011;18(11):1223–6. doi: 10.1111/j.1553-2712.2011.01196.x. [DOI] [PubMed] [Google Scholar]
- 30.Ramasubbu K, Deswal A, Chan W, et al. Echocardiographic changes during treatment of acute decompensated heart failure: insights from the ESCAPE trial. J Card Fail. 2012;18(10):792–8. doi: 10.1016/j.cardfail.2012.08.358. [DOI] [PubMed] [Google Scholar]
- 31.Channer KS, Culling W, Wilde P, et al. Estimation of left ventricular end-diastolic pressure by pulsed Doppler ultrasound. Lancet. 1986;1(8488):1005–7. doi: 10.1016/s0140-6736(86)91273-0. [DOI] [PubMed] [Google Scholar]
- 32.Picano E, Frassi F, Agricola E, et al. Ultrasound lung comets: a clinically useful sign of extravascular lung water. J Am Soc Echocardiogr. 2006;19(3):356–63. doi: 10.1016/j.echo.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 33.Prosen G, Klemen P, Strnad M. Combination of lung ultrasound (a comet-tail sign) and N-terminal pro-brain natriuretic peptide in differentiating acute heart failure from chronic obstructive pulmonary disease and asthma as cause of acute dyspnea in prehospital emergency setting. Crit Care. 2011;15(2):R114. doi: 10.1186/cc10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364(9):797–805. doi: 10.1056/NEJMoa1005419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Konstam MA, Gheorghiade M, Burnett JC, Jr, et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA. 2007;297(12):1319–31. doi: 10.1001/jama.297.12.1319. [DOI] [PubMed] [Google Scholar]
- 36.Peacock WF, Hollander JE, Diercks DB, et al. Morphine and outcomes in acute decompensated heart failure: an ADHERE analysis. Emerg Med J. 2008;25:205. doi: 10.1136/emj.2007.050419. [DOI] [PubMed] [Google Scholar]
- 37.Publication Committee for the VMAC I Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial. JAMA. 2002;287:1531–40. doi: 10.1001/jama.287.12.1531. [DOI] [PubMed] [Google Scholar]
- 38.Peacock WF, IV, Emerman CL, Silver MA, et al. Nesiritide added to standard care favorably reduces systolic blood pressure compared with standard care alone in patients with acute decompensated heart failure. Am J Emerg Med. 2005;157:327. doi: 10.1016/j.ajem.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 39.Sackner-Bernstein JD, Kowalski M, Fox M, et al. Short-term risk of death after treatment with nesiri-tide for decompensated heart failure: a pooled analysis of randomized controlled trials. JAMA. 2005;293(15):1900–5. doi: 10.1001/jama.293.15.1900. [DOI] [PubMed] [Google Scholar]
- 40.Ezekowitz JA, Hernandez AF, O'Connor CM, et al. Assessment of dyspnea in acute decompensated heart failure: insights from ASCEND-HF (Acute Study of Clinical Effectiveness of Nesiritide in De-compensated Heart Failure) on the contributions of peak expiratory flow. J Am Coll Cardiol. 2012;59(16):1441–8. doi: 10.1016/j.jacc.2011.11.061. [DOI] [PubMed] [Google Scholar]
- 41.Peacock WF, IV, Holland R, Gyarmathy R, et al. Observation unit treatment of heart failure with nesiritide: results from the proaction trial. J Emerg Med. 2005;29(3):243–52. doi: 10.1016/j.jemermed.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 42.Ezekowitz JA, Hernandez AF, Starling RC, et al. Standardizing care for acute decompensated heart failure in a large megatrial: the approach for the Acute Studies of Clinical Effectiveness of Nesiritide in Subjects with Decompensated Heart Failure (ASCEND-HF). Am Heart J. 2009;157(2):219–28. doi: 10.1016/j.ahj.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 43.Metra M, Cotter G, Davison BA, et al. Effect of serelaxin on cardiac, renal, and hepatic biomarkers in the Relaxin in Acute Heart Failure (RELAX-AHF) development program: correlation with outcomes. J Am Coll Cardiol. 2013;61(2):196–206. doi: 10.1016/j.jacc.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 44.Teerlink JR, Cotter G, Davison BA, et al. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet. 2013;381(9860):29–39. doi: 10.1016/S0140-6736(12)61855-8. [DOI] [PubMed] [Google Scholar]
- 45.Flaherty JD, Bax JJ, DeLuca L, et al. Acute heart failure syndromes in patients with coronary artery disease: early assessment and treatment. J Am Coll Cardiol. 2009;53:254. doi: 10.1016/j.jacc.2008.08.072. [DOI] [PubMed] [Google Scholar]
- 46.Dickstein K, Cohen-Solal A, Filippatos G, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur Heart J. 2008;29:2388. doi: 10.1093/eurheartj/ehn309. [DOI] [PubMed] [Google Scholar]
- 47.Annane D, Bellissant E, Pussard E, et al. Placebo-controlled, randomized, double-blind study of intravenous enalaprilat efficacy and safety in acute cardiogenic pulmonary edema. Circulation. 1996;94(6):1316–24. doi: 10.1161/01.cir.94.6.1316. [DOI] [PubMed] [Google Scholar]
- 48.Vital FM, Saconato H, Ladeira MT, et al. Non-invasive positive pressure ventilation (CPAP or bilevel NPPV) for cardiogenic pulmonary edema. Cochrane Database Syst Rev. 2008;(3):CD005351. doi: 10.1002/14651858.CD005351.pub2. [DOI] [PubMed] [Google Scholar]
- 49.Masip J, Betbese AJ, Paez J, et al. Non-invasive pressure support ventilation versus conventional oxygen therapy in acute cardiogenic pulmonary edema: a randomized trial. Lancet. 2000;356(9248):2126–32. doi: 10.1016/s0140-6736(00)03492-9. [DOI] [PubMed] [Google Scholar]
- 50.Mak S, Azevedo ER, Liu PP, et al. Effect of hyper-oxia on left ventricular function and filling pressures in patients with and without congestive heart failure. Chest. 2001;120(2):467–73. doi: 10.1378/chest.120.2.467. [DOI] [PubMed] [Google Scholar]
- 51.Schrock JW, Emerman CL. Observation unit management of acute decompensated heart failure. Heart Fail Clin. 2009;5(1):85, vii. doi: 10.1016/j.hfc.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 52.Shah SJ, Gheorghiade M. Heart failure with preserved ejection fraction: treat now by treating comorbidities. JAMA. 2008;300:431. doi: 10.1001/jama.300.4.431. [DOI] [PubMed] [Google Scholar]
- 53.Gheorghiade M, Pang PS. Acute heart failure syndromes. J Am Coll Cardiol. 2009;53(7):557–73. doi: 10.1016/j.jacc.2008.10.041. [DOI] [PubMed] [Google Scholar]
- 54.Gheorghiade M, Abraham WT, Albert NM, et al. Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. JAMA. 2006;296(18):2217–26. doi: 10.1001/jama.296.18.2217. [DOI] [PubMed] [Google Scholar]
- 55.Cotter G, Metra M, Milo-Cotter O, et al. Fluid overload in acute heart failure–re-distribution and other mechanisms beyond fluid accumulation. Eur J Heart Fail. 2008;10(2):165–9. doi: 10.1016/j.ejheart.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 56.Cotter G, Felker GM, Adams KF, et al. The patho-physiology of acute heart failure–is it all about fluid accumulation? Am Heart J. 2008;155(1):9–18. doi: 10.1016/j.ahj.2006.02.038. [DOI] [PubMed] [Google Scholar]
- 57.Adams KF, Jr, Fonarow GC, Emerman CL, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J. 2005;149(2):209–16. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 58.Gheorghiade M, Braunwald E. A proposed model for initial assessment and management of acute heart failure syndromes. JAMA. 2011;305(16):1702–3. doi: 10.1001/jama.2011.515. [DOI] [PubMed] [Google Scholar]
- 59.Smith WR, Poses RM, McClish DK, et al. Prognostic judgments and triage decisions for patients with acute congestive heart failure. Chest. 2002;121(5):1610–7. doi: 10.1378/chest.121.5.1610. [DOI] [PubMed] [Google Scholar]
- 60.Peacock WF, Fonarow GC, Ander DS, et al. Society of Chest Pain Centers Recommendations for the evaluation and management of the observation stay acute heart failure patient: a report from the Society of Chest Pain Centers Acute Heart Failure Committee. Crit Pathw Cardiol. 2008;7(2):83–6. doi: 10.1097/01.hpc.0000317706.54479.a4. [DOI] [PubMed] [Google Scholar]
- 61.Fonarow GC, Adams KF, Jr, Abraham WT, et al. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005;293(5):572–80. doi: 10.1001/jama.293.5.572. [DOI] [PubMed] [Google Scholar]
- 62.Auble TE, Hsieh M, Gardner W, et al. A prediction rule to identify low-risk patients with heart failure. Acad Emerg Med. 2005;12(6):514–21. doi: 10.1197/j.aem.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 63.Hsieh M, Auble TE, Yealy DM. Validation of the acute heart failure index. Ann Emerg Med. 2008;51(1):37–44. doi: 10.1016/j.annemergmed.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 64.Hernandez AF, Greiner MA, Fonarow GC, et al. Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303(17):1716–22. doi: 10.1001/jama.2010.533. [DOI] [PubMed] [Google Scholar]
- 65.Storrow AB, Collins SP, Lyons MS, et al. Emergency department observation of heart failure: preliminary analysis of safety and cost. Congest Heart Fail. 2005;11(2):68–72. doi: 10.1111/j.1527-5299.2005.03844.x. [DOI] [PubMed] [Google Scholar]
- 66.Baugh CW, Venkatesh AK, Hilton JA, et al. Making greater use of dedicated hospital observation units for many short-stay patients could save $3.1 billion a year. Health Aff (Millwood) 2012;31(10):2314–23. doi: 10.1377/hlthaff.2011.0926. [DOI] [PubMed] [Google Scholar]
- 67.Gheorghiade M, De Luca L, Fonarow GC, et al. Pathophysiologic targets in the early phase of acute heart failure syndromes. Am J Cardiol. 2005;96(6A):11G–7G. doi: 10.1016/j.amjcard.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 68.Setoguchi S, Stevenson LW, Schneeweiss S. Repeated hospitalizations predict mortality in the community population with heart failure. Am Heart J. 2007;154(2):260–6. doi: 10.1016/j.ahj.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 69.Graff L, Orledge J, Radford MJ, et al. Correlation of the Agency for Health Care Policy and Research congestive heart failure admission guideline with mortality: peer review organization voluntary hospital association initiative to decrease events (PROVIDE) for congestive heart failure. Ann Emerg Med. 1999;34(4 Pt 1):429–37. doi: 10.1016/s0196-0644(99)80043-2. [DOI] [PubMed] [Google Scholar]
- 70.Collins SP, Pang PS, Fonarow GC, et al. Is hospital admission for heart failure really necessary?: the role of the emergency department and observation unit in preventing hospitalization and rehospitalization. J Am Coll Cardiol. 2013;61(2):121–6. doi: 10.1016/j.jacc.2012.08.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peacock WF, Fonarow GC, Ander DS, et al. Society of Chest Pain Centers recommendations for the evaluation and management of the observation stay acute heart failure patient–parts 1-6. Acute Card Care. 2009;11(1):3–42. doi: 10.1080/02652040802688690. [DOI] [PubMed] [Google Scholar]