Abstract
The objective of this study was to examine the reliability of associations between fat taste, hunger, dietary fat intake, and body mass index (BMI). Detection thresholds for oleic acid (OA) were obtained during each of 7 consecutive visits using a modified staircase procedure. Participants were 48 (N = 17 male; N = 31 female) healthy adults (mean age: 28.5±10.4 years) with BMI’s ranging from 18.9 to 47.2 (≥25 kg·m−2, N = 24). OA detection thresholds and self-reported hunger (100-mm visual analog scale) were assessed at each visit. BMI and dietary fat intake (Block Rapid Fat Screener) were determined at baseline. There was a significant decrease of threshold concentration over repeated trials among lean and overweight (BMI between 25.0 and 29.9 kg·m−2) participants but not in the obese. Combining the lean and overweight and contrasting their responses to the obese revealed the lean plus overweight group to be significantly more sensitive at visits 6 and 7. No change of threshold sensitivity or correlation with fat intake was observed in the obese participants unlike findings in the lean and lean plus overweight participants. Correlations between saturated fat intake and threshold sensitivity were positive (greater intake associated with higher thresholds) at baseline for the group, with additional correlations observed among the lean plus overweight but not in the obese, leaving open questions about the nutritional significance of the association. No significant associations were observed between sensitivity to OA and hunger. Repeated testing is required to assess associations between fat taste and other outcome variables.
Key words: body mass index, dietary fat intake, fat taste, hunger, taste thresholds
Introduction
The evidence supporting human nonesterified fatty acid (NEFA) taste, commonly referred to as “fat taste,” has grown over the past 15 years. The role of taste sensitivity in promoting intake of specific foods or ingredients associated with chronic diseases has long been an area of interest. Examples include the associations between sensitivity to 6-n-propylthiouracil and cruciferous vegetable intake with implications for cancer or goiter risk (Bell and Tepper 2006; Duffy et al. 2010; Tsuji et al. 2012); sensitivity to NaCl and sodium intake posited to promote hypertension and stomach cancer (Yang et al. 2011; Zhang and Zhang 2011); and sensitivity to glucose and sugar intake purportedly leading to obesity and diabetes (Perros et al. 1996). In no case has causality been established because the links between taste, intake, and disease are complex. Indeed, associative claims are made for lower and higher taste sensitivity leading to the same dietary behavior such as increased salt intake. It is argued that lower sensitivity leads to increased intake to achieve a desired level of sensory stimulation and greater sensitivity results in stronger activation of reward centers driving increased intake. Of course, the reverse may also be true where intake alters sensitivity. Nonetheless, with increasing evidence supporting the ability of humans to taste NEFAs (Kamphuis et al. 2003; Chalé-Rush et al. 2007a,b; Mattes 2009a,b; Stewart et al. 2010; Stewart, Feinle-Bisset, et al. 2011; Stewart, Newman, et al. 2011; Stewart, Seimon, et al. 2011; Galindo et al. 2012; Pepino et al. 2012; Stewart and Keast 2012; Tucker et al. 2013; Tucker and Mattes 2013), similar interest in so-called “fat taste” sensitivity, fat consumption, and myriad health risks has arisen (Stewart et al. 2010; Stewart, Newman, et al. 2011; Stewart and Keast 2012). With the touted links between fat intake and many adverse health outcomes such as obesity, diabetes, cardiovascular disease, selected cancers, osteoarthritis, and social stigma, it is imperative this issue be clearly characterized.
Fatty foods are often highly flavorful and associated with obesity, for example, Ryan et al. (2012). This has prompted hypotheses of an association between NEFA taste sensitivity and body mass index (BMI) with mixed experimental support. Some have reported inverse associations between BMI and sensitivity to NEFA (increases in BMI were associated with reduced sensitivity or higher thresholds) (Stewart et al. 2010; Stewart, Newman, et al. 2011; Stewart, Seimon, et al. 2011), whereas others fail to find associations (Kamphuis et al. 2003; Mattes 2009b; Mattes 2011; Stewart and Keast 2012).
Explanations for both sets of findings have been proposed. Based on the recognition that NEFA impart unpleasant or aversive sensations, one explanation for the inverse association between BMI and sensitivity to NEFA holds that decreased sensitivity to fat contributes to increased intake as the unpalatable sensations NEFA impart would be less apparent and less likely to evoke rejection responses. Others propose that habituation to a high-fat diet leads to a requirement for greater exposure to generate an appropriate oral response (Stewart et al. 2010; Stewart, Newman, et al. 2011; Stewart and Keast 2012). This increased fat intake presumably contributes to weight gain. Alternatively, it may be that dietary fat intake or obesity modifies fat taste. In a single 4-week trial, high-fat diet exposure decreased sensitivity to NEFA in lean but not overweight/obese subjects (Stewart and Keast 2012). However, a larger body of evidence indicates there is no significant association between BMI and NEFA sensitivity. The latter findings are not unexpected. First, a lack of association mirrors findings from previous work in other taste qualities (Grinker et al. 1972; Rodin 1975; Grinker 1978; Malcolm et al. 1980; Frijters and Rasmussen-Conrad 1982; Scruggs et al. 1994). Second, thresholds to particular tastes are often not associated with liking or preference for that stimulus at the suprathreshold concentrations commonly encountered in daily eating (Bartoshuk 1979). Third, there may be a methodological basis for a poor association. Evidence of learning effects on tests of threshold sensitivity for NEFA indicate multiple trials must be conducted to establish the lower limits of an individual’s sensory sensitivity to NEFA (Tucker and Mattes 2013). Threshold measurements reported to date do not account for learning effects and likely fail to reflect true fat taste sensitivity. This methodological issue may be especially relevant for NEFA due to a lack of participant familiarity with: 1) the stimuli, 2) descriptors for the sensation, and/or 3) testing procedures. Misclassification of sensitivity could obscure associations between sensitivity and BMI or other variables of interest.
In light of the mixed findings on oral fat detection in lean versus overweight/obese individuals, the present study tested the oleic acid (OA) detection threshold of individuals 7 times to assess differences in sensitivity between lean, overweight, and obese participants. We hypothesized no association between BMI and NEFA detection thresholds for the reasons discussed above. Further, the recent observation that dietary fat intake reportedly influences fat taste sensitivity, at least in lean individuals (Stewart and Keast 2012), prompted a second hypothesis—that high habitual dietary fat intake would correlate with decreased sensitivity. Third, we also measured hunger, as there are reports that hunger improves taste sensitivity (Zverev 2004), and there are mixed reports of differential hunger sensations in lean and obese individuals (Cornier et al. 2004; Mckiernan et al. 2009; Brennan et al. 2012). We hypothesized that sensitivity would be improved with increased levels of hunger, and that hunger may lead to different dietary responses to fat in lean and obese individuals.
Materials and methods
The test stimuli consisted of an emulsion of 5% w/v (1.8×10−1 M) OA (Spectrum Chemicals) in deionized water with 12% gum arabic (Sigma-Aldrich), 0.01% xanthan gum (Jungbunzlauer Inc.), and 0.01% ethylenediaminetetraacetic acid (Spectrum Chemicals). The vehicle was designed to mask textural cues that NEFA may contribute, including viscosity and lubricity (Ramirez 1992; Schiffman et al. 1998). Five percent OA emulsions prepared in 200mL batches were formed by homogenization (IKA T18 Basic Ultra Turrax) for 20min at 15500rpm and then diluted by quarter log steps to create a range of 39 stimulus concentrations to accommodate the most sensitive participant. Five percent was selected as the upper OA concentration as amounts higher than this are rarely found in foods that are not rancid. The vehicle was treated in the same fashion as the OA emulsions. Samples were made less than 24h before testing, stored under nitrogen in polypropylene containers, and served at room temperature. Particle size distributions of the 5% OA emulsion were obtained using a Malvern Instruments Mastersizer 2000 with a Hydro 2000MU dispersion unit. The dispersant was deionized water, and a refractive index of 1.458 and absorption of 0.005 was used for OA. Viscosity was analyzed using an ARG2 Rheometer from TA Instruments equipped with a 40mm, 2° cone and plate geometry with a solvent trap and a Peltier plate for temperature control. Shear rate was increased logarithmically from 1 to 300 s−1 at 37 °C, with 10 data points per decade.
Subjects
Forty-eight (N = 17 male; N = 31 female) adults (mean age: 28.5±10.4 years) gave informed consent and completed the study. Body mass indices (BMI) ranged from 18.9 to 47.2 (lean [≤24.9 kg·m−2]: N = 24; overweight [25.0–29.9 kg·m−2]: N = 11; obese [≥30 kg·m−2]: N = 13). Eight participants were Asian, 5 were Black, 1 was mixed race, 33 were White, and 1 participant failed to provide racial information. Recruitment took place via public advertisements. This study was approved by the University’s Human Subjects Institutional Review Board and was registered at ClinicalTrials.gov (#NCT01550120).
Study design
The study consisted of 7 test visits where OA detection thresholds were determined using a 3-alternative forced choice modified staircase procedure. Previous work showed that significant improvement from baseline was seen over the first 7 visits (Tucker and Mattes 2013). Participants were instructed to abstain from food, beverages other than water, and oral care products for at least an hour before testing. Testing sessions were typically conducted at the same time of day for each participant, but when occasional conflicts arose, visits were scheduled at the convenience of the participant. Prior to each testing session, participants were asked to rate their hunger level on a 100-mm visual analog scale (VAS). Height, weight, and habitual dietary fat intake, as quantified by the Block Rapid Fat Screener (BRFS; NutritionQuest), were measured at baseline. The BRFS, is a one-page, self-administered survey previously validated against a 100-item questionnaire and is reported to correlate significantly with total fat, saturated fat, monounsaturated fat, percent of calories from fat, and cholesterol intake (Spearman r = 0.6–0.72) (Block et al. 2000).
Taste testing methodology
Participants wore blindfolds and nose clips to eliminate visual and olfactory cues. During each trial, 3 samples were presented to participants; 2 samples contained the vehicle, and 1 sample contained the vehicle plus the OA. Participants were instructed to choose the sample that was different from the others following a forced choice format. No more than one testing trial was conducted per day.
The starting concentration at each visit was 3.2mM. A 2-down, 1-up rule was used to determine the concentrations presented to the participants. Once 5 reversals had occurred or when the participant had tasted all concentration levels without 5 reversals, testing ceased for that session. If 5 reversals were not obtained, the threshold was deemed beyond the range of thresholds considered. If 5 reversals were obtained, the last 4 reversals were averaged to obtain the threshold estimate.
Statistics
A linear mixed model that handles censoring (via the NLMIXED procedure in SAS) was used to assess and compare changes in performance over visits for each of the BMI groups. This model was used because of the multiple measurements per participant and the fact that on some visits participants were observed to have thresholds above 5% w/v, the maximal concentration used in this study. We treated these responses above 5% as right censored observations. Correlations were evaluated using Spearman’s rho (ρ). Bonferroni corrections for multiple comparisons were made. Chi-square tests examined the probability of group membership (reliable or unreliable responder). The level of significance was set at P <0.05, 2 tailed. Data were analyzed using IBM SPSS Statistics 20, SAS v9.2, and Microsoft Excel 2010.
Results
Emulsion particle size measurements were made twice using 2 different samples. The average volume weighted mean (D[4,3]) was 2.8 µm. Viscosity measurements at 50 s−1 did not differ between the 5% OA emulsion (0.01024 Pa·s) and the vehicle (0.01005 Pa·s).
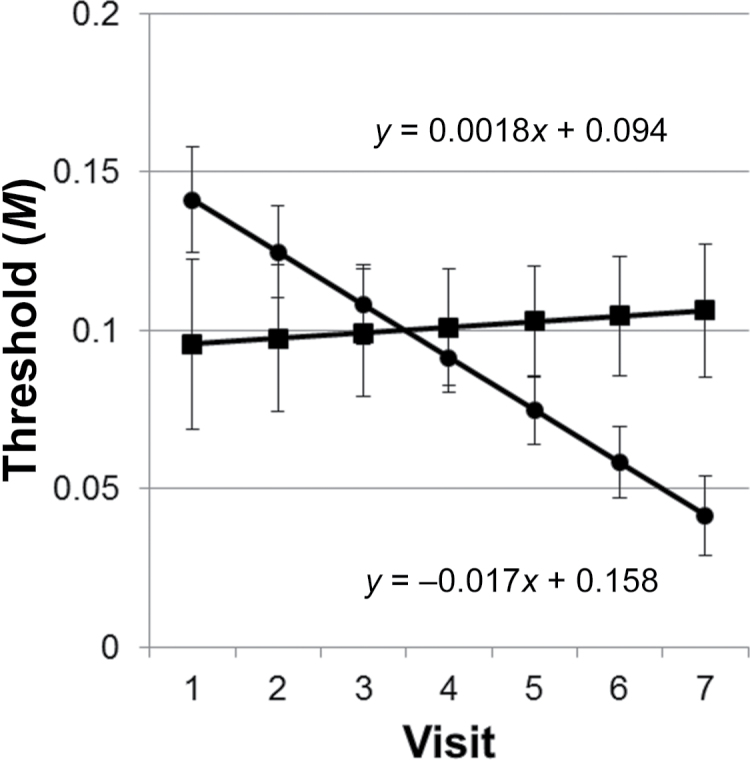
Results from the NLMIXED procedure demonstrated that the intercepts of the lean versus overweight and lean versus obese were not different (P > 0.05). However, the intercepts of the overweight compared with the obese were different (P = 0.040). The slopes of the regression lines between lean and overweight were not different (P > 0.05), but the slope of the obese differed from both the lean (P = 0.013) and the overweight (P = 0.008). As the slopes and intercepts did not differ between the lean and overweight, these 2 groups were combined into 1 group while retaining the obese as a separate group. The fit statistics for the 2-group model (lean plus overweight vs. obese) indicated it was superior to the 3-group model (lean vs. overweight vs. obese) (Bayesian information criterion [BIC] = −249.5 vs. −243.4). Regression lines for the 2-group model are shown in Figure 1. The intercepts are not significantly different (P > 0.05); however, the slopes are still different (P = 0.005). There were also significant differences in threshold sensitivity at visits 6 (P = 0.041) and 7 (P = 0.012).
Figure 1.
Regression lines ± standard error by BMI group (lean and overweight = circles; obese = squares) over the course of 7 visits. Significant differences in thresholds were observed at visits 6 and 7. The slopes of the regression lines were significantly different (P = 0.005).
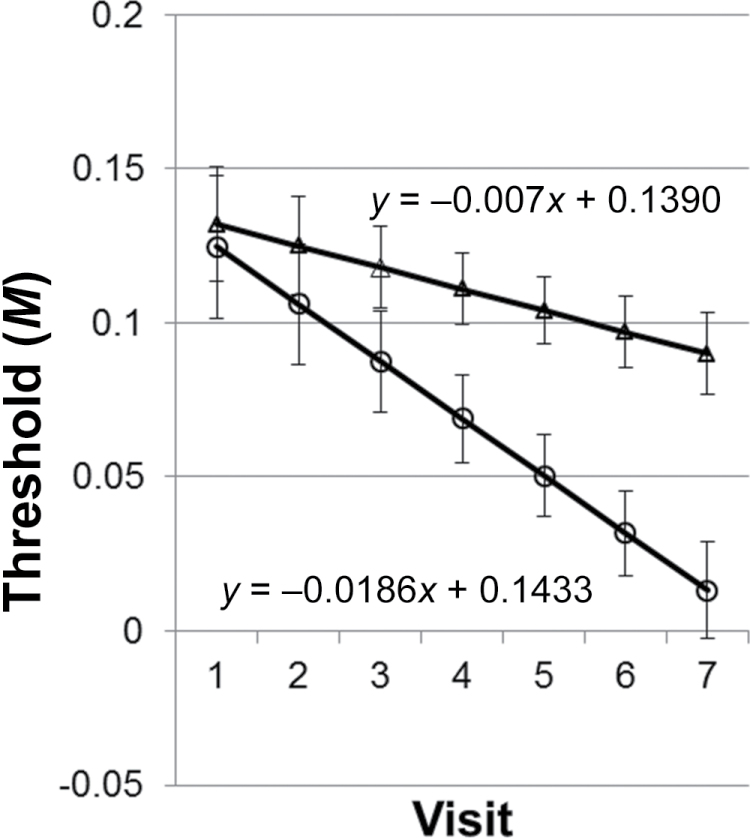
Thresholds that exceeded 0.18M (5% w/v OA) occurred in 47.9% (N = 23) of individuals at baseline and in 16.7% (N = 8) of people at visit 7. All participants achieved a threshold at least once during testing, and 31.3% (N = 15) of participants obtained a threshold at each visit. Chi-square analysis revealed that neither BMI group nor sex was associated with the likelihood of the overall median threshold being greater than 5%, and performance over time did not differ by sex. However, 13 (27.1%) participants had a median threshold greater than 5%, and 9 of these participants were overweight (binomial distribution, P = 0.09). A review of the data revealed 2 response patterns, one where once participants obtained their first threshold they were reliable performers and obtained a threshold at each subsequent visit (reliable; N = 20), and one where those who did not consistently obtain a threshold (unreliable; N = 28). Post hoc analyses demonstrated that BMI did not predict reliability. The intercepts of the 2 groups were not different, but the slopes did show a strong trend for significant difference (P = 0.052) (Figure 2).
Figure 2.
Regression lines ± standard error by reliability grouping over the course of 7 visits. Reliable performers (open circles) obtained their first threshold and obtained a threshold at each subsequent visit (N = 18), compared with those who did not consistently obtain a threshold (unreliable; N = 30; triangles). A trend for significance (P = 0.052) was noted between the 2 slopes.
Correlations were observed between each visit and the mean threshold concentration for the whole group (r = 0.495–0.697; P < 0.001) (Supplementary Table S1). Thresholds appeared to achieve stability, defined as each visit being significantly associated with the visit preceding and following it, at visit 5 (r = 0.477–0.665; P < 0.001). Correlations between baseline and mean threshold concentrations followed different patterns in the lean plus overweight compared with the obese (Table 1). Later visits tended to correlate with the mean threshold in the obese, but the opposite was found in both the lean and lean plus overweight groups with earlier visits associated with mean performance. When tested, the mean of the first 5 visits for the lean plus overweight groups was significantly higher (less sensitive) than the mean of the last 2 visits (P = 0.007). This was not the case for the obese participants. Correlations between visits for lean only and overweight only are shown in Supplementary Table S2.
Table 1.
Spearman’s rho (ρ) correlation coefficients for each visit by BMI group
| Visit 1 | Visit 2 | Visit 3 | Visit 4 | Visit 5 | Visit 6 | Visit 7 | Mean | ||
|---|---|---|---|---|---|---|---|---|---|
| Lean + overweight (N = 35) | |||||||||
| Visit 1 | ρ | 1.000 | 0.565 | 0.498 | 0.285 | 0.397 | 0.158 | 0.358 | 0.763 |
| P value | <0.001 | 0.002 | 0.097 | 0.018 | 0.365 | 0.035 | <0.001 | ||
| Visit 2 | ρ | 1.000 | 0.447 | 0.411 | 0.448 | 0.285 | 0.245 | 0.729 | |
| P value | 0.007 | 0.014 | 0.007 | 0.096 | 0.156 | <0.001 | |||
| Visit 3 | ρ | 1.000 | 0.474 | 0.528 | 0.235 | 0.480 | 0.686 | ||
| P value | 0.004 | 0.001 | 0.175 | 0.004 | <0.001 | ||||
| Visit 4 | ρ | 1.000 | 0.734 | 0.369 | 0.480 | 0.658 | |||
| P value | <0.001 | 0.029 | 0.004 | <0.001 | |||||
| Visit 5 | ρ | 1.000 | 0.407 | 0.455 | 0.728 | ||||
| P value | 0.015 | 0.006 | <0.001 | ||||||
| Visit 6 | ρ | 1.000 | 0.347 | 0.469 | |||||
| P value | 0.041 | 0.005 | |||||||
| Visit 7 | ρ | 1.000 | 0.483 | ||||||
| P value | 0.003 | ||||||||
| Obese (N = 13) | |||||||||
| Visit 1 | ρ | 1.000 | 0.162 | −0.116 | 0.253 | 0.143 | 0.015 | 0.319 | 0.399 |
| P value | 0.598 | 0.705 | 0.404 | 0.642 | 0.962 | 0.289 | 0.177 | ||
| Visit 2 | ρ | 1.000 | −0.288 | 0.181 | 0.268 | 0.545 | 0.491 | 0.507 | |
| P value | 0.340 | 0.555 | 0.376 | 0.054 | 0.088 | 0.077 | |||
| Visit 3 | ρ | 1.000 | −0.145 | 0.020 | 0.007 | 0.060 | −0.080 | ||
| P value | 0.635 | 0.949 | 0.982 | 0.845 | 0.795 | ||||
| Visit 4 | ρ | 1.000 | 0.531 | 0.514 | 0.706 | 0.764 | |||
| P value | 0.062 | 0.072 | 0.007 | 0.002 | |||||
| Visit 5 | ρ | 1.000 | 0.539 | 0.510 | 0.673 | ||||
| P value | 0.058 | 0.075 | 0.012 | ||||||
| Visit 6 | ρ | 1.000 | 0.683 | 0.790 | |||||
| P value | 0.010 | 0.001 | |||||||
| Visit 7 | ρ | 1.000 | 0.875 | ||||||
| P value | <0.001 | ||||||||
Bold values denote a significant association (P ≤ 0.001).
Total fat grams, saturated fat grams, and percent fat were significantly correlated with each other (r = 0.862–0.981; P < 0.001). Regardless of weight category, saturated fat intake was positively correlated with baseline sensitivity among all participants; that is, as intake increased, threshold concentrations increased (sensitivity decreased) (Table 2). The intake of fat, as measured by BRFS, did not differ among lean, overweight, and obese individuals (P > 0.05). Among the lean plus overweight participants, total fat intake in grams at baseline was significantly associated with threshold sensitivity (r = 0.571; P = 0.001). All 3 BRFS fat measures were significant and positively correlated with overall mean thresholds (total fat r = 0.561; P < 0.001; saturated fat grams: r = 0.521; P = 0.001; percent fat: r = 0.573; P < 0.001).
Table 2.
Spearman’s rho (ρ) correlation coefficients for diet-taste relationships and BMI group
| Total fat (g) | Saturated fat (g) | Percent fat (%) | |||||
|---|---|---|---|---|---|---|---|
| Baseline | Mean | Baseline | Mean | Baseline | Mean | ||
| Whole group (N = 48) | ρ | NS | NS | 0.474 | NS | NS | NS |
| P value | 0.001 | ||||||
| Lean plus overweight (N = 35) | ρ | 0.517 | 0.561 | NS | 0.521 | NS | 0.573 |
| P value | 0.001 | <0.001 | 0.001 | <0.001 | |||
| Obese (N = 13) | ρ | NS | NS | NS | NS | NS | NS |
| P value | |||||||
Bold values denote a significant association (P ≤ 0.001).
No consistent pattern of associations between thresholds and hunger were observed. Although the lean reported significantly higher average hunger compared with the obese (45.6±3.20mm vs. 31.0±5.2mm; P = 0.04), the overweight did not differ from the lean or obese (39.0±4.8mm). Average hunger levels were not associated with intake across BMI groups.
Discussion
Threshold sensitivity to OA was measured in lean, overweight, and obese individuals over the course of 7 test visits. Statistical analyses revealed no differences between the lean and overweight group, so the 2 groups were combined (lean plus overweight). The slope of threshold concentrations versus visit was significantly different and negative for the lean plus overweight compared with the positive slope for the obese participants. This indicates that the rate of improvement was significantly greater in the lean plus overweight group compared with the obese. The best estimate of the lower limits of OA acid detection (visit 7) indicated the obese were less sensitive than the lean plus overweight participants.
Although the present data do not permit a determination for the discrepant responses of the obese versus the other groups, BMI-based differences in externality and learning offer possible explanations. The obese may be more attuned to external stimuli (Nisbett 1968; Schachter 1968) and NEFA sensory cues than individuals with lower adiposity. Thus, they were already operating at the limits of their capabilities so did not improve over time. In contrast, the lean and overweight participants were initially less sensitized to NEFA concentrations in their environment (foods) so had greater capacity for improvement. Rodent models suggest deficits in learning and memory in obese animals compared with lean controls (Farr et al. 2008). Weight loss via either Roux-en-Y gastric bypass surgery or caloric restriction resulted in improvements in memory in formerly obese rats (Grayson et al. 2013). In humans, memory deficits were also observed in obese adults compared with lean and overweight individuals, and these findings were independent of age (Gunstad et al. 2006).
The increased sensitivity over time among the lean plus overweight may constitute an improvement in actual sensitivity or an improvement in testing performance. Both explanations have previously been invoked to explain increased sensitivity after repeated testing (Eylam and Kennedy 1998; Kobayashi and Kennedy 2002; Kobayashi et al. 2006; Tucker and Mattes 2013). An improvement in sensitivity could be the result of increased receptor expression (Wysocki et al. 1989), representing a true, biologically mediated improvement in sensitivity. Recent work in rodents suggests that expression levels of one of the putative long chain fatty acid receptors, CD36, is influenced by acute oral exposure to NEFA (Martin et al. 2011). Although fasted lean and obese mice did not differ in terms of expression level (Chevrot et al. 2013), postprandial responses differed between the lean and obese, with a decrease in expression in the lean an hour after the start of exposure. If rodents are an appropriate model for human fat taste, this would suggest that the obese would remain more sensitive during a testing session, which our data do not support.
Understanding of the genetics of fat taste is in its infancy, but previous work in a sample of 21 obese individuals suggests that single nucleotide polymorphisms in the CD36 gene were associated with sensitivity to OA (Pepino et al. 2012). Thus, genetic differences may contribute to sensitivity differences. In the present trial, more than 25% of participants had a median threshold greater than 5%, but BMI did not predict the likelihood of falling into this hyposensitive category. Genetic differences may also explain the reliable and unreliable performer subgroups that were observed as BMI did not predict membership in these categories.
A more likely explanation for the improvement in the lean and overweight is an improvement in performance (lower thresholds) through training. If learning is responsible for the improvement over time in the lean and overweight but not obese, an explanation for this differential response pattern is required. One theory is that a Western-style diet (high fat, high sugar) contributes to neurodegeneration (Francis and Stevenson 2013). Although most studies supporting this hypothesis have been conducted in rodents or rely on epidemiological associations, 1 intervention study fed 20 men a high-fat diet (74% of energy from fat) for 7 days after a 3-day lead-in period (Edwards et al. 2011). Measures of attention and awareness were significantly lower after the high-fat diet intervention. Another study relying on a similar design found diminished attention after 5 days on a high-fat diet (Holloway et al. 2011). High-fat diet exposure resulted in increased OA acid thresholds among lean participants in previous work with no change in thresholds in the overweight participants (Stewart and Keast 2012). While we failed to detect differences in fat intake among the lean, overweight, and obese participants, fat is often regarded as unhealthy and is frequently underreported (Briefel et al. 1997), especially by the obese (Livingstone and Black 2003).
Evidence for detection threshold differences between lean and overweight/obese participants is mixed. In contrast to the present findings, there are other reports that overweight/obese individuals have higher detection thresholds than their lean counterparts with fewer than 4 test visits (Stewart et al. 2010; Stewart, Newman, et al. 2011; Stewart, Seimon, et al. 2011). Prior reports have not clearly described the level of sensory testing experience of study participants; that is, if subjects participate in multiple studies, learning could confound the results. The present work suggests this may be an important methodological factor and could account for the apparently inconsistent reports.
Thresholds for the group appeared to stabilize at visit 5. Limited power likely explains the lack of correlations within the separate BMI groups. However, correlations between threshold concentrations among visits followed different patterns in the lean and lean plus overweight compared with the obese. Later visits tended to correlate with the mean threshold in the obese, but the opposite was found in the lean and lean plus overweight groups with earlier visits associated with mean performance. The fact that the lean plus overweight group had a lower mean threshold for the last 2 visits versus the first 5 suggests they were continuing to improve.
Comparing the detection threshold results of one study to another can be challenging due to differences in methodology, stimulus preparation, vehicle composition, sample characteristics, and so on. This is evidenced by a comparison to our previous work that compared the effect of different testing methodologies on OA detection thresholds (Tucker et al. 2013). Differences in threshold values between this study and the previous are an order of magnitude higher in this study. A number of factors distinguish this study from the first study including more obese participants and the use of censoring rather than assigning a value for those participants without a threshold; each would tend to increase threshold values.
By nearly all accounts, NEFA are aversive stimuli, unlike triacylglycerol (TAG), the predominant form of dietary fat. TAG generally imparts desirable sensory qualities to foods such as creaminess and lubricity and may plausibly be directly related to BMI (Zverev 2004). In this study and previously, participants overwhelmingly described OA, a NEFA, as bitter, although sour was also frequently mentioned (Tucker et al. 2013). Participants also used adjectives associated with flavors like “plastic,” “woody,” or “dirty.” Humans often learn to appreciate tastes and flavors that are initially unpleasant, like those of strong cheeses, fermented products, or alcohol. Participants were asked to describe the taste quality of the OA at each session (data not reported); none of the participants reported developing a liking for the stimulus despite hundreds of exposures, though they also did not receive postingestive feedback from consuming the OA during these trials. While sensitivity is not necessarily a strong predictor of preference and/or consumption (Moskowitz et al. 1974; Lauer et al. 1976; Wise et al. 2007; Donaldson et al. 2009), the presumed antecedents of weight gain, if there is an association between NEFA sensitivity and BMI, it would likely be inverse—reduced intake of an unpleasant, energy-dense stimulus. In agreement with this view, the present data reveal an inverse relationship between threshold sensitivity and fat intake. However, the association is strongest in lean and lean plus overweight individuals, so the nutritional implications of the relationship are questionable.
Our analysis revealed no meaningful associations between hunger and OA thresholds. Others have also failed to find associations between hunger and NEFA sensitivity (Kamphuis et al. 2003). Differences in sensitivity by sex were also not observed in this study.
The relationship between textural attributes and fat content of foods and emulsions has been studied extensively. Particle size and viscosity are frequently cited as predictors of fat content. The particle size of the emulsions presented to participants in this study should not have allowed participants to discriminate between the vehicle and the stimulus based on this potential textural cue. While physical properties do not always accurately translate into consumer experience (Kokini 1987; Akhtar et al. 2005), others have reported that panelists could not discriminate between emulsions with a mean droplet size of 0.5 µm and another with a mean droplet size of 2.3 µm (Akhtar et al. 2005). One study reported that particles must be greater than 5.0 µm to be detected in the oral cavity (Tyle 1993). Greater viscosity may also contribute to the perceived fat content of foods and emulsions (Mela 1988). The viscosities of the vehicle and the highest fat containing stimulus were not different from each other at the shear rate that may correspond most closely to that occurring in the oral cavity (Wood 1968; Richardson et al. 1989), making it unlikely that participants perceived viscosity cues that allowed them to distinguish between the samples presented.
Our work suggests differences of fat taste sensitivity between BMI categories under conditions of repeated testing where thresholds decline for the lean and overweight but remain stable in the obese. Thus, the lean and overweight had lower limits of detection for OA. Increased dietary fat intake was associated with decreased NEFA taste sensitivity, but only in the lean and lean plus overweight participants, so the nutritional implications are questionable. Underreporting of dietary fat intake by obese individuals may have contributed to the failure to observe diet-taste associations in this group. While possibly unrelated to BMI, a subgroup of the population appears to be relatively insensitive to NEFA taste. Further exploration of the genetic contribution to fat taste will likely yield further insight into individual differences in sensory responses.
Supplementary material
Supplementary material can be found at http://www.chemse.oxfordjournals.org/
Funding
This work was supported by NIH/NCATS-Indiana Clin ical and Translational Sciences Institute–TL1 Program [A. Shekhar, PI; 5/01/08-04/30/13] and the United States Department of Agriculture [HATCH IND084055].
Supplementary Material
Acknowledgements
We wish to thank Dr Ganesan Narsimhan, Ms Laura Zimmerer, and Ms Cordelia Running for assistance with the particle size measurements. We also thank Dr Osvaldo Campanella for the use of the rheometer and Ms Cordelia Running for assistance with the viscosity measurements.
References
- Akhtar M, Stenzel J, Murray BS, Dickinson E. 2005. Factors affecting the perception of creaminess of oil-in-water emulsions. Food Hydrocoll. 19:521–526 [Google Scholar]
- Bartoshuk LM. 1979. Methodological problems in psychophysical testing of taste and smell. In: Han SS, Coons DH, editors. Special senses in aging: a current biological assessment. Ann Arbor (MI): University of Michigan [Google Scholar]
- Bell KI, Tepper BJ. 2006. Short-term vegetable intake by young children classified by 6-n-propylthoiuracil bitter-taste phenotype. Am J Clin Nutr. 84:245–251 [DOI] [PubMed] [Google Scholar]
- Block G, Gillespie C, Rosenbaum EH, Jenson C. 2000. A rapid food screener to assess fat and fruit and vegetable intake. Am J Prev Med. 18:284–288 [DOI] [PubMed] [Google Scholar]
- Brennan IM, Luscombe-Marsh ND, Seimon RV, Otto B, Horowitz M, Wishart JM, Feinle-Bisset C. 2012. Effects of fat, protein, and carbohydrate and protein load on appetite, plasma cholecystokinin, peptide YY, and ghrelin, and energy intake in lean and obese men. Am J Physiol Gastrointest Liver Physiol. 303:G129–G140 [DOI] [PubMed] [Google Scholar]
- Briefel RR, Sempos CT, McDowell MA, Chien S, Alaimo K. 1997. Dietary methods research in the third National Health and Nutrition Examination Survey: underreporting of energy intake. Am J Clin Nutr. 65:1203S–1209S [DOI] [PubMed] [Google Scholar]
- Chalé-Rush A, Burgess JR, Mattes RD. 2007a. Evidence for human orosensory (taste?) sensitivity to free fatty acids. Chem Senses. 32:423–431 [DOI] [PubMed] [Google Scholar]
- Chalé-Rush A, Burgess JR, Mattes RD. 2007b. Multiple routes of chemosensitivity to free fatty acids in humans. Am J Physiol Gastrointest Liver Physiol. 292:G1206–G1212 [DOI] [PubMed] [Google Scholar]
- Chevrot M, Bernard A, Ancel D, Buttet M, Martin C, Abdoul-Azize S, Merlin JF, Poirier H, Niot I, Khan NA, et al. 2013. Obesity alters the gustatory perception of lipids in the mouse: Plausible involvement of the lingual CD36. J Lipid Res. 54:2485–2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornier MA, Grunwald GK, Johnson SL, Bessesen DH. 2004. Effects of short-term overfeeding on hunger, satiety, and energy intake in thin and reduced-obese individuals. Appetite. 43:253–259 [DOI] [PubMed] [Google Scholar]
- Donaldson LF, Bennett L, Baic S, Melichar JK. 2009. Taste and weight: is there a link? Am J Clin Nutr. 90:800S–803S [DOI] [PubMed] [Google Scholar]
- Duffy VB, Hayes JE, Davidson AC, Kidd JR, Kidd KK, Bartoshuk LM. 2010. Vegetable Intake in College-Aged Adults Is Explained by Oral Sensory Phenotypes and TAS2R38 Genotype. Chemosens Percept. 3:137–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards LM, Murray AJ, Holloway CJ, Carter EE, Kemp GJ, Codreanu I, Brooker H, Tyler DJ, Robbins PA, Clarke K. 2011. Short-term consumption of a high-fat diet impairs whole-body efficiency and cognitive function in sedentary men. FASEB J. 25:1088–1096 [DOI] [PubMed] [Google Scholar]
- Eylam S, Kennedy LM. 1998. Identification and characterization of human fructose or glucose taste variants with hypogeusia for one monosaccharide but not for the other. Ann N Y Acad Sci. 855:170–174 [DOI] [PubMed] [Google Scholar]
- Farr SA, Yamada KA, Butterfield DA, Abdul HM, Xu L, Miller NE, Banks WA, Morley JE. 2008. Obesity and hypertriglyceridemia produce cognitive impairment. Endocrinology. 149:2628–2636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis H, Stevenson R. 2013. The longer-term impacts of Western diet on human cognition and the brain. Appetite. 63:119–128 [DOI] [PubMed] [Google Scholar]
- Frijters JE, Rasmussen-Conrad EL. 1982. Sensory discrimination, intensity perception, and affective judgment of sucrose-sweetness in the overweight. J Gen Psychol. 107:233–247 [DOI] [PubMed] [Google Scholar]
- Galindo MM, Voigt N, Stein J, van Lengerich J, Raguse JD, Hofmann T, Meyerhof W, Behrens M. 2012. G protein-coupled receptors in human fat taste perception. Chem Senses. 37:123–139 [DOI] [PubMed] [Google Scholar]
- Grayson BE, Fitzgerald MF, Hakala-Finch AP, Ferris VM, Begg DP, Tong J, Woods SC, Seeley RJ, Davidson TL, Benoit SC. 2013. Improvements in hippocampal-dependent memory and microglial infiltration with calorie restriction and gastric bypass surgery, but not with vertical sleeve gastrectomy. Int J Obes (Lond). :10.1038/ijo.2013.100. [DOI] [PubMed] [Google Scholar]
- Grinker J. 1978. Obesity and sweet taste. Am J Clin Nutr. 31:1078–1087 [DOI] [PubMed] [Google Scholar]
- Grinker J, Hirsch J, Smith DV. 1972. Taste sensitivity and susceptibility to external influence in obese and normal weight subjects. J Pers Soc Psychol. 22:320–325 [DOI] [PubMed] [Google Scholar]
- Gunstad J, Paul RH, Cohen RA, Tate DF, Gordon E. 2006. Obesity is associated with memory deficits in young and middle-aged adults. Eat Weight Disord. 11:e15–e19 [DOI] [PubMed] [Google Scholar]
- Holloway CJ, Cochlin LE, Emmanuel Y, Murray A, Codreanu I, Edwards LM, Szmigielski C, Tyler DJ, Knight NS, Saxby BK, et al. 2011. A high-fat diet impairs cardiac high-energy phosphate metabolism and cognitive function in healthy human subjects. Am J Clin Nutr. 93:748–755 [DOI] [PubMed] [Google Scholar]
- Kamphuis MM, Saris WH, Westerterp-Plantenga MS. 2003. The effect of addition of linoleic acid on food intake regulation in linoleic acid tasters and linoleic acid non-tasters. Br J Nutr. 90:199–206 [DOI] [PubMed] [Google Scholar]
- Kobayashi C, Kennedy LM. 2002. Experience-induced changes in taste identification of monosodium glutamate. Physiol Behav. 75:57–63 [DOI] [PubMed] [Google Scholar]
- Kobayashi C, Kennedy LM, Halpern BP. 2006. Experience-induced changes in taste identification of monosodium glutamate (MSG) are reversible. Chem Senses. 31:301–306 [DOI] [PubMed] [Google Scholar]
- Kokini JL. 1987. The physical basis of liquid food texture and texture-taste interactions. J Food Eng. 6:51–81 [Google Scholar]
- Lauer RM, Filer LJ, Reiter MA, Clarke WR. 1976. Blood pressure, salt preference, salt threshold, and relative weight. Am J Dis Child. 130:493–497 [DOI] [PubMed] [Google Scholar]
- Livingstone MB, Black AE. 2003. Markers of the validity of reported energy intake. J Nutr. 133(Suppl 3):895S–920S [DOI] [PubMed] [Google Scholar]
- Malcolm R, O’Neil PM, Hirsch AA, Currey HS, Moskowitz G. 1980. Taste hedonics and thresholds in obesity. Int J Obes. 4:203–212 [PubMed] [Google Scholar]
- Martin C, Passilly-Degrace P, Gaillard D, Merlin JF, Chevrot M, Besnard P. 2011. The lipid-sensor candidates CD36 and GPR120 are differentially regulated by dietary lipids in mouse taste buds: impact on spontaneous fat preference. PLoS One. 6:e24014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattes RD. 2009a. Oral detection of short-, medium-, and long-chain free fatty acids in humans. Chem Senses. 34:145–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattes RD. 2009b. Oral thresholds and suprathreshold intensity ratings for free fatty acids on 3 tongue sites in humans: implications for transduction mechanisms. Chem Senses. 34:415–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattes RD. 2011. Oral fatty acid signaling and intestinal lipid processing: support and supposition. Physiol Behav. 105:27–35 [DOI] [PubMed] [Google Scholar]
- McKiernan F, Hollis JH, McCabe GP, Mattes RD. 2009. Thirst-drinking, hunger-eating; tight coupling? J Am Diet Assoc. 109:486–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mela DJ. 1988. Sensory assessment of fat content in fluid dairy products. Appetite. 10:37–44 [DOI] [PubMed] [Google Scholar]
- Moskowitz HR, Kluter RA, Westerling J, Jacobs HL. 1974. Sugar sweetness and pleasantness: evidence for different psychological laws. Science. 184:583–585 [DOI] [PubMed] [Google Scholar]
- Nisbett RE. 1968. Taste, deprivation, and weight determinants of eating behavior. J Pers Soc Psychol. 10:107–116 [DOI] [PubMed] [Google Scholar]
- Pepino MY, Love-Gregory L, Klein S, Abumrad NA. 2012. The fatty acid translocase gene CD36 and lingual lipase influence oral sensitivity to fat in obese subjects. J Lipid Res. 53:561–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perros P, MacFarlane TW, Counsell C, Frier BM. 1996. Altered taste sensation in newly-diagnosed NIDDM. Diabetes Care. 19:768–770 [DOI] [PubMed] [Google Scholar]
- Ramirez I. 1992. Chemoreception for fat: Do rats sense triglycerides directly? Appetite. 18:193–206 [DOI] [PubMed] [Google Scholar]
- Richardson RK, Morris ER, Ross-Murphy SB, Taylor LJ, Dea ICM. 1989. Characterization of the perceived texture of thickened systems by dynamic viscosity measurements. Food Hydrocoll. 3:175–191 [Google Scholar]
- Rodin J. 1975. Effects of obesity and set point on taste responsiveness and ingestion in humans. J Comp Physiol Psychol. 89:1003–1009 [DOI] [PubMed] [Google Scholar]
- Ryan KK, Woods SC, Seeley RJ. 2012. Central nervous system mechanisms linking the consumption of palatable high-fat diets to the defense of greater adiposity. Cell Metab. 15:137–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter S. 1968. Obesity and eating. Science. 161:751–756 [DOI] [PubMed] [Google Scholar]
- Schiffman SS, Graham BG, Sattely-Miller EA, Warwick ZS. 1998. Orosensory perception of dietary fat. Curr Dir Psychol Sci. 7:137–143 [Google Scholar]
- Scruggs DM, Buffington C, Cowan GS., Jr 1994. Taste acuity of the morbidly obese before and after gastric bypass surgery. Obes Surg. 4:24–28 [DOI] [PubMed] [Google Scholar]
- Stewart JE, Feinle-Bisset C, Golding M, Delahunty C, Clifton PM, Keast RS. 2010. Oral sensitivity to fatty acids, food consumption and BMI in human subjects. Br J Nutr. 104:145–152 [DOI] [PubMed] [Google Scholar]
- Stewart JE, Feinle-Bisset C, Keast RS. 2011. Fatty acid detection during food consumption and digestion: Associations with ingestive behavior and obesity. Prog Lipid Res. 50:225–233 [DOI] [PubMed] [Google Scholar]
- Stewart JE, Keast RSJ. 2012. Recent fat intake modulates fat taste sensitivity in lean and overweight subjects. Int J Obes. 36:834–842 [DOI] [PubMed] [Google Scholar]
- Stewart JE, Newman LP, Keast RS. 2011. Oral sensitivity to oleic acid is associated with fat intake and body mass index. Clin Nutr. 30:838–844 [DOI] [PubMed] [Google Scholar]
- Stewart JE, Seimon RV, Otto B, Keast RS, Clifton PM, Feinle-Bisset C. 2011. Marked differences in gustatory and gastrointestinal sensitivity to oleic acid between lean and obese men. Am J Clin Nutr. 93:703–711 [DOI] [PubMed] [Google Scholar]
- Tsuji M, Nakamura K, Tamai Y, Wada K, Sahashi Y, Watanabe K, Ohtsuchi S, Ando K, Nagata C. 2012. Relationship of intake of plant-based foods with 6-n-propylthiouracil sensitivity and food neophobia in Japanese preschool children. Eur J Clin Nutr. 66:47–52 [DOI] [PubMed] [Google Scholar]
- Tucker RM, Laguna L, Quinn R, Mattes RD. 2013. The effect of short, daily oral exposure on non-esterified fatty acid sensitivity. Chemosens Percept. 6:78–85 [Google Scholar]
- Tucker RM, Mattes RD. 2013. Influences of repeated testing on nonesterified fatty acid taste. Chem Senses. 38:325–332 [DOI] [PubMed] [Google Scholar]
- Tyle P. 1993. Effect of size, shape and hardness of particles in suspension on oral texture and palatability. Acta Psychol. 84:111–118 [DOI] [PubMed] [Google Scholar]
- Wise PM, Hansen JL, Reed DR, Breslin PA. 2007. Twin study of the heritability of recognition thresholds for sour and salty taste. Chem Senses. 32:749–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood FW. 1968. Psychophysical studies on the consistency of liquid foods. In SCI monograph: rheology and texture of foodstuffs. London: Society of Chemical Industry.p. 40–49 [Google Scholar]
- Wysocki CJ, Dorries KM, Beauchamp GK. 1989. Ability to perceive androstenone can be acquired by ostensibly anosmic people. Proc Natl Acad Sci U S A. 86:7976–7978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WG, Chen CB, Wang ZX, Liu YP, Wen XY, Zhang SF, Sun TW. 2011. A case-control study on the relationship between salt intake and salty taste and risk of gastric cancer. World J Gastroenterol. 17:2049–2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Zhang X. 2011. Salt taste preference, sodium intake and gastric cancer in China. Asian Pac J Cancer Prev. 12:1207–1210 [PubMed] [Google Scholar]
- Zverev YP. 2004. Effects of caloric deprivation and satiety on sensitivity of the gustatory system. BMC Neurosci. 5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.