Abstract
The isolation of heat-stable enterotoxin (STa) from Escherichia coli and cholera toxin from Vibrio cholerae has increased our knowledge of specific mechanisms of action that could be used as pharmacological tools to understand the guanylyl cyclase-C and the adenylyl cyclase enzymatic systems. These discoveries have also been instrumental in increasing our understanding of the basic mechanisms that control the electrolyte and water balance in the gut, kidney, and urinary tracts under normal conditions and in disease. Herein, we review the evolution of genes of the guanylin family and STa genes from bacteria to fish and mammals. We also describe new developments and perspectives regarding these novel bacterial compounds and peptide hormones that act in electrolyte and water balance. The available data point toward new therapeutic perspectives for pathological features such as functional gastrointestinal disorders associated with constipation, colorectal cancer, cystic fibrosis, asthma, hypertension, gastrointestinal barrier function damage associated with enteropathy, enteric infection, malnutrition, satiety, food preferences, obesity, metabolic syndrome, and effects on behavior and brain disorders such as attention deficit, hyperactivity disorder, and schizophrenia.
Keywords: Heat-stable enterotoxin, Guanylin, Guanylyl cyclase, Secretory diarrhea, Kidney function, Electrolyte and water balance
Introduction
The heat-stable enterotoxin (Sta) from Escherichia coli is the main agent promoting traveler's diarrhea and is one of the main enterotoxins responsible for diarrhea, dehydration, and death during the first two years of life in many parts of the developing world (1). STa is a peptide that binds to and interacts with two membrane receptors, guanylyl cyclase-C (GC-C) and opossum kidney GC, that were initially described in the intestine. Consideration of the kidney as a large nephron with common ionic transport systems led us to investigate the effects of STa on the kidney in 1983, which initially involved evaluation of the effects of STa in an isolated perfused rat kidney model (2). We had previously performed pilot experiments with an impure STa preparation that resulted in a very potent natriuretic effect in this model (3). This early observation led to the view that an endogenous mediator couples with this orphan receptor. At the time, we also observed that cyclic-GMP (cGMP) was involved in this process (2,4). In 1991, Fonteles et al. (5) described the first effect of STa on kidney function using pure STa toxin. By 1996, we had obtained enough pure guanylin and uroguanylin donated by Professor Leonard Forte's laboratory (Missouri University, Columbia, MO, USA) to test these new STa-like peptides in the isolated rat kidney. Guanylin was isolated by Currie et al. in 1992 (6), 9 years after the first report and when a full paper was published on the natriuretic effects of STa on the isolated perfused kidney (2,4,7).
Evolutionary origin of E. coli
The origin and evolution of E. coli are important in order to define which developed first, the bacterial virulence factor or the evolutionary peptides and their receptors present in the GC system in several other species. For instance, E. coli and Salmonella enterica have a common ancestor from about 100 million years ago (8). E. coli is similar to the genomic and phenotypic species of Yersinia pestis and Vibrio cholerae; however, they have a distant related genus. The genomes of these bacteria have a high degree of plasticity, i.e., they contain several segments that were acquired from different species. This conclusion is based on comparison of non-pathogenic E. coli (K-12 strain) to strains from different laboratories (8). Based on these genetic segments, which encode different virulence factors, E. coli is considered to have at least eight pathological variants that can be associated with human disease, including the enterotoxigenic E. coli (ETEC), which is within the scope of this review. Evolution has conserved the genetic backbone of E. coli with dispersion and variability of virulence genes that can be transmitted between bacteria via the transfer of genetic elements such as plasmids, bacteriophages, and pathogenicity islands. These genetic components can be detected by using DNA sequences that differ from the host bacterial genome by the presence of insertion sequences or sequences flanking the pathogenicity islands. In addition to the vertical acquisition process of these virulence genes by bacteria, the horizontal acquisition implies several mechanisms such as conjugation (via fimbria or pilus links), transduction (phage), and transformation (DNA acquisition from the external medium). Since the genes for endogenous peptides, acting via the GC system, exist in early vertebrate evolution in teleost fish, Anguilla anguilla, birds and mammals, we can conclude that they have been well conserved for about 400 million years from fish to man. As mentioned earlier, it is possible that these genes were acquired by relatively new evolutionary E. coli and other bacterial species that were in close contact with the gut environments of these early vertebrate hosts via transmissible genetic elements throughout these acquisition processes (9).
The survival of these organisms in water, an adaptation developed during evolutionary steps by these bacteria using their virulence factors, is quite impressive. V. cholerae, for example, is known for its capacity to survive in aquatic environments, including sea water, and in the presence of planktons, which allow it to be a reservoir of both endemic and epidemic cholera (10). Recently, ETEC strains were tested for their capacity to survive in sea and fresh water (11). Incubation of these strains in sea water and freshwater showed that genes encoding ETEC (STa, heat-stable and LT, heat-labile enterotoxins) and colonization factors (CS7 and CS17; gapA and 16S RNA, respectively) were expressed over a period of three months in both aqueous media. The conclusion from this study was that ETEC bacteria can also survive for long periods of time in an aquatic environment and have the potential to be infectious and cause most of the endemic enteric diseases seen in developing countries.
Enteric infections with ETEC
The clinical manifestations of ETEC infections are classically associated with watery diarrhea and are particularly important in young children and travelers to endemic areas in developing countries (12,13). Diarrhea caused by different ETEC pathogens is similar to clinical cholera, and may range from mild to life threatening, especially in children, elderly patients, and those with immunocompromised systems. These patients are prone to dehydration, electrolyte imbalance, hypokalemia, acidosis, and malnutrition. Other signs and symptoms include headache, fever, nausea, vomiting, malaise, abdominal cramping, and anorexia. In children, the most common clinical signs and symptoms are vomiting (90%), fever (75%), and mucous stools (60%) (13). The disease is self-limited from 1 to 5 days and rarely extends beyond 10 days (14). ETEC strain infections that produce STa enterotoxin alone or STa plus LT enterotoxins tend to be more severe than those caused by ETEC strains producing LT alone (13).
Major virulence factors and molecular mechanisms of E. coli
The pathogenesis of ETEC infection starts with the ingestion of the organism from contaminated water or food. The balance between host defense and the virulence factors of the infecting organism dictates the potential for symptomatic infection. The bacteria colonize the intestine via the expression of surface adhesins, most of which are known as colonization factors (CFs). About 22 CFs are associated with the adherence of ETEC to the gut mucosa (15). CFs are defined by their structures and their antigenic diversity. The most studied fimbrial structures to date are the CFA/I, fibrillar and helical structures ranging in length from 1 to 20 μm. These CFA/I consist of about 1000 copies of the major fimbrial subunit CfaB and a few copies of CfaE and are located at the tip of the distal ending. The receptors for these CFs are not well known, but some bind to glycoprotein conjugates on the surface of gut mucosa (16).
One of the essential virulence factors found among the phylogenetic diversity of ETEC strains is the acquisition of genes encoding enterotoxins STa and/or LT. Several researchers agree that many other virulence factors may be essential for colonization and successful targeting of enterotoxins to host cells, resulting in symptomatic infection.
ETEC strains produce LT that is biochemically and immunologically related to cholera toxin. This toxin is a heterohexameric molecule with five B subunits and a single A subunit. The A subunit is composed of two domains linked by a disulfide bridge called A1, the active toxin molecule, and A2, a helical structure responsible for anchoring the A subunit to the B subunit (17) (Figure 1). The B subunit binds to the monosialoganglioside GM1 receptor on the host cell surface and triggers endocytosis of the holotoxin. The A1 domain of the A subunit then translocates across the intracellular membrane to interact with ADP-ribosylating factors to the ADP-ribosylate Gsα subunit of a guanine nucleotide protein (G-protein), which inhibits the GTPase (guanosine triphosphate enzyme) activity and leads to continued activation of adenylyl cyclase (AC) (18) (Figure 1). The AC transforms ATP to cAMP, and the accumulation of intracellular cAMP activates protein kinase A (PKA), which inhibits Na+ absorption through sodium-hydrogen (Na+/H+) exchangers (NHE2 and NHE3) and stimulates Cl- secretion by phosphorylation of the cystic fibrosis transmembrane regulator (CFTR) (19). These electrolyte changes cause net loss of water through osmotic diarrhea.
Figure 1. Mechanisms of action of the cholera toxin (CT), heat-labile (LT) and heat-stable enterotoxin (STa) from Escherichia coli, and endogenous peptide ligands of guanylyl cyclase C (GC-C). CT or LT binds to the monosialoganglioside GM1 receptor at the host mucosa surface and triggers endocytosis of the holotoxin. The A1 domain of the A subunit is transported through the Golgi complex and endoplasmic reticulum to activate the Gsα subunit of G-protein. This A1 domain interacts with ADP-ribosylating factors to ADP-ribosylate this Gsα subunit in order to activate G-protein and consequently adenylyl cyclase (AC). The AC cleaves ATP to cAMP and subsequently activates protein kinase A, which inhibits NaCl absorption (NHE transporters) and increases chloride secretion through the cystic fibrosis transmembrane regulator (CFTR). Peptide ligands of the extracellular domain of GC-C activate the intracellular catalytic domain of GC-C resulting in cGMP formation, which activates several pathways: a) inhibition of the NHE3 transporter, which decreases NaCl absorption; b) activation of CFTR, which leads to secretion of chloride; and c) increased calcium influx.
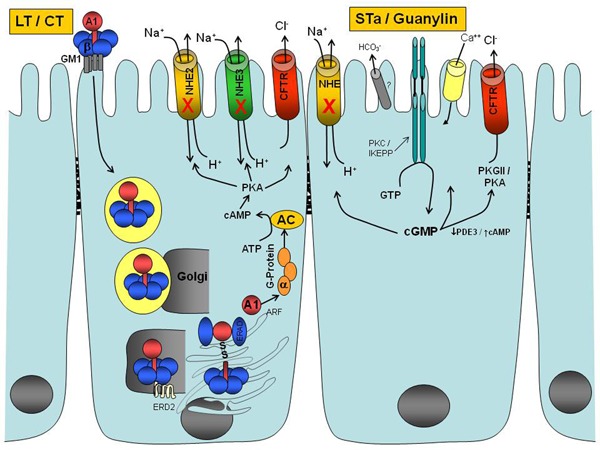
STa enterotoxins are small 18-19 amino acid peptides with three cysteine-based disulfide bonds, encoded by plasmid or chromosomal DNA and secreted by ETEC strains. STa enterotoxins bind to the extracellular domain of GC-C present in the brush border membranes of the intestinal epithelium and renal proximal and distal tubules, as well as other cells and tissues (2,4,6,20-22). These interactions result in the activation of the intracellular catalytic domain of GC-C, which leads to the formation of cGMP from GTP (Figure 1). This intracellular message in turn activates cGMP-dependent protein kinase II (PKG), leading to CFTR phosphorylation (20,21,23,24) (Figure 1). CFTR belongs to the ATP-binding cassette family called the ABC transporter family and works as a Cl- channel by controlling the passive transport of Cl- in either direction across the membrane of the enterocytes (24). cGMP also reduces sodium and chloride absorption via the NHE and is coupled to the chloride/anion exchanger (25). cGMP also inhibits phosphodiesterase (PDE3), a cGMP-regulated cAMP-hydrolyzing phosphodiesterase, leading to a decrease in cAMP hydrolysis, local accumulation of cAMP, and subsequent activation of PKA (26,27). High concentrations of cGMP can also directly activate PKA (28). Phosphorylation of the CFTR leads to increased Cl- secretion and decreased NaCl absorption, resulting in net loss of water through osmotic diarrhea. STa can also relocate from sub-apical vesicles to the apical membrane of CFTR through GC-C stimulation, promoting increased expression of CFTR on the surface of the enterocytes (29). The relocation of CFTR induced by STa is dependent on the intact microtubular network, PKA, and PKG. Experiments with GC-C knockout mice have suggested that there is another receptor for STa, guanylin, and uroguanylin, which might stimulate duodenal HCO- secretion in a CFTR-independent mechanism (30). As mentioned earlier, the discovery of these toxins and their common mechanisms of action has led directly to the isolation and identification of the endogenous STa-like peptides called guanylin and uroguanylin (6,31).
Genome and genes of STa enterotoxins from bacteria and the endogenous mammalian peptide ligands of GC-C
Two genes in the human and mouse genomes, located on chromosomes 1 and 4, express guanylin and uroguanylin, respectively (32). These genes encode preproguanylin (115 amino acids in rats) and preprouroguanylin (109 amino acids in opossums) and contain a signal peptide, propeptide, and an active peptide (see Table 1 for active peptide amino acid sequences). Enteric bacteria have genes encoding different polypeptides called preproST that were found either in plasmids or in chromosomal DNA (33). The ETEC preproST gene encodes a 72-amino acid polypeptide with a signal peptide, propeptide and active peptide amino acid sequence (Table 1). There is a substantial possibility, as described earlier, that bacteria acquired this gene from vertebrates through their continued contact with the gut environment. Recent studies on two ETEC genomes revealed that approximately 4% of the chromosomes are composed of mobile genetic elements (34). Plasmid DNA sequence studies also showed approximately 24% of mobile elements with a few defined insert sequence elements in either the plasmid or chromosome, allowing the integration and resolution of genes and the observed plasticity of this ETEC pathotype (35). In addition, the comparison of ETEC genomes with various pathotypes showed that these species have the highest number of new genes per strain. Many plasmids have been identified in ETEC genome studies (36). These data further suggest that ETEC genomes have a very dynamic flux of genes, including the preproST genes. Transcriptional regulation of STa/LT genes is likely to occur in response to environmental signals in order to co-regulate the expression of virulence factors, including these toxins (36).
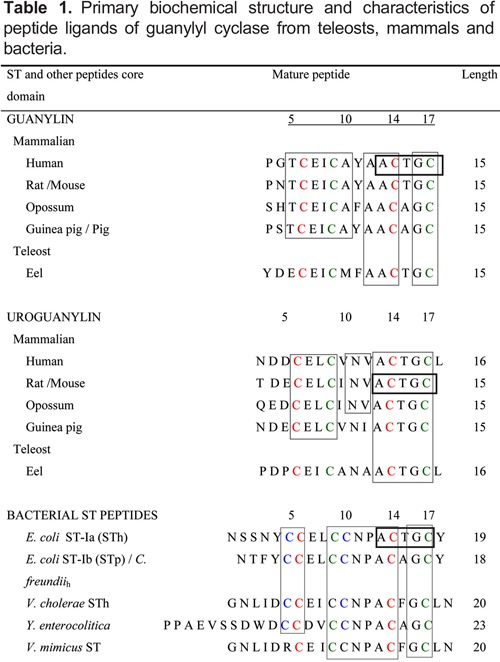
Protein structure and structure-activity of teleost, mammalian, and bacterial peptide ligands of GC
The structure-activity of the related peptide amino acid sequence of ligands to GC from teleost bacteria and mammals is presented in Table 1. The purpose of choosing an earlier evolutionary peptide from teleost eels, mammals, and bacteria was to concentrate on the major evolutionary homology and structure-activity of these important peptides for endogenous functions and diseases associated with the human species. The discovery of renoguanylin, uroguanylin, and guanylin genes in the European teleost eel, A. anguilla, gave us the first clue that these peptides were already in the genome of the early vertebrates probably around 400 million years ago (9,37). The STa enterotoxin from E. coli was isolated and purified first, followed by the endogenous mammalian peptides and their active peptide structures. Sequences of STa obtained from these studies are shown in Table 1 (38-40).
The guanylin and uroguanylin from teleost eels have 15-16 amino acids and four cysteines, which formed two disulfide bonds that are important for their biological activity. This species already had the peptide motif with 5 amino acids, ACTGC, found at the C-termini in all peptide ligands of GC-C, with little variability (Table 1). The GC amino acid at the end of the STa peptide is required in all peptide ligands of GC. It seems clear now that the disulfide bonds, or at least one of them, and this peptide motif as well as the highly conserved dipeptide at the C-terminal were preserved during evolution to maintain the biological activity of these molecules. The size of the active peptides is also important for structure-activity since the core of the 13-amino acid peptides of STa, from the first to the last cysteine, needs to be preserved (41). In addition, the amino acid residues at positions 2-3 at the amino terminal side in both guanylin and uroguanylin are necessary for full activity (42), although the variability of their amino acid composition contributes little to the biological activities of these molecules. The three disulfide bonds found in most bacterial STa peptides represent a further step in the evolution of these molecules since this structure provides a much higher potency and efficacy when compared to other mammalian peptides (6,7,31,43,44). Another interesting aspect of uroguanylin and STa peptides is the presence of an internal asparagine, which confers resistance to the proteolytic action on, and inactivation of these molecules by endoproteases in the kidney and digestive tract, while guanylin is rapidly hydrolyzed and inactivated by these proteases (44).
Gene, protein structure, regulation, and signal transduction of GC-C
GC-C is a unique protein with several domains, including the target receptor for STa peptides and guanylin hormones, as well as the enzymatic activity domain that cleaves GTP to cGMP. Early studies have demonstrated that GC-C activity was present in the apical region of intestinal cells in close association with brush border enzymes such as sucrose, isomaltase, and alkaline phosphatase (45). Although this protein was at first thought to be intestinal tissue specific, there is evidence of STa-specific binding sites in extraintestinal tissues such as the kidney, airway epithelium, perinatal liver, stomach, brain, adrenal glands, epididymis, and corpora carvenosa (46,47). GC-C is conserved evolutionarily in several species, including fish, teleost, birds, and mammals (48), and this is consistent with the functional activities associated with STa peptides and related hormones, as described in the present report. The cDNA derived from human GC-C present in colonic epithelial cells is processed to a 1073-amino acid polypeptide with an N-terminal signal sequence of 23 residues, responsible for the endoplasmic reticulum transport and post-translational proteolysis needed to generate a mature polypeptide of 1050 residues with a theoretical molecular mass of 120 kDa (49,50). In order to maintain the salt and water balance in most of these epithelia, this protein must be located on the apical or luminal side of the membrane. Therefore, the GC-C molecule has a unique region of 11 highly conserved amino acids in the carboxyl terminus of the receptor, which targets it to an unpolarized reporter protein of the apical membrane (51). The GC-C is expressed along the crypt to villus axis in the small intestine with higher expression of GC-C in the crypt of the colonic mucosa compared to the crypt of the small intestine (52).
The holoprotein is a homodimeric protein with a multidomain structure consisting of a) N-terminal extracellular domain (ECD), b) single helical transmembrane region, c) juxtamembrane domain, d) kinase homology domain (KHD), e) linker region, f) GC domain (GCD), and g) C-terminal domain (CTD) (53). The human ECD of GC-C has 70-85% sequence identity with other species, and the receptor side of the molecule binds with higher affinity to the STa bacterial peptide (Kd=0.1 nM) compared to uroguanylin (Kd=1 nM) and guanylin (Kd=10 nM) (54). The mechanism by which STa ligands rearrange the ECD to transmit the signal to the intracellular domains is still unclear. The KHD of the GC-C proteins is significantly similar to protein tyrosine kinase, suggesting that it is an important part of the protein that transmits and regulates the signal of ligand peptides from the ECD (48). The linker region in the GC-C appears to have an important role in repressing GCD activity, which permits ligand peptides to mediate activation of the cyclase domain (55). Based on crystal structure studies of the GC Cya2 from the cyanobacterium Syncheocystis PCC6803 (56) and green alga Chlamydomonas reinhartii (57), it is possible to conclude that the GC-C protein binds to two GTP molecules per dimer. There is a similarity between the GC-C GCD and the polymerases and AC that catalyze analogous reactions. The GCD may act via the aspartic acid residues that coordinate the attack of the metal co-factor ions against the 3′-hydroxyl group of the ribose unit on the α-phosphate of a nucleotide 5′-triphosphate, resulting in the formation of cGMP from the GTP substrate. The carboxyl terminal domain seems to be important for the binding of GC-C to the cytoskeleton of the cell and deletion of this domain results in the loss of ligand-mediated activation of the GC-C protein (58).
Discovery of the natriuretic and diuretic effects of STa enterotoxin from E. coli on renal tubule physiology
Over the last four decades, research on bacterial toxins including cholera toxin from V. cholerae and STa enterotoxin from E. coli has been instrumental in increasing our understanding of the importance of electrolyte and water balance in normal intestine and kidney function (20,21,59). The best example was the discovery of endogenous mammalian ligands of GC, which resulted from early independent studies on STa enterotoxins of ETEC (2-6,22). Recent studies on the virulence factors from enteric bacterial infection have highlighted the complexity of host-microorganism interactions as well as the interaction with intestinal function, especially regarding the mechanisms involved in electrolyte and water transport, epithelial integrity and innate immunity (60). Recent advances in the determination of host and microorganism genomes, as well as the metagenome and metabolome studies of the microbiome in the gut, are important to the understanding of the scientific basis of the complex interaction between hosts and microorganisms (61).
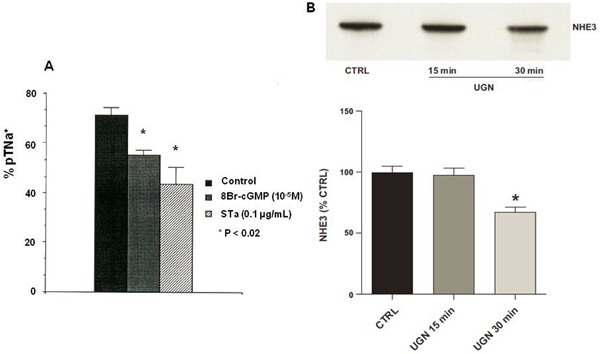
Immediately after the discovery of the mechanism of the effect of STa on ion secretion in the small intestine (20,21), Lima et al. (4) obtained and published information regarding the early effects of this toxin on the decrease of sodium transport in the proximal and distal renal tubules, using a rat kidney perfusion ex vivo model. At that point, they postulated that STa would activate renal GC since 8-bromo-cGMP also mimicked the effects of STa on renal tubules in the perfused ex vivo kidney (Figure 2). At that time, only a few studies by Friedler et al. (62) and Kurokawa et al. (63) had investigated cholera toxin as a pharmacological tool for studying electrolyte as well as phosphate and water transport in canine and rat kidneys. Although these cholera toxin studies were performed many years earlier, an endogenous cholera toxin-like hormone that can act via the monosialoganglioside GM1 receptor, which is also linked to AC activation and is present in the intestine and kidney, had not yet been identified (2,62-64). Since the gut and the nephron have similar electrolyte and water transport systems, it can be expected that the GC system is also present in kidney tissue. Evidence for this postulated mechanism was slow in coming since one of the close collaborators on the STa mechanism had shown a couple of years earlier that the STa effect could not be demonstrated through the activation of GC in kidney tissue slices when incubated with STa (65). However, these proximal renal tubule natriuresis and diuresis observations were validated when Forte et al. (22) clearly demonstrated the presence of the STa receptor and the cGMP signaling mechanism for E. coli enterotoxin in the opossum kidney. Lima et al. (4) further perfused rat kidney with a new purified STa and consistently showed decreased sodium and potassium transportation at proximal and distal renal tubule sites resulting in increased diuresis. In the same report, they clearly raised the point that an unknown yet endogenous hormone could be coupled to the GC system in the kidney. An independent study by Currie et al. (6) published almost at the same time reported an endogenous activator of intestinal GC that had a peptide structure similar to that of the STa enterotoxin; they named this molecule as guanylin. Since then, these three independent groups have worked together and published the first results on the effects of guanylin and uroguanylin on the isolated rat kidney that confirmed the previous results with STa enterotoxin from E. coli.
Figure 2. Effect of heat-stable enterotoxin (STa) from Escherichia coli and endogenous uroguanylin (UGN) mammalian peptide ligand of guanylyl cyclase C on net sodium balance in the kidney. Panel A shows the decreased effect of STa and 8Br-cGMP on proximal sodium tubule transport (pTNa+) in isolated perfused rat kidney (reproduced from Ref. 4, with permission). Panel B also shows the reduced effect of UGN on sodium proton transporter NHE3 in renal proximal tubules, as well as the surface NHE3 expression in proximal tubule cells in culture (reproduced from Ref. 69, with permission). CTRL: control. *P<0.05, compared to UGN 15 min and control (Bonferoni test).
Gastrointestinal and kidney hydrosaline balance is influenced by guanylin family peptides
Guanylin peptides have structural amino acid sequences similar to that of E. coli STa enterotoxin (6). A few years later, Forte's group (7) identified and isolated uroguanylin from opossum and human urine. Guanylin and uroguanylin were first tested in the isolated rat kidney and both presented consistent results as natriuretic and kaliuretic hormones, qualitatively similar to STa (4,7). We also tested a third member of the group, lymphoguanylin, that presented essentially the same effects (natriuresis, kaliuresis, and chlorouresis) in the isolated rat kidney (66). Finally, the most recent member of the group, namely, renoguanylin, was studied using stationary microperfusion by Lessa et al. (67). This peptide was isolated from fish (eel snake-like fish) by Yuge et al. (37). Several advances in recent years have increased our understanding of guanylin's effects on many organs and systems. Fonteles et al. (68) have demonstrated that chronic ingestion of NaCl for 10 days, at a concentration of 2% in drinking water, causes the kidney tissues to respond to a sub-threshold uroguanylin dose during ex vivo renal perfusion, suggesting a sensitization of kidney receptors to this hormone. In a very recent publication, Lessa et al. (69) showed that uroguanylin reduces sodium-proton transport activity (NHE3) in rat renal proximal tubules in a mechanism mediated by both cGMP/PK6 and cAMP/PKA signaling pathways with a parallel reduction in NHE3 surface expression (Figure 2B). Baba et al. (70) demonstrated that uroguanylin, at concentrations that have no effect on normal rats, significantly increases urinary sodium excretion in nephrotic rats, which could be important when treating clinical conditions involving sodium retention.
Novel physiological functions associated with guanylin family peptide GC-C signaling, new drug development, and future research
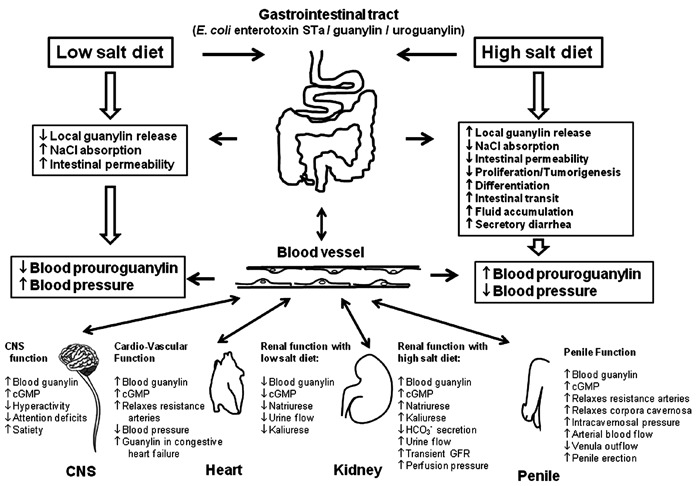
GC-C plays key roles in the regulation of intestinal fluid and electrolyte homeostasis. This is highlighted by the recently identified human mutation in GUCY2C, the gene encoding GC-C and its consequent association with chronic diarrhea and increased risk for inflammatory bowel disease, small-bowel obstruction, and esophagitis (71). Recent studies have also indicated that GC-C signaling has diverse additional functions as reported below and, most importantly, have led to a novel first-in-class GC-C activating peptide, linaclotide, that has been found to be an effective therapeutic intervention for chronic constipation (CC) and constipation associated with irritable bowel syndrome (IBS-C). Figure 3 summarizes the most important pathways associated with GC-C signaling as well as the pathways that agonists such as STa enterotoxin, guanylin, and uroguanylin co-regulate in several tissues and organs.
Figure 3. Major physiological functions associated with the heat-stable enterotoxin (Sta) from Escherichia coli, guanylin, and uroguanylin in guanylate cyclase C (GC-C) signaling pathways. GC-C is expressed on the surface of the intestinal epithelial cells where it is activated by the STa enterotoxin from E. coli and by the endogenous hormones guanylin and uroguanylin. These endogenous hormones are synthesized in the intestine and secreted into the lumen and blood circulation. GC-C is also a receptor for these peptides that activates the catalytic portion of this protein, resulting in the conversion of guanosine triphosphate to cyclic-guanosine-3′,5′-monophosphate (cGMP). Increased cGMP concentration activates protein kinase GII, which phosphorylates and activates the cystic fibrosis transmembrane conductance regulator, leading to chloride/bicarbonate secretion. In addition, cGMP inhibits the sodium-hydrogen exchanger NHE3, decreasing sodium absorption. Local intestinal secretion of guanylin and uroguanylin co-regulates via GC-C signaling the homeostasis of the crypt-villus axis as well as intestinal barrier function and mechanisms underlying colorectal cancer. High or low salt diets co-regulate local intestinal secretion or systemic circulation of these endogenous hormones, which results in electrolyte and water homeostasis influenced by their effects on the gastrointestinal and urinary tracts. The blood circulation of guanylin and uroguanylin alters the functions of several other systems such as the central nervous system (CNS), cardiovascular system, kidney function, and penile function (see text for further details).
Fiskerstrand et al. (71) analyzed genomic DNA isolated from a Norwegian family with a history of chronic diarrhea that started in infancy and reported that a novel autosomal dominant GC showed increased activity. The affected family members had a heterozygous base substitution, c.2519G→T, in exon 22, with the replacement of the amino acid serine at codon 840 with isoleucine. The research group also evaluated normal GC-C and the mutant GC-C in HEK cells and showed that the mutant GC-C had a lower EC50 for STa, guanylin and uroguanylin when compared to normal GC-C cloned cells. Interestingly, the Norwegian family with this mutation had an increased risk for irritable bowel syndrome, inflammatory bowel disease, small-bowel obstruction, and esophagitis later in life.
In a study in Bedouin families that used a genome-wide linkage analysis approach, it was reported that a GUCY2C homozygous autosomal-recessive mutation with a c.2270dupA insertion resulted in a premature stop codon that completely abolished the GC catalytic domain of GC-C, thereby preventing cGMP formation and giving this population an evolutionary advantage against secretory diarrhea (72).
Another unrelated Bedouin family without cystic fibrosis and with normal sweat test results but with a risk for meconium ileus was studied using a genome-wide linkage analysis approach. The best candidate gene identified was GUCY2C, which had a single homozygous mutation, c.1160A>G, leading to a p(Asp387Gly) amino acid substitution in the encoded protein GC-C (72). This mutation is within the extracellular binding domain of GC-C. When GC-C encoding this mutation and normal GC-C were transfected into HEK293 cell lines the STa activation of GC-C showed 60% less cGMP formation in the cells with the mutated GC-C form compared to cells with normal GC-C.
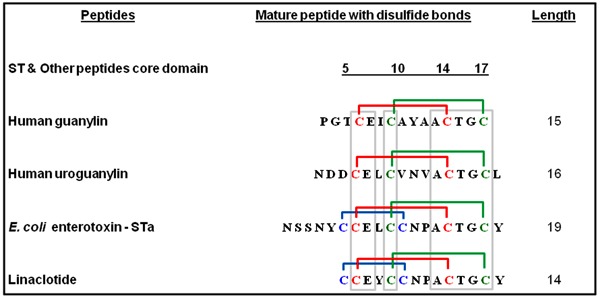
Linaclotide is a first-in-class drug developed for the treatment of IBS-C or CC, based on the guanylin, uroguanylin, and STa peptide structure. It is a peptide with 14 amino acids and three intramolecular disulfide bonds that was determined by nuclear magnetic resonance spectroscopy analysis (73) (Figure 4). The pharmacokinetic and pharmacodynamics were first determined in rat models and T84 cells assayed for GC activity (73,74). Linaclotide is stable at low pH and it is resistant to hydrolysis by pepsin; however, it is completely degraded when incubated in vitro in jejunal fluid for 30 min. Linaclotide has very low oral bioavailability (0.10%) and induces intestinal fluid secretion with accumulation of intraluminal cGMP and increased intestinal transit. Linaclotide exhibited high affinity for GC-C receptors on human colon carcinoma T84 cells and intestinal mucosal membranes. Linaclotide has passed phases I, II, and III clinical trials and is now approved by the US Food and Drug Administration and the European Committee for Medicinal Products for Human Use (CHMP). A recent meta-analysis study carried out to determine the efficacy of linaclotide compared to placebo for patients with IBS-C or CC identified seven trials. Using the endpoint of an increase from baseline of one or more complete spontaneous bowel movement/week and a 30% or more reduction from baseline in the weekly average of daily worst abdominal pain scores for 50% of the treatment weeks, linaclotide improved bowel function and reduced abdominal pain and overall severity of IBS-C or CC compared to placebo (75). Diarrhea was the most common adverse event symptom that was dose-dependently related to these clinical studies. Linaclotide relieves abdominal pain and discomfort in IBS-C or CC, and preliminary in vitro studies suggest that these antinociceptive effects may be mediated by cGMP, which inhibits the mechanical responsiveness of colonic nociceptors (76,77).
Figure 4. Primary peptide structures of human guanylin and uroguanylin, Escherichia coli heat-stable enterotoxin (STa) and linaclotide. The lines connect the disulfide bonds between the cysteine residues. Note that two disulfide bonds are formed in the active conformation of human guanylin and uroguanylin and three disulfide bonds formed the active conformation of STa enterotoxin and linaclotide. The conservative peptide core domains with identical amino acids are marked by boxes.
GC-C-deficient mice are less susceptible to severe dextran sodium sulfate-induced colitis (78). This is associated with minimal production of the resistin-like molecule β via goblet cells, which is important for the induction of TNF-α in this model of inflammation. The homeostasis of the crypt-villus axis in the colon is also dependent on the co-regulation of GC-C signaling. Li et al. (79) reported that GC-C-deficient mice had increased crypt length, which reflected hyperplasia of the proliferating compartment with reduction in differentiated cells, increased migration, and increased apoptosis. These data suggest that GC-C signaling co-regulates homeostasis of the crypt-villus axis and intestinal barrier function, as well as the mechanisms underlying colorectal cancer.
The paracellular permeability of intestinal segments was examined in wild-type, GC-C-deficient, or uroguanylin-deficient mice (80). The GC-C- or uroguanylin-deficient mice showed increased intestinal permeability compared to wild-type mice. GC-C-deficient mice also showed a significant reduction of claudin-2 and JAM-A expression and increased myosin light chain kinase (MLCK) phosphorylation compared to wild-type mice. These data demonstrate that GC-C plays a protective role in the integrity of intestinal mucosa barrier function by regulating MLCK, tight junction claudin-2, and JAM-A expression (80).
Colorectal cancer is one of the most important causes of tumor-related morbidity and mortality worldwide (81). An early report by Pitari et al. (82) suggested that there is an inverse correlation between the incidence of colorectal cancer and secretory diarrhea caused by ETEC. The expression of guanylin or uroguanylin is often lost during colorectal cancer progression whereas GC-C expression is retained. Therefore, GC-C expression could be used as a candidate marker to diagnose or stage the presence of tumor cells in regional lymph nodes. GC-C signaling regulates homeostasis along the crypt-villus axis of the intestinal epithelium. Cytostasis induced by GC-C ligands is mediated by cGMP and is also associated with altered expression of cell cycle mediators, including cyclin D, pRb, and p27, that regulate the delay in transition from the G1-S phase (83). Additional signaling pathways appear to be modulated by GC-C activity. Experiments with GC-C knockout mice show that increased activity of protein kinase B (Akt) results in accelerated cell proliferation, which can be blocked by cGMP (83). It is also an important observation that GC-C signaling disrupts metastasis via inhibition of matrix metalloproteinase-9, which is produced by colorectal cancer cells and is critical for tumor metastasis (84). The data showing how loss of GC-C signaling drives tumorigenesis have led to a number of newly developed GC-C agonists such as linaclotide that may be effective in treating colorectal cancers when used in combination with other drugs.
A paracrine signaling pathway was demonstrated by Kulaksiz and Cetin (85) who detected the presence of uroguanylin and GC-C in human centroacinar cells and the epithelial cells of pancreas ducts, but not in the islets of Langerhans. Kulaksiz et al. (86) also reported molecular evidence for the presence of guanylin, GC-C, GC kinase II, and CFTR in the human parotid and submandibular glands. These data suggest that guanylin is an intrinsic regulator of electrolyte secretion in the salivary glands (86).
Uroguanylin can also be involved in interactions with atriopeptin by potentiating subthreshold doses of both guanylin and atriopeptin when added together to the perfused kidney (87). This interaction could be of importance for the net sodium balance in mammals.
Carrithers et al. (88) observed increased urinary excretion of uroguanylin in patients with congestive heart failure. The discovery of this new class of STa-like compounds has led to a new understanding of signaling pathways that regulate pathological features such as salt-sensitive hypertension resistance to drug treatments for high blood pressure.
The uroguanylin peptide is involved in erectile dysfunction as demonstrated by Sousa et al. (89), who showed that there is a concentration-dependent relaxation in the human corpora cavernosa that is probably dependent on GC-C activation and has no connection with the NO pathway or nitrergic signaling probes.
The regulation of food intake reflects brain regulation of hormones released by most enteroendocrine cells that sense nutrients in the gastrointestinal tract and activate hypothalamic circuits in order to limit meal size. Valentino et al. (90) reported that satiation is disrupted in knockout mice for the GC-C gene (GUCY2C), resulting in hyperphagia and subsequent obesity and metabolic syndrome. They also demonstrated that, after food intake, there is increased prouroguanylin secretion into the circulation, which is activated by proteolytic activity in the hypothalamus, resulting in active uroguanylin, which acts via the GC-C receptors and increases cGMP levels to cause satiation in mice. Another important study showed that mice disrupted for the chemosensory transduction cascade in the social odor carbon disulfide-sensitive olfactory sensory neurons that express the GC-D receptor will be prevented from acquiring these food preferences (91). Olfactory exploration of uroguanylin presented with a food odor similarly produced a preference that was absent when mice were exposed to the food odor alone. These data suggest that this specialized olfactory subsystem acts as a labeled line for a type of associative olfactory learning for food preference in mice.
Motor activity, cognition, motivation and learning are behavioral processes associated with dopamine neurons in the midbrain ventral tegmental area and substantia nigra compacta (VTA/SNc). Gong et al. (92) first demonstrated that GC-C is strongly and selectively expressed throughout the VTA/SNc. They also showed that GC-C mRNA co-localized with tyrosine hydroxylase, an enzyme critical for dopamine synthesis. In addition, their data with knockout mice for GC-C showed attention deficit and hyperactive disorder (ADHD) behavior-like symptoms suggesting that these mice may be used as an ADHD model. Since PKG mediates GC-C signaling, the infusion of 8-Br-cGMP activates PKG in the bilateral VTA/SNc areas, reducing the locomotion of GC-C knockout mice. Guanylin or uroguanylin functionally potentiated the responses evoked by (S)-3,5-dihydroxyphenylglycine, a ligand of group 1 metabotropic glutamate receptors, in adult mouse brain slices using the perforated whole-cell patch-clamp method. These data have shown important behavioral and physiological functions for the GC-C/PKG signaling pathway within the brain and have implications for future drug development for ADHD, schizophrenia, Parkinson's disease, and addiction.
Conclusions
More than thirty years after the identification of the mechanism of E. coli STa enterotoxin and the first discovery of its natriuretic and diuretic effects on kidney tubule functions, STa, guanylin, and uroguanylin studies still reveal new GC-C signaling pathways that suggest novel potentially therapeutic and preventive strategies for CC and IBS-C. These emerging studies on the STa enterotoxin illustrate the success in translation research from the field and laboratory bench to preventive and therapeutic approaches to many diseases (73,74).
There are at least three specific directions for future research that should be addressed in the coming years: a) the mechanisms of GC-C signaling co-regulation of the intestinal crypt-villus axis and preventive as well as therapeutic developments for enteropathy, enteric infection, malnutrition, colorectal tumorigenesis, and metastasis, b) experimental and clinical study of GC-C signaling pathways for the diagnosis and stage evaluation of colorectal cancer, and c) new preventive and therapeutic drugs for the control of conditions and processes associated with GC-C signaling pathways such as satiety, food intake preference, metabolic syndrome, erectile dysfunction, ADHD, schizophrenia, Parkinson's disease, addiction, and hypertension.
Review criteria
For this review, we searched for original articles published between 1970 and 2013 that focused on the effects of the STa enterotoxin from Escherichia coli and the guanylin peptide family on electrolytes and the water net balance in the gastrointestinal tract, renal function, as well as the effects of these STa-like peptides on cardiovascular, penile and central nervous system function. The search was performed using MEDLINE and PubMed databases. The terms used for the search were “diarrheal diseases”, “secretory diarrhea”, “enterotoxigenic Escherichia coli”, “STa enterotoxin”, “guanylin peptide family”, “cGMP”, “guanylyl cyclase”, “hypertension”, “blood pressure and guanylin”, “guanylin and penile erection”, “intestinal permeability”, and “GC-C in central nervous system”, alone or in combination. Full-text English papers and one original Master's dissertation in Portuguese were used. We also searched reference lists of identified articles for obtaining relevant articles. In addition, several papers from the early literature were consulted from full articles available at regular libraries.
Acknowledgments
Research supported by CNPq (#301360/2010-3, #573928/2008-8, and #486044/2007-6).
Footnotes
First published online March 3, 2014.
References
- 1.WHO. Future directions for research on enterotoxigenic Escherichia coli vaccines for developing countries. Wkly Epidemiol Rec. 2006;81:97–104. [PubMed] [Google Scholar]
- 2.Lima AAM. Estudo dos efeitos das toxinas do V. cholerae e E. coli no rim isolado do rato. Pharmacology, Federal University of Ceará; 1983. [Master's degree] [Google Scholar]
- 3.Fonteles MC, Lima AAM. Efeitos das toxinas do V. cholerae e E. coli no rim perfundido; XVII Congresso Brasileiro de Fisiologia; 1983. 46 [Google Scholar]
- 4.Lima AA, Monteiro HS, Fonteles MC. The effects of Escherichia coli heat-stable enterotoxin in renal sodium tubular transport. Pharmacol Toxicol. 1992;70:163–167. doi: 10.1111/j.1600-0773.1992.tb00449.x. [DOI] [PubMed] [Google Scholar]
- 5.Fonteles MC, Lima AAM, Fang G, Guerrant RL. Effect of STa and Cholera toxin on renal electrolyte transport: possible roles of endogenous ST-like compound in the isolated kidney. Twenty-Seventh Joint Conference on Cholera and Related Diarrheal Diseases. 1991:100–105. [Google Scholar]
- 6.Currie MG, Fok KF, Kato J, Moore RJ, Hamra FK, Duffin KL, et al. Guanylin: an endogenous activator of intestinal guanylate cyclase. Proc Natl Acad Sci U S A. 1992;89:947–951. doi: 10.1073/pnas.89.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fonteles MC, Greenberg RN, Monteiro HS, Currie MG, Forte LR. Natriuretic and kaliuretic activities of guanylin and uroguanylin in the isolated perfused rat kidney. Am J Physiol. 1998;275:F191–F197. doi: 10.1152/ajprenal.1998.275.2.F191. [DOI] [PubMed] [Google Scholar]
- 8.Lawrence JG, Ochman H. Molecular archaeology of the Escherichia coli genome. Proc Natl Acad Sci U S A. 1998;95:9413–9417. doi: 10.1073/pnas.95.16.9413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Comrie MM, Cutler CP, Cramb G. Cloning and expression of guanylin from the European eel (Anguilla anguilla) Biochem Biophys Res Commun. 2001;281:1078–1085. doi: 10.1006/bbrc.2001.4485. [DOI] [PubMed] [Google Scholar]
- 10.Huq A, Colwell RR, Rahman R, Ali A, Chowdhury MA, Parveen S, et al. Detection of Vibrio cholerae O1 in the aquatic environment by fluorescent-monoclonal antibody and culture methods. Appl Environ Microbiol. 1990;56:2370–2373. doi: 10.1128/aem.56.8.2370-2373.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lothigius A, Sjoling A, Svennerholm AM, Bolin I. Survival and gene expression of enterotoxigenic Escherichia coli during long-term incubation in sea water and freshwater. J Appl Microbiol. 2010;108:1441–1449. doi: 10.1111/j.1365-2672.2009.04548.x. [DOI] [PubMed] [Google Scholar]
- 12.Sack RB, Gorbach SL, Banwell JG, Jacobs B, Chatterjee BD, Mitra RC. Enterotoxigenic Escherichia coli isolated from patients with severe cholera-like disease. J Infect Dis. 1971;123:378–385. doi: 10.1093/infdis/123.4.378. [DOI] [PubMed] [Google Scholar]
- 13.Moreno AC, Filho AF, Gomes TA, Ramos ST, Montemor LP, Tavares VC, et al. Etiology of childhood diarrhea in the northeast of Brazil: significant emergent diarrheal pathogens. Diagn Microbiol Infect Dis. 2010;66:50–57. doi: 10.1016/j.diagmicrobio.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 14.Yoder JS, Cesario S, Plotkin V, Ma X, Kelly-Shannon K, Dworkin MS. Outbreak of enterotoxigenic Escherichia coli infection with an unusually long duration of illness. Clin Infect Dis. 2006;42:1513–1517. doi: 10.1086/503842. [DOI] [PubMed] [Google Scholar]
- 15.Ansaruzzaman M, Bhuiyan NA, Begum YA, Kuhn I, Nair GB, Sack DA, et al. Characterization of enterotoxigenic Escherichia coli from diarrhoeal patients in Bangladesh using phenotyping and genetic profiling. J Med Microbiol. 2007;56:217–222. doi: 10.1099/jmm.0.46473-0. [DOI] [PubMed] [Google Scholar]
- 16.Jansson L, Tobias J, Lebens M, Svennerholm AM, Teneberg S. The major subunit, CfaB, of colonization factor antigen i from enterotoxigenic Escherichia coli is a glycosphingolipid binding protein. Infect Immun. 2006;74:3488–3497. doi: 10.1128/IAI.02006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sixma TK, Pronk SE, Kalk KH, Wartna ES, van Zanten BA, Witholt B, et al. Crystal structure of a cholera toxin-related heat-labile enterotoxin from E. coli . Nature. 1991;351:371–377. doi: 10.1038/351371a0. [DOI] [PubMed] [Google Scholar]
- 18.Tsai SC, Noda M, Adamik R, Moss J, Vaughan M. Enhancement of choleragen ADP-ribosyltransferase activities by guanyl nucleotides and a 19-kDa membrane protein. Proc Natl Acad Sci U S A. 1987;84:5139–5142. doi: 10.1073/pnas.84.15.5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Viswanathan VK, Hodges K, Hecht G. Enteric infection meets intestinal function: how bacterial pathogens cause diarrhoea. Nat Rev Microbiol. 2009;7:110–119. doi: 10.v1038/nrmicro2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Field M, Graf LH, Jr, Laird WJ, Smith PL. Heat-stable enterotoxin of Escherichia coli: in vitro effects on guanylate cyclase activity, cyclic GMP concentration, and ion transport in small intestine. Proc Natl Acad Sci U S A. 1978;75:2800–2804. doi: 10.1073/pnas.75.6.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hughes JM, Murad F, Chang B, Guerrant RL. Role of cyclic GMP in the action of heat-stable enterotoxin of Escherichia coli . Nature. 1978;271:755–756. doi: 10.1038/271755a0. [DOI] [PubMed] [Google Scholar]
- 22.Forte LR, Krause WJ, Freeman RH. Receptors and cGMP signalling mechanism for E. coli enterotoxin in opossum kidney. Am J Physiol. 1988;255:F1040–F1046. doi: 10.1152/ajprenal.1988.255.5.F1040. [DOI] [PubMed] [Google Scholar]
- 23.Chao AC, de Sauvage FJ, Dong YJ, Wagner JA, Goeddel DV, Gardner P. Activation of intestinal CFTR Cl- channel by heat-stable enterotoxin and guanylin via cAMP-dependent protein kinase. EMBO J. 1994;13:1065–1072. doi: 10.1002/j.1460-2075.1994.tb06355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ostedgaard LS, Baldursson O, Welsh MJ. Regulation of the cystic fibrosis transmembrane conductance regulator Cl- channel by its R domain. J Biol Chem. 2001;276:7689–7692. doi: 10.1074/jbc.R100001200. [DOI] [PubMed] [Google Scholar]
- 25.Schultheis PJ, Clarke LL, Meneton P, Miller ML, Soleimani M, Gawenis LR, et al. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet. 1998;19:282–285. doi: 10.1038/969. [DOI] [PubMed] [Google Scholar]
- 26.Hayashi M, Kita K, Ohashi Y, Aihara E, Takeuchi K. Phosphodiesterase isozymes involved in regulation of HCO3 - secretion in isolated mouse duodenum in vitro . Biochem Pharmacol. 2007;74:1507–1513. doi: 10.1016/j.bcp.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 27.O'Grady SM, Jiang X, Maniak PJ, Birmachu W, Scribner LR, Bulbulian B, et al. Cyclic AMP-dependent Cl secretion is regulated by multiple phosphodiesterase subtypes in human colonic epithelial cells. J Membr Biol. 2002;185:137–144. doi: 10.1007/s00232-001-0120-3. [DOI] [PubMed] [Google Scholar]
- 28.Forte LR, Thorne PK, Eber SL, Krause WJ, Freeman RH, Francis SH, et al. Stimulation of intestinal Cl- transport by heat-stable enterotoxin: activation of cAMP-dependent protein kinase by cGMP. Am J Physiol. 1992;263:C607–C615. doi: 10.1152/ajpcell.1992.263.3.C607. [DOI] [PubMed] [Google Scholar]
- 29.Golin-Bisello F, Bradbury N, Ameen N. STa and cGMP stimulate CFTR translocation to the surface of villus enterocytes in rat jejunum and is regulated by protein kinase G. Am J Physiol Cell Physiol. 2005;289:C708–C716. doi: 10.1152/ajpcell.00544.2004. [DOI] [PubMed] [Google Scholar]
- 30.Sellers ZM, Mann E, Smith A, Ko KH, Giannella R, Cohen MB, et al. Heat-stable enterotoxin of Escherichia coli (STa) can stimulate duodenal HCO3(-) secretion via a novel GC-C- and CFTR-independent pathway. FASEB J. 2008;22:1306–1316. doi: 10.1096/fj.06-7540com. [DOI] [PubMed] [Google Scholar]
- 31.Hamra FK, Forte LR, Eber SL, Pidhorodeckyj NV, Krause WJ, Freeman RH, et al. Uroguanylin: structure and activity of a second endogenous peptide that stimulates intestinal guanylate cyclase. Proc Natl Acad Sci U S A. 1993;90:10464–10468. doi: 10.1073/pnas.90.22.10464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whitaker TL, Steinbrecher KA, Copeland NG, Gilbert DJ, Jenkins NA, Cohen MB. The uroguanylin gene (Guca1b) is linked to guanylin (Guca2) on mouse chromosome 4. Genomics. 1997;45:348–354. doi: 10.1006/geno.1997.4942. [DOI] [PubMed] [Google Scholar]
- 33.So M, McCarthy BJ. Nucleotide sequence of the bacterial transposon Tn1681 encoding a heat-stable (ST) toxin and its identification in enterotoxigenic Escherichia coli strains. Proc Natl Acad Sci U S A. 1980;77:4011–4015. doi: 10.1073/pnas.77.7.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rasko DA, Rosovitz MJ, Myers GS, Mongodin EF, Fricke WF, Gajer P, et al. The pangenome structure of Escherichia coli: comparative genomic analysis of E. coli commensal and pathogenic isolates. J Bacteriol. 2008;190:6881–6893. doi: 10.1128/JB.00619-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Froehlich B, Parkhill J, Sanders M, Quail MA, Scott JR. The pCoo plasmid of enterotoxigenic Escherichia coli is a mosaic cointegrate. J Bacteriol. 2005;187:6509–6516. doi: 10.1128/JB.187.18.6509-6516.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamamoto T, Yokota T. Plasmids of enterotoxigenic Escherichia coli H10407: evidence for two heat-stable enterotoxin genes and a conjugal transfer system. J Bacteriol. 1983;153:1352–1360. doi: 10.1128/jb.153.3.1352-1360.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuge S, Inoue K, Hyodo S, Takei Y. A novel guanylin family (guanylin, uroguanylin, and renoguanylin) in eels: possible osmoregulatory hormones in intestine and kidney. J Biol Chem. 2003;278:22726–22733. doi: 10.1074/jbc.M303111200. [DOI] [PubMed] [Google Scholar]
- 38.Guarino A, Giannella R, Thompson MR. Citrobacter freundii produces an 18-amino-acid heat-stable enterotoxin identical to the 18-amino-acid Escherichia coli heat-stable enterotoxin (ST Ia) Infect Immun. 1989;57:649–652. doi: 10.1128/iai.57.2.649-652.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arita M, Honda T, Miwatani T, Ohmori K, Takao T, Shimonishi Y. Purification and characterization of a new heat-stable enterotoxin produced by Vibrio cholerae non-O1 serogroup Hakata. Infect Immun. 1991;59:2186–2188. doi: 10.1128/iai.59.6.2186-2188.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshino K, Miyachi M, Takao T, Bag PK, Huang X, Nair GB, et al. Purification and sequence determination of heat-stable enterotoxin elaborated by a cholera toxin-producing strain of Vibrio cholerae O1. FEBS Lett. 1993;326:83–86. doi: 10.1016/0014-5793(93)81766-S. [DOI] [PubMed] [Google Scholar]
- 41.Ozaki H, Sato T, Kubota H, Hata Y, Katsube Y, Shimonishi Y. Molecular structure of the toxin domain of heat-stable enterotoxin produced by a pathogenic strain of Escherichia coli. A putative binding site for a binding protein on rat intestinal epithelial cell membranes. J Biol Chem. 1991;266:5934–5941. [PubMed] [Google Scholar]
- 42.Hamra FK, Eber SL, Chin DT, Currie MG, Forte LR. Regulation of intestinal uroguanylin/guanylin receptor-mediated responses by mucosal acidity. Proc Natl Acad Sci U S A. 1997;94:2705–2710. doi: 10.1073/pnas.94.6.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Forte LR, Eber SL, Turner JT, Freeman RH, Fok KF, Currie MG. Guanylin stimulation of Cl- secretion in human intestinal T84 cells via cyclic guanosine monophosphate. J Clin Invest. 1993;91:2423–2428. doi: 10.1172/JCI116476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greenberg RN, Hill M, Crytzer J, Krause WJ, Eber SL, Hamra FK, et al. Comparison of effects of uroguanylin, guanylin, and Escherichia coli heat-stable enterotoxin STa in mouse intestine and kidney: evidence that uroguanylin is an intestinal natriuretic hormone. J Investig Med. 1997;45:276–282. [PubMed] [Google Scholar]
- 45.de Jonge HR. The localization of guanylate cyclase in rat small intestinal epithelium. FEBS Lett. 1975;53:237–242. doi: 10.1016/0014-5793(75)80028-7. [DOI] [PubMed] [Google Scholar]
- 46.Krause WJ, Freeman RH, Fort LR. Autoradiographic demonstration of specific binding sites for E. coli enterotoxin in various epithelia of the North American opossum. Cell Tissue Res. 1990;260:387–394. doi: 10.1007/BF00318641. [DOI] [PubMed] [Google Scholar]
- 47.Forte LR, Krause WJ, Freeman RH. Escherichia coli enterotoxin receptors: localization in opossum kidney, intestine, and testis. Am J Physiol. 1989;257:F874–F881. doi: 10.1152/ajprenal.1989.257.5.F874. [DOI] [PubMed] [Google Scholar]
- 48.Biswas KH, Shenoy AR, Dutta A, Visweswariah SS. The evolution of guanylyl cyclases as multidomain proteins: conserved features of kinase-cyclase domain fusions. J Mol Evol. 2009;68:587–602. doi: 10.1007/s00239-009-9242-5. [DOI] [PubMed] [Google Scholar]
- 49.de Sauvage FJ, Horuk R, Bennett G, Quan C, Burnier JP, Goeddel DV. Characterization of the recombinant human receptor for Escherichia coli heat-stable enterotoxin. J Biol Chem. 1992;267:6479–6482. [PubMed] [Google Scholar]
- 50.Singh S, Singh G, Heim JM, Gerzer R. Isolation and expression of a guanylate cyclase-coupled heat stable enterotoxin receptor cDNA from a human colonic cell line. Biochem Biophys Res Commun. 1991;179:1455–1463. doi: 10.1016/0006-291X(91)91736-V. [DOI] [PubMed] [Google Scholar]
- 51.Hodson CA, Ambrogi IG, Scott RO, Mohler PJ, Milgram SL. Polarized apical sorting of guanylyl cyclase C is specified by a cytosolic signal. Traffic. 2006;7:456–464. doi: 10.1111/j.1600-0854.2006.00398.x. [DOI] [PubMed] [Google Scholar]
- 52.Nandi A, Bhandari R, Visweswariah SS. Epitope conservation and immunohistochemical localization of the guanylin/stable toxin peptide receptor, guanylyl cyclase C. J Cell Biochem. 1997;66:500–511. doi: 10.1002/(SICI)1097-4644(19970915)66:4<500::AID-JCB9>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 53.Basu N, Arshad N, Visweswariah SS. Receptor guanylyl cyclase C (GC-C): regulation and signal transduction. Mol Cell Biochem. 2010;334:67–80. doi: 10.1007/s11010-009-0324-x. [DOI] [PubMed] [Google Scholar]
- 54.Visweswariah SS, Ramachandran V, Ramamohan S, Das G, Ramachandran J. Characterization and partial purification of the human receptor for the heat-stable enterotoxin. Eur J Biochem. 1994;219:727–736. doi: 10.1111/j.1432-1033.1994.tb18551.x. [DOI] [PubMed] [Google Scholar]
- 55.Saha S, Biswas KH, Kondapalli C, Isloor N, Visweswariah SS. The linker region in receptor guanylyl cyclases is a key regulatory module: mutational analysis of guanylyl cyclase C. J Biol Chem. 2009;284:27135–27145. doi: 10.1074/jbc.M109.020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rauch A, Leipelt M, Russwurm M, Steegborn C. Crystal structure of the guanylyl cyclase Cya2. Proc Natl Acad Sci U S A. 2008;105:15720–15725. doi: 10.1073/pnas.0808473105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Winger JA, Derbyshire ER, Lamers MH, Marletta MA, Kuriyan J. The crystal structure of the catalytic domain of a eukaryotic guanylate cyclase. BMC Struct Biol. 2008;8:42. doi: 10.1186/1472-6807-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Deshmane SP, Parkinson SJ, Crupper SS, Robertson DC, Schulz S, Waldman SA. Cytoplasmic domains mediate the ligand-induced affinity shift of guanylyl cyclase C. Biochemistry. 1997;36:12921–12929. doi: 10.1021/bi971077b. [DOI] [PubMed] [Google Scholar]
- 59.Sharp GW, Hynie S. Stimulation of intestinal adenyl cyclase by cholera toxin. Nature. 1971;229:266–269. doi: 10.1038/229266a0. [DOI] [PubMed] [Google Scholar]
- 60.Dean P, Maresca M, Schuller S, Phillips AD, Kenny B. Potent diarrheagenic mechanism mediated by the cooperative action of three enteropathogenic Escherichia coli-injected effector proteins. Proc Natl Acad Sci U S A. 2006;103:1876–1881. doi: 10.1073/pnas.0509451103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ley RE, Hamady M, Lozupone C, Turnbaugh PJ, Ramey RR, Bircher JS, et al. Evolution of mammals and their gut microbes. Science. 2008;320:1647–1651. doi: 10.1126/science.1155725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Friedler RM, Kurokawa K, Coburn JW, Massry SG. Renal action of cholera toxin: I. Effects on urinary excretion of electrolytes and cyclic AMP. Kidney Int. 1975;7:77–85. doi: 10.1038/ki.1975.12. [DOI] [PubMed] [Google Scholar]
- 63.Kurokawa K, Friedler RM, Massry SG. Renal action of cholera toxin: II. Effects on adenylate cyclase-cyclic AMP system. Kidney Int. 1975;7:137–144. doi: 10.1038/ki.1975.21. [DOI] [PubMed] [Google Scholar]
- 64.Lima AA, Monteiro HS, Fonteles MC. Effects of Vibrio cholerae enterotoxin peptide on glomerular filtration rate and renal proximal tubular sodium transport. Braz J Med Biol Res. 1993;26:983–987. [PubMed] [Google Scholar]
- 65.Guerrant RL, Hughes JM, Chang B, Robertson DC, Murad F. Activation of intestinal guanylate cyclase by heat-stable enterotoxin of Escherichia coli: studies of tissue specificity, potential receptors, and intermediates. J Infect Dis. 1980;142:220–228. doi: 10.1093/infdis/142.2.220. [DOI] [PubMed] [Google Scholar]
- 66.Fonteles MC, Carrithers SL, Monteiro HS, Carvalho AF, Coelho GR, Greenberg RN, et al. Renal effects of serine-7 analog of lymphoguanylin in ex vivo rat kidney. Am J Physiol Renal Physiol. 2001;280:F207–F213. doi: 10.1152/ajprenal.2001.280.2.F207. [DOI] [PubMed] [Google Scholar]
- 67.Lessa LM, Amorim JB, Fonteles MC, Malnic G. Effect of renoguanylin on hydrogen/bicarbonate ion transport in rat renal tubules. Regul Pept. 2009;157:37–43. doi: 10.1016/j.regpep.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 68.Fonteles MC, Havt A, Prata RB, Prata PH, Monteiro HS, Lima AA, et al. High-salt intake primes the rat kidney to respond to a subthreshold uroguanylin dose during ex vivo renal perfusion. Regul Pept. 2009;158:6–13. doi: 10.1016/j.regpep.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 69.Lessa LM, Carraro-Lacroix LR, Crajoinas RO, Bezerra CN, Dariolli R, Girardi AC, et al. Mechanisms underlying the inhibitory effects of uroguanylin on NHE3 transport activity in renal proximal tubule. Am J Physiol Renal Physiol. 2012;303:F1399–F1408. doi: 10.1152/ajprenal.00385.2011. [DOI] [PubMed] [Google Scholar]
- 70.Baba A, Fujimoto S, Kikuchi M, Kita T, Kitamura K. Effects of uroguanylin on natriuresis in experimental nephrotic rats. Nephrology. 2009;14:80–85. doi: 10.1111/j.1440-1797.2008.01006.x. [DOI] [PubMed] [Google Scholar]
- 71.Fiskerstrand T, Arshad N, Haukanes BI, Tronstad RR, Pham KD, Johansson S, et al. Familial diarrhea syndrome caused by an activating GUCY2C mutation. N Engl J Med. 2012;366:1586–1595. doi: 10.1056/NEJMoa1110132. [DOI] [PubMed] [Google Scholar]
- 72.Romi H, Cohen I, Landau D, Alkrinawi S, Yerushalmi B, Hershkovitz R, et al. Meconium ileus caused by mutations in GUCY2C, encoding the CFTR-activating guanylate cyclase 2C. Am J Hum Genet. 2012;90:893–899. doi: 10.1016/j.ajhg.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bryant AP, Busby RW, Bartolini WP, Cordero EA, Hannig G, Kessler MM, et al. Linaclotide is a potent and selective guanylate cyclase C agonist that elicits pharmacological effects locally in the gastrointestinal tract. Life Sci. 2010;86:760–765. doi: 10.1016/j.lfs.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 74.Busby RW, Bryant AP, Bartolini WP, Cordero EA, Hannig G, Kessler MM, et al. Linaclotide, through activation of guanylate cyclase C, acts locally in the gastrointestinal tract to elicit enhanced intestinal secretion and transit. Eur J Pharmacol. 2010;649:328–335. doi: 10.1016/j.ejphar.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 75.Videlock EJ, Cheng V, Cremonini F. Effects of linaclotide in patients with irritable bowel syndrome with constipation or chronic constipation: a meta-analysis. Clin Gastroenterol Hepatol. 2013;11:1084–1092. doi: 10.1016/j.cgh.2013.04.032. [DOI] [PubMed] [Google Scholar]
- 76.Castro J, Harrington A, Hughes P, Martin C, Silos-Santiago A, Kurtz C, et al. Mechanism of action for linaclotide induced abdominal pain relief. Gastroenterology. 2012;142(Suppl):S699. [Google Scholar]
- 77.Castro J, Martin C, Hughes PA, Silos-Santiago A, Kurtz CB, Blackshaw LA, et al. A novel role of cyclic GMP in colonic sensory neurotransmission in healthy and TNBS-treated mice. Gastroenterology. 2011;140(Suppl):S538. [Google Scholar]
- 78.Steinbrecher KA, Harmel-Laws E, Garin-Laflam MP, Mann EA, Bezerra LD, Hogan SP, et al. Murine guanylate cyclase C regulates colonic injury and inflammation. J Immunol. 2011;186:7205–7214. doi: 10.4049/jimmunol.1002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li P, Lin JE, Chervoneva I, Schulz S, Waldman SA, Pitari GM. Homeostatic control of the crypt-villus axis by the bacterial enterotoxin receptor guanylyl cyclase C restricts the proliferating compartment in intestine. Am J Pathol. 2007;171:1847–1858. doi: 10.2353/ajpath.2007.070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Han X, Mann E, Gilbert S, Guan Y, Steinbrecher KA, Montrose MH, et al. Loss of guanylyl cyclase C (GCC) signaling leads to dysfunctional intestinal barrier. PLoS One. 2011;6: doi: 10.1371/journal.pone.0016139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lin JE, Li P, Pitari GM, Schulz S, Waldman SA. Guanylyl cyclase C in colorectal cancer: susceptibility gene and potential therapeutic target. Future Oncol. 2009;5:509–522. doi: 10.2217/fon.09.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pitari GM, Zingman LV, Hodgson DM, Alekseev AE, Kazerounian S, Bienengraeber M, et al. Bacterial enterotoxins are associated with resistance to colon cancer. Proc Natl Acad Sci U S A. 2003;100:2695–2699. doi: 10.1073/pnas.0434905100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pitari GM, Di Guglielmo MD, Park J, Schulz S, Waldman SA. Guanylyl cyclase C agonists regulate progression through the cell cycle of human colon carcinoma cells. Proc Natl Acad Sci U S A. 2001;98:7846–7851. doi: 10.1073/pnas.141124698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lubbe WJ, Zuzga DS, Zhou Z, Fu W, Pelta-Heller J, Muschel RJ, et al. Guanylyl cyclase C prevents colon cancer metastasis by regulating tumor epithelial cell matrix metalloproteinase-9. Cancer Res. 2009;69:3529–3536. doi: 10.1158/0008-5472.CAN-09-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kulaksiz H, Cetin Y. Uroguanylin and guanylate cyclase C in the human pancreas: expression and mutuality of ligand/receptor localization as indicators of intercellular paracrine signaling pathways. J Endocrinol. 2001;170:267–275. doi: 10.1677/joe.0.1700267. [DOI] [PubMed] [Google Scholar]
- 86.Kulaksiz H, Rehberg E, Stremmel W, Cetin Y. Guanylin and functional coupling proteins in the human salivary glands and gland tumors: expression, cellular localization, and target membrane domains. Am J Pathol. 2002;161:655–664. doi: 10.1016/S0002-9440(10)64221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Santos-Neto MS, Carvalho AF, Monteiro HS, Forte LR, Fonteles MC. Interaction of atrial natriuretic peptide, urodilatin, guanylin and uroguanylin in the isolated perfused rat kidney. Regul Pept. 2006;136:14–22. doi: 10.1016/j.regpep.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 88.Carrithers SL, Eber SL, Forte LR, Greenberg RN. Increased urinary excretion of uroguanylin in patients with congestive heart failure. Am J Physiol Heart Circ Physiol. 2000;278:H538–H547. doi: 10.1152/ajpheart.2000.278.2.H538. [DOI] [PubMed] [Google Scholar]
- 89.Sousa CM, Havt A, Santos CF, Arnaud-Batista FJ, Cunha KM, Cerqueira JB, et al. The relaxation induced by uroguanylin and the expression of natriuretic peptide receptors in human corpora cavernosa. J Sex Med. 2010;7:3610–3619. doi: 10.1111/j.1743-6109.2009.01672.x. [DOI] [PubMed] [Google Scholar]
- 90.Valentino MA, Lin JE, Snook AE, Li P, Kim GW, Marszalowicz G, et al. A uroguanylin-GUCY2C endocrine axis regulates feeding in mice. J Clin Invest. 2011;121:3578–3588. doi: 10.1172/JCI57925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Arakawa H, Kelliher KR, Zufall F, Munger SD. The receptor guanylyl cyclase type D (GC-D) ligand uroguanylin promotes the acquisition of food preferences in mice. Chem Senses. 2013;38:391–397. doi: 10.1093/chemse/bjt015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gong R, Ding C, Hu J, Lu Y, Liu F, Mann E, et al. Role for the membrane receptor guanylyl cyclase-C in attention deficiency and hyperactive behavior. Science. 2011;333:1642–1646. doi: 10.1126/science.1207675. [DOI] [PubMed] [Google Scholar]


