Abstract
Background
Prior studies suggest that elevated markers of bone turnover are prognostic for poor survival in castration-resistant prostate cancer (CRPC). The predictive role of these markers relative to bone-targeted therapy is unknown. We prospectively evaluated the prognostic and predictive value of bone biomarkers in sera from CRPC patients treated on a placebo-controlled phase III trial of docetaxel with or without the bone targeted endothelin-A receptor antagonist atrasentan (SWOG S0421).
Methods
Markers for bone resorption (N-telopeptide and pyridinoline) and formation (C-terminal collagen propeptide and bone alkaline phosphatase) were assayed in pretreatment and serial sera. Cox proportional hazards regression models were fit for overall survival. Models were fit with main effects for marker levels and with/without terms for marker–treatment interaction, adjusted for clinical variables, to assess the prognostic and predictive value of atrasentan. Analysis was adjusted for multiple comparisons. Two-sided P values were calculated using the Wald test.
Results
Sera from 778 patients were analyzed. Elevated baseline levels of each of the markers were associated with worse survival (P < .001). Increasing marker levels by week nine of therapy were also associated with subsequent poor survival (P < .001). Patients with the highest marker levels (upper 25th percentile for all markers) not only had a poor prognosis (hazard ratio [HR] = 4.3; 95% confidence interval [CI] = 2.41 to 7.65; P < .001) but also had a survival benefit from atrasentan (HR = 0.33; 95% CI = 0.15 to 0.71; median survival = 13 [atrasentan] vs 5 months [placebo]; P interaction = .005).
Conclusions
Serum bone metabolism markers have statistically significant independent prognostic value in CRPC. Importantly, a small group of patients (6%) with highly elevated markers of bone turnover appear to preferentially benefit from atrasentan therapy.
Bone metabolism is characterized by new bone formation mediated by osteoblasts and bone resorption mediated by osteoclasts. These processes are homeostatically regulated, with ultimate bone mass determined by the balance between these two activities. In patients with metastatic prostate cancer, this balance is often disrupted, with predominance of osteoblastic activity resulting in sclerotic bone metastases. This balance is further compromised by androgen deprivation therapy, a standard palliative treatment for advanced prostate cancer, which has been associated with acceleration of osteopenia and osteoporosis.
Unfortunately, bone metastasis is an extremely common event in patients with advanced prostate cancer, particularly those with castration-resistant prostate cancer (CRPC). In fact, more than 90% of patients with metastatic prostate cancer will have evidence of skeletal involvement. This predilection for bony spread becomes a frequent source of morbidity in this terminally ill population, with increased rates of fracture and bone pain.
Clinical evaluation of bone metastases is currently done with imaging modalities such as nuclear medicine bone scans, but these scans are not specific nor do they reliably detect areas of bone degradation that are commonly seen in bony metastases (1,2). Importantly, patients who are responding to systemic therapy (eg, patients with declining prostate specific antigen [PSA] levels coincident with a reduction in pain) may not always have a corresponding improvement seen in the bone scan. It is in this context that circulating markers of bone metabolism have been explored for their prognostic and/or predictive value (3,4).
We previously evaluated the prognostic and predictive significance of markers of bone metabolism in the context of a randomized National Cancer Institute–sponsored clinical trial of a matrix metalloproteinase inhibitor in CRPC patients (5). Markers of bone formation (osteocalcin, procollagen N-terminal propeptides: PINP & PIIINP) and resorption (N-telopeptide, pyridinoline, deoxypyridinoline) in serum were measured using commercial enzyme immunoassays. Of 80 patients enrolled, 69 had evaluable baseline serum specimens. We found that patients with lower baseline levels (less than the median) of N-telopeptide, deoxypyridinoline, alkaline phsophatase, PINP, and PIIINP had statistically significant better overall and/or progression-free survival compared with those with higher levels (greater than the median). These survival results were independent of PSA and bisphosphonate use. Further, serial evaluation of these same markers showed that decreasing levels were also associated with improved overall survival (OS) at 18 months. In addition, data from trials using the novel endothelin-A antagonist atrasentan in prostate cancer patients showed that levels of total and bone alkaline phosphastase were statistically significantly associated with time to progression and that atrasentan slowed the increase in bone alkaline phosphastase levels (6,7).
We hypothesized that baseline levels of bone metabolism markers in sera collected from patients with metastatic CRPC would be of high prognostic value and would be predictive of benefit from an osteoblast targeted agent such as atrasentan. This hypothesis is supported by observations that the endothelin pathway, which includes the secreted ET-1 protein and the ET-A and B receptors, contributes to the progression of prostate cancer bone metastases (8–11). In preclinical models, atrasentan inhibited prostate cancer cell–related paracrine mitogenic stimulation of cocultured osteoblasts, although it may likely have additional effects beyond osteoblastic cells (12,13).
We therefore sought to prospectively validate the encouraging results of our initial bone biomarker study in a larger CRPC patient cohort: a randomized phase III SWOG trial (S0421) of docetaxel/prednisone with or without atrasentan.
Methods
Clinical Trial Design and Eligibility
S0421 was a two-arm, randomized study, open label for docetaxel and double-blind placebo-controlled for atrasentan. This trial was registered with ClinicalTrials.gov (NCT00134056). Patients assigned to arm 1 received docetaxel 75mg/m2 intravenously over 1 hour every 21 days with prednisone 10 mg orally daily and an oral placebo daily. Patients on arm 2 received the same regimen as arm 1 except atrasentan 10mg daily orally in place of placebo. Treatment with docetaxel, prednisone, and atrasentan/placebo continued until disease progression or unacceptable toxicity. (Docetaxel was given for a maximum of 12 cycles.) The clinical results of the S0421 trial have been reported previously; in the overall study population, atrasentan failed to improve progression-free survival or OS in this trial (14).
Patients enrolled in S0421 were specifically consented to provide serial serum specimens for the bone marker studies described herein. All patients must have metastatic CRPC with imaging evidence of bone metastasis or cytological or histopathological bone marrow or bone biopsy evidence of involvement with prostate cancer. Bisphosphonate therapy was permitted but must have commenced before registration and continued at standard doses for the first four cycles of trial therapy. Commencement of bisphosphonates was not permitted within the first four cycles of study therapy. Other eligibility requirements were as described in the parent study (14). The study protocol was approved by the institutional review board or the National Cancer Institute Central Institutional Review Board or both.
Specimen Collection
Blood (serum) samples were collected using standard venipuncture techniques. Fifteen milliliters of whole blood were drawn pretreatment (after registration but before receiving the first dose of protocol therapy) and on the day of docetaxel infusion in cycles 2–4, referred to as weeks 4, 7, and 9. Whole blood was collected in red-top vacutainer tubes and allowed to clot for approximately 30 minutes. Serum was separated from cells within 45 to 60 minutes of venipuncture by centrifugation at 3000× for 10 minutes. Serum was equally aliquoted into four cryotubes and shipped to the SWOG repository.
Biomarker Assays
Bone Formation.
C-Terminal of type 1 collagen propeptide (CICP) was measured by a sandwich enzyme-linked immunosorbent assay (Quidel Corp, San Diego, CA) using a microtiter plate coated with monoclonal anti-C1CP antibody. A spectrophotomer (Biotek Corp, Winooski, VT) measured the absorbance of the samples at a wavelength of 405nm to determine results. Bone-specific alkaline phosphatase (BAP) activity in serum was measured using the Microvue BAP enzyme-linked immunosorbent assay (Quidel Corp) with a monoclonal anti-BAP antibody coated on a microtiter plate to capture BAP in the sample. Enzyme activity was detected using a pNPP substrate; optical density was measured at 405nm using a spectrophotometer (Biotek Corp).
Bone Resorption.
N-telopeptide (NTx), a marker of bone resorption from N-telopeptides of type 1 collagen, was quantified by a competitive enzyme-linked immunosorbent assay (Wampoles Laboratories, Princeton, NJ) using a 96-well microplate. Absorbance was determined spectrophotometrically (Biotek Corp) at a wavelength of 450nm. Pyridinoline (PYD), one of two nonreducible pyridinium cross-links present in the mature form of collagen (15,16), was measured in serum using a competitive enzyme immunoassay in a microtiter plate format (Quidel Corp). The optical density was measured at 405nm by a spectrophotomer (Biotek Corp).
Statistical Analysis
Associations Between Bone Marker Levels and OS.
We evaluated baseline bone marker concentrations for prognosis of OS by Cox regression, stratified for bisphosphonate usage and adjusted for baseline PSA levels. We extended this model to adjust for the following variables (measured at study entry): age, race (black vs all other), performance status, Gleason score (<7, 7, >7), PSA progression only vs progression of measurable and/or nonmeasureable disease, worst pain score (<4 vs ≥4), and presence of extraskeletal metastases. In patients surviving longer than 9 weeks, we used Cox regression to evaluate the association between OS and a change in bone marker concentrations from baseline to week 9, adjusted for baseline bone marker concentrations (fit as a natural cubic spline with 4 degrees of freedom), stratified for bisphosphonate usage, and adjusted for baseline PSA levels, with and without the extended panel of variables. The analysis plan was prespecified, and we made Bonferroni corrections to control the overall type I error rate at 0.05 for each primary question. For example, for the baseline analysis we accounted for eight tests based on four markers and two models (ie, with and without the extended panel of clinical covariables) by determining statistical significance at a P value less than or equal to .006. We further explored the interaction between bisphosphonates and bone markers on OS in the model that included the extended panel of variables. The distribution of bone marker concentrations were skewed with a wide dynamic range (eg, BAP ranged from 1.9 to 1761 u/L); therefore, to normalize the distribution and minimize overly influential datapoints, we log2 transformed all bone marker concentrations.
We evaluated baseline bone markers for prediction of a treatment effect on OS by dichotomizing bone markers and including an interaction term between bone markers and treatment in the Cox regression model; bone markers were dichotomized at the 50th percentile and, alternatively, at the upper 25th percentile. We evaluated bone markers separately and collectively (ie, elevated bone marker vs not, and all elevated bone markers vs not all elevated). We elected for a simple dichotomization as opposed to higher order cutpoints (eg, trichotomization) to facilitate analyzing bone markers as a composite measure (ie, all elevated bone markers vs not all elevated bone markers). We chose the median as a cutpoint to maximize the size of comparison groups. Alternatively, we chose the upper 25th percentile to identify a group with more extreme marker concentrations and, therefore, potentially more responsive to treatment, yet sufficiently large as a percentage of the patient population to be of clinical interest (ie, the all elevated marker group represented 6% of the patients). In the high-marker group, we explored the effect modification by bisphosphonates of the treatment effect on OS and (upon discovery of a treatment effect in patients with all elevated markers in the upper 25th percentile but not in patients with all elevated markers in the upper 50th percentile) prediction of a treatment effect dichotomizing at the 66th percentile.
The analyses for predictive markers were adjusted for statistically significant covariables discovered from the prognostic analysis. The plan was specified before the analyses, up to the selection of these clinical covariables. We made Bonferroni corrections to control the type I error rate at 0.05 across 10 tests (ie, 4 markers analyzed individually and collectively using 2 alternative dichotomizations). All P values were two-sided and calculated using the Wald test from the Cox regression model.
Effect of Treatment on Week 9 Marker Levels and Marker Dynamics.
We evaluated the effect of treatment and bisphosphonate usage on the change in bone marker levels from baseline to week 9 using linear regression (of the change in log2 bone marker concentrations on treatment and bisphosphonate usage). P values were based on robust standard errors to accommodate unequal variance across groups. Bone marker concentrations were log2 transformed for all regression models to minimize overly influential datapoints from skewed distributions. We made Bonferroni corrections to control the overall type I error rate at 0.05 due to multiple comparisons (ie, there were 4 tests, one for each bone marker, related to effect of treatment on the change in bone marker concentrations from baseline to week 9 and, similarly, there were 4 tests related to bisphosphonate usage).
Exploratory Analysis: Subgroup Cox Regressions and Receiver Operating Characteristic (ROC) Curves.
To further understand the importance of each bone marker we explored ROC curves. We evaluated ROC curves for prognosis of 2-year survival in both the placebo arm and the atrasentan arm accounting for the censoring (17). ROC curves were estimated using the R package survivalROC.
Note on Verification of Proportionality for Cox Models.
No deviations were observed in the Schoenfeld residuals that would indicate a violation of the Cox proportional hazards assumption.
Results
S0421 enrolled 1038 eligible patients; of those, 855 submitted serum for the bone biomarkers. Of these, 778 patients had usable specimens at baseline. Patient characteristics are summarized in Table 1. These characteristics mirrored that of the overall study cohort. Bone marker concentrations were skewed left; to minimize overly influential datapoints, we log2 transformed bone marker concentrations for all regression models. To provide additional comparative information, Supplementary Table 1 (available online) displays demographic information on the number of patients who were included in (n = 778) and excluded from the analysis (n = 260), as well as the corresponding number and rate of events (deaths) in each subgroup.
Table 1.
Patient characteristics and demographics*
| Variable | Docetaxel/ atrasentan | Docetaxel/placebo | Combined |
|---|---|---|---|
| 382 | 396 | 778 | |
| Race, No. (%) | |||
| Black | 53 (0.14) | 47 (0.12) | 100 (0.13) |
| Other | 9 (0.02) | 8 (0.02) | 17 (0.02) |
| Unknown | 6 (0.02) | 7 (0.02) | 13 (0.02) |
| White | 314 (0.82) | 334 (0.84) | 648 (0.83) |
| Type of progression, No. (%) | |||
| Measureable/evaluable | 316 (0.83) | 311 (0.79) | 627 (0.81) |
| PSA only | 66 (0.17) | 85 (0.21) | 151 (0.19) |
| Bisphosphonate usage, No. (%) | |||
| No | 145 (0.38) | 159 (0.40) | 304 (0.39) |
| Yes | 237 (0.62) | 237 (0.60) | 474 (0.61) |
| Worst pain, No. (%) | |||
| <4 | 228 (0.60) | 235 (0.59) | 463 (0.60) |
| ≥4 | 154 (0.40) | 161 (0.41) | 315 (0.40) |
| Extraskeletal metastases, No. (%) | |||
| No | 170 (0.45) | 182 (0.46) | 352 (0.45) |
| Yes | 212 (0.55) | 214 (0.54) | 426 (0.55) |
| Performance status, No. (%) | |||
| 0 | 167 (0.44) | 172 (0.43) | 339 (0.44) |
| 1 | 185 (0.48) | 188 (0.47) | 373 (0.48) |
| 2 | 28 (0.07) | 33 (0.08) | 61 (0.08) |
| 3 | 1 (0.00) | 0 (0.00) | 1 (0.00) |
| Missing | 1 (0.00) | 3 (0.01) | 4 (0.01) |
| Gleason score, No. (%) | |||
| <7 | 46 (0.12) | 48 (0.12) | 94 (0.12) |
| 7 | 108 (0.28) | 111 (0.28) | 219 (0.28) |
| >7 | 214 (0.56) | 222 (0.56) | 436 (0.56) |
| Missing | 14 (0.04) | 15 (0.04) | 29 (0.04) |
| Age at registration | |||
| Median (range) | 69 (41–92) | 69 (43–88) | 69 (41–92) |
| Baseline PSA | |||
| Median (range) | 78 (0–7417) | 60 (0–10414) | 68 (0–10414) |
* PSA = prostate specific antigen.
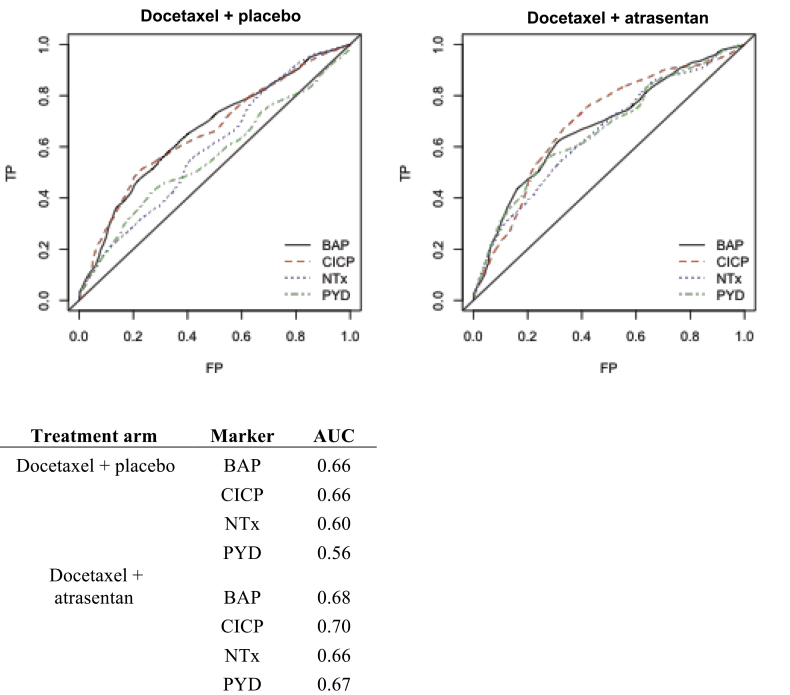
The ROC curves for baseline bone marker concentrations and 2-year survival are provided in Figure 1 for the placebo arm and the atrasentan arm. In the atrasentan arm, estimates of the area under the curve across all markers ranged from 0.66 to 0.70. In the placebo arm, estimates of the area under the curve were slightly diminished, ranging from 0.56 to 0.66. The biomarkers for bone formation CICP and BAP had the highest area under the curve across both arms. We explored whether there was any incremental improvement in prediction for these markers above and beyond baseline PSA and total alkaline phosphatase levels in the placebo and atrasentan arms. Taking PSA and total alkaline phosphatase into account, baseline bone markers did not contribute statistically significantly to the prognostication of survival in the placebo group (P = .92), but it did contribute statistically significantly to those on the atrasentan arm (P = .04).
Figure 1.
Unadjusted receiver operating characteristic curves for bone markers in the placebo and atrasentan arms, respectively, with corresponding areas under the curve (AUC). BAP = bone alkaline phosphatase; CICP = C-terminal of type 1 collagen; FP = False Positive; NTx = N-telopeptide of type 1 collagen; PYD = pyridinoline; TP = True Positive.
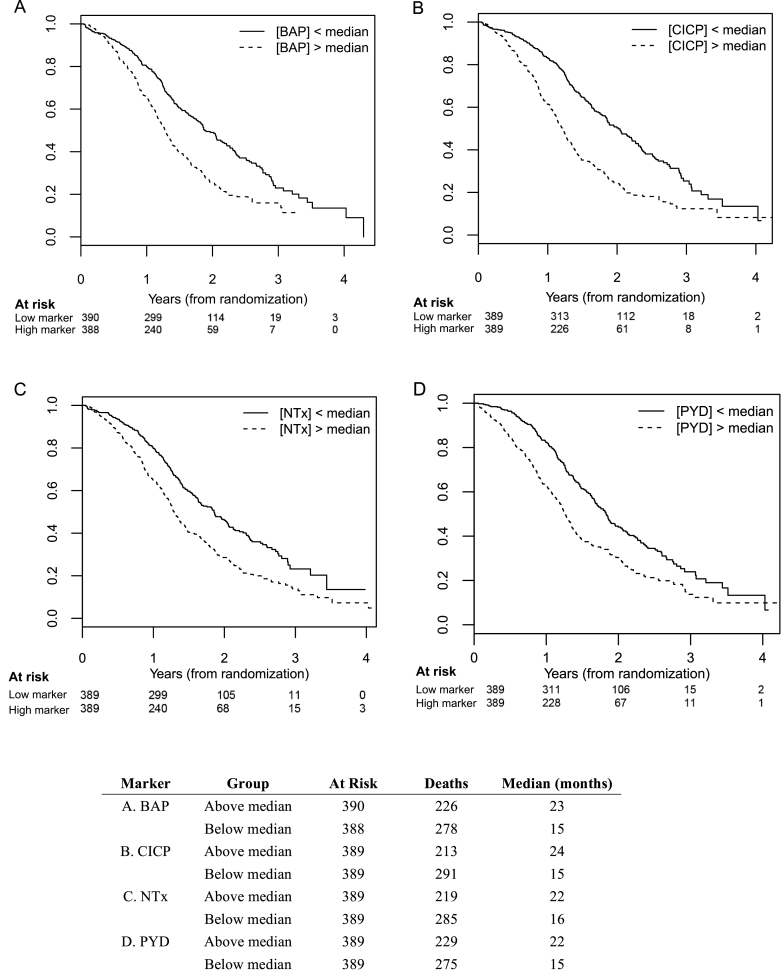
Across all four bone markers, the Kaplan–Meier curves for OS show a clear separation between patients stratified by high vs low baseline bone marker concentrations (ie, greater than the median vs less than or equal to the median) (Figure 2). There is statistically significant evidence that all bone markers measured at baseline are associated with OS (ie, higher levels portend poorer survival) based on Cox regression, both with and without adjustments for clinical variables (Table 2). For BAP, higher levels were associated with worse survival (hazard ratio [HR] = 1.23; 95% confidence interval [CI] = 1.14 to 1.32; with median survival times (MST) of 23 vs 15 months for values less than and greater than the median, respectively). For CICP, the hazard ratio was 1.38 (95% CI = 1.26 to 1.51) with median survival times of 24 vs 15 months. For NTx, the hazard ratio was 1.40 (95% CI = 1.27 to 1.54), with median survival times of 22 vs 16 months. Finally for pyridinoline, the hazard ratio was 1.52 (95% CI = 1.28, 1.81), with median survival times of 22 vs 15 months. All two-sided P values for these comparisons were less than .001. We found no evidence the association was modified by bisphosphonate usage. There was evidence of an association between the change in bone marker concentrations from baseline to week 9 and subsequent OS for all bone markers, even after adjusting for baseline bone marker levels and clinical variables. Specifically, increasing bone marker levels at week 9 were associated with a statistically significant increased risk of death (Table 3). Finally, we found a greater reduction in pyridinoline levels by week 9 in the atrasentan arm than in the placebo arm (P < .001), and marginal evidence for a greater reduction in CICP levels (P = .02); we did not find evidence of an association between bisphosphonate usage and changes in bone marker levels from baseline to week 9 (Table 3; Supplementary Table 2, available online).
Figure 2.
Baseline serum markers of bone metabolism in castration-resistant prostate cancer (CRPC) patients: association with overall survival. A) Bone alkaline phosphatase (BAP). B) C-terminal of type 1 collagen (CICP). C) N-telopeptides of type 1 collagen (NTx). D) Pyridinoline (PYD). Kaplan–Meier curves are shown for each of the biomarkers. Y-axis represents survival probability. All P values are two-sided. Each of these comparisons has a P value less than .001.
Table 2.
Baseline bone marker levels and overall survival*
| Biomarker† | Hazard ratio‡ (95% CI) | P§ |
|---|---|---|
| BAP, u/L | 1.23 (1.14 to 1.32) | <.001 |
| CICP, ng/mL | 1.38 (1.26 to 1.51) | <.001 |
| NTx, nM | 1.40 (1.27 to 1.54) | <.001 |
| PYD, nmol/L | 1.52 (1.28 to 1.81) | <.001 |
* BAP = bone alkaline phosphatase; CI = confidence interval; CICP = C-terminal of type 1 collagen; NTx = N-telopeptides of type 1 collagen; PYD = pyridinoline.
† Models adjusted for clinical covariates (see text).
‡ Hazard ratios for a twofold increase in bone marker concentrations.
§ The threshold for statistical significance was ≤.0125 to control overall error rate across four tests at ≤.05. All tests were two-sided.
Table 3.
Effect of atrasentan on bone marker concentrations from baseline to week 9
| Marker† (log2- transformed) | Baseline, mean (SD) Placebo arm‡ Atrasentan arm‡ | Week 9, mean (SD) Placebo arm‡ Atrasentan arm‡ | Change from baseline | Estimate§ (P||) |
|---|---|---|---|---|
| BAP, u/L | 6.38 (1.52) 6.26 (1.49) |
5.58 (1.49) 5.62 (1.36) |
−0.80 −0.64 |
0.16 (.03) |
| CICP, ng/mL | 3.43 (1.10) 3.43 (1.08) |
2.97 (0.96) 2.82 (0.99) |
−0.45 −0.61 |
−0.16 (.02) |
| NTx, nM | 3.98 (1.15) 3.92 (1.13) |
3.83 (1.13) 3.83 (1.10) |
−0.15 −0.09 |
0.05 (.31) |
| PYD, nmol/L | 1.49 (0.63) 1.56 (0.69) |
1.17 (0.68) 1.10 (0.64) |
−0.31 −0.46 |
−0.15 (<.001) |
* BAP = bone alkaline phosphatase; CICP = C-terminal of type 1 collagen; NTx = N-telopeptides of type 1 collagen; PYD = pyridinoline; SD = standard deviation
† Increasing bone marker levels at week 9 were associated with a statistically significant increased risk of death: for BAP, the hazard ratio was 1.28 (95% confidence interval [CI] = 1.11 to 1.47; P < .001); for CICP, the hazard ratio was 1.35 (95% CI = 1.14 to 1.59; P < .001); for NTx, the hazard ratio was 1.36 (95% CI = 1.12 to 1.66; P = .002); and for PYD, the hazard ratio was 1.36 (95% CI = 1.12 to 1.66; P = .002).
‡ n = 311 in the placebo arm and n = 307 in the atrasentan arm for all markers at both baseline and week 9.
§ Estimates for the difference between arms (atrasentan – placebo) in the mean change in log2 bone marker concentrations (from baseline to week9), adjusted for bisphosphonate usage.
|| The threshold for statistical significance was ≤.0125 to control overall error rate across four tests at ≤.05. All tests were two-sided.
For individual baseline bone markers dichotomized at the upper 25th percentile, improved survival was observed in the atrasentan arm compared with the placebo arm for two of the markers (CICP and BAP). These results are summarized in Table 4. Although none of these interactions were statistically significant based on highly conservative Bonferroni correction, P values for the association of CICP and BAP with a survival benefit from atrasentan were .04 and .02, respectively. We observed similar, although attenuated, trends when bone markers were dichotomized at the median.
Table 4.
Interaction of individual bone markers with treatment: impact on survival*
| Biomarker | Ratio of Hazard ratios† (95% CI) | P‡ |
|---|---|---|
| BAP, u/L | 0.65 (0.42 to 0.99) | .04 |
| CICP, ng/mL | 0.61 (0.41 to 0.92) | .02 |
| NTx, nM | 0.92 (0.60 to 1.40) | .69 |
| PYD, nmol/L | 0.97 (0.64 to 1.48) | .90 |
* Models adjusted for treatment main effects and clinical covariables (see text). BAP = bone alkaline phosphatase; CI = confidence interval; CICP = C-terminal of type 1 collagen; NTx = N-telopeptides of type 1 collagen; PYD = pyridinoline.
† Ratio of hazard ratios was used to show impact of marker × treatment interaction on survival using the following formula: [death events in high marker group/low marker group]Atrasemtam Arm / [death events in high marker group/low marker group]Placebo arm. Ratio of hazard ratios were calculated from separate Cox regression models for overall survival and baseline bone marker level (dichotomized as ≤25 percentile vs >25 percentile) by treatment interaction.
‡ All tests are two-sided.
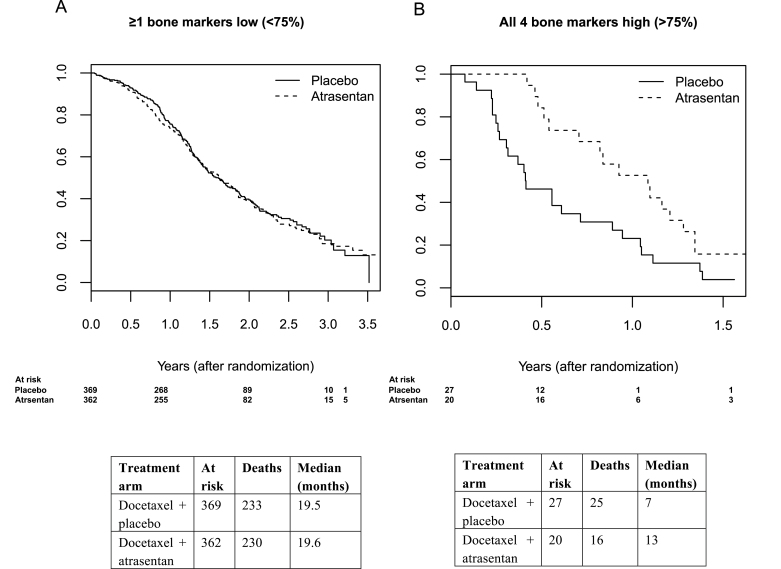
For patients with a high bone marker profile (ie, all markers in the upper 25th percentile) the Kaplan–Meier survival curves show a clear separation for patients stratified by treatment arm; however, there does not appear to be separation by treatment arm for patients with one or more low bone markers (Figure 3). Based on a Cox regression analysis, there is clear evidence that atrasentan increased OS in patients with a high bone marker profile (Table 5). These patients not only had a poor prognosis (HR = 4.3; 95% CI = 2.41 to 7.65; P < .001) but also had a survival benefit from atrasentan (HR = 0.33; 95% CI = 0.15 to 0.71; median survival = 13 [atrasentan] vs 5 months [placebo]; P interaction = .005). This group represented 6% of the patients that were assayed for bone markers. There was no evidence that bisphosphonate usage modified the treatment effect of atrasentan on OS in this subgroup of patients (data not shown). We did not find evidence for a treatment effect in a larger cohort of patients with all markers in the upper 50th percentile or, in more exploratory analysis, in a cohort of patients with all markers in the upper 66th percentile.
Figure 3.
Kaplan–Meier survival curves of castration-resistant prostate cancer (CRPC) patients with low (<25th percentile; left image) and high (≥25th percentile; right image) bone marker levels. Y-axis represents overall survival probability. Patients with the highest levels of bone biomarkers not only have a very poor prognosis (hazard ratio [HR] = 4.3; 95% confidence interval [CI] = 2.41 to 7.65; P < .001) but have a statistically significant survival benefit from atrasentan (HR = 0.33; 95% CI = 0.15 to 0.71; P interaction = .005). Patients whose bone biomarkers were not in the upper 25th percentile did not benefit from atrasentan therapy, with a median survival time of approximately 19.5 months in both arms (P = .83). P values were calculated using the Wald test and are two-sided.
Table 5.
Cox regression analysis* of overall survival based on treatment arm, bone marker profile, and treatment × bone marker interaction
| Variable | Upper 50th percentile | Upper 25th percentile | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Atrasentan arm | 1.05 (0.85 to 1.28) | .66 | 1.04 (0.86 to 1.25) | .69 |
| High marker profile† | 2.7 (1.96 to 3.72) | <.001 | 4.3 (2.41 to 7.65) | <.001 |
| Atrasentan × high marker profile interaction | 0.78 (0.50 to 1.22) | .28 | 0.33 (0.15 to 0.71) | .005 |
* Analysis was adjusted for clinical covariables as defined in text. All P values are two-sided. HR = hazard ratio; CI = confidence interval.
† Indicates patients in whom all four markers are greater than the median or in the highest quartile, respectively.
Discussion
Deaths from prostate cancer are commonly due to CRPC, the virulent end-stage form of the disease. The vast majority of patients with CRPC will suffer from skeletal metastases, a frequent source of morbidity, including bone pain or fracture. Because there are no established biomarkers relevant to bone metabolism that are currently used in clinic to prognosticate survival or to select therapy, research on candidate bone biomarkers remains critically warranted (3,4).
Several bone markers have been used to monitor cancer patients’ responses to therapy and include blood and urine products of bone collagen breakdown and serum markers of osteoblast activity and bone collagen synthesis (18–23). Circulating serum biomarkers are attractive to use in clinic because of their practicality and ease of availability. These markers also reflect the totality of the cancer’s biologic heterogeneity, instead of molecular readouts from small tumor biopsies or archival specimens that capture only a fragment of an otherwise highly heterogeneous tumor.
Some markers of bone turnover—specifically urine NTx and serum bone alkaline phosphatase—have previously shown prognostic significance in the context of bisphosphonate use (24,25). In a pooled analysis of phase III trials of zoledronic acid (25), an elevated baseline BAP level in prostate cancer patients was associated with an increased risk for a skeletal related event (odds ratio [OR] = 1.53; P = .03) and progression (OR = 2.64; P < .001). High baseline urine NTx levels were associated with higher rates of progression (relative risk [RR] = 2.2; P < .001) and death (RR = 5.72; P < .001). Serial reductions in serum BAP and urinary NTx during protocol therapy for either hormone-sensitive prostate cancer or CRPC were associated with better survival (26). However, there have been no prospective studies before this report to show that elevated baseline markers of bone metabolism in CRPC are predictive of benefit from bone-targeted therapy.
In CRPC patients with nonmetastatic disease, atrasentan appeared to have modest effects on time to disease progression (TTP), with a reported 93-day delay in the median TTP with atrasentan; however, the TTP difference compared with placebo was not statistically significant (P = .29) (7). In two other prior randomized trials of atrasentan vs placebo in the metastatic CRPC setting, a trend was detected for a TTP benefit in favor of atrasentan. In one of those trials (a phase III study involving >800 patients), atrasentan therapy was reported to yield a TTP hazard ratio of 0.88 (95% CI = 0.75 to 1.03) with a P value of .12 (6). In a post hoc subset analysis of that trial, patients with bone metastases at baseline were found to have an even better hazard ratio for TTP—0.8 with a 95% confidence interval (0.67 to 0.95) that did not cross unity. These hints of atrasentan activity in the phase III setting suggest that only a small subset of patients preferentially benefit from this agent and that perhaps a biologic marker can be used to detect those patients a priori. The results we report herein not only validate the strong prognostic value of serum markers of bone resorption and formation but also appear to identify the likely subset of CRPC patients who benefit from treatment with atrasentan. These data also provide proof of principle for the concept of bone marker–driven patient and treatment selection to be tested with other osteoblast milieu-targeted agents in prostate cancer.
These results are limited by issues related to generalizability because only 6% of patients appear to preferentially benefit and by the fact that the predictive value of bone biomarkers may not necessarily be applicable to all bone-directed treatments. It is also uncertain whether additional bone biomarkers can improve the prognostic and predictive performance of the existing biomarker group.
In conclusion, our results show serum baseline levels of bone turnover can serve as biomarkers for both prognosis and prediction in metastatic CRPC. Future studies of bone-targeted therapies in this disease should consider using bone marker levels (dichotomized at the median) as a stratification factor and designing cohort enrichment strategies using these markers.
Funding
This investigation was supported by National Institutes of Health/National Cancer Institute grant 5R01-CA120469 and in part by the following Public Health Service or PHS Cooperative Agreement grant numbers awarded by the National Cancer Institute: CA32102, CA38926. The parent trial (S0421) had accrual participation from other US Cooperative Groups (Eastern Cooperative Oncology Group and Cancer and Leukemia Group B). ClinicalTrials.gov: NCT00134056.
The study sponsors (ie, R01 grant sponsor) had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication. The corresponding author had full access to all the data and the final responsibility to submit for publication.
These data were presented in part at the 43rd Annual Meeting of the American Society of Clinical Oncology, June 1–5, 2012, Chicago, Illinois.
References
- 1. Pollen JJ, Gerber K, Ashburn WL, et al. Nuclear bone imaging in metastatic cancer of the prostate. Cancer. 1981;47 (11):2585–2594 [DOI] [PubMed] [Google Scholar]
- 2. Maeda H, Koizumi M, Yoshimura K, et al. Correlation between bone metabolic markers and bone scan in prostatic cancer. J Urol. 1997;157 (2):539–543 [PubMed] [Google Scholar]
- 3. Fontana A, Delmas PD. Markers of bone turnover in bone metastases. Cancer. 2000;88 (12 suppl):295–2960 [DOI] [PubMed] [Google Scholar]
- 4. Ali SM, Demers LM, Leitzel K, et al. Baseline serum NTx levels are prognostic in metastatic breast cancer patients with bone-only metastasis. Ann Oncol. 2004;15 (3):455–459 [DOI] [PubMed] [Google Scholar]
- 5. Lara PN, Jr, Stadler WM, Longmate J, et al. A randomized phase II trial of the matrix metalloproteinase inhibitor BMS-275291 in hormone-refractory prostate cancer patients with bone metastases. Clin Cancer Res. 2006;12(5):1556–1563 [DOI] [PubMed] [Google Scholar]
- 6. Carducci MA, Saad F, Abrahamsson PA, et al. A phase 3 randomized controlled trial of the efficacy and safety of atrasentan in men with metastatic hormone-refractory prostate cancer. Cancer. 2007;110 (9):1959–1966 [DOI] [PubMed] [Google Scholar]
- 7. Nelson JB, Love W, Chin JL, et al. Phase 3, randomized, controlled trial of atrasentan in patients with nonmetastatic, hormone-refractory prostate cancer. Cancer. 2008;113 (9):2478–2487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nelson J, Bagnato A, Battistini B, et al. The endothelin axis: emerging role in cancer. Nat Rev Cancer. 2003;3(2):110–116 [DOI] [PubMed] [Google Scholar]
- 9. Nelson JB, Lee WH, Nguyen SH, et al. Methylation of the 5’ CpG island of the endothelin B receptor gene is common in human prostate cancer. Cancer Res. 1997;57 (1):35–37 [PubMed] [Google Scholar]
- 10. Yin JJ, Mohammad KS, Kakonen SM, et al. A causal role for endothelin-1 in the pathogenesis of osteoblastic bone metastases. Proc Natl Acad Sci U S A. 2003;100(19):10954–10959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mundy GR. Endothelin-1 and osteoblastic metastasis. Proc Natl Acad Sci U S A. 2003;100 (19):10588–10589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fizazi K, Yang J, Peleg S, et al. Prostate cancer cells-osteoblast interaction shifts expression of growth/survival-related genes in prostate cancer and reduces expression of osteoprotegerin in osteoblasts. Clin Cancer Res. 2003;9 (7):2587–2597 [PubMed] [Google Scholar]
- 13. Pirtskhalaishvili G, Nelson JB. Endothelium-derived factors as paracrine mediators of prostate cancer progression. Prostate. 2000;44 (1):77–87 [DOI] [PubMed] [Google Scholar]
- 14. Quinn DI, Tangen CM, Hussain M, et al. Docetaxel and atrasentan versus docetaxel and placebo for men with advanced castration-resistant prostate cancer (SWOG S0421): a randomised phase 3 trial. Lancet Oncol. 2013;14 (9):893–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sano M, Kushida K, Takahashi M, et al. Urinary pyridinoline and deoxypyridinoline in prostate carcinoma patients with bone metastasis. Brit J Cancer. 1994;70 (4):701–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pecherstorfer M, Zimmer-Roth I, Schilling T, et al. The diagnostic value of urinary pyridinium cross-links of collagen, serum total alkaline phosphatase, and urinary calcium excretion in neoplastic bone disease. J Clin Endocrinol Metab. 1995;80 (1):97–103 [DOI] [PubMed] [Google Scholar]
- 17. Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000; 56 (2):337–344 [DOI] [PubMed] [Google Scholar]
- 18. Demers LM, Costa L, Chinchilli VM, et al. Biochemical markers of bone turnover in patients with metastatic bone disease. Clin Chem. 1995; 41 (10):1489–1494 [PubMed] [Google Scholar]
- 19. Houze P, Bellik B, Extra JM, et al. Urinary carboxyterminal telopeptide of collagen I as a potential marker of bone metastases chemotherapy monitoring in breast cancer. Clin Chim Acta. 1999;281 (1–2):77–88 [DOI] [PubMed] [Google Scholar]
- 20. Blomqvist C, Risteli L, Risteli J, et al. Markers of type I collagen degradation and synthesis in the monitoring of treatment response in bone metastases from breast carcinoma. Brit J Cancer. 1996;73 (9):1074–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Berruti A, Piovesan A, Torta M, et al. Biochemical evaluation of bone turnover in cancer patients with bone metastases: relationship with radiograph appearances and disease extension. Brit J Cancer. 1996;73 (12):1581–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Takeuchi S, Arai K, Saitoh H, et al. Urinary pyridinoline and deoxypyridinoline as potential markers of bone metastasis in patients with prostate cancer. J Urol. 1996;156 (5):1691–1695 [PubMed] [Google Scholar]
- 23. Yoshida K, Sumi S, Arai K, et al. Serum concentration of type I collagen metabolites as a quantitative marker of bone metastases in patients with prostate carcinoma. Cancer. 1997;80(9):1760–1767 [PubMed] [Google Scholar]
- 24. Cook RJ, Coleman R, Brown J, et al. Markers of bone metabolism and survival in men with hormone-refractory metastatic prostate cancer. Clin Cancer Res. 2006;12(11 Pt 1):3361–3367 [DOI] [PubMed] [Google Scholar]
- 25. Coleman RE, Major P, Lipton A, et al. Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. J Clin Oncol. 2005;23 (22):4925–4935 [DOI] [PubMed] [Google Scholar]
- 26. Som A, Tu SM, Liu J, et al. Response in bone turnover markers during therapy predicts overall survival in patients with metastatic prostate cancer: analysis of three clinical trials. Brit J Cancer. 2012;107 (9):1547–1553 [DOI] [PMC free article] [PubMed] [Google Scholar]