Abstract
Platelets contain an invaginated, tubular membranous structure called the surface-connected open canalicular system (SCCS or OCS), which is contiguous with the plasma membrane and serves as a site for granule fusion and as a reservoir of membrane for platelet spreading. According to ultrastructural studies, platelets from some species lack OCS. In an attempt to correlate biochemical and functional attributes with the presence of an OCS, platelets from human, mouse and dog (OCS+), and from cow, camel and horse (OCS−) were analysed for differential protein expression and aggregation in response to thrombin. Among the 18 different cytoskeletal and regulatory proteins examined, five (Rac1, RhoA, Ras, calmodulin and Src) were expressed at higher levels in OCS+ platelets (p<0.05). Given the role of Arf6 in the formation of tubular invaginations in nucleated cells, the levels of Arf6-GTP were analysed in OCS+ and OCS− platelets. There was no significant correlation between the presence of OCS and total Arf6 or Arf6-GTP levels. Comparison of platelet aggregation between different species suggests that OCS− platelets have delayed responses. This comparison of platelets from six different species, which differ in their OCS, shows the differential expression of known signaling components and foreshadows future studies focusing on OCS formation and function.
Keywords: Membrane structures, OCS, small GTPase, aggregation, cytoskeleton
Introduction
Resting platelets contain a unique membrane structure called surface-connected open canalicular system (OCS). It is an invaginated, tubular extension of the plasma membrane generally absent in nucleated cells. The OCS is thought to serve at least two purposes: first, as a reservoir of plasma membrane for a rapid, activation-dependent increase in surface area; and second, as a conduit for granule content release. Upon activation, platelet granules are thought to fuse with the OCS to release their cargo. This results in an enlargement of the OCS, a loss of its tubular shape and an increase in platelet surface area [1]. The OCS membrane contains many platelet plasma membrane proteins, such as integrins, but may sequester some proteins, presumably through internalization processes related to endocytosis [1, 2]. Since its discovery ~40 years ago, many morphological studies of OCS have been carried [3], yet it is unclear how the OCS is formed or why it is present in only some species (e.g. human, mouse and dog) but not others (e.g. cow, camel and horse) [3–7].
A number of proteins could play a role in OCS formation or function. The Ras superfamily of small GTPases are ‘on-off’ switches that act as intracellular transducers to facilitate interpretation of extracellular stimuli. There are four major families in platelets: Ras, Rho, Rab and Arf [8]. They exist in two forms: the GTP-bound, active form, that interacts with downstream effectors and the GDP-bound inactive form [9]. Studies have focused on the functions of Rho and Ras family GTPases in platelet activation. Rho family GTPases (RhoA, Rac1 and Cdc42) are essential regulators of the platelet cytoskeleton: RhoA for focal adhesion and stress fiber formation, Rac1 for lamellipodia and Cdc42 for filopodia formation [10–13]. Ras family GTPases (Ras, Ral and Rap) are required for the initiation of signaling events that lead to platelet secretion (Ras and Ral) [14, 15] and integrin activation (Rap) [16].
In resting platelets, most of the small GTPases exist predominantly in the GDP-bound state and are transiently activated to their GTP-bound state when the platelets are stimulated. However, ADP-Ribosylation Factor 6 (Arf6) is an exception; it is present in the GTP-bound state in resting platelets and is converted to the GDP-bound form upon platelet stimulation [17, 18]. This suggests a function for Arf6-GTP in resting platelets, which is yet unknown. Recent studies show that expression of high levels of Arf6-GTP in nucleated cells causes the formation of tubular membrane invaginations by inducing positive curvature in the plasma membrane [19–21]. These invaginations bear a striking resemblance to the platelet OCS. Given the roles of Arf6 in nucleated cells, the invaginated structures could originate from its effects on the actin cytoskeleton or on membrane trafficking. Alternatively, experiments done with purified liposomes show that Arf6-GTP directly inserts into lipid bilayers and promotes positive membrane curvature [21]. Due to the current lack of biochemical or genetic information about OCS formation and regulation, it is difficult to determine what is important for the maintenance of the OCS; however, based on its reported effects, Arf6 could play some role.
Comparative ultrastructural studies of platelets show that certain species lack the invaginated tubular membrane structures defined as OCS [5–7]. The present study seeks to delineate some of the biochemical differences associated with the presence or absence of the OCS by examining the expression of 18 different proteins in platelets from three species that have obvious OCS (OCS+; human, mouse and dog) and three that do not (OCS−; cow, camel and horse) [3–7, 22]. Specific focus was placed on the small GTPases (Rho, Ras and Arf families) and their regulators, which are known to play roles in cytoskeleton and membrane dynamics. Given the known function of Arf6 in the formation of membrane invaginations that are morphologically similar to OCS, the levels of Arf6-GTP in resting platelets from different species were given more scrutiny. Finally, aggregation, following stimulation with thrombin, of OCS+ and OCS− platelets was compared. Data in this study should provide a useful foundation for future biochemical analyses of the OCS in mammalian platelets.
Materials and methods
Antibodies and reagents
Antibodies used in this study (see Table I) are as follows: an anti-Arf6 rabbit polyclonal antibody produced, under contract, by Bethyl Laboratory (Montgomery, TX, USA) and described in [17], an anti-RabGDIα rabbit polyclonal antibody generated and characterized in [23]; anti-Srcpp60, anti-IQGAP1, anti-Cdc42, anti-RalA, anti-Rap1A and anti-GIT1 mouse monoclonal antibodies (BD Biosciences, San Jose, CA, USA); anti-IQGAP2 mouse monoclonal antibody (GeneTex, San Antonio, TX), USA; anti-Rac1, anti-K/H/N-Ras and anti-calmodulin (CaM) mouse monoclonal antibodies (Upstate Biotechnology, Lake Placid, NY, USA); anti-β3 integrin polyclonal antibody (Cell Signaling, Danvers, MA, USA); anti-α-actinin mouse monoclonal antibody and anti-Arf1/3 sheep polyclonal antibody (Sigma, St Louis, MO, USA); anti-focal adhesion kinase (FAK) rabbit polyclonal antibody (EMD Chemicals Inc., Gibbstown, NJ, USA); and anti-RhoA mouse monoclonal antibody (Cytoskeleton, Denver, CO, USA). The anti-ASAP1 rabbit polyclonal antibody was a generous gift from Dr. Paul A. Randazzo (NIH, Bethesda, MD, USA).
Table I.
The antibodies used and the sequence identities of the proteins examined.
| Proteins | Ab Typea | Immunogenb | Human | Murine | Canine | Bovine | Camelidc | Equine |
|---|---|---|---|---|---|---|---|---|
| Arf6 | R, poly | 164–175 | 100 | 100 | 100 | 100 | N/A | 97 |
| RabGDIα | R, poly | FL | 100 | 98 | 97 | 98 | N/A | 97 |
| β3 integrin | R, poly | FL | 100 | 90 | 95 | 95 | 95 | 95 |
| actinin | M, mono | FL | 100 | 99 | 99 | 99 | N/A | 98 |
| FAK | R, poly | 853–1502 | 100 | 97 | 95 | 95 | N/A | 93 |
| Srcpp60 | M, mono | 82–169 | 100 | 98 | 98 | 97 | N/A | 93 |
| IQGAP1 | M, mono | 1348–1490 | 100 | 95 | 97 | 94 | N/A | 96 |
| IQGAP2 | M, mono | 343–450 | 100 | 89 | 90 | 87 | N/A | 91 |
| RhoA | M, mono | FL | 100 | 99 | 99 | 100 | N/A | 100 |
| Rac1 | M, mono | FL | 100 | 100 | 100 | 100 | N/A | 95 |
| Cdc42 | M, mono | FL | 100 | 100 | 100 | 95 | N/A | 95 |
| Arf1 | S, poly | 98–112 | 100 | 100 | 96 | 100 | N/A | 100 |
| Arf3 | 100 | 100 | 100 | 100 | N/A | 100 | ||
| K-Ras | 100 | 98 | –d | –d | N/A | 95 | ||
| H-Ras | M, mono | FL H-Ras | 100 | 99 | 100 | 100 | N/A | 100 |
| N-Ras | 100 | 100 | 97 | –d | N/A | 97 | ||
| RalA | M, mono | 35–206 | 100 | 99 | 99 | 99 | N/A | 99 |
| Rap1A | M, mono | FL | 100 | 100 | 100 | 100 | N/A | 98 |
| CaM | M, mono | 128–148 | 100 | 100 | 100 | 100 | N/A | 100 |
| GIT1 | M, mono | 664–770 | 100 | 97 | 97 | 98 | N/A | 93 |
| ASAP1 | R, poly | 905–921 | 100 | 94 | 96 | 95 | N/A | 95 |
The amino acid sequence identities for the proteins analysed in this study were examined using a web-based, search tool, BLASTP (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The human homologs are set as the query and the subjects from different species are represented as a percent identity.
The types of antibodies used in this study are monoclonal (mono) or polyclonal (poly) immunoglobulins from mouse (M), rabbit (R) or sheep (S).
The immunogens, used to generate the antibodies used, were either full-length proteins (FL) or small fragments (corresponding start/end positions are indicated).
N/A, information is not currently available.
Some homologs are absent in canine and bovine sequence databases.
The reagents used were: apyrase, benzamidine and glutathione-conjugated agarose (Sigma); complete, EDTA-free protease cocktail (Roche, Indianapolis, IN, USA); acid citrate dextrose (ACD) Vacutainer® blood collection tubes (BD Diagnostics, Sparks, MD, USA); human plasma thrombin (Chrono-log, Havertown, PA, USA); and prostaglandin I2 (PGI2) (Cayman Chemical, Ann Arbor, MI, USA). A prokaryotic expression plasmid encoding human GGA3VHS-GAT(1-313) was kindly provided by Dr. Julie G. Donaldson (NIH).
Preparation of platelets
Washed platelets from different species were prepared from freshly drawn whole blood using ACD collection tubes. Whole blood was diluted 3 : 1 with HEPES-Tyrode’s buffer (20 mM HEPES/KOH, pH 6.5, 128 mM NaCl, 2.8 mM KCl, 1 mM MgCl2, 5 mM D-glucose, 12 mM NaHCO3, 0.4 mM NaH2PO4) containing 0.37 U/mL apyrase, 100 ng/mL PGI2 and 1 mM EGTA and centrifuged at 150 ×g (human, canine, camelid), 250 ×g (murine) and 80 ×g (bovine, equine) for 20 min at room temperature to prepare platelet rich plasma (PRP). Plasma was removed by centrifugation at 900 ×g (human, canine, camelid), 1200 ×g (murine) and 400 ×g (bovine, equine) for 10 min, and the platelets were resuspended in HEPES-Tyrode’s buffer (pH 6.5) containing 0.37 U/mL apyrase, 100 ng/mL PGI2 and 1 mM EGTA. Platelets were washed one additional time by centrifugation at 900 ×g (human, canine, camelid), 1200 ×g (murine) and 400 ×g (bovine, equine) for 7 min and resuspended in HEPES-Tyrode’s buffer (pH 7.4). Platelets were counted and their sizes were measured using a Z2 Coulter Counter (Beckman Coulter, Inc., Miami, FL, USA). Their concentration was adjusted to 4 ×108/mL in HEPES-Tyrode’s buffer (pH 7.4). To minimize the inhibitory effect of any residual PGI2 in the preparation, platelet suspensions were incubated at 37°C for 30 min prior to use.
Platelet aggregometry
The platelet suspension (500 μL) was warmed to 37°C in a siliconized glass cuvette (Chrono-log) containing a metal stir bar (stirring at 800 rpm) using a Model 460 Vs Lumi-Dual aggregometer (Chrono-log) for 5 min. Subsequently, thrombin (0.1 U/mL) was added to initiate platelet activation. Aggregation traces were monitored for 3 min using a Model 810 Aggro/Link computer interface and Aggro/Link software (Chrono-log).
Western blotting
Protein samples were prepared by boiling resting platelet pellets (2 ×108) in 1 ×SDS-PAGE sample buffer (0.5 mL) for 10 min. Equal number of platelets (2 ×107 platelets per lane) were loaded, separated by SDS-PAGE and transferred to PVDF membrane (Milipore, Bedford, MA, USA) with constant voltage (100 V) for 1 h. The membranes were blocked with 5% nonfat dried milk in TBS-T (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.1% Tween-20) containing 0.02% NaN3 for 1 h at room temperature. The blocking solution was replaced with a dilution of primary antibody in blocking buffer and membranes were incubated at 4°C overnight. Membranes were then washed with TBS-T three times and incubated with the appropriate alkaline phosphatase-conjugated, anti-mouse, anti-rabbit or anti-sheep antibodies diluted in blocking buffer without NaN3 for 1 h at room temperature. Following three washes with TBS-T, membranes were incubated with Vistra™ ECF substrate (GE Healthcare, Piscataway, NJ, USA) and the fluorescence was detected using Typhoon™ 9400 Phosphoimager (GE Healthcare). Quantification of immuno-decorated proteins was performed using ImageQuant 5.2 software (GE Healthcare).
Assay for Arf6-GTP
Resting platelet lysates were obtained by adding an equal volume of 2 ×ice-cold HEPES-lysis buffer (20 mM HEPES/KOH, pH 7.4, 128 mM NaCl, 9 mM MgCl2, 2% Triton X-100 (Tx-100), 0.2% SDS, 1% deoxycholic acid, 20% glycerol, 2 mM benzamidine and 2 ×EDTA-free protease inhibitor cocktail) to platelet suspensions. The lysates were flash-frozen in an ethanol-dry ice chamber and kept in liquid N2 until analysis. Frozen lysates were quickly thawed at room temperature and transferred to 1.5 mL tubes on ice. The lysates were cleared by centrifugation at 15,000 ×g for 5 min to remove the Tx-100-insoluble cytoskeleton pellet (under these conditions all Arf6 is soluble [17, 18]) and the supernatants were incubated for 30 min at 4°C with 20 μL glutathione-agarose beads pre-bound to 50 μg of glutathione-S-transferase (GST)-fusion protein containing human GGA3VHS-GAT(1-313), which selectively binds Arf-GTP [24, 25]. The bead-bound complexes were recovered at 500 ×g for 10 s and washed three times with HEPES-wash buffer (20 mM HEPES, pH 7.4, 128 mM NaCl, 2 mM MgCl2, 1% Tx-100, 10% glycerol). The bound proteins were eluted with 2 ×SDS sample buffer by boiling for 10 min and the Arf6 protein was detected by western blotting using an anti-Arf6 antibody.
Statistics
Data from OCS+ and OCS− species (Figure 2B–F) were grouped and compared to ensure the difference in protein expression were significant. Statistical significance was determined by unpaired Student’s t-test using GraphRad Prism 4.0 (GraphPad Software, Inc., La Jolla, CA, USA) and p<0.05 was considered to be statistically significant. These data are included in Table II.
Figure 2.
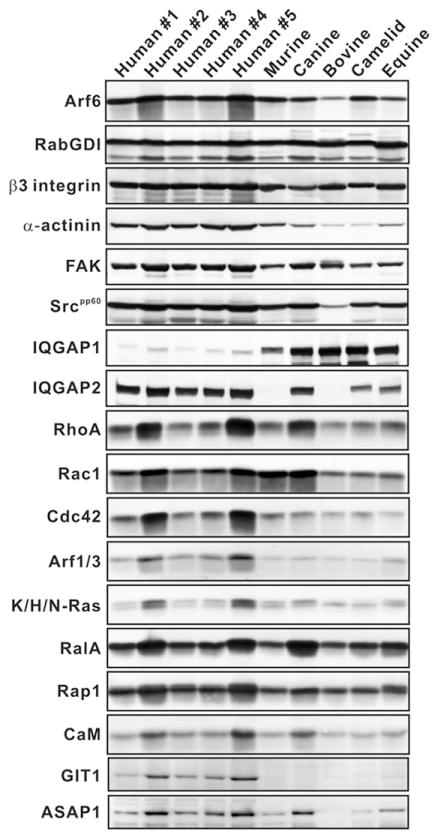
The expression of platelet proteins from different species. Samples from an equal number of platelets (2 ×107 platelets per lane) from different species were boiled in sample buffer, separated by SDS-PAGE, and probed by immunoblotting for the indicated proteins.
Table II.
Protein expression in platelets from different species.
| Proteins Species |
OCS+
|
OCS−
|
p-value# | ||||
|---|---|---|---|---|---|---|---|
| Human | Murine | Canine | Bovine | Camelid | Equine | ||
| Arf6 | 1.74 (±0.45) | 1.19 | 0.84 | 0.37 | 1.03 | 0.52 | 0.1336 |
| β-Actinin | 0.43 (±0.02) | 0.27 | 0.16 | 0.06 | 0.06 | 0.12 | 0.0627 |
| FAK | 0.64 (±0.07) | 0.37 | 0.54 | 0.43 | 0.31 | 0.29 | 0.1258 |
| Arf1/3 | 0.81 (±0.25) | 0.50 | 0.47 | 0.39 | 0.38 | 0.46 | 0.1734 |
| Cdc42 | 0.58 (±0.30) | 0.30 | 0.23 | 0.14 | 0.16 | 0.08 | 0.0881 |
| Rac1 | 1.79 (±0.28) | 2.44 | 2.29 | 1.07 | 1.14 | 1.01 | 0.0055 |
| RhoA | 0.56 (±0.28) | 0.38 | 0.62 | 0.25 | 0.31 | 0.31 | 0.0368 |
| Rap1 | 2.28 (±0.30) | 1.67 | 1.83 | 1.41 | 1.50 | 1.39 | 0.0586 |
| RalA | 1.12 (±0.25) | 0.72 | 1.62 | 0.67 | 0.95 | 0.96 | 0.3487 |
| Ras-K, H, or N | 0.31 (±0.15) | 0.27 | 0.26 | 0.18 | 0.20 | 0.21 | 0.0093 |
| CaM | 0.40 (±0.11) | 0.26 | 0.40 | 0.20 | 0.21 | 0.20 | 0.0332 |
| Src(pp60) | 0.93 (±0.05) | 0.69 | 0.76 | 0.21 | 0.51 | 0.49 | 0.0315 |
| β3 integrin | 0.77 (±0.03) | 0.59 | 0.37 | 0.59 | 0.49 | 0.53 | 0.7691 |
| IQGAP1 | 0.09 (±0.03) | 0.35 | 0.71 | 0.84 | 0.85 | 0.60 | 0.1276 |
| IQGAP2 | 0.62 (±0.08) | 0.04 | 0.49 | 0.03 | 0.28 | 0.29 | 0.3932 |
| GIT1 | 0.08 (±0.03) | 0.03 | 0.02 | 0.00 | 0.00 | 0.01 | 0.1328 |
| ASAP1 | 0.19 (±0.09) | 0.07 | 0.18 | 0.02 | 0.03 | 0.07 | 0.0554 |
The ratios of different proteins to RabGDIα were calculated from the fluorescent intensities of the immuno-decorated proteins detected in Figure 2. The human values were averaged from the five samples. The standard deviations from the five human samples (Figure 2) were calculated and shown in parenthesis.
To evaluate correlations, the ratios were grouped as OCS+ and OCS− species, averaged, and the significance of the differences between averages were tested by unpaired Student’s t-test. p values<0.05 were considered to be significant (bold).
Results
Platelet size, normalization standard, and sequence conservation of the proteins analysed
Previous studies of the structure of platelets from different species show that platelets from some species lack recognizable OCS structures and do not have the distinctive pattern of internal membrane staining when membrane-impermeant tannic acid is used as a stain [3–7, 22]. Based on previous reports and observations from our laboratory, we separated the platelets from six different species into OCS+ (human, mouse and dog) and OCS− (horse, cow and camel) groups (Table III). Washed platelets from each species were prepared from whole blood. The mean platelet volumes (MPV) were measured using a Z2 Coulter Counter and were comparable to reported values (see Table III). By this metric, human, canine and equine platelets are larger than murine, bovine and camelid platelets. Since human, canine, and murine platelets have an obvious OCS, these data imply that the presence of OCS does not correlate with platelet volume.
Table III.
Characteristics of the platelets examined in the study.
| Species (subspecies) | No. of donors | OCSa | RabGDIαb | MPV ±SDc |
|---|---|---|---|---|
| Human (Homo s. sapiens) | 5 | +[3] | 5.29 | 7.1 ±3.09 [43] |
| Murine (Mus m. musculus) | 10 | +[4] | 5.06 | 5.0 ±2.18 [43, 44] |
| Canine (Canis l. familiaris) | 2 | +[5] | 5.66 | 8.1 ±3.32 [43, 45] |
| Bovine (Bos p. taurus) | 3 | −[22] | 5.47 | 5.2 ±2.43 [45] |
| Camelid (Camelus dromedarius)d | 1 | −[6] | 5.30 | 4.0 ±2.05 [6] |
| Equine (Equus f. caballus) | 2 | −[7] | 6.08 | 7.2 ±2.97[45] |
The presence (+) or absence (−) of OCS in platelets from each species was determine from published studies (Supporting references are indicated);
The RabGDIα-specific bands in Figure 2 were represented as the raw fluorescent intensity per platelet (AU);
The mean platelet volume (MPV) for each species analysed in this study (unit: femtoliter; fL). The published studies for MPV are indicated;
Currently, there is no subspecies.
RabGDI α was selected for normalizing the western blotting analysis due to the following properties: (1) its molecular weight is ~50 kDa; it migrates in the middle of SDS-PAGE gels used and therefore its transfer efficiency should represent the average for all antigens examined; (2) it is a cytoplasmic protein [26] readily solubilized in detergent-containing lysis buffer; (3) its sequence is highly homologous between all the species examined (≥97%, Table I); and (4) it is expressed at similar levels among all the species used in this analysis (Figure 2 and Table III).
A focus of this study was to determine whether the expression levels of small GTPases and their effectors correlate to the presence or absence of an OCS in platelets. Since small GTPases function in remodeling the platelet cytoskeleton and in regulating membrane dynamics they are potential candidates for regulating the OCS. Shown in Table I are the representative members of small GTPases families (Arf, Rho and Ras), regulators (i.e. ArfGAPs) and functional effectors (β3 integrin-related and IQGAP related), which were investigated in this study. The sequence identities of each protein were compared between each species using a web-based, search tool (BLASTP, http://blast.ncbi.nlm.nih.gov/Blast.cgi) and the results showed that there is a high degree of sequence identity between the homologs from each species (Table I). Based on this sequence homology, it was assumed that most of the polyclonal immuno-reagents used for our analysis would recognize the specific proteins across the six species examined. For the proteins that were detected by monoclonal antibodies, the sequences of the specific immunogens were compared and they showed identities greater than 99% (data not shown).
Correlation of Arf6-GTP with OCS
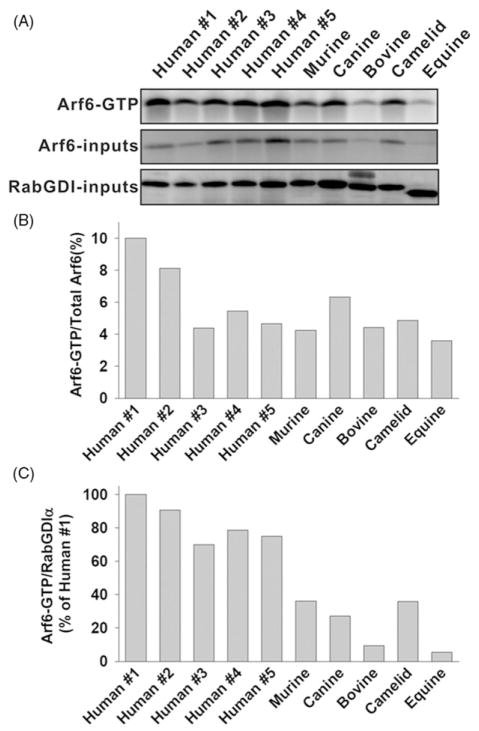
Chinese Hamster Ovary (CHO) cells expressing either constitutively active Arf6 (Arf6-GTP) or a guanine-nucleotide exchange factor (GEF) for Arf6 (EFA6A) show extensive tubular invaginations of the plasma membrane [19, 20]. Purified myristoylated Arf6 induces positive curvature of artificial liposomes, which is essential for tubule formation [21]. We first examined the hypothesis that Arf6-GTP levels correlated with the presence of OCS in resting platelets. Figure 1A shows that both the levels of Arf6-GTP and total Arf6 differed among species and also among individual human donors. However, when the ratios of Arf6-GTP to total Arf6 were calculated, the variation was minimal with the exception of two human samples (Figure 1B). Arf6-GTP was between 5 to 10% of the total Arf6 in all samples tested. To correlate the levels of Arf6-GTP to the presence or absence of OCS, we normalized Arf6-GTP to RabGDIα. Figure 1C shows that human platelets had the highest levels of Arf6-GTP; bovine and equine platelets had the lowest level of Arf6-GTP, while the murine, canine and camelid platelets showed similar levels of Arf6-GTP. Although it does not rule out the functional importance of Arf6 as a regulator of platelet OCS, the data in Figure 1 suggest that there is no clear correlation between the level of Arf6-GTP and the presence of OCS.
Figure 1.
The levels of Arf6-GTP in platelets from different species. (A) Washed platelets (2 ×108) were incubated at 37°C for 5 min, lysed, and analysed for Arf6-GTP as in Methods. An aliquot of each lysate (5%) was immunoblotted for Arf6 or RabGDIα(Total). (B) The ratios of Arf6-GTP to Arf6 total were calculated from fluorescent intensities. (C) The ratios of Arf6-GTP to RabGDIα totals were calculated and normalized to human #1.
Correlation of platelet protein expression with OCS
Since there was no correlation between Arf6-GTP and the presence of OCS in platelets, the expression levels of other candidate proteins were examined to determine whether they had any correlation with the presence of OCS. Figure 2 shows the immunoblots for 18 proteins, including RabGDIα, in platelet extracts from the six different species examined. Gel loading was based on equal platelet numbers (2 ×107/lane) and the immuno-decorated proteins were detected using enhanced chemi-fluorescence. The fluorescence intensity from individual proteins was normalized using that for RabGDIα from the corresponding species and the ratios are presented in Table II. It should be noted that the platelets from each non-human species were pooled from different donors, with the exception of the camel, and thus represent an average of several individuals (see Table III). When the ratios taken from OCS+ (human, murine and canine) platelets were compared to those from OCS− (bovine, camelid and equine) platelets using an unpaired t-test, the levels of Rac1, RhoA, Ras, calmodulin and Src were shown to be significantly higher in OCS+ species (p-values less than 0.05, Table II). Rap1 showed a similar trend but did not reach statistical significance. Although the expression of individual isoforms did not correlate with the presence of OCS, there was a striking difference in the expression of IQGAP1 and IQGAP2 (Figure 2, Table II). Human platelets expressed IQGAP2 as a major isoform whereas IQGAP1 was the predominant isoform in the other species. IQGAP1 levels were eight times higher than those in humans. In summary, the data from Figure 2 and Table II show: (1) most proteins examined are present in platelets from the different species; (2) some protein expression levels differ among species; and (3) the levels of a subset of proteins correlate with the presence of OCS (Table II).
Comparison of platelet aggregation
Given the potential role of OCS in granule release and as a membrane reservoir for platelet spreading, we sought to compare platelet aggregation between the different species, using human platelets as a reference sample. Figure 3 shows the aggregation traces for human platelets and platelets from the other species. No difference was observed in platelets from OCS+ species while the platelets from OCS− species showed delayed responses when activated with human plasma thrombin (0.1 U/mL). Equine platelets showed slower kinetics of aggregation while bovine platelets showed a biphasic kinetic with an initial delayed response followed by a rapid secondary phase. The extents of aggregation for both bovine and equine platelets were the same as for human platelets. The response of camelid platelets was strikingly different. While the platelets did undergo shape change, as evidenced by the increase in absorbance seen upon thrombin addition, no aggregation was observed. This indicates that platelet responses, as measured by aggregometry, were different among OCS+ and OCS− platelets and supports a potential role for OCS in platelet activation. Due to the limited availability of platelet samples, other agonists such as collagen or ADP were not tested.
Figure 3.
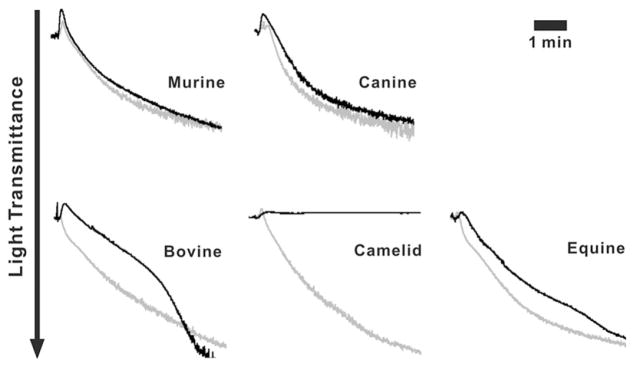
Differences in aggregation among platelets from different species. Washed platelets (4 ×108/mL) were incubated at 37°C for 5 min then stimulated with thrombin (0.1 U/mL) and the aggregation traces were obtained. The aggregation traces from different species (black) were superimposed on those of a human platelet reference sample (grey).
These differences were unlikely due to the use of human thrombin. The sequences of thrombin from the different species show a high degree of homology to human thrombin (murine; 89%, bovine; 88%, equine; 86%, canine and camelid were not available). Additionally, human thrombin has been shown to stimulate platelets from all of these species (except camel) though to differing extents [27, 28]. Consistent with our results, Gader et al. [32] showed that camel platelets are less responsive to human thrombin, despite being sensitive to other agonists.
Discussion
In this study, we examined the expression levels of 18 different proteins found in platelets from six different species. Those species were chosen based on the presence (human, murine, canine) or absence (bovine, camelid, equine) of an obvious OCS in their platelets. Given the previous reports that Arf6 can control membrane curvature and that an increase in Arf6-GTP level results in tubular membrane invagination [19–21], our initial hypothesis was that high levels of Arf6-GTP in resting platelets might control platelet OCS structure. To address this hypothesis, we examined the levels of Arf6-GTP in washed platelets from three OCS+ and three OCS− species. Collectively, the data did not support our hypothesis, though human, murine and canine platelets did have higher levels of Arf6-GTP than did bovine or equine platelets. The correlation was broken in the camelid platelets, which do not have an obvious OCS but have levels of Arf6-GTP comparable to those of OCS+ platelets (Figure 1).
Given that OCS is likely regulated by remodeling of the cytoskeleton and/or the plasma membrane, we next sought to extend our analysis to regulators of actin cytoskeleton and membrane dynamics. The expression levels of 5 different proteins (Rac1, RhoA, Ras, calmodulin and Src) were higher in OCS+ platelets and they showed statistically significant correlations to the presence of OCS (p<0.05, Table II). Of specific interest was the distribution of IQGAP isoforms. IQGAP1 is present in all species but is eight times higher in the non-human species. IQGAP2 is not detectible in murine and bovine platelets. Finally, the platelet responses to thrombin (0.1 U/mL), as measured by aggregometry, were different. Consistent with previous studies [7, 27–32], OCS− platelets showed markedly altered aggregation kinetics as compared to OCS+ platelets.
Since its discovery as an invaginated plasma membrane structure, research has focused on the morphological properties of the OCS structure. In resting platelets, it exists predominantly as a tubular structure that dilates upon activation and may facilitate granular exocytosis [1]. However, it is still not clear how the OCS is maintained in resting platelets or how it is regulated during platelet activation. It has been noted that the amount of membrane invaginations (termed demarcation membrane system; DMS) may dictate the presence or absence of OCS in the platelets [33]. Bovine (OCS−) megakaryocytes have fewer DMS in their pseudopodia, and significant amounts of DMS remain in guinea pig and rat platelet fragments (both are OCS+) [34, 35]. The functional role of OCS has also been difficult to address since little is known about why some species that lack OCS maintain normal hemostasis. Few studies have included a comparative analysis of platelets from OCS+ and OCS− species. Meyers et al. showed that bovine platelets respond to thrombin and arachidonic acid more weakly than do human platelets [29]. Equine and camelid platelets aggregate less than human platelets upon stimulation with ADP [30, 32]. Interestingly, adrenaline and arachidonic acid did not cause aggregation of platelets from either species [31, 32]. These data taken together with the aggregation traces in Figure 3 are at least consistent with OCS− platelets being less responsive to stimulation than are the OCS+ platelets.
The data from our analysis suggest a role for OCS in a platelet’s response to stimuli. The only other correlation that might be relevant is with plasma viscosity (a determinant for shear stress); horse and cow (OCS−) have a slightly higher plasma viscosity than the others (OCS+) with the exception of cat [36]. However, the data are insufficient for further speculations. Future identification of factors regulating platelet OCS will aid in understanding the role of the OCS. Since murine platelets are OCS+, the use of murine knockout models could result in the conversion of murine platelets to an OCS− phenotype, which would be useful for direct functional analyses.
The comparison of total protein levels in different species identified five proteins that are apparently higher in OCS+ species: Rac1, RhoA, Ras, calmodulin and Src (Table II). Rac1 and RhoA are members of Rho family GTPase that control lamellipodia and focal adhesion/stress fiber through regulation of actin cytoskeleton [37]. Given that sub-membrane cytoskeleton remodeling might play roles in OCS dynamics, Rac1 and RhoA may be upstream regulators for this process. Recent studies have shown that extensive cross-talk exists between Ras and Rho GTPase families [38]. A well-characterized pathway involves activation of phosphatidylinositide 3-kinase (PI3K) by Ras and the subsequent stimulation of the Rac and Rho [39]. Thus, one could speculate that OCS might be formed or regulated by coordinated functions of these small GTPases. Since many of calmodulin (CaM)-dependent processes are linked to the regulation of cytoskeleton and adhesion molecules [40, 41], the positive correlation of CaM to the presence of OCS is perhaps not surprising. Src is a protein tyrosine kinase that is involved in many processes including cell-to-matrix adhesion [42]. Src could play a role in OCS dynamics since it can regulate the activation cycle of Rho family GTPases via signaling through integrins [42]. Since the integrins are also present in OCS membranes [1], the formation or regulation of OCS could be, in part, mediated by a Src-dependent pathway.
The measurements in this manuscript address the distribution of different antigens in platelets from various species and demonstrate correlations between protein expression and the presence of OCS. The proteins that are higher in OCS+ species suggest the potential roles of actin cytoskeleton and membrane trafficking in regulation of platelet OCS. Future studies focusing on those proteins that correlate with platelet OCS will provide insights into its regulation and function. The role of OCS in platelet function will be important to understand since certain species lack OCS structure and there appears to be kinetic differences between the responses of OCS+ and OCS− platelets. Appreciating these differences may be useful in dissecting how platelets function in cardiovascular disease.
Acknowledgments
The authors are indebted to the members of the Whiteheart laboratory for their careful reading of and comments on this manuscript. The authors wish to thank Dr. Roy B. Burns DVM for his help and advice and the Louisville Zoological Gardens for access to their collection. We also wish to thank Dr. Karen J. McDowell, Department of Veterinary Sciences, Dr. Glen E. Aiken, Department of Plant and Soil Sciences, and the staff of the Department of Laboratory Animal Resources, University of Kentucky for their help in gathering samples. This work was supported by grants HL56652 and HL091893 from the National Institutes of Health (NIH) to SWW, and a predoctoral fellowship 0615112B from the AHA Great Rivers Affiliate to WC.
Footnotes
Disclosure
The authors have no financial conflicts and thus nothing to disclose.
References
- 1.Klinger MH. The storage lesion of platelets: Ultrastructural and functional aspects. Ann Hematol. 1996;73:103–112. doi: 10.1007/s002770050210. [DOI] [PubMed] [Google Scholar]
- 2.Leistikow EA. Platelet internalization in early thrombogenesis. Semin Thromb Hemost. 1996;22:289–294. doi: 10.1055/s-2007-999021. [DOI] [PubMed] [Google Scholar]
- 3.White JG. A search for the platelet secretory pathway using electron dense tracers. Am J Pathol. 1970;58:31–49. [PMC free article] [PubMed] [Google Scholar]
- 4.Behnke O. Coated pits and vesicles transfer plasma components to platelet granules. Thromb Haemost. 1989;62:718–722. [PubMed] [Google Scholar]
- 5.Kudo S, Onai H, Ogawa R. Response of blood cells to hemorrahagic shock in the dog. J Anesth. 1987;1:51–61. doi: 10.1007/s0054070010051. [DOI] [PubMed] [Google Scholar]
- 6.Gader AG, Ghumlas AK, Hussain MF, Haidari AA, White JG. The ultrastructure of camel blood platelets: A comparative study with human, bovine, and equine cells. Platelets. 2008;19:51–58. doi: 10.1080/09537100701627151. [DOI] [PubMed] [Google Scholar]
- 7.Segura D, Monreal L, Perez-Pujol S, Pino M, Ordinas A, Brugues R, White JG, Escolar G. Assessment of platelet function in horses: Ultrastructure, flow cytometry, and perfusion techniques. J Vet Intern Med. 2006;20:581–588. doi: 10.1892/0891-6640(2006)20[581:aopfih]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 8.Lichtman MA, Kaushansky K, Kipps TJ, Seligsohn U, Prchal JT. Williams Hematology. 8. New York: McGraw-Hill Medical; In press. [Google Scholar]
- 9.Wennerberg K, Rossman KL, Der CJ. The Ras superfamily at a glance. J Cell Sci. 2005;118:843–846. doi: 10.1242/jcs.01660. [DOI] [PubMed] [Google Scholar]
- 10.Soulet C, Gendreau S, Missy K, Benard V, Plantavid M, Payrastre B. Characterisation of Rac activation in thrombin-and collagen-stimulated human blood platelets. FEBS Lett. 2001;507:253–258. doi: 10.1016/s0014-5793(01)02984-2. [DOI] [PubMed] [Google Scholar]
- 11.Vidal C, Geny B, Melle J, Jandrot-Perrus M, Fontenay-Roupie M. Cdc42/Rac1-dependent activation of the p21-activated kinase (PAK) regulates human platelet lamellipodia spreading: Implication of the cortical-actin binding protein cortactin. Blood. 2002;100:4462–4469. doi: 10.1182/blood.V100.13.4462. [DOI] [PubMed] [Google Scholar]
- 12.Soulet C, Hechler B, Gratacap MP, Plantavid M, Offermanns S, Gachet C, Payrastre B. A differential role of the platelet ADP receptors P2Y1 and P2Y12 in Rac activation. J Thromb Haemost. 2005;3:2296–2306. doi: 10.1111/j.1538-7836.2005.01588.x. [DOI] [PubMed] [Google Scholar]
- 13.Leng L, Kashiwagi H, Ren XD, Shattil SJ. RhoA and the function of platelet integrin aIIbb3. Blood. 1998;91:4206–4215. [PubMed] [Google Scholar]
- 14.Wolthuis RM, Franke B, van Triest M, Bauer B, Cool RH, Camonis JH, Akkerman JW, Bos JL. Activation of the small GTPase Ral in platelets. Mol Cell Biol. 1998;18:2486–2491. doi: 10.1128/mcb.18.5.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawato M, Shirakawa R, Kondo H, Higashi T, Ikeda T, Okawa K, Fukai S, Nureki O, Kita T, Horiuchi H. Regulation of platelet dense granule secretion by the Ral GTPase-exocyst pathway. J Biol Chem. 2008;283:166–174. doi: 10.1074/jbc.M705340200. [DOI] [PubMed] [Google Scholar]
- 16.Chrzanowska-Wodnicka M, Smyth SS, Schoenwaelder SM, Fischer TH, White GC., 2nd Rap1b is required for normal platelet function and hemostasis in mice. J Clin Invest. 2005;115:680–687. doi: 10.1172/JCI22973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi W, Karim ZA, Whiteheart SW. Arf6 plays an early role in platelet activation by collagen and convulxin. Blood. 2006;107:3145–3152. doi: 10.1182/blood-2005-09-3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karim ZA, Choi W, Whiteheart SW. Primary platelet signaling cascades and integrin-mediated signaling control ADP-ribosylation factor (Arf) 6-GTP levels during platelet activation and aggregation. J Biol Chem. 2008;283:11995–12003. doi: 10.1074/jbc.M800146200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D’Souza-Schorey C, van Donselaar E, Hsu VW, Yang C, Stahl PD, Peters PJ. ARF6 targets recycling vesicles to the plasma membrane: Insights from an ultrastructural investigation. J Cell Biol. 1998;140:603–616. doi: 10.1083/jcb.140.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franco M, Peters PJ, Boretto J, van Donselaar E, Neri A, D’Souza-Schorey C, Chavrier P. EFA6, a sec7 domain-containing exchange factor for ARF6, coordinates membrane recycling and actin cytoskeleton organization. EMBO J. 1999;18:1480–1491. doi: 10.1093/emboj/18.6.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lundmark R, Doherty GJ, Vallis Y, Peter BJ, McMahon HT. Arf family GTP loading is activated by, and generates, positive membrane curvature. Biochem J. 2008;414:189–194. doi: 10.1042/BJ20081237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zucker-Franklin D, Benson KA, Myers KM. Absence of a surface-connected canalicular system in bovine platelets. Blood. 1985;65:241–244. [PubMed] [Google Scholar]
- 23.Rutledge TW, Whiteheart SW. SNAP-23 is a target for calpain cleavage in activated platelets. J Biol Chem. 2002;277:37009–37015. doi: 10.1074/jbc.M204526200. [DOI] [PubMed] [Google Scholar]
- 24.Dell’Angelica EC, Puertollano R, Mullins C, Aguilar RC, Vargas JD, Hartnell LM, Bonifacino JS. GGAs: A family of ADP ribosylation factor-binding proteins related to adaptors and associated with the Golgi complex. J Cell Biol. 2000;149:81–94. doi: 10.1083/jcb.149.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takatsu H, Yoshino K, Toda K, Nakayama K. GGA proteins associate with Golgi membranes through interaction between their GGAH domains and ADP-ribosylation factors. Biochem J. 2002;365:369–378. doi: 10.1042/BJ20020428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfeffer SR, Dirac-Svejstrup AB, Soldati T. Rab GDP dissociation inhibitor: Putting rab GTPases in the right place. J Biol Chem. 1995;270:17057–17059. doi: 10.1074/jbc.270.29.17057. [DOI] [PubMed] [Google Scholar]
- 27.Nylander S, Mattsson C, Lindahl TL. Characterisation of species differences in the platelet ADP and thrombin response. Thromb Res. 2006;117:543–549. doi: 10.1016/j.thromres.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 28.Callan MB, Shofer FS, Wojenski C, Giger U. Chrono-lume and magnesium potentiate aggregation of canine but not human platelets in citrated platelet-rich plasma. Thromb Haemost. 1998;80:176–180. [PubMed] [Google Scholar]
- 29.Meyers KM, Katz JB, Clemmons RM, Smith JB, Holmsen H. An evaluation of the arachidonate pathway of platelets from companion and food-producing animals, mink, and man. Thromb Res. 1980;20:13–24. doi: 10.1016/0049-3848(80)90052-3. [DOI] [PubMed] [Google Scholar]
- 30.Mateos-Trigos G, Evans RJ, Heath MF. Effects of a P2Y12 receptor antagonist on the response of equine platelets to ADP. Comparison with human platelets. Res Vet Sci. 2002;73:171–175. doi: 10.1016/s0034-5288(02)00096-6. [DOI] [PubMed] [Google Scholar]
- 31.Evans R. Blood platelets and their role in the genesis and sequelae of intestinal ischaemia. Equine Vet J. 1992;13:31–37. [Google Scholar]
- 32.Abdel Gader A, Al Ghumlas A, Hussain M, Al Haidary A. Platelet aggregation and platelet function analyzer 100 (PFA-100) closure time in camels – A comparative study with humans. Comparative Clinical Pathology. 2006;15:31–37. [Google Scholar]
- 33.Topp KS, Tablin F, Levin J. Culture of isolated bovine megakaryocytes on reconstituted basement membrane matrix leads to proplatelet process formation. Blood. 1990;76:912–924. [PubMed] [Google Scholar]
- 34.Tablin F, Castro M, Leven RM. Blood platelet formation in vitro. The role of the cytoskeleton in megakaryocyte fragmentation. J Cell Sci. 1990;97(Pt 1):59–70. doi: 10.1242/jcs.97.1.59. [DOI] [PubMed] [Google Scholar]
- 35.Leven RM, Tablin F. Megakaryocyte and platelet ultrastructure in the Wistar Furth rat. Am J Pathol. 1988;132:417–426. [PMC free article] [PubMed] [Google Scholar]
- 36.Windberger U, Bartholovitsch A, Plasenzotti R, Korak KJ, Heinze G. Whole blood viscosity, plasma viscosity and erythrocyte aggregation in nine mammalian species: Reference values and comparison of data. Exp Physiol. 2003;88:431–440. doi: 10.1113/eph8802496. [DOI] [PubMed] [Google Scholar]
- 37.Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem J. 2000;348(Pt 2):241–255. [PMC free article] [PubMed] [Google Scholar]
- 38.Bar-Sagi D, Hall A. Ras and Rho GTPases: A family reunion. Cell. 2000;103:227–238. doi: 10.1016/s0092-8674(00)00115-x. [DOI] [PubMed] [Google Scholar]
- 39.Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol Rev. 2001;81:153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- 40.Briggs MW, Sacks DB. IQGAP proteins are integral components of cytoskeletal regulation. EMBO Rep. 2003;4:571–574. doi: 10.1038/sj.embor.embor867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sobue K, Kanda K, Adachi J, Kakiuchi S. Calmodulin-binding proteins that interact with actin filaments in a Ca2+-dependent flip-flop manner: Survey in brain and secretory tissues. Proc Natl Acad Sci U S A. 1983;80:6868–6871. doi: 10.1073/pnas.80.22.6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huveneers S, Danen EH. Adhesion signaling – crosstalk between integrins, Src and Rho. J Cell Sci. 2009;122:1059–1069. doi: 10.1242/jcs.039446. [DOI] [PubMed] [Google Scholar]
- 43.Nakeff A, Ingram M. Platelet count: Volume relationships in four mammalian species. J Appl Physiol. 1970;28:530–533. doi: 10.1152/jappl.1970.28.4.530. [DOI] [PubMed] [Google Scholar]
- 44.Jackson CW, Steward SA, Chenaille PJ, Ashmun RA, McDonald TP. An analysis of megakaryocytopoiesis in the C3H mouse: An animal model whose megakaryocytes have 32N as the modal DNA class. Blood. 1990;76:690–696. [PubMed] [Google Scholar]
- 45.Clemmons RM, Bliss EL, Dorsey-Lee MR, Seachord CL, Meyers KM. Platelet function, size and yield in whole blood and in platelet-rich plasma prepared using differing centrifugation force and time in domestic and food-producing animals. Thromb Haemost. 1983;50:838–843. [PubMed] [Google Scholar]