Abstract
Background
Senescent cells, which express p16 INK4a, accumulate with aging and contribute to age-related pathology. To understand whether cytotoxic agents promote molecular aging, we measured expression of p16 INK4a and other senescence markers in breast cancer patients treated with adjuvant chemotherapy.
Methods
Blood and clinical information were prospectively obtained from 33 women with stage I to III breast cancer at four time points: before anthracycline-based chemotherapy, immediately after anthracycline-based chemotherapy, 3 months after anthracycline-based chemotherapy, and 12 months after anthracycline-based chemotherapy. Expression of senescence markers p16 INK4a and ARF mRNA was determined using TaqMan quantitative reverse-transcription polymerase chain reaction in CD3+ T lymphocytes, telomere length was determined by Southern analysis, and senescence-associated cytokines were determined by enzyme-linked immunosorbent assay. Findings were independently assessed in a cross-sectional cohort of 176 breast cancer survivors enrolled a median of 3.4 years after treatment; 39% previously received chemotherapy. All statistical tests were two-sided.
Results
In prospectively analyzed patients, expression of p16 INK4a and ARF increased immediately after chemotherapy and remained elevated 12 months after treatment. Median increase in log2 p16 INK4a was 0.81 (interquartile range = 0.28–1.62; Wilcoxon signed-rank P < .001), or a 75% absolute increase in expression, equivalent to the increase observed over 14.7 years of chronological aging. ARF expression was comparably increased (P < .001). Increased expression of p16 INK4a and ARF was associated with dose-dense therapy and hematological toxicity. Expression of two senescence-associated cytokines (VEGFA and MCP1) was durably increased by adjuvant chemotherapy. Telomere length was not affected by chemotherapy. In a cross-sectional cohort, prior chemotherapy exposure was independently associated with a log2-increase in p16 INK4a expression of 0.57 (repeated measures model, P < .001), comparable with 10.4 years of chronological aging.
Conclusions
Adjuvant chemotherapy for breast cancer is gerontogenic, inducing cellular senescence in vivo, thereby accelerating molecular aging of hematopoietic tissues.
With the aging of the American population, the incidence of new cancer diagnoses is projected to increase 45% from 2010 to 2030 (1). Coupled with the growing proportion of cancer patients who are cured (2), we face a new challenge: a large population of aging cancer survivors (3). Long-term survivors of childhood and adult cancer can exhibit substantial late sequelae, including endocrine dysfunction, cognitive impairment, cardiovascular morbidity, secondary neoplasms, and neuromuscular impairment (4–8). Little is known about how chemotherapy causes long-term adverse effects and whether it alters the pace of physiologic aging.
Human aging is characterized by a steady decline in organ function, which leads to loss of physiologic reserve and frailty (9). This loss of function is characterized by a decline in the replicative capacity of certain self-renewing cells and the accumulation of cells that have undergone cellular senescence (10,11). Cellular senescence is triggered by the activation of tumor-suppressor mechanisms in response to varied cellular stresses such as oncogene activation, tissue injury, telomere dysfunction, and persistent DNA damage. Senescence is strongly associated with activation of the INK4/ARF (CDKN2a) locus on human chromosome 9p21.3, which encodes the p16INK4a and ARF tumor suppressor proteins. Several lines of evidence suggest senescence influences mammalian aging: 1) expression of p16 INK4a increases exponentially with chronological aging (12–14) and causes reduced replicative capacity of some cell types (15–19); 2) regulatory polymorphisms of senescence regulators (eg, CDKN2a and TERT) have been linked through unbiased genome-wide studies with many age-associated conditions such as cancer, pulmonary fibrosis, atherosclerosis, and type II diabetes (20); and 3) therapies to decrease the production of or increase the clearance of senescent cells in mice ameliorate certain age-associated phenotypes (21–23).
Because of the intimate links between senescence and aging, markers of cellular senescence, including leukocyte telomere length (LTL), expression of senescence-associated (SA) cytokines such as interleukin 6 (IL-6), and expression of INK4a/ARF transcripts, have been tested as potential biomarkers of molecular aging. Decreased LTL has been linked with chronological age and age-promoting stressors such as cigarette smoking in several studies in human populations (24–26). Senescent cells elaborate a host of potent cytokines (ie, the senescence-associated secretory phenotype) (27), which promote a proinflammatory tissue microenvironment. Expression of SA-cytokines, such as IL-6, has been reported to increase with aging and to predict age-associated morbidities and mortality (28–32). More recently, expression of p16 INK4a, and to a lesser extent ARF, in defined tissues such as peripheral blood T cells (PBTLs) have been described as biomarkers of aging. Using the p16 INK4a assay, we have shown that smoking, physical inactivity, and chronic human immunodeficiency virus infection accelerate expression of this biomarker of molecular age in the PBTL compartment (14,33). Given the apparent long-term toxicities of DNA-damaging agents, we sought to determine whether cytotoxic chemotherapy given with curative intent accelerates molecular aging in humans.
Methods
Patients
Human studies were approved by the University of North Carolina Institutional Review Board (08-0823; 09-2344). All patients gave written informed consent before undergoing any study-related procedures. The adjuvant cohort (LCCC0810) (Figure 1A) consisted of women with newly diagnosed American Joint Committee on Cancer 6th edition (34) stage I to III breast cancer planning to receive either neoadjuvant or adjuvant anthracycline-containing chemotherapy. Women were consented before treatment to undergo phlebotomy before chemotherapy, at count recovery immediately after chemotherapy, and at their 3- and 12 month regularly scheduled visits. Patients with prior pelvic radiation or a clonal bone marrow disorder were excluded.
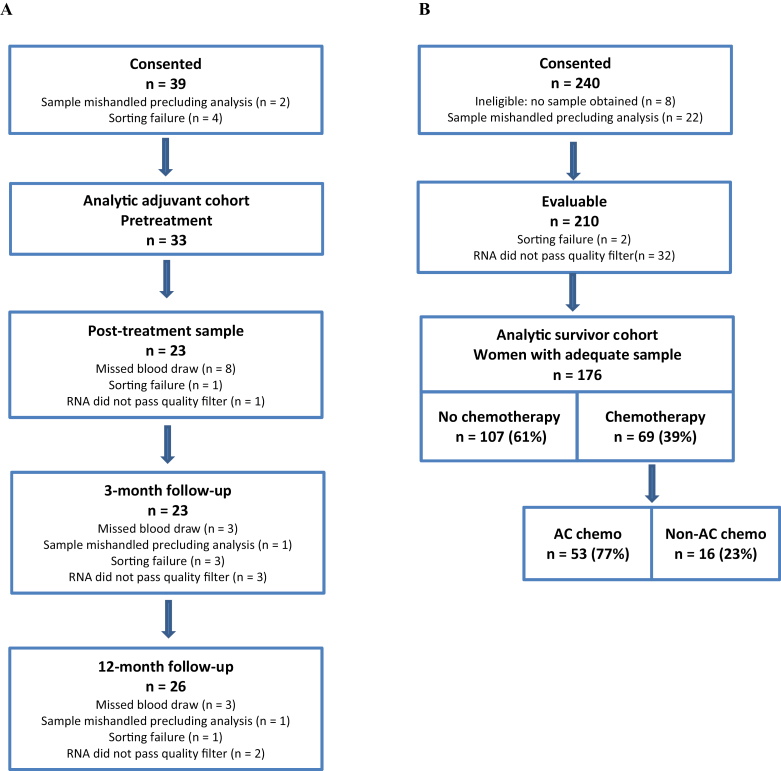
Figure 1.
Cohort assembly. The consort diagram for LCCC0810, the adjuvant cohort (A) shows number of evaluable patients at each time point. Thirty-nine women consented to participation. Results from the baseline sample were not available for six of the 39 patients. These six could not be included in the paired analysis; thus the analytic cohort is comprised of the 33 women for whom baseline results are available. All 33 women had at least one post-treatment sample collected. Fourteen had results at all post-treatment points, 11 were missing one time point, and 8 were missing two time points. The consort diagram for LCCC0924, the survivor cohort (B) shows 34 of 210 evaluable patients were excluded because of sorting or RNA failure (see Supplementary Methods, available online). AC = doxorubicin and cyclophosphamide.
The survivor cohort (LCCC0924) (Figure 1B) consisted of women aged 50 years and older with a history of a surgically resected stage I to III breast cancer. Those who had received chemotherapy needed to enroll at least 3 months from completion of chemotherapy. Those taking endocrine therapy were eligible after 3 months of treatment. Women with recurrent cancer, a clonal bone marrow disorder, or a life expectancy less than 12 months were excluded. A single sample was obtained from women in the survivor cohort at time of consent. Women in both cohorts received standard-of-care chemotherapy regimens or treatment on institutional review board–approved clinical trials. Standard-of-care chest wall radiotherapy and adjuvant hormonal therapy were given as clinically indicated. Health behaviors and demographics were evaluated by patient questionnaire. Medical history and treatment were extracted from the medical record. For each time point, a single lavender (EDTA) tube (5–10mL) was collected and placed immediately on ice. Molecular and serologic analyses were performed by investigators blinded to patient data, and investigators collecting clinical information were blinded to laboratory results until data collection was complete.
Analysis of INK4a/ARF Expression, Telomere Length, and SA-Cytokine Expression
Analysis of p16 INK4a and ARF expression in CD3+ PBTLs was performed as described (14,33). In brief, on the day of collection, PBTLs were isolated using anti-CD3 microbeads and an AutoMACSPRO separator (Miltenyi Biotec, San Diego, CA). Total RNA and cDNA were prepared (14,33), followed by TaqMan quantitative reverse-transcription polymerase chain reaction specific for p16 INK4a (exon 1alpha-exon 2; custom order ID: AII1L5T; Life Technologies USA, Carlsbad, CA) or ARF (exon 1beta-exon 2; custom assay order ID: AIKAKB1; Life Technologies USA) with normalization to 18S housekeeping gene (order number 4352655; Life Technologies USA). Using this method, 15.4% of samples in the adjuvant cohort and 16.2% of samples in the survivor cohort failed analysis because of sorting failure, insufficient nucleic acid yield, poor quality RNA, or failure to pass a data replicate filter (Figure 1; see also Supplementary Methods, available online, for details). There was no statistically significant difference in age between patients whose samples failed analysis and patients with results who were included in either cohort. Expression of p16 INK4a with aging in reporter mice was assayed as described (see also Supplementary Methods, available online) (35).
Analysis of senescence-associated cytokines and LTL was performed on patient sera collected before and 12 months after chemotherapy in the adjuvant cohort (in 3 patients without a 12-month sample, the 3-month sample was used). Analysis of vascular endothelial growth factor A (VEGFA; R&D systems DVE00, Minneapolis, MN), IL-6 (R&D systems LUH206), interleukin 7 (IL-7; R&D systems HS750), interleukin 8 (IL-8; R&D systems LUH208) and monocyte chemotactic protein-1 (MCP1, also referred to as chemokine CCL2; R&D systems DCP00) was performed by enzyme-linked immunosorbent assay. IL-6 was detectable before and after chemotherapy in only three patients; therefore it was not further analyzed. LTL was measured by Southern analysis as described (36).
p16INK4a Expression With Reporter Mice
All murine studies were done under a protocol approved by the University of North Carolina Institutional Animal Care and Use Committee. Albino hairless SKH1-E p16+/Luc mice developed in the Sharpless lab at the University of North Carolina as described in Burd et al. (35) were serially housed in the University of North Carolina LCCC Mouse Phase I Unit. A cohort of 32 female mice were aged (6, 23, 55, and 73 weeks) and imaged. This was repeated five times with different sets of mice from this cohort. Animals were imaged using an IVIS LUMINA imaging system (PerkinElmer, Waltham, MA). The image shown is a 2-minute exposure taken 8 minutes after luciferin injection. For the full protocol, see Burd et al. (35).
Statistical Analysis
In brief (see Supplementary Methods, available online, for additional details), because p16 INK4a and ARF expression increase exponentially with age (14,37), results were logarithmically transformed. For the adjuvant cohort, change in INK4a/ARF expression, SA-cytokine, and LTL from baseline to post-treatment time points were compared overall and across age strata using the Wilcoxon signed-rank, rank-sum, and Kruskal–Wallis tests. Because each woman had at least one post-treatment sample, to maximize sample size we used the average post-treatment p16 INK4a and ARF expression to test for association of clinical factors with magnitude of change in expression. In the survivor cohort, p16 INK4a was measured twice in all patients. Rather than arbitrarily choose a single or mean value for each patient, we assumed that measures within a batch were not independent, and thus multivariable repeated measures models, which account for this nonindependence, were used to evaluate the association between clinical factors and p16 INK4a expression. ARF expression was measured in a single batch and analyzed by linear regression. Predicted p16 INK4a for age according to prior treatment is shown graphically. In both cohorts, the association between age, race, and key clinical factors with p16 INK4a expression was evaluated. Results for the Adjuvant cohort are shown in Supplementary Table 1 (available online). In the survivor cohort, few clinical factors were associated with expression; therefore only factors either statistically significantly associated with expression or of key clinical relevance were included in multivariable models. Data were analyzed by H. K. Sanoff, A. M. Deal, and J. G. Ibrahim using SAS version 9.2 (SAS, Cary, NC) and STATA version 12 (StataCorp, College Station, TX). To quantitate the age-promoting effects of chemotherapy, the fold-change in p16 INK4a expression induced by chemotherapy was compared with the effect of chronologic aging on p16 INK4a in healthy donors from previously published, independent cohorts (14,35).
All tests of statistical significance were two-sided. P values of .05 or less were considered statistically significant.
Results
To test the hypothesis that chemotherapy accelerates the activation of cellular senescence in vivo, we measured expression of senescence markers in two cohorts of women with breast cancer treated with curative intent.
The prospective adjuvant cohort consisted of 33 women, with a median age of 49 years (range = 32–69) (Table 1; Supplementary Table 1, available online). The women had few comorbid conditions, although nearly half were obese (body mass index ≥30kg/m2; n = 15; 45.5%). The majority (n = 29) of women received adjuvant doxorubicin and cyclophosphamide and a taxane. Most women (n = 27; 81.8%) received adjuvant doxorubicin and cyclophosphamide in a dose-dense fashion (38). Baseline p16 INKa and ARF expression were not associated with age in this small sample with narrow age range (Supplementary Table 1, available online).
Table 1.
Patient and treatment characteristics*
| Patient and treatment characteristics | Adjuvant cohort (n = 33) | Survivor cohort (n = 176) |
|---|---|---|
| Age, y, median (range) | 49 (32–69) | 67 (50–93) |
| <50 | 17 (51.5%) | 0 |
| 50–64 | 12 (36.4%) | 63 (35.8%) |
| 65–74 | 4 (12.1%) | 80 (45.5%) |
| ≥75 | 0 | 33 (18.8%) |
| Race/ethnicity | ||
| White | 26 (78.8%) | 148 (84.1%) |
| Black | 7 (21.2%) | 26 (14.8%) |
| Asian/Other | 0 | 2 (1.1%) |
| Comorbidities | ||
| Diabetes | 5 (15.2%) | 24 (13.9%) |
| Coronary heart disease | 0 | 6 (3.5%) |
| Stroke | 0 | 4 (2.3%) |
| BMI, kg/m2, median (range) | 29 (18–50) | 28 (18–58) |
| <18.5, underweight | 1 (3.0%) | 1 (0.6%) |
| 18.5–24.9, normal | 6 (18.2%) | 59 (33.5%) |
| 25.0–29.9, overweight | 11 (33.3%) | 55 (31.3%) |
| ≥30, obese | 15 (45.5%) | 61 (34.7%) |
| Smoking | ||
| Never | 20 (62.5%) | 102 (58.3%) |
| Former | 7 (21.9%) | 63 (36.0%) |
| Current | 5 (15.6%) | 10 (5.7%) |
| AJCC summary stage† | Not collected | |
| Stage I | 6 (18.2%) | — |
| Stage II | 18 (54.5%) | — |
| Stage III | 9 (27.3%) | — |
| ER or ER/PR positive | 20 (60.6%) | — |
| HER2 positive | 7 (21.2%) | — |
| Triple-negative | 9 (27.3%) | — |
| Adjuvant radiotherapy | 25 (75.8%) | 103 (58.5%) |
| Adjuvant hormonal therapy | 20 (60.6%) | 124 (70.5%) |
| Adjuvant chemotherapy | 33 (100%) | 69 (39.2%) |
| AC | 3 (9.4%) | 12 (17.4%) |
| AC → T | 29 (87.9%)‡ | 41 (59.4%)§ |
| TC | 0 | 9 (13.0%) |
| CMF | 0 | 3 (4.3%) |
| Other|| | 1 (3.0%) | 4 (5.8%) |
| Years from chemotherapy, median (range) | NA | 3.4 (0.3–18. 8) |
* Data are No. (%) unless otherwise noted. AC = doxorubicin and cyclophosphamide; AJCC = American Joint Committee on Cancer; BMI = body mass index; CMF = cyclophosphamide, methotrexate, 5-fluorouracil; HER2 = human epidermal growth factor receptor 2; ER = estrogen receptor; PR = progesterone receptor; T = paclitaxel; TC = docetaxel and cyclophosphamide.
† Ten women were treated neoadjuvantly; summary stage is pretreatment clinical stage. For the 23 adjuvantly treated women, summary stage is the pathological stage. Stage, ER/PR, and HER2 could not be confirmed from pathology reports for the survivor cohort.
‡ One woman received nab-paclitaxel.
§ One woman received 2 AC/2 TC; one women received docetaxel instead of paclitaxel; two women received capecitabine after completing AC-T; one woman received post-AC+T bevacizumab and metronomic chemotherapy.
|| One woman in the adjuvant cohort received fluorouracil, epirubicin, cyclophosphamide. One woman in the survivor cohort received navelbine only, and three women in the survivor cohort received paclitaxel only.
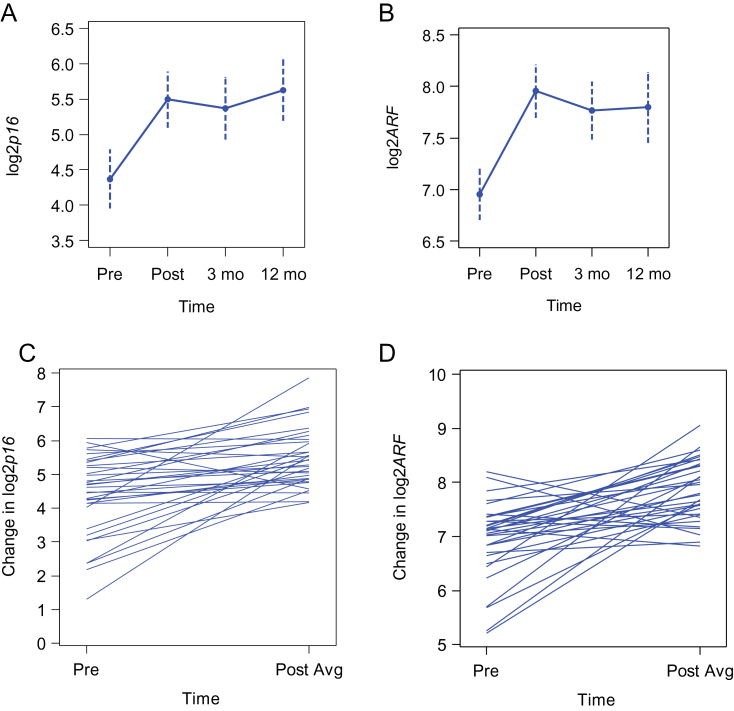
In the adjuvant cohort, chemotherapy led to a statistically significant and persistent rise in both p16 INKa and ARF expression at all post-treatment time points (Figure 2; Supplementary Table 2, available online). Expression of p16 INK4a decreased with chemotherapy in only one patient, who was later found to have congenital p53 deficiency (Li-Fraumeni syndrome; see Supplementary Methods, available online). In the entire cohort, a median increase in the log2 p16 INKa expression of 0.81 (interquartile range [IQR] = 0.28–1.62; Wilcoxon signed-rank P < .001) and in the log2 ARF expression of 0.89 (IQR = 0.29–1.27; Wilcoxon signed-rank P < .001) was seen from baseline to the average post-treatment value. In non-log-transformed terms, this reflects median increases of 75% and 85% in p16 INK4a and ARF, respectively. Using a murine reporter system (Supplementary Figure 1, available online) and in prior studies of normal human volunteers (14,35), we have noted a characteristic exponential increase in p16 INK4a expression with aging that is associated with species’ lifespans. In independent cohorts of healthy human donors, log2 p16 INKa exhibits a mean increase of 0.055 units per year (or 4% per year) (14,35). Therefore, the increase in PBTL p16 INK4a expression associated with adjuvant breast cancer chemotherapy is equivalent to increases seen with 14.7 years of chronological aging.
Figure 2.
Chemotherapy-induced increase in p16 INK4a and ARF expression in the prospective adjuvant cohort. Mean values (dots) with 95% confidence intervals (dotted lines) of log2p16 INK4a (A) and log2ARF (B) from pretreatment through three post-treatment time points: immediately upon count recovery after chemotherapy and 3 months and 12 months after chemotherapy. The change from pretreatment (Pre) to the average post-treatment (Post avg) value is shown for individual patients for log2p16 INK4a (median change = 0.81; interquartile range [IQR] = 0.28–1.62; Wilcoxon signed-rank P < .001) (C) and log2ARF (median change = 0.89; IQR = 0.29–1.27; Wilcoxon signed-rank P < .001) (D). All statistical tests were two-sided.
Cytotoxic chemotherapy also increased the in vivo levels of other markers of senescence. Plasma concentrations of two SA-cytokines, VEGFA and MCP1/CCL2 (27,39), were statistically significantly increased in postchemotherapy compared with prechemotherapy samples (median VEGFA = 118 vs 80 pg/mL, Wilcoxon signed-rank P = .001; median MCP1/CCL2 = 166 vs 117 pg/mL, Wilcoxon signed-rank P < .001). Expression of other senescence markers (eg, IL-7, IL-8, and LTL) (Supplementary Figure 2, available online) did not statistically significantly change after chemotherapy. These data suggest that cytotoxic chemotherapy increases expression of transcriptional senescence markers in PBTLs, as well as serologic levels of two well-described secreted markers of senescence activation.
We also considered as secondary endpoints the effect of other treatment-related factors on change in PBTL p16 INK4a expression with cytotoxic chemotherapy. Neither adjuvant chest wall radiotherapy (n = 25) nor taxane use (n = 29) was independently associated with change in p16 INK4a expression, although caution is warranted given the small number of patients. Dose-dense (every 2 weeks) chemotherapy was associated with a greater rise in log2 p16 INKa than a standard schedule of adjuvant doxorubicin and cyclophosphamide every 3 weeks (median = 1.13 vs 0.32; Wilcoxon rank-sum P = .04) (Supplementary Figure 3, available online). A trend toward greater median change in p16 INKa expression was observed in women experiencing grade 3 and 4 hematologic toxicity than in women without severe hematologic toxicity (median = 1.31 vs 0.57; Wilcoxon rank-sum P = .07). These results suggest that more intensive chemotherapy and more severe myelosuppression are associated with a greater acceleration of molecular age in hematopoietic tissues.
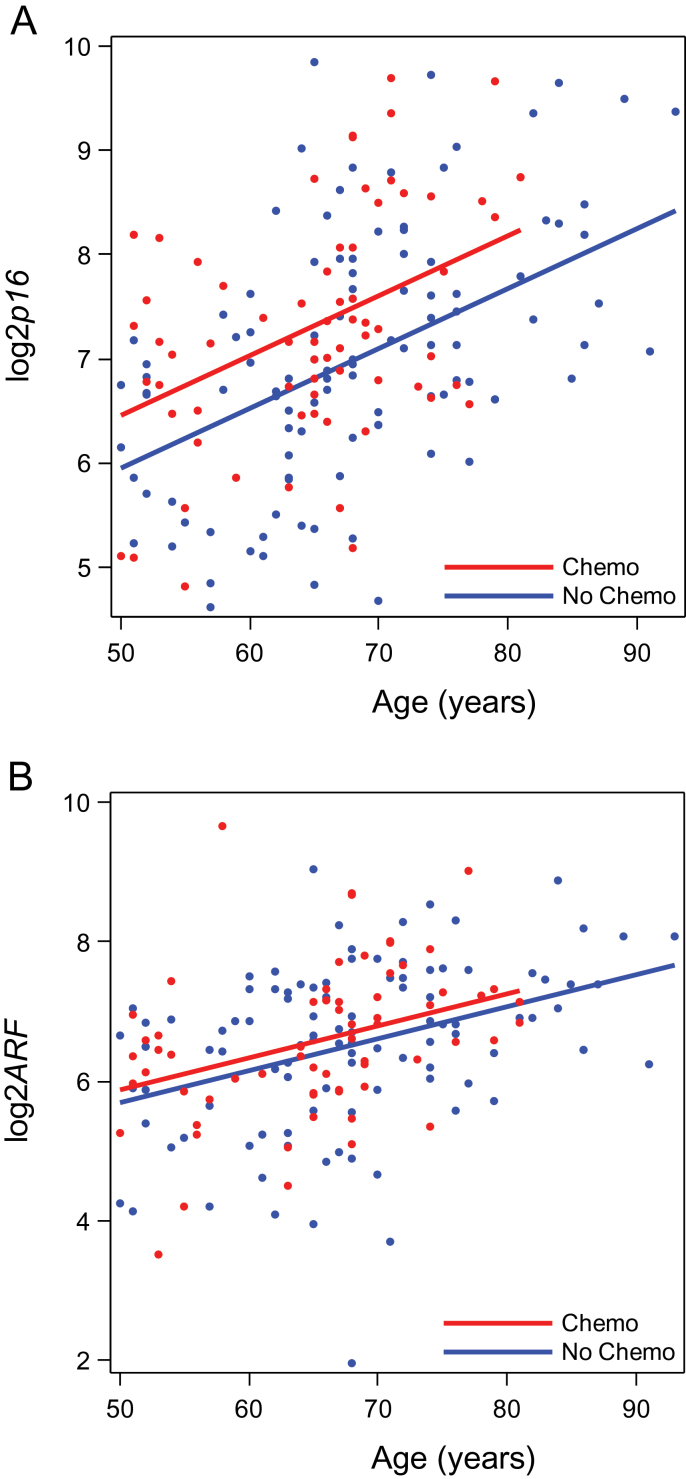
Given the statistically significant induction of p16 INK4a for up to 1 year after chemotherapy (Figure 2A), we examined the effect of cytotoxic chemotherapy on p16 INK4a expression at later time points in an independent cross-sectional survivor cohort (Figure 1B and Table 1). Of the 176 evaluable breast cancer survivors, 69 (39.2%) had received adjuvant chemotherapy. In this cohort, smoking, comorbidity, and body mass index were not independently associated with p16 INK4a expression. Age was statistically significantly associated with p16 INK4a expression, with an estimated p16 INK4a doubling time of 16.7 years (ie, a 0.06 unit per year increase in log2p16 INK4a; repeated measures model P < .001). In adjusted analyses, log2p16 INK4a expression in chemotherapy-treated women was 0.57 higher (repeated measures model P < .001) than in women not treated with chemotherapy. Compared with the increase seen in independent cohorts of healthy human donors [0.055 per year (14, 35)], this increase is equivalent to 10.4 years of chronological aging (Figure 3). ARF expression was not statistically significantly associated with prior chemotherapy use in the survivor cohort. As in the adjuvant cohort, chest wall radiotherapy and adjuvant hormonal therapy were not independently associated with p16 INK4a expression (Supplementary Table 3, available online). Because patients in this cohort were a median 3.4 years from the completion of chemotherapy (some up to 18 years from treatment), these results suggest that the sharp increase in p16 INK4a expression observed after chemotherapy persists for at least several years, if not indefinitely, after chemotherapy exposure.
Figure 3.
Expression of p16 INK4a and ARF by age and prior chemotherapy exposure in the cross-sectional survivor cohort. Log2p16 INK4a (A) and log2ARF (B) at each age according to receipt of adjuvant therapy. Dots represent the average value for each patient among the three batches. Lines represent the predicted values for chemotherapy-treated (red) and non-chemotherapy-treated (blue) patients based on the results of the repeated measures models. Log2p16 INK4a is statistically significantly higher in chemotherapy-treated vs non-chemotherapy-treated patients (repeated measures model P < .001). Log2ARF is not statistically significantly higher in chemotherapy-treated patients vs non-chemotherapy-treated patients (linear regression model P = .10). All statistical tests were two-sided.
Discussion
We have shown that adjuvant breast cancer chemotherapy leads to a substantial increase in molecular markers of cellular senescence, including CDKN2a expression in PBTLs, as well as serologic levels of two senescence-associated cytokines. In these prospectively followed patients treated with standard adjuvant chemotherapy, a near doubling of p16 INK4a and ARF expression was seen in T cells after adjuvant doxorubicin and cyclophosphamide chemotherapy, an increase comparable to 14.7 years of normal chronological aging (14,35). More aggressive dose-dense chemotherapy schedules and greater hematologic toxicity were associated with a greater in vivo induction of markers of cellular senescence. The effect of chemotherapy on p16 INK4a expression was confirmed in an independent, cross-sectional cohort of long-term breast cancer survivors. In aggregate, results from these two analyses demonstrate that adjuvant cytotoxic chemotherapy durably promotes expression of markers of cellular senescence in breast cancer patients.
The principal limitation of this study is the reliance on markers that can be only easily assayed in the peripheral blood. Although data from both cohorts showed an increase in p16 INK4a expression after systemic chemotherapy, we can only conclusively state that chemotherapy promotes expression of senescence markers in CD3+ lymphocytes. Age-related increases in p16 INK4a expression occur across a wide variety of mammalian tissue compartments (Supplementary Figure 1, available online) (see also [12,13,35,40)], yet the amplitude of age-related change varies by tissue compartment as does its response to interventions that modify aging such as caloric restriction (13). Likewise, the tissue source of increased senescence-associated cytokines after chemotherapy is unknown. Thus, it is uncertain whether measurement of senescence-associated cytokines or p16 INK4a expression in a hematopoietic compartment can serve as a proxy for generalized organismal age, and our data do not speak to the pro-aging effects of chemotherapy in nonhematopoietic compartments.
We were able to reproducibly show that breast cancer chemotherapy leads to a durable increase in PBTL p16 INK4a expression, but we were unable to show an effect of chemotherapy on LTL and IL-6. Both of these biomarkers have been extensively studied as aging biomarkers, and unequivocally change (decrease for LTL and increase for IL-6) across the lifespan when measured in large populations (24–26,41). However, neither assay is well suited for individual risk prediction: the absolute level/length vary substantially among same-aged individuals, and the absolute change is relatively small over an individual’s lifespan (24,32,41–43). Longitudinal studies of LTL have shown an expected annual decrease in length of 25 to 45 base pairs, although 15% to 20% of prospectively followed people demonstrated telomere elongation over the studied period (41). Such variation has also been observed in studies of patients receiving chemotherapy (44,45). This wide variation in individual LTL over time in concert with the finding that administration of granulocyte colony stimulating factor may increase LTL through upregulation of telomerase (46,47), suggest LTL is unlikely to be a reliable biomarker of the effect of chemotherapy on molecular aging. Further, others have shown that the chemotherapy-induced cellular senescence in a variety of cancer cell lines and murine models is independent of telomere length and telomerase activity, suggesting LTL is a poor marker of chemotherapy-induced aging [reviewed in (48)]. Likewise IL-6 is suboptimal for this purpose because baseline expression is low but shows substantial age-independent confounding from circadian variation and induction by viral infection and other stressors (32,49). We believe that p16 INK4a expression in PBTLs offers new potential as a diagnostic marker of senescence induction in vivo given its very large dynamic range (approximately 16-fold change over a human lifespan), ease and low cost of measure, and strong correlation with chronological age (R 2 with chronological age is approximately 0.6 for p16 INK4a vs <0.2 for LTL or IL-6) (14,26,41,42).
Based on murine analyses, we believe the PBTL increase in p16 INK4a expression likely reflects heritable damage to hematopoietic stem cells (17,50) but may also reflect damage to the thymic milieu (21) or direct damage to self-renewing, post-thymic T cells (18). Adjuvant anthracycline-based chemotherapy unequivocally saves lives, markedly decreasing the relative risk of breast cancer recurrence by 27% and death by 21% (51). Therefore, we believe the finding of durable, age-promoting effects of anthracyclines and alkylating agents must be weighed against these established benefits with regard to relapse risk. The ability to serially measure markers of molecular age in patients may provide a means to identify beneficial adjuvant approaches that are less “pro-aging.” For example, these results suggest that dose-dense therapy may induce greater long-term hematologic damage, whereas taxanes may be associated with less long-term risk. Further work to more directly quantify the contribution of each component of adjuvant therapy on certain known long-term sequelae may allow for more balanced treatment decisions and perhaps greater use of less gerontogenic regimens for low-risk cancers.
In summary, we have shown that cytotoxic chemotherapy potently induces the expression of markers of cellular senescence in the hematologic compartment in vivo, comparable with the effects of 10 to 15 years of chronologic aging in independent cohorts of healthy donors. We are currently enrolling in two prospective trials: one evaluating whether changing PBTL p16 INK4a expression is a biomarker of acute toxicity of various chemotherapy regimens (LCCC1027, NCT01305954); and a second focused exclusively on older patients evaluating the combination of a validated geriatric assessment and PBTL p16 INK4a for prediction of treatment-related toxicity and change in function (NCT01472094). Although refining the clinical utility of this biomarker of molecular aging will take further study, we have shown that chemotherapy accelerates molecular aging in the hematopoietic compartment of breast cancer survivors.
Funding
This work was supported by the National Institutes of Health (K07CA106722 to HKS; AG024379 to NES); the Paul Glenn Foundation (to NES); the Burroughs Wellcome Fund (to NES); and the Breast Cancer Research Foundation (to HM and LAC).
The study sponsors had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
J. Krishnamurthy and N. E. Sharpless are co-inventors on a University of North Carolina–owned patent related to this work (US PCT/US2005/034542 “Determination of Molecular Age by Detection of INK4a/ARF Expression”).
We wish to thank Christin Burd, David Darr, and the University of North Carolina LCCC Mouse Phase I Unit for assistance with animal handling, and the LCCC Biostatistics core.
Data from the survivor cohort were presented in part at the 2011 meeting of the American Society for Clinical Oncology (Muss HB, Krishnamurthy J, Alston SM, et al. J Clin Oncol. 2011;29(Suppl):abstract 9002).
References
- 1. Smith BD, Smith GL, Hurria A, et al. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol. 2009;27(17):2758–2765 [DOI] [PubMed] [Google Scholar]
- 2. Eheman C, Henley SJ, Ballard-Barbash R, et al. Annual report to the nation on the status of cancer, 1975–2008, featuring cancers associated with excess weight and lack of sufficient physical activity. Cancer. 2012;118(9):2338–2366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62(4):220–241 [DOI] [PubMed] [Google Scholar]
- 4. Chow EJ, Liu W, Srivastava K, et al. Differential effects of radiotherapy on growth and endocrine function among acute leukemia survivors: a childhood cancer survivor study report. Pediatr Blood Cancer. 2013;60(1):110–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Castellino SM, Geiger AM, Mertens AC, et al. Morbidity and mortality in long-term survivors of Hodgkin lymphoma: a report from the Childhood Cancer Survivor Study. Blood. 2011;117(6):1806–1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Krull KR, Sabin ND, Reddick WE, et al. Neurocognitive function and CNS integrity in adult survivors of childhood hodgkin lymphoma. J Clin Oncol. 2012;30(29):3618–3624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ness KK, Hudson MM, Pui CH, et al. Neuromuscular impairments in adult survivors of childhood acute lymphoblastic leukemia: associations with physical performance and chemotherapy doses. Cancer. 2012;118(3):828–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pachman DR, Barton DL, Swetz KM, et al. Troublesome symptoms in cancer survivors: fatigue, insomnia, neuropathy, and pain. J Clin Oncol. 2012;30(30):3687–3696 [DOI] [PubMed] [Google Scholar]
- 9. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156 [DOI] [PubMed] [Google Scholar]
- 10. Sharpless NE, DePinho RA. How stem cells age and why this makes us grow old. Nat Rev Mol Cell Biol. 2007;8(9):703–713 [DOI] [PubMed] [Google Scholar]
- 11. Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol. 2011;192(4):547–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zindy F, Quelle DE, Roussel MF, et al. Expression of the p16INK4a tumor suppressor versus other INK4 family members during mouse development and aging. Oncogene. 1997;15(2):203–211 [DOI] [PubMed] [Google Scholar]
- 13. Krishnamurthy J, Torrice C, Ramsey MR, et al. Ink4a/Arf expression is a biomarker of aging. J Clin Invest. 2004;114(9):1299–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu Y, Sanoff HK, Cho H, et al. Expression of p16(INK4a) in peripheral blood T-cells is a biomarker of human aging. Aging Cell. 2009;8(4):439–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Krishnamurthy J, Ramsey MR, Ligon KL, et al. p16(INK4a) induces an age-dependent decline in islet regenerative potential. Nature. 2006;443(7110):453–457 [DOI] [PubMed] [Google Scholar]
- 16. Molofsky AV, Slutsky SG, Joseph NM, et al. Increasing p16(INK4a) expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 2006;443(7110):448–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Janzen V, Forkert R, Fleming HE, et al. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16(INK4a). Nature. 2006;443(7110):421–426 [DOI] [PubMed] [Google Scholar]
- 18. Liu Y, Johnson SM, Fedoriw Y, et al. Expression of p16(INK4a) prevents cancer and promotes aging in lymphocytes. Blood. 2011;117(12):3257–3267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Signer RA, Montecino-Rodriguez E, Witte ON, et al. Aging and cancer resistance in lymphoid progenitors are linked processes conferred by p16Ink4a and Arf. Genes Dev. 2008;22(22):3115–3120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jeck WR, Siebold AP, Sharpless NE. Review: a meta-analysis of GWAS and age-associated diseases. Aging Cell. 2012;11(5):727–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Berent-Maoz B, Montecino-Rodriguez E, Signer RA, et al. Fibroblast growth factor-7 partially reverses murine thymocyte progenitor aging by repression of Ink4a. Blood. 2012;119(24):5715–5721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Baker DJ, Wijshake T, Tchkonia T, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479(7372):232–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen H, Gu X, Liu Y, et al. PDGF signalling controls age-dependent proliferation in pancreatic beta-cells. Nature. 2011;478(7369):349–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Valdes AM, Andrew T, Gardner JP, et al. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366(9486):662–664 [DOI] [PubMed] [Google Scholar]
- 25. Mirabello L, Huang WY, Wong JY, et al. The association between leukocyte telomere length and cigarette smoking, dietary and physical variables, and risk of prostate cancer. Aging Cell. 2009;8(4):405–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Song Z, von Figura G, Liu Y, et al. Lifestyle impacts on the aging-associated expression of biomarkers of DNA damage and telomere dysfunction in human blood. Aging Cell. 2010;9(4):607–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Coppe JP, Desprez PY, Krtolica A, et al. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ferrucci L, Corsi A, Lauretani F, et al. The origins of age-related proinflammatory state. Blood. 2005;105(6):2294–2299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cesari M, Penninx BW, Pahor M, et al. Inflammatory markers and physical performance in older persons: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2004;59(3):242–248 [DOI] [PubMed] [Google Scholar]
- 30. Taaffe DR, Harris TB, Ferrucci L, et al. Cross-sectional and prospective relationships of interleukin-6 and C-reactive protein with physical performance in elderly persons: MacArthur studies of successful aging. J Gerontol A Biol Sci Med Sci. 2000;55(12):M709–M715 [DOI] [PubMed] [Google Scholar]
- 31. Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Res Rev. 2011;10(3):319–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maggio M, Guralnik JM, Longo DL, et al. Interleukin-6 in aging and chronic disease: a magnificent pathway. J Gerontol A Biol Sci Med Sci. 2006;61(6):575–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nelson JA, Krishnamurthy J, Menezes P, et al. Expression of p16(INK4a) as a biomarker of T-cell aging in HIV-infected patients prior to and during antiretroviral therapy. Aging Cell. 2012;11(5):916–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. American Joint Committee on Cancer (AJCC) C, Illinois AJCC Cancer Staging Manual. 6th ed. New York: Springer-Verlag; 2002 [Google Scholar]
- 35. Burd CE, Sorrentino JA, Clark KS, et al. Monitoring tumorigenesis and senescence in vivo with a p16(INK4a)-luciferase model. Cell .2013;152(1–2):340–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wiemann SU, Satyanarayana A, Tsahuridu M, et al. Hepatocyte telomere shortening and senescence are general markers of human liver cirrhosis. FASEB J. 2002;16(9):935–942 [DOI] [PubMed] [Google Scholar]
- 37. Herbig U, Ferreira M, Condel L, et al. Cellular senescence in aging primates. Science. 2006;311(5765):1257. [DOI] [PubMed] [Google Scholar]
- 38. Citron ML, Berry DA, Cirrincione C, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003;21(8):1431–1439 [DOI] [PubMed] [Google Scholar]
- 39. Coppe JP, Kauser K, Campisi J, et al. Secretion of vascular endothelial growth factor by primary human fibroblasts at senescence. J Biol Chem. 2006;281(40):29568–29574 [DOI] [PubMed] [Google Scholar]
- 40. Nielsen GP, Stemmer-Rachamimov AO, Shaw J, et al. Immunohistochemical survey of p16INK4A expression in normal human adult and infant tissues. Lab Invest. 1999;79(9):1137–1143 [PubMed] [Google Scholar]
- 41. Muezzinler A, Zaineddin AK, Brenner H. A systematic review of leukocyte telomere length and age in adults. Ageing Res Rev. 2013;12(2):509–519 [DOI] [PubMed] [Google Scholar]
- 42. Hager K, Machein U, Krieger S, et al. Interleukin-6 and selected plasma proteins in healthy persons of different ages. Neurobiol Aging. 1994;15(6):771–772 [DOI] [PubMed] [Google Scholar]
- 43. Longo DL. Telomere dynamics in aging: much ado about nothing? J Gerontol A Biol Sci Med Sci. 2009;64(9):963–964 [DOI] [PubMed] [Google Scholar]
- 44. Schroder CP, Wisman GB, de Jong S, et al. Telomere length in breast cancer patients before and after chemotherapy with or without stem cell transplantation. Br J Cancer. 2001;84(10):1348–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ben-Josef E, Schipper M, Francis IR, et al. A phase I/II trial of intensity modulated radiation (IMRT) dose escalation with concurrent fixed-dose rate gemcitabine (FDR-G) in patients with unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2012;84(5):1166–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Szyper-Kravitz M, Uziel O, Shapiro H, et al. Granulocyte colony-stimulating factor administration upregulates telomerase activity in CD34+ haematopoietic cells and may prevent telomere attrition after chemotherapy. Br J Haematol. 2003;120(2):329–336 [DOI] [PubMed] [Google Scholar]
- 47. Engelhardt M, Kumar R, Albanell J, et al. Telomerase regulation, cell cycle, and telomere stability in primitive hematopoietic cells. Blood. 1997;90(1):182–193 [PubMed] [Google Scholar]
- 48. Roninson IB. Tumor cell senescence in cancer treatment. Cancer Res. 2003;63(11):2705–2715 [PubMed] [Google Scholar]
- 49. Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2000;51:245–270 [DOI] [PubMed] [Google Scholar]
- 50. Rossi DJ, Bryder D, Seita J, et al. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447(7145):725–729 [DOI] [PubMed] [Google Scholar]
- 51. Early Breast Cancer Trialists’ Collaborative Group. Peto R, Davies C, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379(9814):432–444 [DOI] [PMC free article] [PubMed] [Google Scholar]