Abstract
Background
Previous studies have hypothesized that tumor blood flow may be elevated or reduced during exercise, which could impact the tumor microenvironment. However, to date technical limitations have precluded the measurement of tumor blood flow during exercise. Using an orthotopic preclinical model of prostate cancer, we tested the hypotheses that during exercise tumors would experience 1) diminished vascular resistance, 2) augmented blood flow, 3) increased numbers of perfused vessels, and 4) decreased tissue hypoxia and, furthermore, that the increased perfusion would be associated with diminished vasoconstriction in prostate tumor arterioles.
Methods
Dunning R-3327 MatLyLu tumor cells were injected into the ventral prostate of male Copenhagen rats aged 4 to 6 months randomly assigned to tumor-bearing (n = 42) or vehicle control (n = 14) groups. Prostate tumor blood flow, vascular resistance, patent vessel number, and hypoxia were measured in vivo in conscious rats at rest and during treadmill exercise, and vasoconstrictor responsiveness of resistance arterioles was investigated in vitro.
Results
During exercise there was a statistically significant increase in tumor blood flow (approximately 200%) and number of patent vessels (rest mean ± standard deviation [SD] = 12.7±1.3; exercise mean ± SD = 14.3±0.6 vessels/field; Student t test two-sided P = .02) and decreased hypoxia compared with measurements made at rest. In tumor arterioles, the maximal constriction elicited by norepinephrine was blunted by approximately 95% vs control prostate vessels.
Conclusions
During exercise there is enhanced tumor perfusion and diminished tumor hypoxia due, in part, to a diminished vasoconstriction. The clinical relevance of these findings are that exercise may enhance the delivery of tumor-targeting drugs as well as attenuate the hypoxic microenvironment within a tumor and lead to a less aggressive phenotype.
It is well established that exercise elicits several health benefits and is an important component of comprehensive health-care strategies (1). Despite the recommendation of exercise for cancer patients (2,3), the effects of exercise on tumor blood flow or oxygenation are unknown. This is surprising given that the direction (eg, increase, decrease, or unchanged) and magnitude of any alterations in tumor blood flow are fundamental for interpreting any effects of exercise training on the growth, progression, or microenvironment of a tumor. Furthermore, without knowing how tumor perfusion responds to physical activity, it is difficult to predict how treatments that influence physical function may impact treatment outcomes. During exercise, there is a reduction in blood flow to most organs in the body to shunt blood flow to active muscle (4,5). Given that the mechanisms regulating tumor blood flow are complex, predicting directional changes in tumor perfusion or oxygenation during exercise, when regional blood flow is changing, is challenging. If tumor blood flow is diminished during exercise, the presence of hypoxic microenvironments may be potentiated within the tumor [eg, increased HIF-1α expression (6)], which is associated with the adoption of a more aggressive phenotype (7). Although in some animal models exercise results in an earlier appearance of tumors compared with sedentary groups (8) and high-intensity exercise may increase the incidence of metastases (9), the majority of studies show exercise training suppresses tumorigenesis with chronic training (10,11). Conversely, if tumor flow is elevated during exercise, a reduction in tumor hypoxia would be expected and may have a benefic ial effect on the tumor microenvironment [eg, slow tumor growth (11)] and potentially increase the delivery of tumor-targeting intravascular compounds.
To our knowledge, there have been no reports of tumor blood flow measurement in humans or animals during exercise. Furthermore, the majority of measurements of tumor blood flow in animals have been made under anesthesia, which alters tumor blood flow and tumor-to-tumor variability (12), making it difficult to extrapolate such measures to the conscious resting state, let alone the exercising condition. Therefore, the purpose of this investigation was to test the hypotheses, in an established orthotopic model of prostate cancer (13), that an acute bout of low-to-moderate intensity exercise (similar in intensity to most daily activities) will 1) enhance prostate tumor blood flow because of a diminished tumor vascular resistance, 2) increase the number of perfused blood vessels in the tumor, and 3) diminish tumor hypoxia. Furthermore, we conducted additional mechanistic studies to measure tumor resistance arteriole vasomotor control to determine whether contractile deficits in the tumor vasculature are associated with the tumor perfusion response to exercise.
Methods
Experimental Model
All procedures were approved by the Institutional Animal Care and Use Committee at the University of Florida and complied fully with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (14). Male Copenhagen rats aged 4 to 6 months (n = 56; Charles River Laboratories, Wilmington, MA) were studied. The parental tumor from which the cell line is derived is the original Dunning R-3327 discovered in Copenhagen rats (15). The rats were housed at 23°C and maintained on a 12-hour light/12-hour dark cycle and provided rat chow and water ad libitum.
The Dunning R3327-MatLyLu (MLL) rat prostate carcinoma cell line (Sigma Aldrich, USA) was used in this study and is a well-established model of prostate cancer (16) with similar characteristics to progressive human prostate cancers (17). MLL cells were cultured in Roswell Park Memorial Institute 1640 medium (supplemented with 2mM glutamine, 250nM dexamethasone, 10% fetal bovine serum, and 1% penicillin/streptomyocin; Sigma Aldrich) and maintained in a humidified incubator at 5% carbon dioxide. At approximately 80% to 90% confluence, viable cells were counted and a cell stock solution was prepared with sterile saline (PSS) and separated into aliquots of 0.1mL containing approximately 104 MLL cells each.
While under anesthesia (isofluorane; 2%/oxygen [O2] balance), the bladder and prostate complex of the male Copenhagen rats were exposed and isolated through a small abdominal incision (<2cm) lateral to the midline of the abdomen. Rats were randomly assigned to a tumor-bearing (n = 42) or vehicle control (n = 14) group. Using sterile insulin syringes (28 G), 0.1mL of cell/PSS solution was injected into the ventral lobe of the prostate. After the injection of the cells, the abdominal wall (3-0, polyglycolic acid coated; DemeTECH, Miami Lakes, FL) and overlying skin/fascia (3-0 nylon monofilament; DemeTECH) incisions were closed. Vehicle control rats were subjected to the same surgical procedure, with 0.1mL of PSS injected into the prostate. All procedures were performed under aseptic conditions. Postoperative monitoring of the rats was performed daily until experimental protocols were performed at approximately 21 days postinjection.
Experimental Protocol
The detailed experimental protocol can be found in the Supplementary Methods (available online).
Study 1: Tumor Blood Flow and Vascular Resistance During Acute Exercise.
Blood flow and vascular resistance in the prostate tissue (control; n = 6) and prostate tumor (tumor-bearing; n = 7) at rest and during exercise were determined using the radionuclide-tagged microsphere technique (18). Detailed methods regarding the surgery and microsphere technique have been previously published (18–20) and are described in the Supplementary Methods (available online). Before measurements, rats were familiarized with treadmill exercise, during which they walked for 5 minutes per day for 5 days. At least 24 hours after the last bout of the familiarization period, catheters were implanted in the ascending aorta (for microsphere infusion and blood pressure determination) and the caudal tail artery (for reference withdrawal samples), as we have previously described (18).
After surgical recovery (>4 hours), the rat was placed on the treadmill, and blood flow in the conscious condition was measured at rest and 5 minutes after the onset of exercise because circulatory hemodynamics are stable by this time during exercise (21). Specifically, either at rest or after 5 minutes of total exercise, time, radiolabeled (57Co or 85Sr) microspheres (approximately 2.5×105; 15 μm diameter; PerkinElmer/NEN, Waltham, MA) were infused into the ascending aorta concomitant with blood withdrawal from the caudal artery. After microsphere infusions, rats were killed (pentobarbital sodium, 100mg/kg intraperitoneally), the prostate tissue, prostate tumor, and soleus muscle were removed, and tissue radioactivity level was determined by a gamma scintillation counter. Total blood flow to each tissue was calculated by the reference sample method (22) and expressed in milliliters per minute per 100g of tissue. Vascular resistance was calculated and expressed as millimeters of mercury per milliliter of blood flow per 100g of tissue. Oxygen delivery was calculated using an average of 17.8mL of O2 per 100mL of blood for the rat (23) and the measured blood flow.
Study 2: Prostate Tumor Perfused Vessels During Exercise.
Perfused vessels in prostate tumors were determined at rest (n = 8) and during exercise (n = 7) using the Hoechst-33342 staining procedure (24). Detailed methods are provided in the Supplementary Methods (available online). The surgical procedure and exercise protocol used were identical to those used for blood flow measurements in study 1. Hoechst-33342 (40mg/kg) was infused during the final minute of exercise (or during a 5-minute resting period) and allowed to circulate throughout the body for 1 minute, before the rats were killed (pentobarbital, 100mg/kg). The excised tumor was sectioned on slides and viewed with a fluorescent microscope, and perfused vessels were identified by the surrounding halo of the Hoechst-33342 labeled cells (8,25). Under ×10 objective magnification, a 25-point Chalkley grid was positioned randomly over a field of view, and the points falling within the fluorescent halo were scored as positive (24,26). The mean vessel density per field was determined from 30 random fields across a minimum of 10 sections per tumor.
Study 3: Prostate Tumor Hypoxia During Exercise.
Tumor hypoxia was assessed in a separate group of rats at rest (n = 7) or during exercise (n = 7) by immunohistochemical identification of EF5, designed and provided by Dr C. J. Koch (University of Pennsylvania, Philadelphia, PA) (27). Detailed methods regarding EF 5 administration, fixation, and quantification are described in the Supplementary Methods (available online). One hour before being killed, the rats were injected with EF5 (30mg/kg intraperitoneally) and immediately subjected to treadmill exercise (60 minutes), or they remained in resting conditions. Tumors were excised and sliced in five consecutive 10-µm sections at three different regions within the tumor (approximately 750 µm apart). To determine the area of EF5 bound, section-by-section images were analyzed using public domain National Institutes of Health Image software (ImageJ; available at http://rsb.info.nih.gov/ij) (28). Images were converted to a binary format, and pixels with a higher intensity than the threshold were treated as areas with positive uptake of EF5. Hypoxic fraction was quantified by dividing the EF5-bound area by the total viable tissue area, as measured by area of 4',6-diamidino-2-phenylindole (DAPI) staining within the same slide.
Study 4: Tumor Vascular Function.
In a separate set of rats, alpha (α)–adrenergic and myogenic vasoconstrictor responsiveness of prostate arterioles (control; n = 8) and prostate tumor arterioles (tumor-bearing; n = 6) were investigated in vitro using the isolated microvessel technique, which we have described previously (20,29) and is detailed in the Supplementary Methods (available online). Resistance arterioles (<200 µm) from the tumor or healthy prostate were isolated, cannulated, and imaged by an inverted microscope (Olympus IX70, Olympus America, Center Valley, PA) equipped with a video camera and caliper for recording luminal diameter. To evaluate vasoconstrictor responsiveness, arterioles were exposed to 1) cumulative additions of the α-adrenoreceptor agonist norepinephrine (10−9 to 10−4 M) and 2) myogenic response to increases in intraluminal pressure from 0 to 135cm water (H2O) in 15-cm H2O increments.
Statistical Analysis
A Student t test was used to determine differences in body mass, vessel characteristics, perfused vessel density, and regions of hypoxia between groups. Dose–response and pressure–diameter curves were analyzed by two-way analysis of variance (ANOVA) with repeated measures. A two-way ANOVA with repeated measures was used to compare within-group (rest vs exercise) and between-group (control vs tumor-bearing) differences in arterial pressure, blood flow, and vascular resistance. A Wilcoxon signed-rank test was used to compare percentage changes in vascular resistance from rest to exercise. Post hoc analyses were performed using Duncan’s multiple range test. All statistical tests were two-tailed and considered statistically significant if P was less than or equal to .05.
Results
Tumor Blood Flow and Vascular Resistance
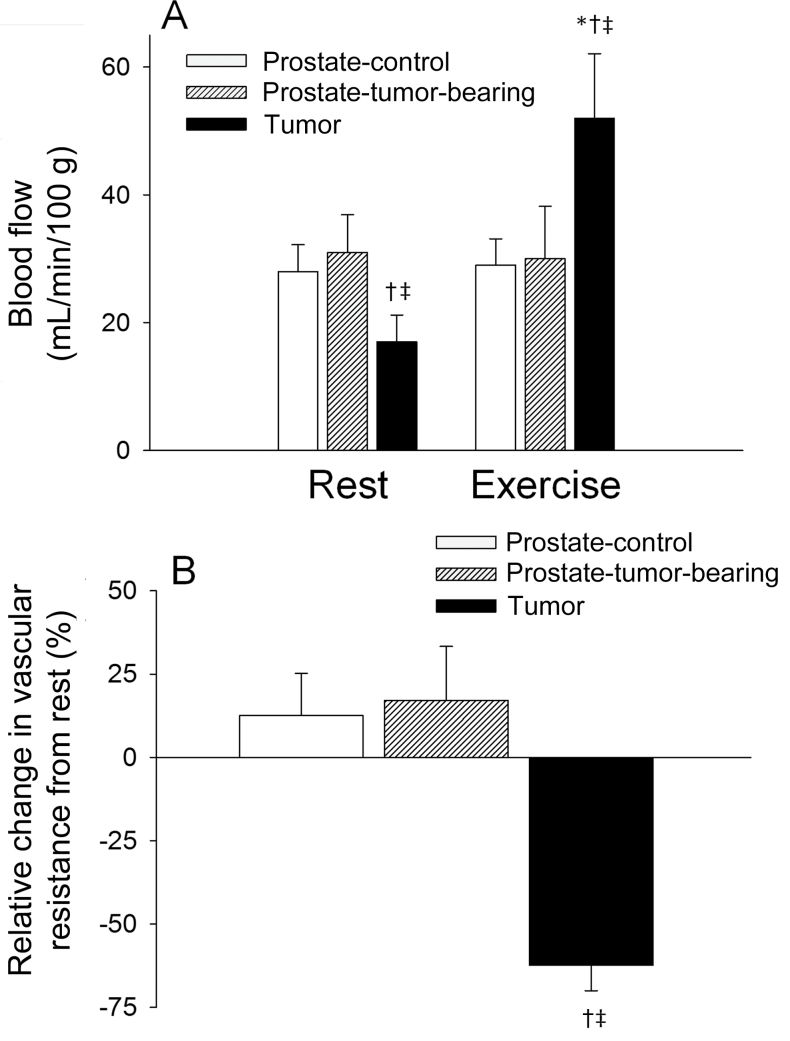
Tumor and body weights are reported in Table 1. During exercise, mean arterial pressure and soleus muscle blood flow were statistically significantly elevated vs resting values in all groups (Table 1). In the tumor-bearing rats, there was a lower blood flow to the prostatic tumor compared with the surrounding prostate tissue at rest (Figure 1A), whereas there was no difference in blood flow between prostate tissue vs control values (Figure 1A). Exercise resulted in an approximately 200% increase in prostate tumor blood flow (Figure 1A), which led to an increase in O2 delivery from a resting value of 3.0mL O2/min/100g to 9.3mL O2/min/100g during exercise. Vascular resistance within the prostate tumor was statistically significantly greater at rest when compared with the prostate tissue of control rats and the host prostate of tumor-bearing rats (Table 1). During the rest–exercise transition, prostate tumor vascular resistance decreased approximately 65%, whereas resistance increased slightly in the host prostate and prostate from the control group (Figure 1B).
Table 1.
Body mass, tissue weights, arterial blood pressure, muscle blood flow, and prostate and prostate tumor vascular resistance and arteriolar characteristics*
| Animal characteristics | Control, Mean (95% CI) | Tumor-bearing, Mean (95% CI) | P |
|---|---|---|---|
| No. | 14 | 42 | ___ |
| Body weight, g | 282 (262 to 301) | 312 (289 to 336) | .09† |
| Prostate weight, g | 0.58 (0.45 to 0.71) | 0.31 (0.29 to 0.32) | .005† |
| Prostate tumor weight, g | — | 2.0 (1.3 to 2.7) | ___ |
| Blood flow and vascular resistance, No. | 6 | 7 | ___ |
| Mean arterial pressure, mm Hg | |||
| Rest | 118 (112 to 123) | 117 (112 to 122) | .43‡ |
| Exercise | 131 (127 to 135)§ | 133 (125 to 140)§ | .37‡ |
| Soleus blood flow, mL/min/100 g | |||
| Rest | 131 (114 to 148) | 129 (117 to 140) | .39‡ |
| Exercise | 313 (272 to 357)§ | 335 (302 to 367)§ | .20‡ |
| Soleus vascular resistance, mm Hg/mL/min/100 g | |||
| Rest | 0.91 (0.79 to 1.08) | 0.92 (0.83 to 1.02) | .45‡ |
| Exercise | 0.43 (0.35 to 0.51)§ | 0.40 (0.37 to 0.43)§ | .23‡ |
| Prostate vascular resistance, mm Hg/mL/min/100 g | |||
| Rest | 4.24 (3.61 to 4.87) | 3.89 (3.29 to 4.48) | .17‡ |
| Exercise | 4.65 (4.02 to 5.27)¶ | 4.51 (3.79 to 5.23)|| | .41‡ |
| Prostate tumor vascular resistance, mm Hg/mL/min/100 g | |||
| Rest | 7.24 (5.71 to 8.77) ‡,|| | ||
| Exercise | 2.65 (2.20 to 3.10) §,# | ||
| Isolated microvessels, No. | Prostate arteriole, 8 | Tumor arteriole, 6 | |
| Maximal diameter, µm | 177 (144 to 210) | 160 (112 to 209) | .28† |
| Spontaneous tone, % | 16.7 (10.0 to 23.6) | 16.2 (11.6 to 21.0) | .46† |
| Wall thickness, µm | 17.1 (14.2 to 20.1) | 16.9 (14.1 to 19.7) | .45† |
| Wall-to-lumen ratio | 10.1 (8.3 to 11.7) | 11.7 (7.8 to 16.2) | .20† |
* Maximal diamater was determined in the absence of extracellular Ca2+ in resistance arterioles from the healthy prostate and prostate tumor. Spontaneous tone is expressed as a relative percentage of tone established following pressurization at 90cm water. All statistical tests were two-sided. CI = confidence interval.
† Student t test.
‡ Analysis of variance with repeated measures.
§ P ≤ .001 vs. resting value within group.
|| P = 0.06 vs resting value within group.
¶ P = 0.20 vs resting value within group.
# P < .001 vs prostate from tumor-bearing group during same condition (ie, at rest or during exercise).
Figure 1.
A) Blood flow at rest and during the steady-state of exercise in the prostate of control (n = 6) rats and prostate and prostate tumor of tumor-bearing (n = 7 per group) rats. B) Relative change in vascular resistance in the exercising vs resting condition in the prostate of control (n = 6) rats, and prostate and prostate tumor of tumor-bearing (n = 7 per group) rats. Error bars represent the standard deviation. *P ≤ .001 vs resting conditions within the same tissue. †P ≤ .001 vs prostate of control group during same condition. ‡P ≤ 0.001 vs prostate of the tumor-bearing group during same condition. All statistical tests were two-sided. A two-way analysis of variance with repeated measures was used to compare within-group (rest vs exercise) and between-group (control vs tumor-bearing) differences in blood flow. A Wilcoxon signed-rank test was used to compare percentage changes in vascular resistance from rest to exercise.
Patent Tumor Blood Vessels and Oxygenation
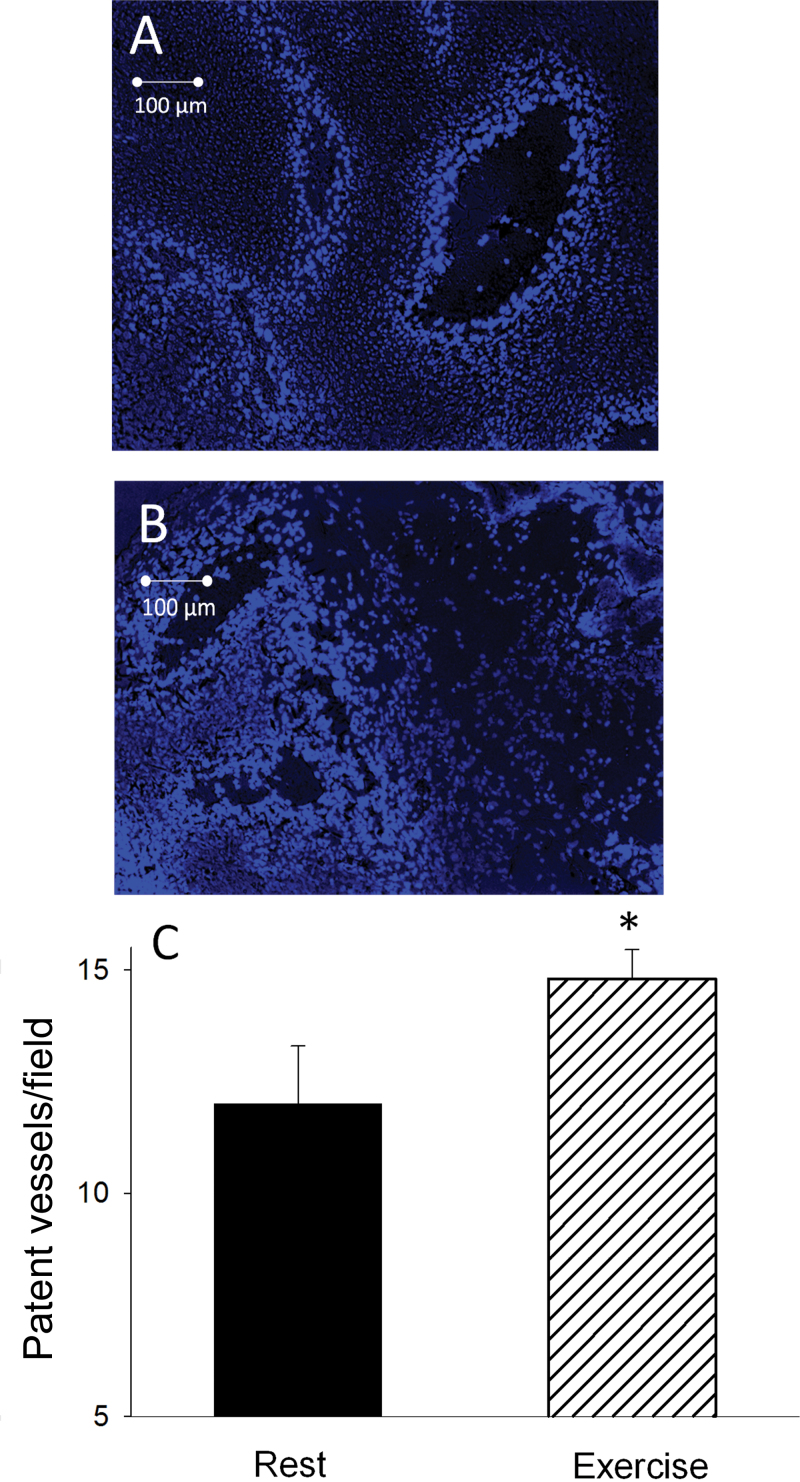
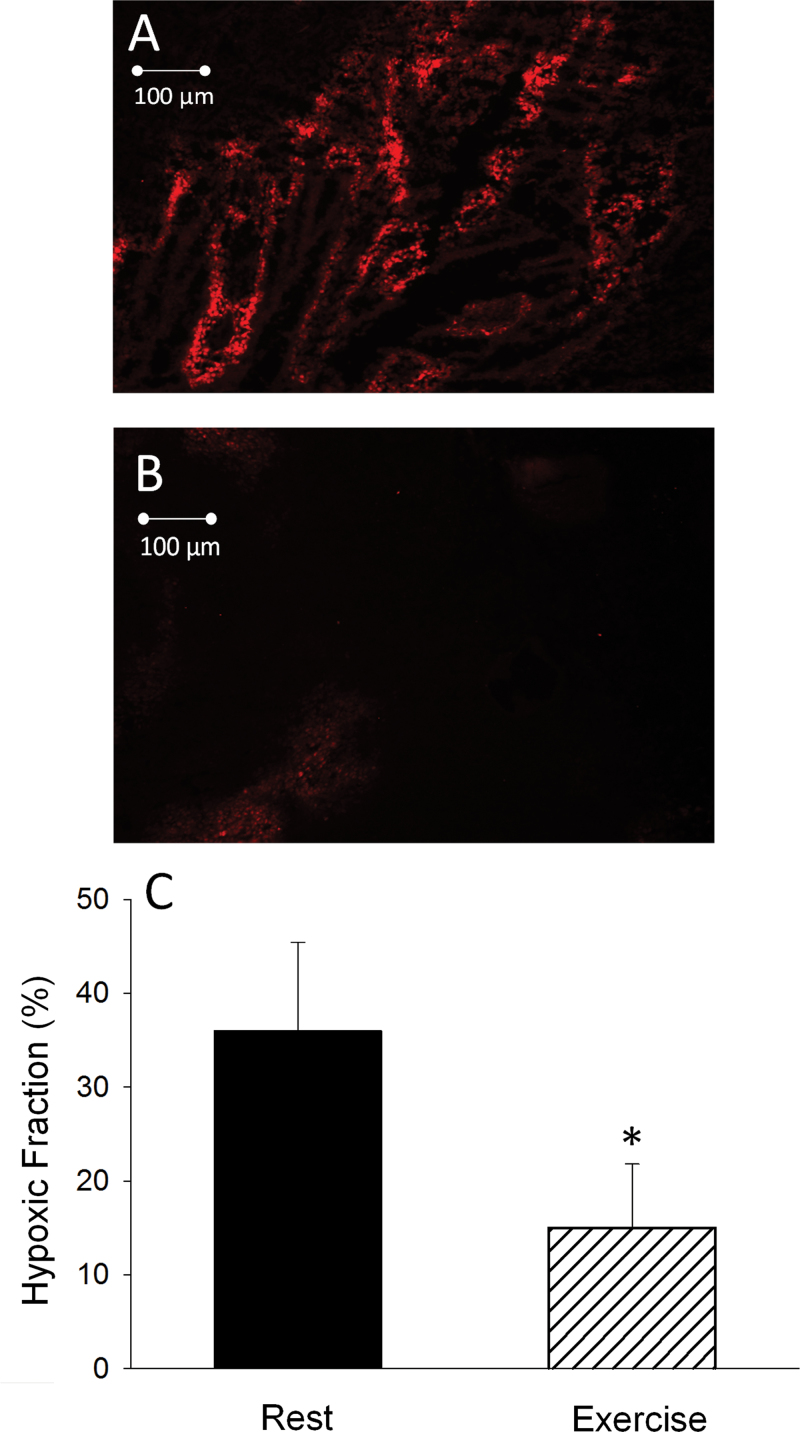
Representative images of perfused vessels in a prostate tumor at rest vs at exercise are shown in Figure 2, A and B. During exercise, there was a statistically significant increase in the average number of patent vessels per field in the tumor compared with that measured in the resting condition (rest mean ± standard deviation [SD] = 12.7±1.3; exercise mean ± SD = 14.3±0.6 vessels/field; Student t test two-sided P = .02) (Figure 2C). Representative images of the spatial distribution of hypoxia, as demonstrated by presence of EF5, during rest and exercise are illustrated in Figure 3, A and B. During exercise, the fraction of EF5-bound cells was statistically significantly reduced (15.4±8.1% [SD]) compared with resting conditions (35.5±10.4% [SD]; P < .001) (Figure 3C), demonstrating a substantial reduction in tumor hypoxia during exercise.
Figure 2.
Fluorescence photomicrograph of a prostate tumor cross-sectional field, obtained from a MatLyLu Copenhagen rat prostate carcinoma after intravenous injection of Hoechst-33342 (40mg/kg) at rest (A) and during acute exercise (B). The cells of the vessels perfused at the time of injection are fluoresced. C) Effect of an acute bout of exercise on patent prostatic tumor blood vessels per field (at least 30 fields per tumor were measured; see Methods for further information). Rats were injected with Hoechst-33342 at rest (n = 8) or during exercise (n = 7). Error bars represent the standard deviation. *P = .02 vs. resting condition. All statistical tests were two-sided. A Student t test was used to determine differences in perfused vessel density.
Figure 3.
Fluorescence photomicrograph of a prostate tumor cross-section, obtained from a MatLyLu Copenhagen rat prostate carcinoma after intraperitoneal injection of EF5 (30mg/kg) at rest (A) and during acute exercise (B). The EF5 binds to hypoxic regions and is fluoresced. C) Graphical representation of the fraction of tissues bound by EF5 (ie, hypoxic fraction). EF5 was injected and allowed to circulate for 1 hour during rest (n = 7) or exercise (n = 7). Error bars represent the standard deviation. *P < .001 vs resting condition. All statistical tests were two-sided. A Student t test was used to determine differences in hypoxic fraction.
Tumor Vascular Contractile Responsiveness
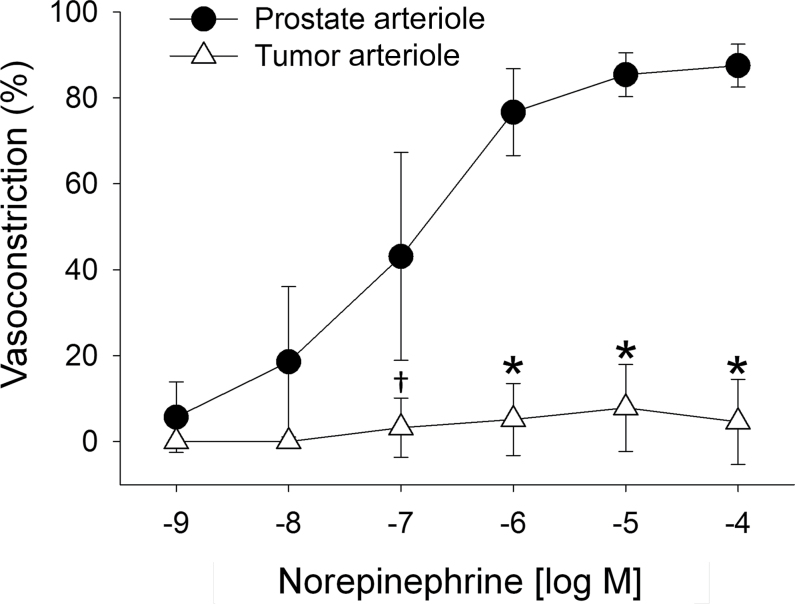
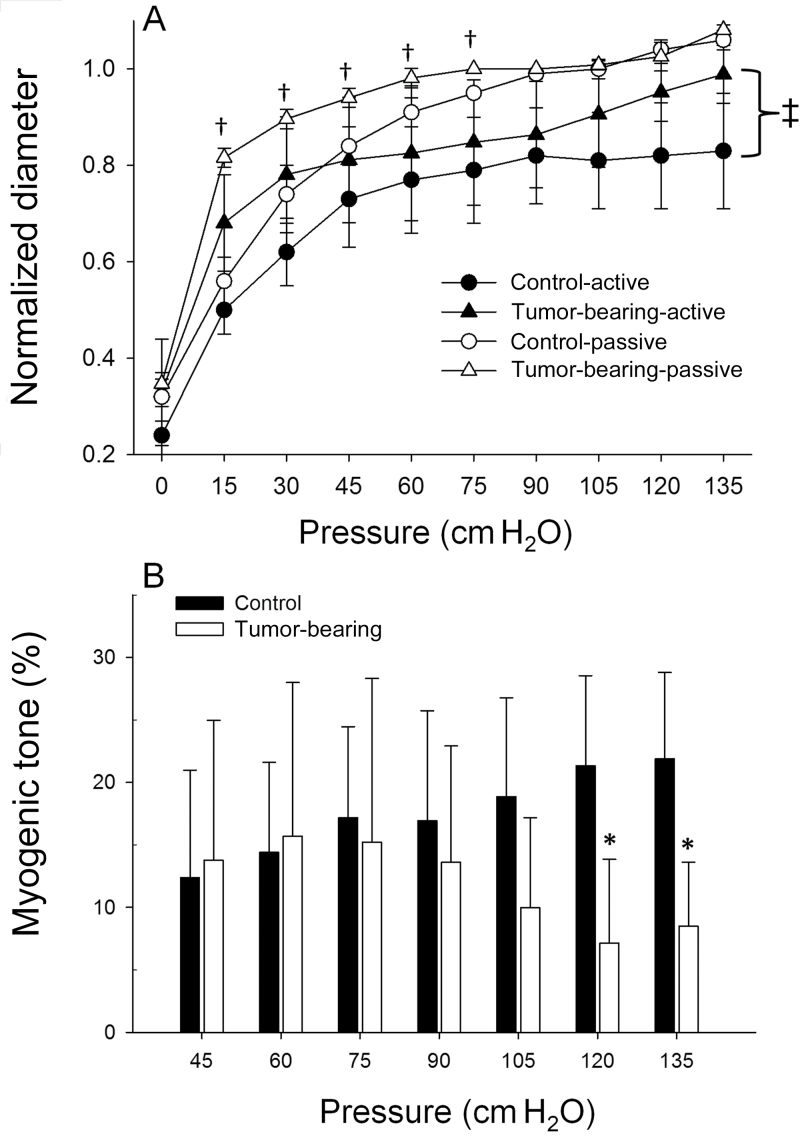
There were no differences in maximum diameter, spontaneous tone, and wall thickness of resistance arterioles between groups (Table 1). There were no differences in wall-to-lumen ratio between vessels (Table 1), indicating no gross structural differences between vessels. In response to the norepinephrine, there was a diminished constriction in prostate tumor (tumor-bearing) resistance arterioles vs control prostate (Figure 4), with the maximal constriction blunted by approximately 95% in tumor-bearing vessels (3.4±6.3% [SD]) compared with healthy prostate arterioles (87.5±3.3% [SD]; P ≤ .001). Figure 5A illustrates active and passive pressure–diameter relations. Active contractile responses from the tumors were statistically significantly less than those of vessels from the healthy prostate (Figure 5A). For example, at an intraluminal pressure of 135cm H2O, the tumor arterioles generated a statistically significantly diminished vasoconstriction (2.3±3.6% [SD]) vs the healthy prostate arterioles (17.5±12.5% [SD]; P < .001). Acute myogenic tone (ie, ability to constrict with elevations in intraluminal pressure) was not different between groups from 0 to 105cm H2O (Figure 5B), demonstrating that tumor vessels display a functional ability to vasoconstrict to physical stimuli. However, at higher intraluminal pressures (ie, > 120cm H2O) acute myogenic tone was diminished in tumor-bearing arterioles compared with control vessels (Figure 5B), which suggests a diminished ability to develop myogenic tone when the tumor vessels are exposed to higher arterial pressures (eg, during exercise).
Figure 4.
Dose–response relations to adrenergic receptor agonist norepinephrine in arterioles perforating the prostate from control (n = 8) rats and the prostatic tumor of tumor-bearing rats (n = 6). Error bars represent the standard deviation. *P < .001 vs prostate arteriolar constriction at a given does of norepinephrine. †P = .006 versus prostate arteriolar constriction at matched dose of norepinephrine. All statistical tests were two-sided. Dose–response curves were analyzed by two-way analysis of variance with repeated measures.
Figure 5.
A) Active and and passive diameter responses to increasing intraluminal pressure in arterioles perforating the prostate from control (n = 8) rats and the prostatic tumor of tumor-bearing rats (n = 6). Values are normalized to diameter at 90cm water (H2O). B) Resting myogenic tone across the physiological range of pressures arteries are subjected to. Values are normalized to diameter at 90cm H2O and expressed as a percentage change in diameter relative to passive myogenic tone. Error bars represent the standard deviation. *P < .001 vs control myogenic tone at a matched intraluminal pressure. groups. †P < .001 vs control passive at that given intraluminal pressure. ‡P = .06 between group responses for active myogenic response. All statistical tests were two-sided. Pressure–diameter curves were analyzed by two-way analysis of variance with repeated measures.
Discussion
This is the first study to measure blood flow, perfused vessels, and hypoxia in experimental tumors during exercise. Of importance, tumor blood flow was measured in the conscious animal, which avoids the well-known effect of anesthetics on central hemodynamics and tumor blood flow (30) and provides an accurate evaluation of the resting vs exercising condition. Compared with measurements made in the resting condition, tumor blood flow increased approximately 200% during acute exercise and resulted in an increase in the number of perfused vessels. Furthermore, there was an approximately 50% reduction in tumor hypoxia during exercise. The large decrease in tumor vascular resistance during exercise was due, in part, to an inability of the tumor vasculature to vasoconstrict. Clinically, this suggests that exercise has the potential to enhance tumor oxygenation and may mitigate the hypoxic microenvironments that are associated with conventional anticancer agent treatment failures and with an aggressive tumor phenotype (31–33). Furthermore, the delivery of intravascular compounds to a tumor is compromised by a low resting blood flow. Thus, exercise may provide a unique opportunity to enhance compound delivery to tumors.
In healthy tissue, local regulatory mechanisms within vascular beds ensure that metabolic demands are coupled with adequate tissue perfusion (35). In tumors, oxygenation may decrease as a result of diminished blood flow (36) and/or an increased oxygen demand (37). In solid tumors, the unique vascular morphology in combination with aberrant tumor metabolism (38) results in heterogeneous perfusion across the tumor tissue and subsequent hypoxia (39). Clinical investigations have shown that the prevalence of hypoxic tissue areas contributes to the pathophysiology of locally advanced solid tumors (36) and is a critical determinant of their malignant progression (31), metastasis (40) and radiosensitivity (41).
Our data demonstrate that, in addition to elevated blood flow, there is enhancement of the functional vasculature within the tumor and reduction of hypoxia during exercise when compared with rest. Furthermore, the reduction in tumor hypoxia suggests that elevations in tumor blood flow were sustained above resting values for the 60-minute bout of exercise on the treadmill. Overall, these data demonstrate that exercise augments tumor oxygenation, which, considering hypoxia is associated with a more aggressive phenotype (7), provides a potential mechanism for the reduced rate of metastasis and tumor growth observed in most studies with chronic exercise (9,30,42) [see Colbert et al. for exception; (43)] and the beneficial effects of exercise after diagnosis of prostate cancer (44). A recent study did demonstrate an HIF-1α upregulation in orthotopic breast tumors after exercise training, suggesting the tumors became more hypoxic (6). The result was surprising given the increase in patent vessels observed after training in that study (6) would be expected to diminish the diffusion distance of O2 to hypoxic regions of the tumor, although we recently found no changes in patent vessels after exercise training (45). It should be noted that HIF-1α can be upregulated by many mechanisms not directly related to hypoxia, including vascular mechanical stretch (46,47), a stimulus likely to occur within a tumor during exercise because of the elevated arterial pressure (Table 1) and diminished arterial myogenic autoregulation. Conversely, we have recently demonstrated that long-term, chronic exercise training mitigates prostate tumor hypoxia in the resting state (45). In both of these previous studies (6,45), there were no measurements of tumor blood flow, patent vessels, vascular resistance, or tumor hypoxia during exercise, which are fundamental to determine how either acute or chronic exercise may affect the tumor microenvironment and/or drug delivery. Given previous studies have provided conflicting data regarding patent vessels after long-term exercise training (6,45), our data demonstrating enhanced patent vessels concurrent with a large increase in tumor blood flow during exercise suggest that the most efficacious time to modulate tumor drug delivery is during aerobic exercise vs in the resting state.
In response to aerobic exercise, the distribution of cardiac output changes substantially vs rest, with a shunting of blood flow from nonactive tissues (eg, splanchnic organs) to the contracting musculature, with a similar blood flow distribution pattern in rats (5) and humans (48). This shunting is due, in part, to an enhanced sympathetic nerve activity and release of norepinephrine, which can bind to α-adrenergic receptors on the vascular smooth muscle and induce vasoconstriction. Although prostate tumors are innervated with sympathetic nerve fibers (49), the severely diminished α-adrenergic constriction of tumor arterioles would diminish the ability of the sympathetic nervous system to regulate tumor blood flow. In addition, the tumor arterioles demonstrate a diminished myogenic vasoconstriction at higher intraluminal pressures, which occur during exercise (increase arterial pressure) (Table 1). Overall, the diminished α-adrenergic vasoconstriction concurrent with the loss of myogenic tone at higher intraluminal pressures are likely the predominant mechanisms responsible for the enhanced tumor flow during exercise.
These data have clinical implications regarding the potential for exercise to modify the tumor microenvironment in response to low-to-moderate intensity exercise. However, it is unknown whether the same response is observed in other solid tumors or at different intensities of exercise. We chose a low intensity because more intense exercise has the potential to enhance metastases (9), and, therefore, our results may not be representative of the effects of more strenuous exercise on tumor blood flow and oxygenation. Although physical activity is considered to be a critical component of individual health status, a discrepancy in the effects of chronic exercise on tumor progression exists (6,50,51). However, repeated elevations in shear stress (as experienced with exercise) have been shown to promote angiogenesis and arteriogenesis (28,52) and may serve as a mechanism to normalize the tumor microenvironment through improvements in the vascular phenotype and stabilization of perfusion patterns, a conclusion that is supported by findings of a recent study in mice subjected to voluntary wheel running exercise (53).
Our study is not without limitations. For example, only α-adrenergic and pressure stimuli were tested in the isolated vessels to assess two potential pathways of vascular function. Whether exercise affects vasodilator activity or whether the tumor responds differently to other vasoconstrictor agonists (eg, angiotensin II) is unknown and may contribute to the hyperemic response in the tumor during exercise. Furthermore, although there was an increase in the number of patent vessels during exercise, we cannot discriminate between vessels that are supporting a large number of red blood cells from those that may only be supporting a higher proportion of plasma (which has a low capacitance of O2). Therefore, without measures of intravascular hemodynamics (eg, capillary tube hematocrit), it is unknown how tumor diffusional characteristics, which help facilitate O2 transfer (54), may change with exercise and alter tumor oxygenation.
This is the first study to demonstrate that tumor blood flow increases during acute exercise in a preclinical orthotopic model of prostate cancer due, in part, to a diminished contractile responsiveness of the tumor vasculature. The implications of this enhanced tumor perfusion may include improved tumor oxygenation and mitigation of the “hypoxic tumor phenotype” (55). In addition, the combination of the tumor dysfunctional vasculature and systemic events with exercise (eg, elevated arterial pressure) may provide a mechanism to enhance the efficacy of intravascular tumor drug delivery. Conversely, a greater tumor perfusion may also lead to an enhanced tumor survival and progression and exacerbation of the hostile tumor microenvironment (56), although recent studies in mice suggest no detrimental effect of long-term voluntary exercise on tumor growth (53). Whether similar hemodynamic profiles occur during exercise in tumors growing in other tissues is currently unknown and cannot be generalized from our data. It is likely, however, that tumor blood flow and oxygenation (as well as growth and metastasis) with exercise are dependent on regional blood flow responses to exercise as well as tumor resistance vascular function. How exercise affects these systems is an important question for future studies.
Funding
This work was supported by the National Institute of Health (AG-31317 to BJB) and the Florida Biomedical Research Program (1BN-02 to BJB).
The study sponsors had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
References
- 1. Mereles D, Ehlken N, Kreuscher S, et al. Exercise and respiratory training improve exercise capacity and quality of life in patients with severe chronic pulmonary hypertension. Circulation. 2006;114(14):1482–1489 [DOI] [PubMed] [Google Scholar]
- 2. National Cancer Institute Physical Activity and Cancer. http://www.cancer.gov/cancertopics/factsheet/prevention/physicalactivity Accessed February 12, 2014 [Google Scholar]
- 3. Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62(4):243–274 [DOI] [PubMed] [Google Scholar]
- 4. Musch TI, Friedman DB, Pitetti KH, et al. Regional distribution of blood flow of dogs during graded dynamic exercise. J Appl Physiol. 1987;63(6):2269–2277 [DOI] [PubMed] [Google Scholar]
- 5. Rowell L. Human Cardiovascular Control. Oxford: Oxford University Press; 1993 [Google Scholar]
- 6. Jones LW, Viglianti BL, Tashjian JA, et al. Effect of aerobic exercise on tumor physiology in an animal model of human breast cancer. J Appl Physiol. 2010;108(2):343–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vaupel P. Hypoxia and aggressive tumor phenotype: implications for therapy and prognosis. Oncologist. 2008;13(Suppl 3):21–26 [DOI] [PubMed] [Google Scholar]
- 8. Woods JA, Davis JM, Kohut ML, et al. Effects of exercise on the immune response to cancer. Med Sci Sports Exerc. 1994;26(9):1109–1115 [PubMed] [Google Scholar]
- 9. Cohen LA, Boylan E, Epstein M, et al. Voluntary exercise and experimental mammary cancer. Adv Exp Med Biol. 1992;322:41–59 [DOI] [PubMed] [Google Scholar]
- 10. Thompson HJ, Westerlind KC, Snedden JR, et al. Inhibition of mammary carcinogenesis by treadmill exercise. J Natl Cancer Inst. 1995;87(6):453–455 [DOI] [PubMed] [Google Scholar]
- 11. Westerlind KC, McCarty HL, Schultheiss PC, et al. Moderate exercise training slows mammary tumour growth in adolescent rats. Eur J Cancer Prev. 2003;12(4):281–287 [DOI] [PubMed] [Google Scholar]
- 12. Menke H, Vaupel P. Effect of injectable or inhalational anesthetics and of neuroleptic, neuroleptanalgesic, and sedative agents on tumor blood flow. Radiat Res. 1988;114(1):64–76 [PubMed] [Google Scholar]
- 13. Lubaroff DM, Canfield L, Feldbush TL, et al. R3327 adenocarcinoma of the Copenhagen rat as a model for the study of the immunologic aspects of prostate cancer. J Natl Cancer Inst. 1977;58(6):1677–1689 [DOI] [PubMed] [Google Scholar]
- 14. National Institutes of Health. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Research Council; 1995 [Google Scholar]
- 15. Dunning WF. Prostate cancer in the rat. Natl Cancer Inst Monogr 1963;12:351–369 [PubMed] [Google Scholar]
- 16. Kiessling F, Huber PE, Grobholz R, et al. Dynamic magnetic resonance tomography and proton magnetic resonance spectroscopy of prostate cancers in rats treated by radiotherapy. Invest Radiol. 2004;39(1):34–44 [DOI] [PubMed] [Google Scholar]
- 17. Isaacs JT, Heston WD, Weissman RM, et al. Animal models of the hormone-sensitive and -insensitive prostatic adenocarcinomas, Dunning R-3327-H, R-3327-HI, and R-3327-AT. Cancer Res. 1978;38(11 Pt 2):4353–4359 [PubMed] [Google Scholar]
- 18. Behnke BJ, Prisby RD, Lesniewski LA, et al. Influence of ageing and physical activity on vascular morphology in rat skeletal muscle. J Physiol. 2006;575(Pt 2):617–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Davis RT, 3rd, Stabley JN, Dominguez JM, 2nd, et al. Differential effects of aging and exercise on intra-abdominal adipose arteriolar function and blood flow regulation. J Appl Physiol. 2013;114(6):808–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McCullough DJ, Davis RT, 3rd, Dominguez JM, 2nd, et al. Effects of aging and exercise training on spinotrapezius muscle microvascular PO2 dynamics and vasomotor control. J Appl Physiol. 2011;110(3):695–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Laughlin MH, Armstrong RB. Rat muscle blood flows as a function of time during prolonged slow treadmill exercise. Am J Physiol. 1983;244(6):H814–H824 [DOI] [PubMed] [Google Scholar]
- 22. Ishise S, Pegram BL, Yamamoto J, et al. Reference sample microsphere method: cardiac output and blood flows in conscious rat. Am J Physiol. 1980;239(4):H443–H449 [DOI] [PubMed] [Google Scholar]
- 23. Behnke BJ, McDonough P, Padilla DJ, et al. Oxygen exchange profile in rat muscles of contrasting fibre types. J Physiol. 2003;549(Pt 2):597–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smith KA, Hill SA, Begg AC, et al. Validation of the fluorescent dye Hoechst 33342 as a vascular space marker in tumours. Br J Cancer. 1988;57(3):247–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Salmon HW, Siemann DW. Effect of the second-generation vascular disrupting agent OXi4503 on tumor vascularity. Clin Cancer Res. 2006;12(13):4090–4094 [DOI] [PubMed] [Google Scholar]
- 26. Siemann DW, Rojiani AM. The vascular disrupting agent ZD6126 shows increased antitumor efficacy and enhanced radiation response in large, advanced tumors. Int J Radiat Oncol Biol Phys. 2005;62(3):846–853 [DOI] [PubMed] [Google Scholar]
- 27. Koch CJ, Hahn SM, Rockwell K, Jr, et al. Pharmacokinetics of EF5 [2-(2-nitro-1-H-imidazol-1-yl)-N-(2,2,3,3,3-pentafluoropropyl) acetamide] in human patients: implications for hypoxia measurements in vivo by 2-nitroimidazoles. Cancer Chemother Pharmacol. 2001;48(3):177–187 [DOI] [PubMed] [Google Scholar]
- 28. Yuan H, Schroeder T, Bowsher JE, et al. Intertumoral differences in hypoxia selectivity of the PET imaging agent 64Cu(II)-diacetyl-bis(N4-methylthiosemicarbazone). J Nucl Med. 2006;47(6):989–998 [PubMed] [Google Scholar]
- 29. Behnke BJ, Ramsey MW, Stabley JN, et al. Effects of aging and exercise training on skeletal muscle blood flow and resistance artery morphology. J Appl Physiol. 2012;113(11):1699–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Steiner JL, Davis JM, McClellan JL, et al. Effects of voluntary exercise on tumorigenesis in the C3(1)/SV40Tag transgenic mouse model of breast cancer. Int J Oncol. 2013;42(4):1466–1472 [DOI] [PubMed] [Google Scholar]
- 31. Hockel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001;93(4):266–276 [DOI] [PubMed] [Google Scholar]
- 32. Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11(6):393–410 [DOI] [PubMed] [Google Scholar]
- 33. Brahimi-Horn MC, Chiche J, Pouyssegur J. Hypoxia and cancer. J Mol Med (Berl). 2007;85(12):1301–1307 [DOI] [PubMed] [Google Scholar]
- 34. Bertout JA, Patel SA, Simon MC. The impact of O2 availability on human cancer. Nat Rev Cancer. 2008;8(12):967–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Saltin B, Radegran G, Koskolou MD, et al. Skeletal muscle blood flow in humans and its regulation during exercise. Acta Physiol Scand. 1998;162(3):421–436 [DOI] [PubMed] [Google Scholar]
- 36. Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49(23):6449–6465 [PubMed] [Google Scholar]
- 37. Secomb TW, Hsu R, Ong ET, et al. Analysis of the effects of oxygen supply and demand on hypoxic fraction in tumors. Acta Oncol. 1995;34(3):313–316 [DOI] [PubMed] [Google Scholar]
- 38. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vaupel P, Harrison L. Tumor hypoxia: causative factors, compensatory mechanisms, and cellular response. Oncologist 2004;9(Suppl 5):4–9 [DOI] [PubMed] [Google Scholar]
- 40. De Jaeger K, Kavanagh MC, Hill RP. Relationship of hypoxia to metastatic ability in rodent tumours. Br J Cancer. 2001;84(9):1280–1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vaupel P, Briest S, Hockel M. Hypoxia in breast cancer: pathogenesis, characterization and biological/therapeutic implications. Wien Med Wochenschr. 2002;152(13–14):334–342 [DOI] [PubMed] [Google Scholar]
- 42. Zheng X, Cui XX, Huang MT, et al. Inhibition of progression of androgen-dependent prostate LNCaP tumors to androgen independence in SCID mice by oral caffeine and voluntary exercise. Nutr Cancer. 2012;64(7):1029–1037 [DOI] [PubMed] [Google Scholar]
- 43. Colbert LH, Westerlind KC, Perkins SN, et al. Exercise effects on tumorigenesis in a p53-deficient mouse model of breast cancer. Med Sci Sports Exerc. 2009;41(8):1597–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kenfield SA, Stampfer MJ, Giovannucci E, et al. Physical activity and survival after prostate cancer diagnosis in the health professionals follow-up study. J Clin Oncol. 2011;29(6):726–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McCullough DJ, Nguyen LM, Siemann DW, et al. Effects of exercise training on tumor hypoxia and vascular function in the rodent preclinical orthotopic prostate cancer model. J Appl Physiol (1985). 2013;115(12):1846–1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lim CS, Qiao X, Reslan OM, et al. Prolonged mechanical stretch is associated with upregulation of hypoxia-inducible factors and reduced contraction in rat inferior vena cava. J Vasc Surg. 2011;53(3):764–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chang H, Shyu KG, Wang BW, et al. Regulation of hypoxia-inducible factor-1alpha by cyclical mechanical stretch in rat vascular smooth muscle cells. Clin Sci (Lond). 2003;105(4):447–456 [DOI] [PubMed] [Google Scholar]
- 48. Rowell LB. Human cardiovascular adjustments to exercise and thermal stress. Physiol Rev. 1974;54(1):75–159 [DOI] [PubMed] [Google Scholar]
- 49. Magnon C, Hall SJ, Lin J, et al. Autonomic nerve development contributes to prostate cancer progression. Science. 2013;341(6142):1236361. [DOI] [PubMed] [Google Scholar]
- 50. Verma VK, Singh V, Singh MP, et al. Effect of physical exercise on tumor growth regulating factors of tumor microenvironment: implications in exercise-dependent tumor growth retardation. Immunopharmacol Immunotoxicol. 2009;31(2):274–282 [DOI] [PubMed] [Google Scholar]
- 51. MacNeil B, Hoffman-Goetz L. Exercise training and tumour metastasis in mice: influence of time of exercise onset. Anticancer Res. 1993;13(6A):2085–2088 [PubMed] [Google Scholar]
- 52. Hudlicka O, Brown M, Egginton S. Angiogenesis in skeletal and cardiac muscle. Physiol Rev. 1992;72(2):369–417 [DOI] [PubMed] [Google Scholar]
- 53. Jones LW, Antonelli J, Masko EM, et al. Exercise modulation of the host-tumor interaction in an orthotopic model of murine prostate cancer. J Appl Physiol. 2012;113(2):263–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wagner PD. Muscle O2 transport and O2 dependent control of metabolism. Med Sci Sports Exerc. 1995;27(1):47–53 [PubMed] [Google Scholar]
- 55. Goliasova T, denko NC. Hypoxia-inducible factor 1 (HIF1) mediated adaptive responses in the solid tumor. In: Siemann DW, ed. Tumor Microenvironment. Chichester, West Sussex, UK: John Wiley & Sons; 2011:271–290 [Google Scholar]
- 56. Kallinowski F, Schlenger KH, Runkel S, et al. Blood flow, metabolism, cellular microenvironment, and growth rate of human tumor xenografts. Cancer Res. 1989;49(14):3759–3764 [PubMed] [Google Scholar]