Abstract
Notch signaling plays an important role in development and cell fate determination, and it is deregulated in human hematologic malignancies and solid tumors. This review includes a brief introduction of the relevant pathophysiology of Notch signaling pathway and primarily focuses on the clinical development of promising agents that either obstruct Notch receptor cleavages such as γ-secretase inhibitors (GSIs) or interfere with the Notch ligand–receptor interaction by monoclonal antibodies (mAbs). Antitumor activity by GSIs and mAbs administered as single agent in early phases of clinical trials has been observed in advanced or metastatic thyroid cancer, non-small cell lung cancer, intracranial tumors, sarcoma or desmoid tumors, colorectal cancer with neuroendocrine features, melanoma and ovarian cancer. A number of mechanism-based adverse events particularly gastrointestinal toxicities emerged and mitigation strategies are developed after testing multiple GSIs and Notch targeting mAbs. We also discuss pharmacodynamic biomarkers in conjunction with methods of assessment of the molecular target inhibition validation. Biomarkers of efficacy or benefit may be of importance for a successful development of this class of drugs.
Keywords: Clinical trials, Biomarkers, Diarrhea, Notch signaling, Monoclonal antibodies (mAbs), γ-Secretase inhibitors
1. Introduction
The Notch pathway is a highly evolutionally conserved molecular pathway that plays an important role for cell fate determination, proliferation, differentiation and survival in development, neurogenesis and homeostasis (Ranganathan et al., 2011; Takebe et al., 2011). Increasing evidence demonstrates that Notch signaling is deregulated in human hematological malignancies and solid tumors (Nickoloff et al., 2003; Aster & Blacklow, 2012) and implicated in tumor/tissue angiogenesis (Li & Harris, 2005; Dufraine et al., 2008). A role for Notch signaling in the maintenance of cancer stem cells (CSCs), also known as tumor initiating cells (TICs), has been described in preclinical models and recently in clinical studies (Pannuti et al., 2010; Takebe et al., 2011). Based on but not limited to the above-mentioned mechanisms, targeting the Notch pathway either with small molecule inhibitors primarily with γ-secretase inhibitors (GSIs) or large molecule monoclonal antibodies (mAbs) to Notch ligands and Notch receptors are currently in clinical development. However, clinical development has encountered significant challenges with dose limiting intestinal adverse events. In this article, we focus on clinical development advances and limitations as well as strategies of mitigating toxicities associated with Notch inhibitors. We also discuss Notch pathway targets/biomarkers in tissues or plasma that serve as the pharmacodynamics (PD) biomarkers towards validation of Notch signaling inhibition, and/or potentially as markers of efficacy or benefit in the context of development of Notch signaling pathway targeting drugs.
2. Notch signaling pathway
Notch signaling is vital to embryo development through regulation of cell-to-cell communication by controlling cell proliferation, differentiation, and apoptosis. It is also implicated in the postnatal hematopoiesis, breast development, gastrointestinal epithelial maturation, immune regulation, vascular development and neural stem cell survival (Dontu et al., 2004; Androutsellis-Theotokis et al., 2006). Moreover, Notch signaling outcome determines whether promotion or restriction of differentiation occurs, which largely depends on the cell-context, microenvironment and crosstalk with other signaling pathways. Notch regulates normal and cancer stem cell renewal and differentiation, while in cooperation with the Wnt pathway, it provides cues for intestinal epithelial cell fate decisions (Fre et al., 2005; van Es et al., 2005; Ohlstein & Spradling, 2006; Nakamura et al., 2007). In mouse models, pan Notch inhibition by GSIs can turn rapidly proliferative cells in the intestinal crypts into goblet cells, leading to secretory diarrhea (van Es et al., 2005).
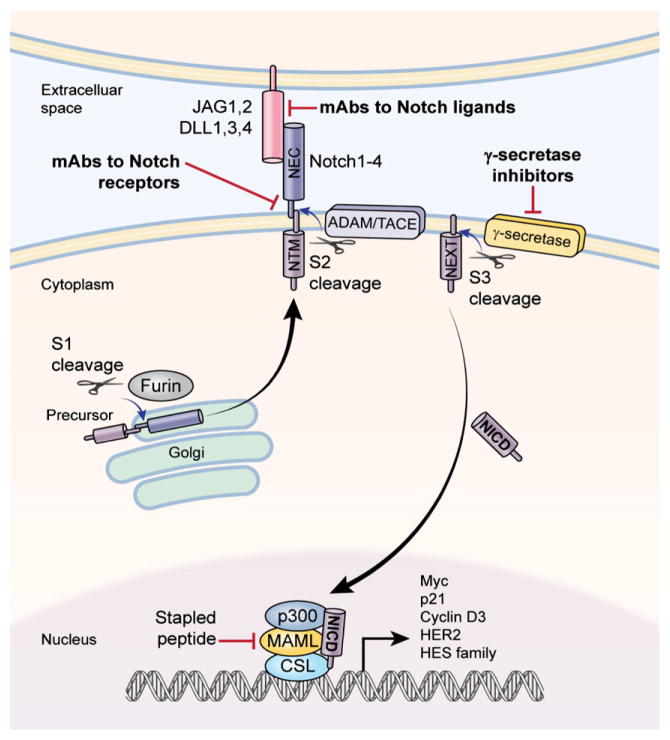
Notch receptors are synthesized in the endoplasmic reticulum as an inactive single peptide precursor, which is proteolytically cleaved by a furin-like convertase in the trans-Golgi network before it reaches the plasma membrane (Fig. 1). The first cleavage (S1) produces non-covalently bound heterodimers comprising a N-terminal ligand-accessible Notch extracellular subunit (NEC), and a C-terminal Notch transmembrane fragment (NTM) that includes an extracellular stub, transmembrane domain and Notch intracellular domain (NICD). There are four Notch receptors (Notch1, 2, 3 and 4) and five ligands including delta-like ligand (DLL) 1, 3, and 4, and Jagged (JAG) 1 and 2 in mammals. All Notch receptors and their ligands are single-pass transmembrane proteins featuring multiple epidermal growth factor (EGF)-like repeats in the extracellular region. The extent of EGF-like repeat fucosylation by the glycosyltransferases (Lunatic Fringe, Radical Fringe and Manic Fringe) determines the affinity strength between the receptors and their ligands (Rampal et al., 2005).
Fig. 1.
Notch signaling pathway and agents in clinical development. Two major classes of Notch inhibitors are currently in early clinical development: γ-secretase inhibitors (GSIs) and monoclonal antibodies (mAbs) against Notch receptors or ligands.
The characteristic of Notch signaling is juxtacrine signaling between neighboring cells. That is, the signaling is initiated by binding of a Notch ligand expressed on one cell to a Notch receptor on an adjacent cell (Fig. 1). Upon ligand binding to the receptors, they undertake conformational change followed by the second cleavage (S2) catalyzed by a member of a disintegrin and metalloproteases (ADAM) family (ADAM17 or ADAM10), also known as the metalloproteinase tumor necrosis factor-α-converting enzyme (TACE). This cleavage leads to the dissociation of the extracellular subunit and generates a membrane-associated Notch extracellular truncated (NEXT) intermediate. The third cleavage (S3) is mediated by a presenilin-dependent γ-secretase protease complex (an integral membrane protein complex), which consists of presenilin 1 (PSEN1) or PSEN2, nicastrin, presenilin enhancer 2 (PEN2) and anterior pharynx-defective 1 (APH1) (Fortini, 2002; Ranganathan et al., 2011). This results in the release of active NICD into the cytoplasm. After translocation into the nucleus, NICD binds to ubiquitous transcription factor CSL (CBF1/Suppressor of Hairless, and Longevity-Assurance Gene-1), and converts a large co-repressor complex into a transcriptional activating complex. The complex primarily contains NICD, CSL, mastermind-like protein (MAML; a transcriptional coactivator), SKIP (Ski-interacting protein as a CBF1 binding protein) and p300, and activates the transcription of Notch target genes. These include genes encoding Hairy Enhancer of Split (HES) family proteins, HES-related proteins (HEY), and Notch-regulated ankyrin repeat protein (Kao et al., 1998) as well as p21cip1/waf1 (Rangarajan et al., 2001), cyclin D1 and 3 (Ronchini & Capobianco, 2001; Sicinska et al., 2003), c-myc (Weng et al., 2006), and HER2 (Chen et al., 1997)(Fig. 1). The transcriptional complex also activates some members of NF-κB family (Cheng et al., 2001) and PPAR family (Garces et al., 1997; Nickoloff et al., 2002), and the ubiquitin ligase SKP2, which promotes p27KIP1 degradation (Sarmento et al., 2005). Therefore, Notch signaling is broadly implicated in cell differentiation, proliferation, survival, and cell fate determinations in a context-dependent fashion. Particularly, some of the Notch target genes are well known by having significant roles in carcinogenesis and tumor progression. In addition, the association between Notch extracellular domain and Notch trans-membrane domain is calcium-dependent (Rand et al., 2000). Notch signaling can be activated through the calcium depletion by EDTA in vitro, which the Notch extracellular domain dissociation relieves its inhibition on the intrinsically active Notch intracellular subunit.
3. Notch signaling pathway and cancer
Notch signaling is primarily oncogenic in many cellular contexts although it can be tumor-suppressive, for instance, inducing keratinocyte growth arrest in the skin (Ellisen et al., 1991; Rangarajan et al., 2001; Weng et al., 2004). The role of Notch signaling in human malignancy was first unraveled in T-cell acute lymphoblastic leukemia/lymphoma (T-ALL/T-LL), in which the t(7;9) chromosomal translocation that produces a truncated Notch1 lacking most of the extracellular subunit was discovered (Reynolds et al., 1987; Ellisen et al., 1991). Notch1 gain-of-function mutations were subsequently found in approximately 50% of T-ALL (Weng et al., 2004).
Since discovery of Notch1 gene alterations in T-ALL, aberrant Notch signaling was subsequently identified in many solid tumors (Ranganathan et al., 2011). However, mutations are infrequent in the members of the Notch family in solid tumors that include breast cancer, glioblastoma multiforme (GBM), head and neck squamous cell carcinoma (HNSCC), non-small cell lung cancer (NSCLC), and ovarian cancer. After compiling the above-mentioned solid tumors, mutation rates are only 4.7% for Notch1, 1.5% for Notch2 and 1.3% for Notch3. Notch4 and Notch ligand genes were rarely mutated (Egloff & Grandis, 2012). Nonetheless, deregulated expression of wild-type Notch receptors and Notch ligands has been found in a growing number of human solid tumors including pancreatic, prostate, breast, lung, GBM, sarcomas, cervical, melanoma, head and neck, and renal cancers as well as gastroenreropancreatic neuroendocrine tumors (Zagouras et al., 1995; Hendrix et al., 2002; Collins et al., 2004; Santagata et al., 2004; Reedijk et al., 2005; Kanamori et al., 2007; De La & Murtaugh, 2009; Rota et al., 2012; Wang et al., 2013). Aberrant activation of Notch1 signaling was associated with loss of Numb activity, a negative regulator of Notch pathway, in about 40% of breast cancer and 30% of lung cancer (Stylianou et al., 2006). High level of JAG1 or Notch1 was seen in about 25% of human breast tumors by in situ hybridization; and high versus low levels of co-expression of Notch1 and JAG1 were associated with reduced overall survival (Reedijk et al., 2005).
Oncogenic Ras can increase level and activity of the intracellular form of wild-type Notch1, and up-regulate DLL1 and presenilin-1. The oncogenic phenotype transformed by Ras was maintained by activated Notch1 signaling (Weijzen et al., 2002), and this may confer the necessity of co-inhibiting both pathways to revert the oncogenic phenotype. Moreover, GBM cell growth was markedly reduced by expressing a dominant negative form of a Notch coactivator, mastermind-like (DN-MAML) or treatment with GSI MRK-003 (Chen et al., 2010). The preclinical report revealed the aberrant Notch signaling in GBM cell lines and human GBM-derived neurospheres. Furthermore, microarray analysis identifies that several members (MAML2 and 3, JAG1) of the Notch family were upregulated in human papillary thyroid cancer (Vasko et al., 2007); and Notch1 expression was higher in cancer cells than normal thyroid tissues (Geers et al., 2011). In addition, Notch pathway in connection with the MAPK pathway influences thyroid tumor cell proliferation (Yamashita et al., 2013).
Notch signaling is also linked to the mesenchymal phenotype, especially epithelial–mesenchymal transition (EMT) during tumor progression and metastasis (Timmerman et al., 2004; Z. Wang et al., 2010). Recently, Notch3 was identified as a regulator of motility in neuroblastoma. A subset of neuroblastoma with high expression of Notch3 and its downstream-regulated genes is associated with an increased incidence of metastases and poor prognosis (van Nes et al., 2013).
4. Notch signaling pathway and angiogenesis
Notch signaling is involved in the specification, proliferation, and migration of endothelial tip and stalk cells including the coordination of multiple aspects of endothelial behavior during vessel patterning (Phng & Gerhardt, 2009). Notch1 and Notch4, and their ligands DLL1, DLL4, and JAG1 are most prevalently expressed on endothelial cells (Villa et al., 2001; Favre et al., 2003; Claxton & Fruttiger, 2004; Hofmann & Iruela-Arispe, 2007). Dll4/Notch1 signaling regulates the specification of endothelial cells into tip and stalk cells in multiple animal models (Noguera-Troise et al., 2006; Hellstrom et al., 2007; Leslie et al., 2007; Lobov et al., 2007; Siekmann & Lawson, 2007; Suchting et al., 2007). GSI treatment or genetically knockout of one Dll4 allele augmented sprouting, branching, and hyperfusion of the capillary network by excessive tip cell formation (Hellstrom et al., 2007). Thus, Dll4-Notch1 signaling between the endothelial cells within the angiogenesis sprout serves to restrict tip-cell formation in response to VEGF, thereby establishing an adequate ratio between tip and stalk cells required for correct sprouting and branching patterns. Mouse tumor models have also shown that Notch signaling controls the branching frequency of tumor blood vessels by the tip-stalk specification (Noguera-Troise et al., 2006; Ridgway et al., 2006). The in vivo data suggest that inhibition of DLL4-Notch signaling in endothelial cells led to an increased sprouting angiogenesis and surprisingly reduced tumor growth, which is referred to as so-called non-functional angiogenesis. However, continuous dosing with anti-DLL4 mAb has resulted in new vessel formation and angiomas in humans (Dufraine et al., 2008; Yan et al., 2010). This effect was overcome by combining with VEGF inhibitors, which added anti-tumor efficacy (Li & Harris, 2009).
In human ovarian cancer, objective response to aflibercept/ bevacizumab therapy was observed in patients whose tumors had lower levels of tumor cell DLL4 expression compared to those with high levels of DLL4 (Hu et al., 2011). In human breast cancer, low versus high expression of Dll4 examined by immunohistochemistry was associated with an improvement in progression-free survival (PFS) in patients who received bevacizumab plus capecitabine chemotherapy (Jubb et al., 2011). High expression of DLL4 in the tumor-associated endothelium was considered to invoke resistance to anti-VEGF-A therapy probably by maintaining angiogenesis through the DLL4-Notch1 signaling. The data further provide the rationale of simultaneously targeting VEGF and DLL4 as a sound approach for a more efficacious anti-angiogenesis therapy.
Expression of JAG1 in tumor cells positively affects tumor angiogenesis and was up-regulated in head and neck squamous cell carcinoma (Funahashi et al., 2008). In addition, Notch1 decoy, a soluble form of Notch1 receptor, inhibited tumor angiogenesis and its mechanism of action appears to be different from those reported on DLL4 blockade, in which Notch1 decoy did not cause blood vessel overgrowth (Funahashi et al., 2008).
5. Notch signaling and cancer stem cells
Stem cells have features of self-renewal and perhaps capacity of initiating cancer, therefore also named as TICs. CSCs are characterized by the cell surface markers of CD133-positive (CD133+), CD44-positive and CD24-low (CD44+/CD24−) as well as aldehyde dehydrogenase-positive (ALDH+) (Dontu & Wicha, 2005; Ginestier et al., 2007). In vitro tumorsphere assay or mammosphere-forming efficiency (MSFE) assay, serving as surrogate readout of the CSC function, was used to demonstrate sphere formation inhibition by GSIs (Fan et al., 2010; Grudzien et al., 2010). It has been widely hypothesized that CSCs may confer resistance to many types of treatment modalities such as targeted therapy, chemotherapy or radiation therapy. Breast cancer and GBM are the most extensively studied tumor models that have elucidated the mechanisms of Notch signaling in the CSC maintenance (Farnie & Clarke, 2007; J. Wang et al., 2010). The formation of MSFE in ductal carcinoma in situ was markedly reduced when inhibition of Notch signaling by a Notch 4-neutralizing antibody, suggesting the role of Notch in breast cancer CSC maintenance. Notch pathway blockade by GSIs depletes stem-like cells in GBMs and inhibits the growth of neurosphere and established xenograft tumors. Recently, Notch activity was identified as a marker for cells with stem cell-like properties and is associated with poor overall survival in lung cancer patients (Hassan et al., 2013). A study has found a crosstalk between the Hedgehog signaling and Notch signaling as HES1, a Notch signaling target, directly modulates the Hedgehog signaling transcription factor Gli-1 expression in GBM neurospheres (Schreck et al., 2010). These data suggest that Notch signaling is implicated in the CSC maintenance in some solid tumors and further justifies this pathway as a therapeutic target for cancer therapy.
6. Agents targeting Notch signaling pathway
Given the substantial roles of Notch signaling in cancer, CSC maintenance and angiogenesis, targeting Notch signaling with various investigational agents including small molecule inhibitors and large molecule mAbs has been moved from preclinical research into early clinical development (Table 1). Here, we discuss clinical activities, treatment-associated-toxicities, PD and pharmacokinetics (PK) of Notch targeting agents in an order of clinical development advances (Table 2).
Table 1.
Drugs in clinical development.
| Class | Drug(s) | Target | Phase | Enrollment or estimated | Primary endpoint; subjects | Trial status | Trial IDa |
|---|---|---|---|---|---|---|---|
| γ-Secretase inhibitors | MK0752 | γ-Secretase | I | 50 | Safety/efficacy; relapsed or refractory T-ALL/T-LL | Terminated | NCT00100152 |
| MK0752 | I | 103 | Safety/MTD; advanced breast cancer/advanced solid tumors | Completed | NCT00106145 | ||
| MK0752 | I | 33 | Safety; recurrent or refractory CNS malignancies | Terminated | NCT00572182 | ||
| MK0752 + Docetaxel | I/II | 30 | DLT; locally advanced or metastatic breast cancer | Completed | NCT00645333 | ||
| MK0752 + Gemcitabine | I/IIa | 60 | Safety/MTD; stage IV pancreatic cancer | Recruiting | NCT01098344 | ||
| MK0752 | I | 30 | Notch response signature; healthy young adults | Completed | NCT00803894 | ||
| MK0752 | Pre-surgical | 20 | Safety/tolerability; early stage ER-positive breast cancer | Unknown | NCT00756717 | ||
| RO4929097b | - | - | - | Terminated | - | ||
| PF03084014 | I | 60 | Safety/DLT; advanced solid Tumors/T-ALL/T-LL | Ongoing | NCT00878189 | ||
| BMS-906024 | I | 95 | Safety/tolerability; advanced solid tumors | Recruiting | NCT01292655 | ||
| BMS-906024 | I | 42 | Safety/tolerability; T-ALL/T-LL | Recruiting | NCT01363817 | ||
| BMS906024 + chemotherapy c | Ib | 60 | Safety; advanced or metastatic solid tumors | Recruiting | NCT01653470 | ||
| LY3039478 | I | 80 | DLTs; advanced or metastatic cancer | Recruiting | NCT01695005 | ||
| mAbs to Notch receptors or ligands | OMP-59R5 | Notch2/3 | I | 44 | Safety; advanced solid tumors | Recruiting | NCT01277146 |
| OMP-59R5 + Nab-P & Gemcitabine | Notch2/3 | Ib/II | 154 | DLT/MTD; first line for stage IV pancreatic cancer | Recruiting | NCT01647828 | |
| OMP21M18 | DLL4 | I | 30 | Safety; advanced solid tumors | Recruiting | NCT00744562 | |
| OMP21M18 + FOLFIRI c | DLL4 | Ib | 32 | MTD; first or second line for advanced colorectal cancer | Recruiting | NCT01189942 | |
| OMP21M18 + CP c | DLL4 | Ib | 32 | MTD; first line for advanced non-squamous NSCLC | Recruiting | NCT01189968 | |
| OMP21M18 + Gemcitabine | DLL4 | Ib | 40 | MTD; first line for advanced or metastatic pancreatic cancer | Recruiting | NCT01189929 | |
| REGN421 | DLL4 | I | 80 | Safety/tolerability; advanced solid tumors | Recruiting | NCT00871559 | |
| OMP52M51 | Notch1 | I | 33 | Safety; relapsed or refractory solid tumors | Recruiting | NCT01778439 | |
| OMP52M51 | Notch1 | I | 53 | Safety; relapsed or refractory lymphoid malignancies | Recruiting | NCT01703572 | |
| γ-Secretase modifier | MPC-7869/R-flurbiprofen | γ-Secretase | IIb | Unknown | TTP c; localized prostate cancer at risk of recurrence following radiation therapy and/or prostatectomy | Unknown | NCT00045123 |
Clinical trial registration and information at www.clinicaltrials.gov.
Clinical trials with RO4929097 are not shown due to the termination of its development.
Chemotherapy, weekly paclitaxel, FOLFIRI or carboplatin plus paclitaxel; FOLFIRI, FOLolinic acid (leucovorin), 5-Fluorouracil (5-FU) plus IRInotecan (irinotecan) or carboplatin plus paclitaxel; CP, carboplatin and pemetrexed; Nab-P, Nab-paclitaxel; TTP, time to progression.
Table 2.
Single agent results in early phases of clinical trials.
| Drug | Patients treated | Disease control rate a | Major treatment-related toxicities | Reference |
|---|---|---|---|---|
| MK0752 | 103 | 1 CR in anaplastic astrocytoma, 15 SDs in mostly GBM | GI disorders, fatigue, AST increase, rash | Krop et al., 2012; |
| RO4929097 | 110 | 1 PR in colorectal adenocarcinoma with neuroendocrine features, 26 SDs in sarcoma, melanoma or ovarian cancer | GI events, fatigue, hypophosphatemia, rash | Tolcher et al, 2012; |
| PF03084014 | 41 | 1 CR in papillary thyroid cancer, 4 PRs in desmoid tumors | Diarrhea, nausea, rash and fatigue | Messersmith et al, 2012 |
| OMP-59R5 | 39 | SDs in adenoid cystic adenocarcinoma, sarcomas, rectal cancer, or triple-negative breast cancer | Diarrhea | Smith et al, 2012 |
| REGN421 | 53 | 2 PRs in NSCLC and ovarian cancer, 16 SDs | Fatigue, headache, hypertension, nausea | Jimeno et al, 2013 |
Disease control rate includes complete and partial responses, and stable disease.
6.1. γ-Secretase inhibitors
As discussed earlier, the interaction between Notch ligand(s) and receptor(s) occurs within a tight cell-to-cell compartment where their engagement takes place. Followed by the second receptor cleavage by the ADAM/TACE, the third cleavage catalyzed by γ-secretase is a crucial activation step of Notch signaling, and is argeted by GSIs with which currently we have the most experience in early clinical development (Table 1). GSI types, their chemical structures and pharmacological modes of action have been reviewed by Olsauskas-Kuprys and colleagues recently (Olsauskas-Kuprys et al., 2013). GSIs are either competitive inhibitors of the catalytic activity of presenilins through competitively binding to the active cleavage site in the γ-secretase complex (transition state analogs) or non-transition state inhibitors. GSIs target not only all four Notch receptors but also the other substrates such as delta-like, Jagged, amyloid precursor protein (APP), CSC marker CD44, ErbB4, E-cadherin, N-cadherin, and syndecan-3 largely dependent of cellular expression levels of substrates, and GSI types and doses utilized (Nickoloff et al., 2003; Kopan & Ilagan, 2004; Maetzel et al., 2009). Nevertheless, it is important to point out that GSIs exhibit their effects predominantly on the Notch receptors, mostly Notch1 (De Strooper et al., 1999). Additionally, GSIs target CSCs and deregulate angiogenesis as the proposed mechanisms of action in cancer treatment.
6.1.1. MK-0752
6.1.1.1. Single agent trials
MK-0752 (MERK) is a potent non-competitive oral GSI and has good preclinical activity in T-ALL and breast cancer. It can induce G0/G1 arrest and apoptosis of T-ALL cell lines harboring Notch activating mutations. MK-0752 was clinically investigated in T-ALL initially and more recently in solid tumors (Deangelo et al., 2006; Krop et al., 2012). In a leukemia phase I trial, MK-0752 was orally administered by once daily dosing in 28-day cycles. Dose limiting toxicity (DLT) was grade 3 or 4 diarrhea at 300 mg/m2 dose level. No objective response was seen in eight patients treated in this study, even the four T-ALL patients with Notch1 mutations. The best response of this trial was one T-ALL patient with Notch1 activating mutation who achieved a 45% reduction in a mediastinal mass at 28 days but subsequently progressed at day 56. The reasons for the lack of activity in T-ALL patients with Notch1 mutations were unclear. No evaluation was available for the potential effects of MK-0752 on the Notch1 NICD and Notch target genes in tumor cells. The continuous dosing regimen was apparently too toxic as the treatment duration only ranged from 2 to 56days either due to the drug-related toxicities or disease progression. This has resulted in subsequent optimization of dosing schedules to mitigate the drug-associated toxicities.
Of 23 patients enrolled in a pediatric brain tumor trial, two patients had stable disease (SD) by 3 cycles of therapy – one with ependymoma and the other with GBM (Fouladi et al., 2011). The recommended phase II dose was intermittent schedule of once daily for 3 days followed by 4 days off at 260 mg/m2. Further, in a trial of 103 adult patients with advanced solid tumors, a RECIST (response evaluation criteria in solid tumors) complete response (CR) was observed in a patient with anaplastic astrocytoma and SD in 12 patients with GBM (Krop et al., 2012). No single agent activity was observed in patients with other types of solid tumors including 24 patients with advanced breast cancer and 16 with colorectal cancer. Three escalating dosing schedules were tested in this trial, which includes a continuous once daily dosing at 450 and 600mg, an intermittent schedule of 3 out of 7days at 450 and 600mg, and once a week at 600, 900, 1200, 1500, 1800, 2400, 3200 and 4200 mg. The dose and schedule associated with the CR was 1800 mg weekly in this study.
6.1.1.2. MK-0752 in combination with chemotherapy
Very recently, a phase Ib clinical trial tested MK-0752 in combination with docetaxel chemotherapy in locally advanced or metastatic breast cancer (Schott et al., 2013). Eleven patients had partial response (PR), 9 had SD and 3 had progressive disease (PD) by RECIST in 23 patients evaluable for response. DLTs in this combination trial were pneumonitis, hand–foot syndrome, elevated liver function tests, and diarrhea. Interestingly, expression of CD44+/CD24−, ALDH + CSCs and MSFE were reduced after multiple cycles of combination treatment in 6 patients who had a series of biopsies. Among the six patients, four had PRs and 2 had SDs with all but one receiving 600mg dose. This trial recommends the intermittent schedule of 600 mg MK-0752 for 3 days on and 4 days off and 80 mg/m2 docetaxel administered on day 8 of each 21-day cycle for a proposed randomized phase II study. The findings validate the concept of inhibiting CSCs and bulk of tumors by Notch targeted therapy in combination with chemotherapy in the clinical setting.
MK-0752 in combination with chemotherapy or hormonal therapy is currently evaluated in phase I/II clinical trials (Table 1). A trial with MK-0752 in combination with tamoxifen or letrozole is assessed for the treatment of early stage hormone receptor-positive breast cancer (Albain et al., 2011). MK-0752 plus gemcitabine is being conducted for the treatment of patients with stage IV surgically unresectable stage IV pancreatic cancer (Table 1).
6.1.2. RO-4929097
RO-4929097 (Roche), a potent competitive oral GSI, was evaluated by Roche and the National Cancer Institute Cancer Treatment and Evaluation Program to find the best dosing and least toxic schedules during past several years. There was a hint of single agent activity in one case each of colorectal adenocarcinoma with neuroendocrine features, epithelioid sarcoma and metastatic melanoma in 92 patients with advanced solid tumors (Tolcher et al., 2012). In addition, prolonged SD was frequently observed in patients with advanced melanoma, sarcoma and ovarian cancer (Table 2). However, high levels of IL-6 and IL-8 in serum at baseline have been shown to negate RO4929097 activity by diminishing its effect on angiogenesis and infiltration of tumor associated fibroblasts (He et al., 2011).
The drug was also tested in combination with several chemotherapy regimens or targeted agents such as a hedgehog signaling inhibitor GDC-0449 in patients with breast cancer and sarcoma (Gounder & Schwartz, 2012). In a recent phase I trial report, 20 patients with advanced solid tumors were treated with RO4929097 in combination with cediranib. One patient with endometrial stromal cell tumor had PR and 11 patients had SD as their best response. Prolonged SD was achieved in 7 patients with 1 uterine leiomyosarcoma, 2 colorectal cancer, 2 renal cell carcinoma, and 1 cholangiocarcinoma. The most frequent treatment-related adverse events were diarrhea, hypertension, fatigue and nausea (Sahebjam et al., 2013). In a phase Ib combination trial of RO4929097 and temsirolimus, a mTOR inhibitor, 17 patients with refractory advanced solid tumors were enrolled (Diaz-Padilla et al., 2013). In this study, SD was observed in 11 (73%) patients; grade 3 toxicities were fatigue, mucositis, neutropenia, anemia and hypertriglyceridemia. Of particular note, co-administration of RO4929097 was associated with an increased clearance and reduced exposure to temsirolimus, suggesting a CYP3A4-mediated drug–drug interaction. In another combination study, 18 patients with advanced solid tumors were treated with RO4929097 plus gemcitabine. One patient with naso-pharyngeal cancer had PR and three patients (pancreatic, tracheal and breast cancers) had SD >4 months (Richter et al., in press). After an extensive clinical trial testing, RO-4929097 development was terminated due to a limited PK rise caused by CYP3A4 induction and the CYP3A4-mediated drug to drug interaction in some combination studies (Table 1).
6.1.3. PF-03084014
PF-03084014 (Pfizer Oncology), a small molecule reversible, non-competitive and selective GSI, inhibits growth of several T-ALL cell lines by inducing cell cycle arrest and induction of apoptosis (Samon et al., 2012). A significant antitumor activity has been shown in 10 of the 18 breast cancer xenograft models including the triple-negative breast cancer models by inducing apoptosis, inhibition of CSC self-renewal, anti-proliferation and anti-angiogenesis (Zhang et al., 2012). Interestingly, Notch1 mutant HCC1599 models both in vitro and in vivo were shown to have highest responses to PF-03084014 treatment.
It is currently in a phase 1 dose escalation trial in patients with advanced solid tumors (Messersmith et al., 2012). Forty-one patients were dosed continuously for 21days followed by 7days off. One patient with papillary thyroid cancer had CR and 4 with desmoid tumors had PR. Maximum tolerated dose (MTD) was defined as 220 mg twice daily (Table 2). Most treatment-related grade1/2 adverse events were diarrhea (34%), nausea (32%), rash (27%) and fatigue (27%). Grade 3 toxicities were diarrhea (12%), hypophosphatemia (12%), rash (2%) and hypokalemia (2%).
Synergistic activity of PF-03084014 with docetaxel was shown in breast cancer animal models (Zhang et al., 2013). Several mechanisms of action have been linked to its antitumor activity. First, the combination reverses docetaxel activated Notch pathway with up-regulation of NICD and suppression of expression of NUMB, an endogenous Notch signaling inhibitor. Second, PF-03084014 was able to reverse mesenchymal transition phenotype caused by docetaxel and reducing CSCs.
6.1.4. Other GSIs
BMS-708163 (Bristol-Myers Squibb) is a second generation GSI currently in phase 1 development in oncology and late-stage phase III development for Alzheimer’s disease. BMS-906024 is another pan-Notch inhibitor currently in phase I clinical trial both administered as a single agent or in combination with chemotherapy in oncology (www.clinicaltrials.gov). Patients with advanced colorectal, lung, breast and leukemia are receiving intravenous doses of the compound to determine its safety and tolerability, and optimal dose ranges. LY3039478 (Eli Lilly) is an oral GSI in phase I single drug trial to determine its safety profile and DLTs (Table 1).
Taken together, phase I trials have identified the safety profiles of several GSIs especially with the intermittent administration schedule. Limited clinical activity as single agent was noted in GSI trials. In this context and toxicity issues, Gounder and Schwartz have proposed to interrogate the Notch pathway inhibition in tumor cells after treatment to define a minimally biological effective dose (Gounder & Schwartz, 2012). Nonetheless, disease control including CR, PR and SD has been observed in patients with intracranial tumors, sarcomas or desmoid tumors, colorectal cancer with neuroendocrine features, thyroid cancer, melanoma and ovarian cancer in early clinical trials (Table 2). Particularly, MK-0752 in combination with docetaxel demonstrated a high disease control rate or clinical benefit rate in metastatic and locally advanced breast cancer. However, it is noteworthy to mention that single agent activity has not been observed in breast cancer despite the substantial preclinical evidence of activity.
6.1.5. Major GSI treatment-related toxicities
The most serious and prominent toxicities associated with GSIs are gastrointestinal toxicities such as intractable diarrhea, especially by continuous dosing schedules (Wei et al., 2010). It is due to the rapid differentiation of progenitor cells into secretory goblet cells in the intestinal crypts (goblet cell metaplasia) after GSI administration as Notch signaling is required to maintain the normal architecture of the intestinal epithelium (Fre et al., 2005). In vivo models were used to test how to ameliorate the toxicity and improve efficacy by administration of GSI with intermittent dosing schedule and co-administration of corti-costeroids (Real et al., 2009). This strategy has been proved to effectively mitigate treatment-related toxicities especially diarrhea clinically and appears not to compromise efficacy (Krop et al., 2012; Schott et al., 2013). The other GSI-associated adverse events are skin disorders (erythema, rash and pruritus), gastrointestinal toxicities for example nausea and vomiting, fatigue, hypophosphatemia, and headache (Tolcher et al., 2012). These symptoms are likely attributable to the inhibition of physiological functions of the Notch signaling in keratinocyte differentiation and metabolism.
6.2. Monoclonal antibodies targeting Notch pathway
Another class of agents under development is the mAb either against Notch receptors or Notch ligands. Experimental evidence demonstrates that Notch inhibition by either mAb against Notch1 or Norch2 alleviates the toxicities while simultaneous inhibition of Notch1 and 2 leads to severe gastrointestinal toxicities (Wu et al., 2010). It is hoped that this category of drugs could reduce or spare some of the toxicities associated with pan-Notch inhibition by GSIs. Table 1 lists Notch targeting mAbs that have entered into early phase clinical development and Table 2 summarizes the preliminary results of some of those agents.
6.2.1. OMP-21M18 and REGN421
OMP-21M18 (OncoMed Pharmaceuticals) is a humanized mAb against DLL4, which blocks the ligand to interact with Notch1 and Notch4 in the Notch signaling pathway. OMP-21M18 (demcizumab) has shown activity in patient derived xenograft model without promoting angiogenesis (Reynolds et al., 2011). Currently, several clinical trials are testing OMP-21M18 as single agent or in combination with chemotherapy in advanced or metastatic solid tumors (Table 1). A phase Ib trial with OMP-21M18 in combination with standard-of-care gemcitabine is being conducted in patients with advanced pancreatic cancer. OMP-21M18 plus pemetrexed and carboplatin phase 1b trial has reported a 44% PR and 94% disease control rate (PR + SD) among 17 evaluable advanced NSCLC patients. Fatigue and hypertension were the most common drug-related toxicities (McKeage et al., 2012).
Very recently, REGN421/SAR153192 (Regeneron Pharmaceuticals), a fully humanized mAb against DLL4 demonstrated a single agent activity in advanced solid tumors (Jimeno et al., 2013). With 53 patients on trial, two patients with advanced ovarian cancer and a beta-catenin mutant NSCLC had PR and 16 patients with SD by RECIST. It would be of interest to validate whether the Wnt pathway mutation status signals clinical efficacy to Notch inhibition. Most frequent treatment related toxicities were fatigue, headache, hypertension and nausea. Two DLTs were grade 3 nausea at 0.5mg/kg every 3weeks and grade 3 abdominal pain at 1 mg/kg every 3 weeks. Noticeably, six treatment related severe adverse events occurred in 4 patients; these were grade 1 brain natriuretic peptide (BNP) and grade 3 troponin I increases, one event each of right and left ventricular dysfunction (grade 3), and two events of grade 3 pulmonary hypertension. Recommended phase 2 doses are 4 mg/kg every 3 weeks and 3 mg/kg every 2 weeks.
6.2.2. OMP-59R5 and OPM-52M51
A fully humanized monoclonal antibody OMP-59R5 selectively targeting Notch 2/3 receptors was tested in a phase I dose escalation study given as a single agent. It was generally well tolerated with diarrhea as the main treatment-related toxicity (Table 2). Prolonged SD was observed in sarcomas, rectal cancer and triple-negative breast cancer (www.oncomed.com). The established MTDs were at doses of 2.5 mg/kg weekly and 7.5 mg/kg every three weeks. OPM-52M51 is an anti-Notch1 antibody currently tested in a phase 1 clinical trial (Table 1).
6.3. Agents in preclinical development
Other notable investigational drugs include a class of agents called “stapled peptide” which are in pre-clinical development (Fig. 1). The stapled peptide blocks the interaction of MAML with the Notch intracellular domain (Moellering et al., 2009). Development of a fusion protein consisted of antennapedia-DN-MAML-like construct has also been reported to target Notch signaling by interrupting NICD and MAML (Epenetos et al., 2012). A5226A is a novel mAb against nicastrin and targets the fully glycosylated extracellular domain within the active γ-secretase complex. It has been shown to inhibit γ-secretase activity in T-ALL cell lines and xenograft models (Hayashi et al., 2012). Moreover, some nicastrin mAb was shown to have anti-CSC and anti-tumor activity in breast cancer models (Lombardo et al., 2012). Targeting nicastrin to indirectly inhibit γ-secretase may be better tolerated in patients largely due to less off-target effects than GSIs.
7. Biomarkers and pharmacodynamics assays
It is believed that PD biomarkers are critical for early phases of cancer drug development in terms of target inhibition validation. As such, PD markers can be used to find the doses that lead to target inhibition and degree of target inhibition, instead of the conventional determination of MTD. Application of this strategy in early oncology drug trials may be especially valuable for the determination of the doses of two or more targeted agents or a targeted agent in combination with chemotherapy. The doses determined by the traditional MTDs are in general too toxic and frequently require dose reduction encountered in many clinical trials (Kummar et al., 2010, 2011). In addition, there are some agents for which an MTD cannot be readily defined (LoRusso et al., 2012). As a whole, MTD is less frequently established in trials investigating molecularly targeted agents alone (51.3%) or in combinations (42.8%) as compared to chemotherapy agents (75.6%) (Ferte et al., 2011).
In a phase I GSI trial, several Notch PD markers – Aβ-40 protein (a peptide of 36–43 amino acids that is processed from the APP) and VEGFR2 in plasma, and HES1 mRNA expression in hair follicles were evaluated (Tolcher et al., 2012). There was a weak to moderate relationship between PK and the PD biomarkers. RO-4929097 at higher plasma concentrations led to the decreases in Aβ-40 protein and HES1 mRNA and a compensatory increase in plasma VEGFR2. However, none of the markers explored at baseline predicted treatment responses. Krop et al. reported a set of surrogate PD markers obtained from hair follicles from a healthy volunteer study (Krop et al., 2012). A nine-gene signature of ADAM19, CCND1, DVL1, HES4, HES5, HEY1, HEYL, NOTCH1, and NRARP was generated by gene expression microarray approach, and was used to associate with Notch inhibition. The composite score based on the mRNA expression of the nine Notch-related genes exhibited an inverse linear correlation with plasma MK-0752 concentrations 4 h after treatment, confirming its utility as a PD biomarker. However, the relationship between the signature and clinical responses was unclear. Thus far, the effects of Notch inhibitors on tumor cell proliferation and apoptosis in paired biopsies have not been assessed or reported in clinical trials. Moreover, expression of NICD, the Notch pathway signaling mediator, has not been reported in clinical studies and may serve as a PD marker and an efficacy indicator in the clinical setting. As such, evaluation of the NICD expression levels in tumor, endothelial and CSC cells at baseline and after Notch inhibition by immunohistochemistry could facilitate to monitor drug activities and particularly correlate with treatment efficacy (Gounder & Schwartz, 2012; Nguyen et al., 2012).
8. Conclusions and perspectives
Substantial evidence demonstrates the roles of Notch signaling in cancer, maintenance of CSCs, and angiogenesis in a context-dependent manner. As highlighted, single agent GSI activity has been observed in some patients with advanced or metastatic solid tumors, and unexpectedly no activity seen in T-ALL/T-LL from available clinical data. GSI clinical development was hampered by treatment-related toxicities especially gastrointestinal adverse events and by complex optimizing process of drug dosing schedules. The intermittent schedule with 3 days on and 4 days off in the 21- or 28-day cycle appears to be a solution to mitigate treatment-related toxicities without significantly compromising efficacy. Nonetheless, Notch signaling is a clinically proved druggable target and agents targeting the pathway could potentially evolve as successful cancer therapeutics. Especially, identification and validation of efficacy biomarkers should be prioritized during the process of early and late phases of clinical development. Future incorporation of the validated biomarkers into clinical trials may help identify the patient subpopulations who likely respond to and/or benefit from this class of drugs. In addition, back to bench research on characterization of Notch-driven disease(s) in the context of cancer environment among members of Notch pathway or in connection with other pathways could re-enforce identification of responding and/or benefiting patient population(s) in the clinic. Finally, a Notch inhibitor in combination with chemotherapy at the forefronts of both elimination/reduction of CSCs and treatment of bulk of tumors holds great potential in this arena of cancer drug development.
Acknowledgments
We thank Dr. Richard Swerdlow (PSI International, Inc.) for his support on the review article references. The Division of Cancer Treatment and Diagnosis, National Cancer Institute, National Institutes of Health, Bethesda, Maryland, United States in part supported this work.
Abbreviations
- ADAM
a disintegrin and metalloproteases
- CR
complete response
- CSCs
cancer stem cells
- DLL
Delta-like ligand
- DLT
dose limiting toxicity
- EDTA
ethylenediaminetetraacetic acid
- JAG
Jagged
- GBM
glioblastoma multiforme
- mAb
monoclonal antibody
- GSI
γ-secretase inhibitor
- MAML
mastermind-like
- MSFE
mammosphere-forming efficiency
- MTD
maximum tolerated dose
- NICD
Notch intracellular domain
- NSCLC
non-small cell lung cancer
- PD
pharmacodynamics
- PK
pharmacokinetics
- PR
partial response
- RECIST
response evaluation criteria in solid tumors
- SD
stable disease
- T-ALL
T-cell acute lymphoblastic leukemia
- T-LL
T-cell lymphoblastic lymphoma
- TACE
metalloproteinase tumor necrosis factor-α-converting enzyme
- TICs
tumor initiating cells
Footnotes
Conflict of interest statement
The authors declare no conflicts of interest.
References
- Albain K, Czerlanis C, Zlobin A, Covington K, Rajan P, Godellas C, et al. Modulation of cancer and stem cell biomarkers by the Notch Inhibitor MK-0752 added to endocrine therapy for early stage ER+ breast cancer. Cancer Res (Thirty-Fourth Annual CTRC-AACR San Antonio Breast Cancer Symposium) 2011:S1–S5. [Google Scholar]
- Androutsellis-Theotokis A, Leker RR, Soldner F, Hoeppner DJ, Ravin R, Poser SW, et al. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature. 2006;442:823–826. doi: 10.1038/nature04940. [DOI] [PubMed] [Google Scholar]
- Aster JC, Blacklow SC. Targeting the Notch pathway: Twists and turns on the road to rational therapeutics. J Clin Oncol. 2012;30:2418–2420. doi: 10.1200/JCO.2012.42.0992. [DOI] [PubMed] [Google Scholar]
- Chen Y, Fischer WH, Gill GN. Regulation of the ERBB-2 promoter by RBPJkappa and NOTCH. J Biol Chem. 1997;272:14110–14114. doi: 10.1074/jbc.272.22.14110. [DOI] [PubMed] [Google Scholar]
- Chen J, Kesari S, Rooney C, Strack PR, Chen J, Shen H, et al. Inhibition of notch signaling blocks growth of glioblastoma cell lines and tumor neurospheres. Genes Cancer. 2010;1:822–835. doi: 10.1177/1947601910383564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P, Zlobin A, Volgina V, Gottipati S, Osborne B, Simel EJ, et al. Notch-1 regulates NF-kappaB activity in hemopoietic progenitor cells. J Immunol. 2001;167:4458–4467. doi: 10.4049/jimmunol.167.8.4458. [DOI] [PubMed] [Google Scholar]
- Claxton S, Fruttiger M. Periodic Delta-like 4 expression in developing retinal arteries. Gene Expr Patterns. 2004;5:123–127. doi: 10.1016/j.modgep.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Collins BJ, Kleeberger W, Ball DW. Notch in lung development and lung cancer. Semin Cancer Biol. 2004;14:357–364. doi: 10.1016/j.semcancer.2004.04.015. [DOI] [PubMed] [Google Scholar]
- De La OJ, Murtaugh LC. Notch and Kras in pancreatic cancer: At the crossroads of mutation, differentiation and signaling. Cell Cycle. 2009;8:1860–1864. doi: 10.4161/cc.8.12.8744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, et al. A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature. 1999;398:518–522. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- Deangelo DJ, Stone RM, Silverman LB, Stock W, Attar EC, Fearen I, et al. A phase I clinical trial of the notch inhibitor MK-0752 in patients with T-cell acute lymphoblastic leukemia/lymphoma (T-ALL) and other leukemias. J Clin Oncol (ASCO Annual Meeting Proceedings) 2006:Abst. 6585. [Google Scholar]
- Diaz-Padilla I, Hirte H, Oza AM, Clarke BA, Cohen B, Reedjik M, et al. A phase Ib combination study of RO4929097, a gamma-secretase inhibitor, and temsirolimus in patients with advanced solid tumors. Invest New Drugs. 2013;31:1182–1191. doi: 10.1007/s10637-013-0001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dontu G, Jackson KW, McNicholas E, Kawamura MJ, Abdallah WM, Wicha MS. Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells. Breast Cancer Res. 2004;6:R605–R615. doi: 10.1186/bcr920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dontu G, Wicha MS. Survival of mammary stem cells in suspension culture: Implications for stem cell biology and neoplasia. J Mammary Gland Biol Neoplasia. 2005;10:75–86. doi: 10.1007/s10911-005-2542-5. [DOI] [PubMed] [Google Scholar]
- Dufraine J, Funahashi Y, Kitajewski J. Notch signaling regulates tumor angiogenesis by diverse mechanisms. Oncogene. 2008;27:5132–5137. doi: 10.1038/onc.2008.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egloff AM, Grandis JR. Molecular pathways: Context-dependent approaches to Notch targeting as cancer therapy. Clin Cancer Res. 2012;18:5188–5195. doi: 10.1158/1078-0432.CCR-11-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellisen LW, Bird J, West DC, Soreng AL, Reynolds TC, Smith SD, et al. TAN-1, the human homolog of the Drosophila notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell. 1991;66:649–661. doi: 10.1016/0092-8674(91)90111-b. [DOI] [PubMed] [Google Scholar]
- Epenetos A, Kousparou C, Deonarain M, Stylianou S. Development of cancer stem cells therapeutics. Ann Oncol (Proceedings of 37th EMSO Congress) 2012;23:160. (abstr 609) [Google Scholar]
- Fan X, Khaki L, Zhu TS, Soules ME, Talsma CE, Gul N, et al. NOTCH pathway blockade depletes CD133-positive glioblastoma cells and inhibits growth of tumor neurospheres and xenografts. Stem Cells. 2010;28:5–16. doi: 10.1002/stem.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnie G, Clarke RB. Mammary stem cells and breast cancer—role of Notch signalling. Stem Cell Rev. 2007;3:169–175. doi: 10.1007/s12015-007-0023-5. [DOI] [PubMed] [Google Scholar]
- Favre CJ, Mancuso M, Maas K, McLean JW, Baluk P, McDonald DM. Expression of genes involved in vascular development and angiogenesis in endothelial cells of adult lung. Am J Physiol Heart Circ Physiol. 2003;285:H1917–H1938. doi: 10.1152/ajpheart.00983.2002. [DOI] [PubMed] [Google Scholar]
- Ferte C, Soria JC, Penel N. Dose-levels and first signs of efficacy in contemporary oncology phase 1 clinical trials. PLoS One. 2011;6:e16633. doi: 10.1371/journal.pone.0016633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortini ME. Gamma-secretase-mediated proteolysis in cell-surface-receptor signalling. Nat Rev Mol Cell Biol. 2002;3:673–684. doi: 10.1038/nrm910. [DOI] [PubMed] [Google Scholar]
- Fouladi M, Stewart CF, Olson J, Wagner LM, Onar-Thomas A, Kocak M, et al. Phase I trial of MK-0752 in children with refractory CNS malignancies: A pediatric brain tumor consortium study. J Clin Oncol. 2011;29:3529–3534. doi: 10.1200/JCO.2011.35.7806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fre S, Huyghe M, Mourikis P, Robine S, Louvard D, Artavanis-Tsakonas S. Notch signals control the fate of immature progenitor cells in the intestine. Nature. 2005;435:964–968. doi: 10.1038/nature03589. [DOI] [PubMed] [Google Scholar]
- Funahashi Y, Hernandez SL, Das I, Ahn A, Huang J, Vorontchikhina M, et al. A notch1 ectodomain construct inhibits endothelial notch signaling, tumor growth, and angiogenesis. Cancer Res. 2008;68:4727–4735. doi: 10.1158/0008-5472.CAN-07-6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garces C, Ruiz-Hidalgo MJ, Font de Mora J, Park C, Miele L, Goldstein J, et al. Notch-1 controls the expression of fatty acid-activated transcription factors and is required for adipogenesis. J Biol Chem. 1997;272:29729–29734. doi: 10.1074/jbc.272.47.29729. [DOI] [PubMed] [Google Scholar]
- Geers C, Colin IM, Gerard AC. Delta-like 4/Notch pathway is differentially regulated in benign and malignant thyroid tissues. Thyroid. 2011;21:1323–1330. doi: 10.1089/thy.2010.0444. [DOI] [PubMed] [Google Scholar]
- Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gounder MM, Schwartz GK. Moving forward one Notch at a time. J Clin Oncol. 2012;30:2291–2293. doi: 10.1200/JCO.2012.42.3277. [DOI] [PubMed] [Google Scholar]
- Grudzien P, Lo S, Albain KS, Robinson P, Rajan P, Strack PR, et al. Inhibition of Notch signaling reduces the stem-like population of breast cancer cells and prevents mammosphere formation. Anticancer Res. 2010;30:3853–3867. [PubMed] [Google Scholar]
- Hassan KA, Wang L, Korkaya H, Chen G, Maillard I, Beer DG, et al. Notch pathway activity identifies cells with cancer stem cell-like properties and correlates with worse survival in lung adenocarcinoma. Clin Cancer Res. 2013;19:1972–1980. doi: 10.1158/1078-0432.CCR-12-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi I, Takatori S, Urano Y, Miyake Y, Takagi J, Sakata-Yanagimoto M, et al. Neutralization of the gamma-secretase activity by monoclonal antibody against extracellular domain of nicastrin. Oncogene. 2012;31:787–798. doi: 10.1038/onc.2011.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Luistro L, Carvajal D, Smith M, Nevins T, Yin X, et al. High tumor levels of IL6 and IL8 abrogate preclinical efficacy of the gamma-secretase inhibitor, RO4929097. Mol Oncol. 2011;5:292–301. doi: 10.1016/j.molonc.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrom M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- Hendrix MJ, Seftor RE, Seftor EA, Gruman LM, Lee LM, Nickoloff BJ, et al. Transendothelial function of human metastatic melanoma cells: Role of the microenvironment in cell-fate determination. Cancer Res. 2002;62:665–668. [PubMed] [Google Scholar]
- Hofmann JJ, Iruela-Arispe ML. Notch signaling in blood vessels: Who is talking to whom about what? Circ Res. 2007;100:1556–1568. doi: 10.1161/01.RES.0000266408.42939.e4. [DOI] [PubMed] [Google Scholar]
- Hu W, Lu C, Dong HH, Huang J, Shen DY, Stone RL, et al. Biological roles of the Delta family Notch ligand Dll4 in tumor and endothelial cells in ovarian cancer. Cancer Res. 2011;71:6030–6039. doi: 10.1158/0008-5472.CAN-10-2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimeno A, LoRusso P, Strother RM, Diamond JR, Plato L, Younger A, et al. Phase I study of REGN421 (R)/SAR153192, a fully-human delta-like ligand 4 (Dll4) monoclonal antibody (mAb), in patients with advanced solid tumors. J Clin Oncol. 2013;31(Suppl, Abst 2502) [Google Scholar]
- Jubb AM, Miller KD, Rugo HS, Harris AL, Chen D, Reimann JD, et al. Impact of exploratory biomarkers on the treatment effect of bevacizumab in metastatic breast cancer. Clin Cancer Res. 2011;17:372–381. doi: 10.1158/1078-0432.CCR-10-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamori M, Kumabe T, Watanabe M, Tominaga T. Anaplastic astrocytoma and anaplastic oligodendroglioma occurring 6 years after subtotal resection of a central neurocytoma. Case report. J Neurosurg. 2007;107:185–189. doi: 10.3171/JNS-07/07/0185. [DOI] [PubMed] [Google Scholar]
- Kao HY, Ordentlich P, Koyano-Nakagawa N, Tang Z, Downes M, Kintner CR, et al. A histone deacetylase corepressor complex regulates the Notch signal trans-duction pathway. Genes Dev. 1998;12:2269–2277. doi: 10.1101/gad.12.15.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopan R, Ilagan MX. Gamma-secretase: Proteasome of the membrane? Nat Rev Mol Cell Biol. 2004;5:499–504. doi: 10.1038/nrm1406. [DOI] [PubMed] [Google Scholar]
- Krop I, Demuth T, Guthrie T, Wen PY, Mason WP, Chinnaiyan P, et al. Phase I pharmacologic and pharmacodynamic study of the gamma secretase (Notch) inhibitor MK-0752 in adult patients with advanced solid tumors. J Clin Oncol. 2012;30:2307–2313. doi: 10.1200/JCO.2011.39.1540. [DOI] [PubMed] [Google Scholar]
- Kummar S, Chen A, Ji J, Zhang Y, Reid JM, Ames M, et al. Phase I study of PARP inhibitor ABT-888 in combination with topotecan in adults with refractory solid tumors and lymphomas. Cancer Res. 2011;71:5626–5634. doi: 10.1158/0008-5472.CAN-11-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummar S, Chen HX, Wright J, Holbeck S, Millin MD, Tomaszewski J, et al. Utilizing targeted cancer therapeutic agents in combination: Novel approaches and urgent requirements. Nat Rev Drug Discov. 2010;9:843–856. doi: 10.1038/nrd3216. [DOI] [PubMed] [Google Scholar]
- Leslie JD, Ariza-McNaughton L, Bermange AL, McAdow R, Johnson SL, Lewis J. Endothelial signalling by the Notch ligand Delta-like 4 restricts angiogenesis. Development. 2007;134:839–844. doi: 10.1242/dev.003244. [DOI] [PubMed] [Google Scholar]
- Li JL, Harris AL. Notch signaling from tumor cells: A new mechanism of angiogenesis. Cancer Cell. 2005;8:1–3. doi: 10.1016/j.ccr.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Li JL, Harris AL. Crosstalk of VEGF and Notch pathways in tumour angiogenesis: Therapeutic implications. Front Biosci. 2009;14:3094–3110. doi: 10.2741/3438. [DOI] [PubMed] [Google Scholar]
- Lobov IB, Renard RA, Papadopoulos N, Gale NW, Thurston G, Yancopoulos GD, et al. Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc Natl Acad Sci U S A. 2007;104:3219–3224. doi: 10.1073/pnas.0611206104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo Y, Filipovic A, Molyneux G, Periyasamy M, Giamas G, Hu Y, et al. Nicastrin regulates breast cancer stem cell properties and tumor growth in vitro and in vivo. Proc Natl Acad Sci U S A. 2012;109:16558–16563. doi: 10.1073/pnas.1206268109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoRusso PM, Canetta R, Wagner JA, Balogh EP, Nass SJ, Boerner SA, et al. Accelerating cancer therapy development: The importance of combination strategies and collaboration. Summary of an Institute of Medicine workshop. Clin Cancer Res. 2012;18:6101–6109. doi: 10.1158/1078-0432.CCR-12-2455. [DOI] [PubMed] [Google Scholar]
- Maetzel D, Denzel S, Mack B, Canis M, Went P, Benk M, et al. Nuclear signalling by tumour-associated antigen EpCAM. Nat Cell Biol. 2009;11:162–171. doi: 10.1038/ncb1824. [DOI] [PubMed] [Google Scholar]
- McKeage M, Kotasek D, Millward M, Markman B, Jameson M, Hidalgo M, et al. A Phase 1b study of demcizumab plus pemetrexed and carboplatin in patients with 1st line non-small cell lung cancer (NSCLC) Eur J Cancer. 2012;48:183–184. (Abst 598) [Google Scholar]
- Messersmith W, LoRusso P, Cleary J, Dasari A, Huang B, Shaik N, et al. A first-in-patient Phase I study of the novel gamma secretase inhibitor PF-03084014 in patients with advanced solid tumor malignancies. Eur J Cancer. 2012;48:Abst 588. [Google Scholar]
- Moellering RE, Cornejo M, Davis TN, Del Bianco C, Aster JC, Blacklow SC, et al. Direct inhibition of the NOTCH transcription factor complex. Nature. 2009;462:182–188. doi: 10.1038/nature08543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Tsuchiya K, Watanabe M. Crosstalk between Wnt and Notch signaling in intestinal epithelial cell fate decision. J Gastroenterol. 2007;42:705–710. doi: 10.1007/s00535-007-2087-z. [DOI] [PubMed] [Google Scholar]
- Nguyen D, Witter S, Tomaszewski JE, Ivy P, Doroshow JH, Yang SX. Notch-1 expression in human cancer cell lines and solid tumors by validated immunohistochemistry. Suppl Cancer Res. 2012;72 (Abstr 746) [Google Scholar]
- Nickoloff BJ, Osborne BA, Miele L. Notch signaling as a therapeutic target in cancer: A new approach to the development of cell fate modifying agents. Oncogene. 2003;22:6598–6608. doi: 10.1038/sj.onc.1206758. [DOI] [PubMed] [Google Scholar]
- Nickoloff BJ, Qin JZ, Chaturvedi V, Denning MF, Bonish B, Miele L. Jagged-1 mediated activation of notch signaling induces complete maturation of human keratinocytes through NF-kappaB and PPARgamma. Cell Death Differ. 2002;9:842–855. doi: 10.1038/sj.cdd.4401036. [DOI] [PubMed] [Google Scholar]
- Noguera-Troise I, Daly C, Papadopoulos NJ, Coetzee S, Boland P, Gale NW, et al. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature. 2006;444:1032–1037. doi: 10.1038/nature05355. [DOI] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–474. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- Olsauskas-Kuprys R, Zlobin A, Osipo C. Gamma secretase inhibitors of Notch signaling. OncoTargets Ther. 2013;6:943–955. doi: 10.2147/OTT.S33766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannuti A, Foreman K, Rizzo P, Osipo C, Golde T, Osborne B, et al. Targeting Notch to target cancer stem cells. Clin Cancer Res. 2010;16:3141–3152. doi: 10.1158/1078-0432.CCR-09-2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phng LK, Gerhardt H. Angiogenesis: A team effort coordinated by notch. Dev Cell. 2009;16:196–208. doi: 10.1016/j.devcel.2009.01.015. [DOI] [PubMed] [Google Scholar]
- Rampal R, Arboleda-Velasquez JF, Nita-Lazar A, Kosik KS, Haltiwanger RS. Highly conserved O-fucose sites have distinct effects on Notch1 function. J Biol Chem. 2005;280:32133–32140. doi: 10.1074/jbc.M506104200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand MD, Grimm LM, Artavanis-Tsakonas S, Patriub V, Blacklow SC, Sklar J, et al. Calcium depletion dissociates and activates heterodimeric notch receptors. Mol Cell Biol. 2000;20:1825–1835. doi: 10.1128/mcb.20.5.1825-1835.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan P, Weaver KL, Capobianco AJ. Notch signalling in solid tumours: A little bit of everything but not all the time. Nat Rev Cancer. 2011;11:338–351. doi: 10.1038/nrc3035. [DOI] [PubMed] [Google Scholar]
- Rangarajan A, Talora C, Okuyama R, Nicolas M, Mammucari C, Oh H, et al. Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation. EMBO J. 2001;20:3427–3436. doi: 10.1093/emboj/20.13.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Real PJ, Tosello V, Palomero T, Castillo M, Hernando E, de Stanchina E, et al. Gamma-secretase inhibitors reverse glucocorticoid resistance in T cell acute lymphoblastic leukemia. Nat Med. 2009;15:50–58. doi: 10.1038/nm.1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reedijk M, Odorcic S, Chang L, Zhang H, Miller N, McCready DR, et al. High-level coexpression of JAG1 and NOTCH1 is observed in human breast cancer and is associated with poor overall survival. Cancer Res. 2005;65:8530–8537. doi: 10.1158/0008-5472.CAN-05-1069. [DOI] [PubMed] [Google Scholar]
- Reynolds ND, Lukacs NW, Long N, Karpus WJ. Delta-like ligand 4 regulates central nervous system T cell accumulation during experimental autoimmune encephalomyelitis. J Immunol. 2011;187:2803–2813. doi: 10.4049/jimmunol.1100160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds TC, Smith SD, Sklar J. Analysis of DNA surrounding the breakpoints of chromosomal translocations involving the beta T cell receptor gene in human lymphoblastic neoplasms. Cell. 1987;50:107–117. doi: 10.1016/0092-8674(87)90667-2. [DOI] [PubMed] [Google Scholar]
- Richter S, Bedard PL, Chen EX, Clarke BA, Tran B, Hotte SJ, et al. A phase I study of the oral gamma secretase inhibitor R04929097 in combination with gemcitabine in patients with advanced solid tumors (PHL-078/CTEP 8575) Invest New Drugs. 2013 doi: 10.1007/s10637-013-9965-4. http://dx.doi.org/10.1007/s10637-013-9965-4 (in press) [DOI] [PMC free article] [PubMed]
- Ridgway J, Zhang G, Wu Y, Stawicki S, Liang WC, Chanthery Y, et al. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006;444:1083–1087. doi: 10.1038/nature05313. [DOI] [PubMed] [Google Scholar]
- Ronchini C, Capobianco AJ. Induction of cyclin D1 transcription and CDK2 activity by Notch(ic): Implication for cell cycle disruption in transformation by Notch(ic) Mol Cell Biol. 2001;21:5925–5934. doi: 10.1128/MCB.21.17.5925-5934.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rota R, Ciarapica R, Miele L, Locatelli F. Notch signaling in pediatric soft tissue sarcomas. BMC Med. 2012;10:141. doi: 10.1186/1741-7015-10-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahebjam S, Bedard PL, Castonguay V, Chen Z, Reedijk M, Liu G, et al. A phase I study of the combination of ro4929097 and cediranib in patients with advanced solid tumours (PJC-004/NCI 8503) Br J Cancer. 2013;109:943–949. doi: 10.1038/bjc.2013.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samon JB, Castillo-Martin M, Hadler M, Ambesi-Impiobato A, Paietta E, Racevskis J, et al. Preclinical analysis of the gamma-secretase inhibitor PF-03084014 in combination with glucocorticoids in T-cell acute lymphoblastic leukemia. Mol Cancer Ther. 2012;11:1565–1575. doi: 10.1158/1535-7163.MCT-11-0938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santagata S, Demichelis F, Riva A, Varambally S, Hofer MD, Kutok JL, et al. JAGGED1 expression is associated with prostate cancer metastasis and recurrence. Cancer Res. 2004;64:6854–6857. doi: 10.1158/0008-5472.CAN-04-2500. [DOI] [PubMed] [Google Scholar]
- Sarmento LM, Huang H, Limon A, Gordon W, Fernandes J, Tavares MJ, et al. Notch1 modulates timing of G1-S progression by inducing SKP2 transcription and p27 Kip1 degradation. J Exp Med. 2005;202:157–168. doi: 10.1084/jem.20050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott AF, Landis MD, Dontu G, Griffith KA, Layman RM, Krop I, et al. Preclinical and clinical studies of gamma secretase inhibitors with docetaxel on human breast tumors. Clin Cancer Res. 2013;19:1512–1524. doi: 10.1158/1078-0432.CCR-11-3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreck KC, Taylor P, Marchionni L, Gopalakrishnan V, Bar EE, Gaiano N, et al. The Notch target Hes1 directly modulates Gli1 expression and Hedgehog signaling: A potential mechanism of therapeutic resistance. Clin Cancer Res. 2010;16:6060–6070. doi: 10.1158/1078-0432.CCR-10-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicinska E, Aifantis I, Le Cam L, Swat W, Borowski C, Yu Q, et al. Requirement for cyclin D3 in lymphocyte development and T cell leukemias. Cancer Cell. 2003;4:451–461. doi: 10.1016/s1535-6108(03)00301-5. [DOI] [PubMed] [Google Scholar]
- Siekmann AF, Lawson ND. Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature. 2007;445:781–784. doi: 10.1038/nature05577. [DOI] [PubMed] [Google Scholar]
- Smith DC, Chugh R, Patnaik A, Papadopoulos K, Chambers G, Thorpe V, et al. A first-in-human phase I study to evaluate the fully human monoclonal antibody OMP-59R5 (anti-Notch2/3) administered intravenously to patients with advanced solid tumors. Eur J Cancer. 2012;48:3319–3514. (Abst 28) [Google Scholar]
- Stylianou S, Clarke RB, Brennan K. Aberrant activation of notch signaling in human breast cancer. Cancer Res. 2006;66:1517–1525. doi: 10.1158/0008-5472.CAN-05-3054. [DOI] [PubMed] [Google Scholar]
- Suchting S, Freitas C, le Noble F, Benedito R, Breant C, Duarte A, et al. The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc Natl Acad Sci U S A. 2007;104:3225–3230. doi: 10.1073/pnas.0611177104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebe N, Harris PJ, Warren RQ, Ivy SP. Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways. Nat Rev Clin Oncol. 2011;8:97–106. doi: 10.1038/nrclinonc.2010.196. [DOI] [PubMed] [Google Scholar]
- Timmerman LA, Grego-Bessa J, Raya A, Bertran E, Perez-Pomares JM, Diez J, et al. Notch promotes epithelial–mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev. 2004;18:99–115. doi: 10.1101/gad.276304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolcher AW, Messersmith WA, Mikulski SM, Papadopoulos KP, Kwak EL, Gibbon DG, et al. Phase I study of RO4929097, a gamma secretase inhibitor of Notch signaling, in patients with refractory metastatic or locally advanced solid tumors. J Clin Oncol. 2012;30:2348–2353. doi: 10.1200/JCO.2011.36.8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, et al. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- van Nes J, Chan A, van Groningen T, van Sluis P, Koster J, Versteeg R. A NOTCH3 transcriptional module induces cell motility in neuroblastoma. Clin Cancer Res. 2013;19:3485–3494. doi: 10.1158/1078-0432.CCR-12-3021. [DOI] [PubMed] [Google Scholar]
- Vasko V, Espinosa AV, Scouten W, He H, Auer H, Liyanarachchi S, et al. Gene expression and functional evidence of epithelial-to-mesenchymal transition in papillary thyroid carcinoma invasion. Proc Natl Acad Sci U S A. 2007;104:2803–2808. doi: 10.1073/pnas.0610733104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa N, Walker L, Lindsell CE, Gasson J, Iruela-Arispe ML, Weinmaster G. Vascular expression of Notch pathway receptors and ligands is restricted to arterial vessels. Mech Dev. 2001;108:161–164. doi: 10.1016/s0925-4773(01)00469-5. [DOI] [PubMed] [Google Scholar]
- Wang H, Chen Y, Fernandez-Del Castillo C, Yilmaz O, Deshpande V. Heterogeneity in signaling pathways of gastroenteropancreatic neuroendocrine tumors: A critical look at notch signaling pathway. Mod Pathol. 2013;26:139–147. doi: 10.1038/modpathol.2012.143. [DOI] [PubMed] [Google Scholar]
- Wang Z, Li Y, Kong D, Sarkar FH. The role of Notch signaling pathway in epithelial–mesenchymal transition (EMT) during development and tumor aggressiveness. Curr Drug Targets. 2010;11:745–751. doi: 10.2174/138945010791170860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wakeman TP, Lathia JD, Hjelmeland AB, Wang XF, White RR, et al. Notch promotes radioresistance of glioma stem cells. Stem Cells. 2010;28:17–28. doi: 10.1002/stem.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei P, Walls M, Qiu M, Ding R, Denlinger RH, Wong A, et al. Evaluation of selective gamma-secretase inhibitor PF-03084014 for its antitumor efficacy and gastrointestinal safety to guide optimal clinical trial design. Mol Cancer Ther. 2010;9:1618–1628. doi: 10.1158/1535-7163.MCT-10-0034. [DOI] [PubMed] [Google Scholar]
- Weijzen S, Rizzo P, Braid M, Vaishnav R, Jonkheer SM, Zlobin A, et al. Activation of Notch-1 signaling maintains the neoplastic phenotype in human Ras-transformed cells. Nat Med. 2002;8:979–986. doi: 10.1038/nm754. [DOI] [PubMed] [Google Scholar]
- Weng AP, Ferrando AA, Lee W, Morris JPT, Silverman LB, Sanchez-Irizarry C, et al. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science. 2004;306:269–271. doi: 10.1126/science.1102160. [DOI] [PubMed] [Google Scholar]
- Weng AP, Millholland JM, Yashiro-Ohtani Y, Arcangeli ML, Lau A, Wai C, et al. c-Myc is an important direct target of Notch1 in T-cell acute lymphoblastic leukemia/lymphoma. Genes Dev. 2006;20:2096–2109. doi: 10.1101/gad.1450406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Cain-Hom C, Choy L, Hagenbeek TJ, de Leon GP, Chen Y, et al. Therapeutic antibody targeting of individual Notch receptors. Nature. 2010;464:1052–1057. doi: 10.1038/nature08878. [DOI] [PubMed] [Google Scholar]
- Yamashita AS, Geraldo MV, Fuziwara CS, Kulcsar MA, Friguglietti CU, da Costa RB, et al. Notch pathway is activated by MAPK signaling and influences papillary thyroid cancer proliferation. Transl Oncol. 2013;6:197–205. doi: 10.1593/tlo.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan M, Callahan CA, Beyer JC, Allamneni KP, Zhang G, Ridgway JB, et al. Chronic DLL4 blockade induces vascular neoplasms. Nature. 2010;463:E6–E7. doi: 10.1038/nature08751. [DOI] [PubMed] [Google Scholar]
- Zagouras P, Stifani S, Blaumueller CM, Carcangiu ML, Artavanis-Tsakonas S. Alterations in Notch signaling in neoplastic lesions of the human cervix. Proc Natl Acad Sci U S A. 1995;92:6414–6418. doi: 10.1073/pnas.92.14.6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CC, Pavlicek A, Zhang Q, Lira ME, Painter CL, Yan Z, et al. Biomarker and pharmacologic evaluation of the gamma-secretase inhibitor PF-03084014 in breast cancer models. Clin Cancer Res. 2012;18:5008–5019. doi: 10.1158/1078-0432.CCR-12-1379. [DOI] [PubMed] [Google Scholar]
- Zhang CC, Yan Z, Zong Q, Fang DD, Painter C, Zhang Q, et al. Synergistic effect of the gamma-secretase inhibitor PF-03084014 and docetaxel in breast cancer models. Stem Cells Transl Med. 2013;2:233–242. doi: 10.5966/sctm.2012-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]