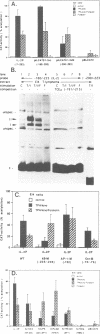
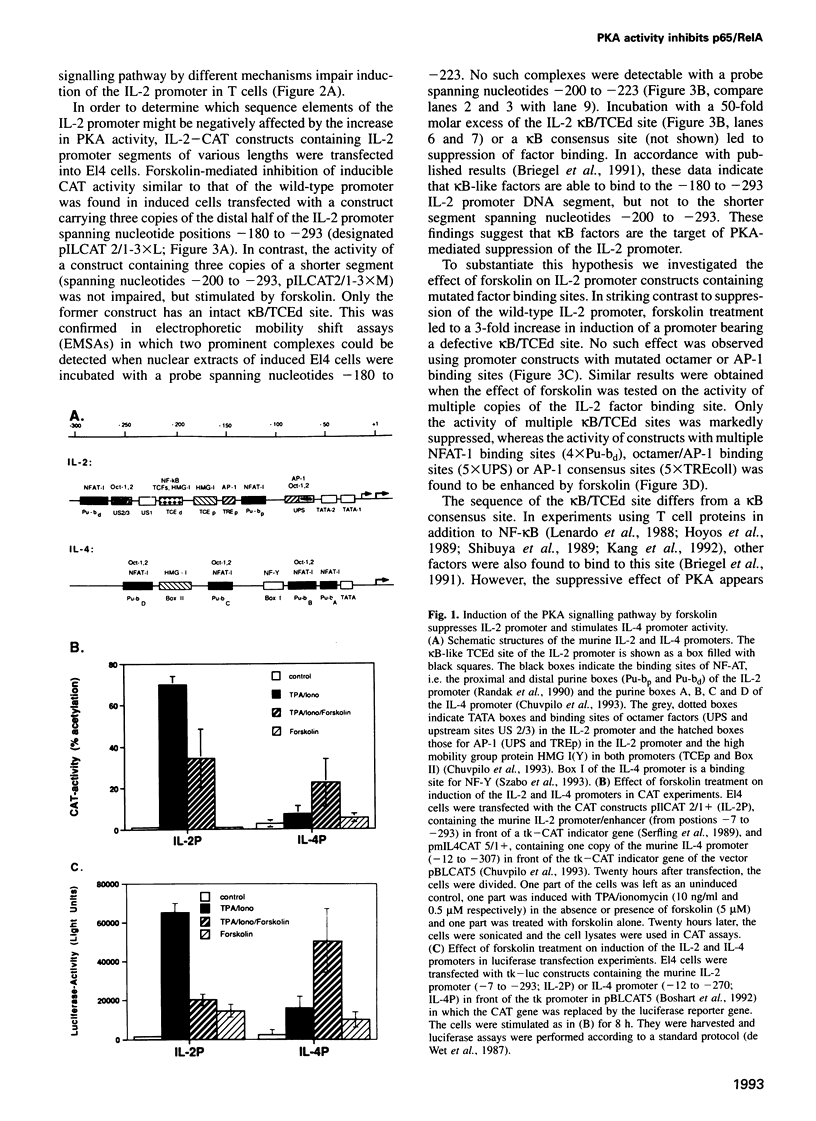
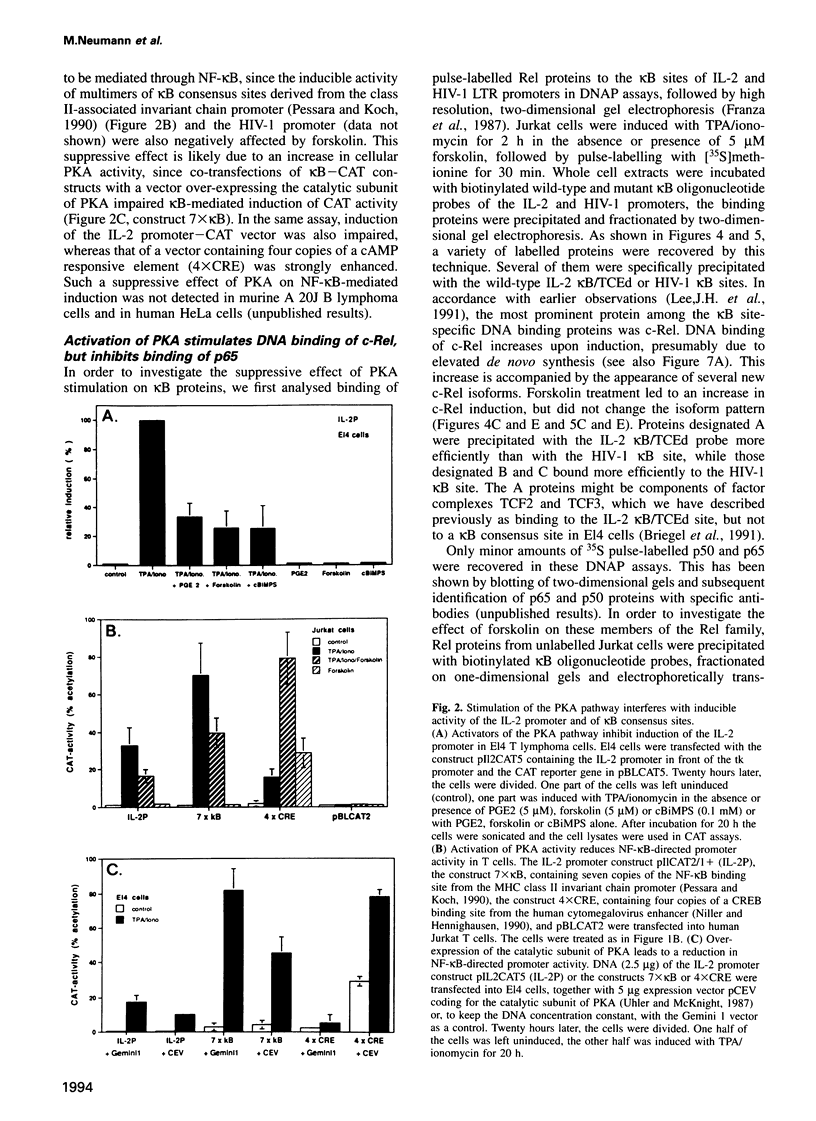
Abstract
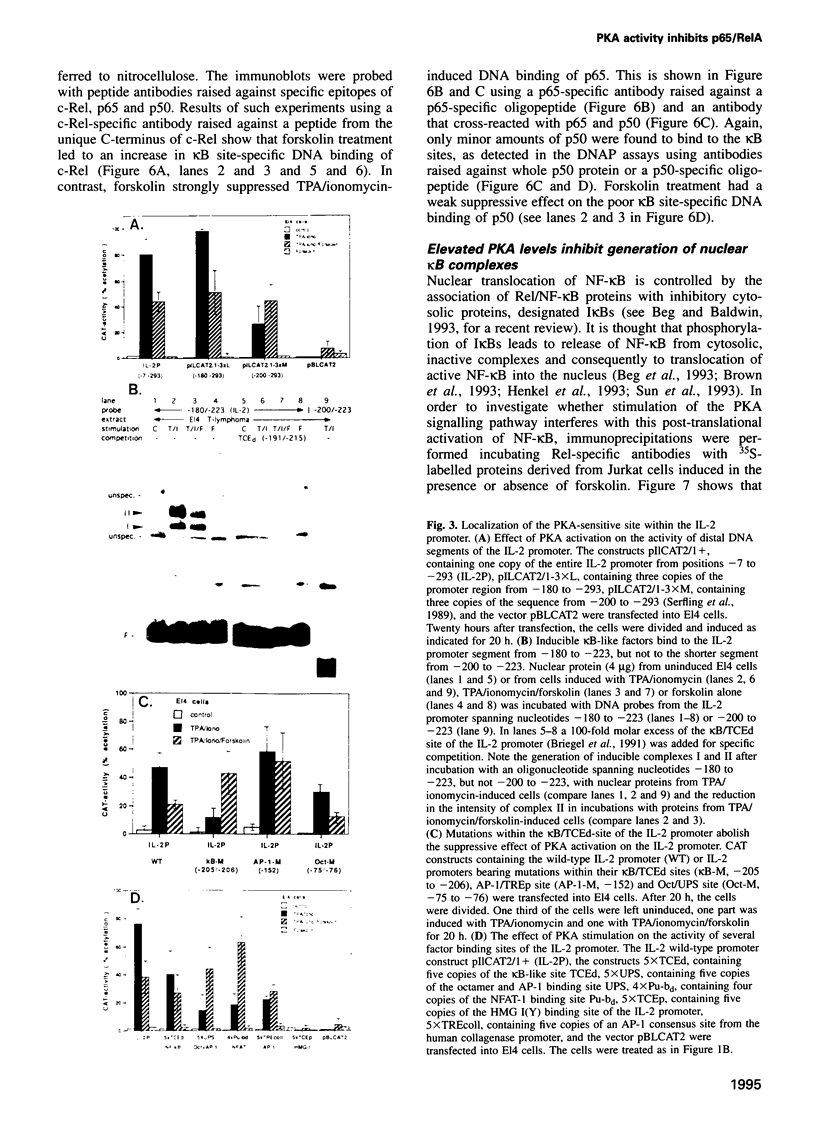
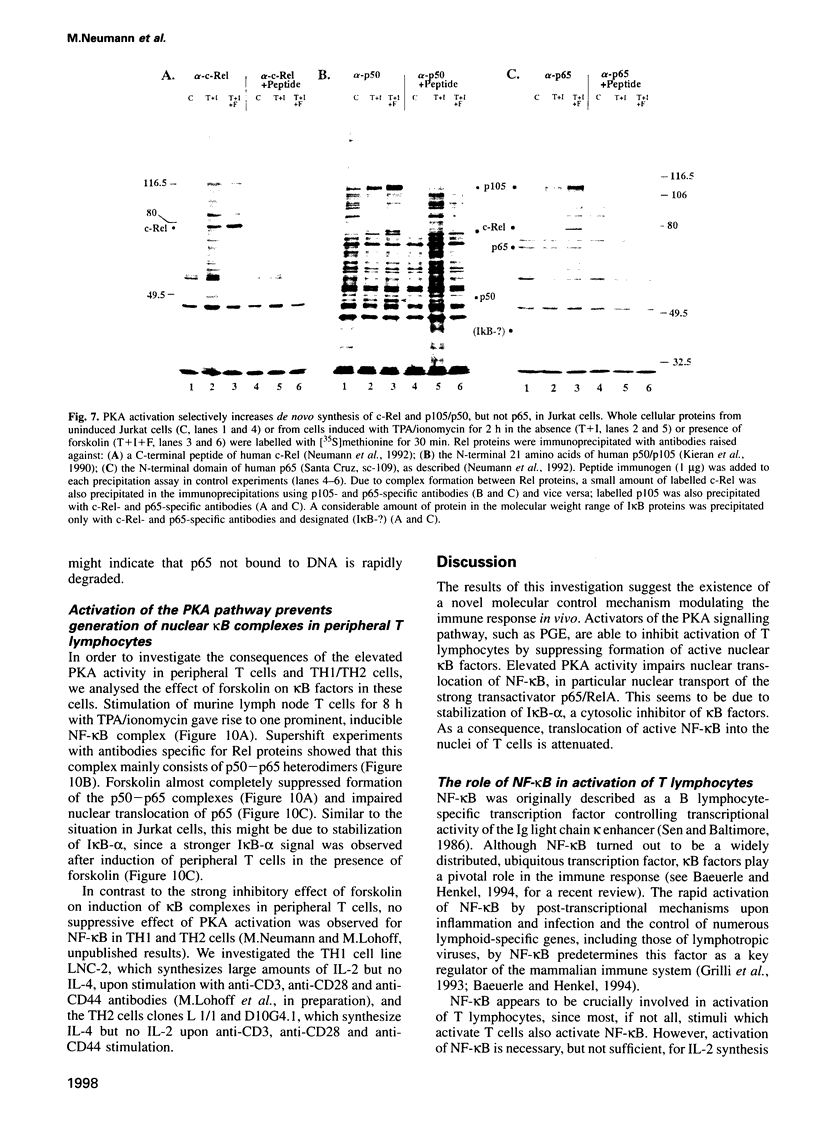
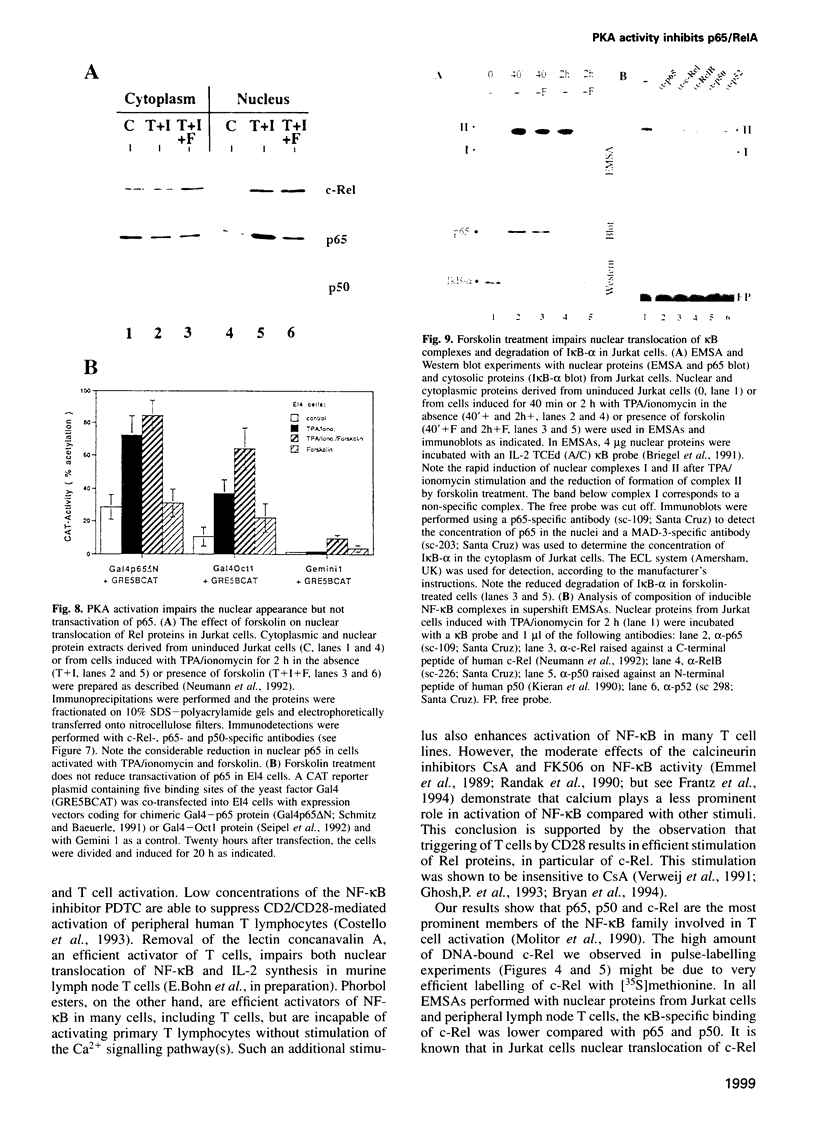
Stimulation of the protein kinase A (PKA) signalling pathway exerts an inhibitory effect on the proliferation of numerous cells, including T lymphocytes. In CD4+ T helper cells, stimulation of PKA leads to suppression of interleukin 2 (IL-2) induction, while induction of the genes coding for the lymphokines IL-4 and IL-5 is enhanced. We show that the differential effect of PKA activity on induction of the IL-2 and IL-4 genes is mediated through their promoters. One major target of the suppressive effect of PKA is the kappa B site in the IL-2 promoter. A kappa B site is missing in the IL-4 promoter. Mutations preventing factor binding to the IL-2 kappa B site result in a loss of PKA-mediated suppression of IL-2 promoter activity. Furthermore, activation of the PKA signalling pathway impairs the inducible activity of multiple kappa B sites of the IL-2 promoter, but not of other factor binding sites. The reduction in activity of kappa B sites in activated and PKA-stimulated T cells is accompanied by changes in the concentration and DNA binding of Rel/NF-kappa B factors. Stimulation of the PKA pathway in Jurkat T cells with the PKA activator forskolin leads to an increase in synthesis of c-Rel and p105/p50, while synthesis of p65/RelA remains unchanged. However, nuclear translocation and DNA binding of p65 is distinctly impaired, probably due to a retarded degradation of I kappa B-alpha. In a similar way, stimulation of the PKA signalling pathway inhibits nuclear translocation of p65 and generation of nuclear kappa B complexes in peripheral T lymphocytes from murine lymph nodes. These results indicate that PKA-mediated suppression of NF-kappa B activity plays an important role in the control of activation of peripheral T lymphocytes.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baeuerle P. A., Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- Baxter J. D., Forsham P. H. Tissue effects of glucocorticoids. Am J Med. 1972 Nov;53(5):573–589. doi: 10.1016/0002-9343(72)90154-4. [DOI] [PubMed] [Google Scholar]
- Beg A. A., Baldwin A. S., Jr The I kappa B proteins: multifunctional regulators of Rel/NF-kappa B transcription factors. Genes Dev. 1993 Nov;7(11):2064–2070. doi: 10.1101/gad.7.11.2064. [DOI] [PubMed] [Google Scholar]
- Beg A. A., Finco T. S., Nantermet P. V., Baldwin A. S., Jr Tumor necrosis factor and interleukin-1 lead to phosphorylation and loss of I kappa B alpha: a mechanism for NF-kappa B activation. Mol Cell Biol. 1993 Jun;13(6):3301–3310. doi: 10.1128/mcb.13.6.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz M., Fox B. S. Prostaglandin E2 inhibits production of Th1 lymphokines but not of Th2 lymphokines. J Immunol. 1991 Jan 1;146(1):108–113. [PubMed] [Google Scholar]
- Boshart M., Klüppel M., Schmidt A., Schütz G., Luckow B. Reporter constructs with low background activity utilizing the cat gene. Gene. 1992 Jan 2;110(1):129–130. doi: 10.1016/0378-1119(92)90456-y. [DOI] [PubMed] [Google Scholar]
- Briegel K., Hentsch B., Pfeuffer I., Serfling E. One base pair change abolishes the T cell-restricted activity of a kB-like proto-enhancer element from the interleukin 2 promoter. Nucleic Acids Res. 1991 Nov 11;19(21):5929–5936. doi: 10.1093/nar/19.21.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K., Park S., Kanno T., Franzoso G., Siebenlist U. Mutual regulation of the transcriptional activator NF-kappa B and its inhibitor, I kappa B-alpha. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2532–2536. doi: 10.1073/pnas.90.6.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce J., Symington F. W., McKearn T. J., Sprent J. A monoclonal antibody discriminating between subsets of T and B cells. J Immunol. 1981 Dec;127(6):2496–2501. [PubMed] [Google Scholar]
- Bruder J. T., Heidecker G., Tan T. H., Weske J. C., Derse D., Rapp U. R. Oncogene activation of HIV-LTR-driven expression via the NF-kappa B binding sites. Nucleic Acids Res. 1993 Nov 11;21(22):5229–5234. doi: 10.1093/nar/21.22.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan R. G., Li Y., Lai J. H., Van M., Rice N. R., Rich R. R., Tan T. H. Effect of CD28 signal transduction on c-Rel in human peripheral blood T cells. Mol Cell Biol. 1994 Dec;14(12):7933–7942. doi: 10.1128/mcb.14.12.7933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgering B. M., Pronk G. J., van Weeren P. C., Chardin P., Bos J. L. cAMP antagonizes p21ras-directed activation of extracellular signal-regulated kinase 2 and phosphorylation of mSos nucleotide exchange factor. EMBO J. 1993 Nov;12(11):4211–4220. doi: 10.1002/j.1460-2075.1993.tb06105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuvpilo S., Schomberg C., Gerwig R., Heinfling A., Reeves R., Grummt F., Serfling E. Multiple closely-linked NFAT/octamer and HMG I(Y) binding sites are part of the interleukin-4 promoter. Nucleic Acids Res. 1993 Dec 11;21(24):5694–5704. doi: 10.1093/nar/21.24.5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clipstone N. A., Crabtree G. R. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature. 1992 Jun 25;357(6380):695–697. doi: 10.1038/357695a0. [DOI] [PubMed] [Google Scholar]
- Cook S. J., McCormick F. Inhibition by cAMP of Ras-dependent activation of Raf. Science. 1993 Nov 12;262(5136):1069–1072. doi: 10.1126/science.7694367. [DOI] [PubMed] [Google Scholar]
- Costello R., Lipcey C., Algarté M., Cerdan C., Baeuerle P. A., Olive D., Imbert J. Activation of primary human T-lymphocytes through CD2 plus CD28 adhesion molecules induces long-term nuclear expression of NF-kappa B. Cell Growth Differ. 1993 Apr;4(4):329–339. [PubMed] [Google Scholar]
- Cupps T. R., Fauci A. S. Corticosteroid-mediated immunoregulation in man. Immunol Rev. 1982;65:133–155. doi: 10.1111/j.1600-065x.1982.tb00431.x. [DOI] [PubMed] [Google Scholar]
- Daum G., Eisenmann-Tappe I., Fries H. W., Troppmair J., Rapp U. R. The ins and outs of Raf kinases. Trends Biochem Sci. 1994 Nov;19(11):474–480. doi: 10.1016/0968-0004(94)90133-3. [DOI] [PubMed] [Google Scholar]
- Doerre S., Sista P., Sun S. C., Ballard D. W., Greene W. C. The c-rel protooncogene product represses NF-kappa B p65-mediated transcriptional activation of the long terminal repeat of type 1 human immunodeficiency virus. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):1023–1027. doi: 10.1073/pnas.90.3.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downward J., Graves J. D., Warne P. H., Rayter S., Cantrell D. A. Stimulation of p21ras upon T-cell activation. Nature. 1990 Aug 23;346(6286):719–723. doi: 10.1038/346719a0. [DOI] [PubMed] [Google Scholar]
- Emmel E. A., Verweij C. L., Durand D. B., Higgins K. M., Lacy E., Crabtree G. R. Cyclosporin A specifically inhibits function of nuclear proteins involved in T cell activation. Science. 1989 Dec 22;246(4937):1617–1620. doi: 10.1126/science.2595372. [DOI] [PubMed] [Google Scholar]
- Finco T. S., Baldwin A. S., Jr Kappa B site-dependent induction of gene expression by diverse inducers of nuclear factor kappa B requires Raf-1. J Biol Chem. 1993 Aug 25;268(24):17676–17679. [PubMed] [Google Scholar]
- Firpo E. J., Koff A., Solomon M. J., Roberts J. M. Inactivation of a Cdk2 inhibitor during interleukin 2-induced proliferation of human T lymphocytes. Mol Cell Biol. 1994 Jul;14(7):4889–4901. doi: 10.1128/mcb.14.7.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz B., Nordby E. C., Bren G., Steffan N., Paya C. V., Kincaid R. L., Tocci M. J., O'Keefe S. J., O'Neill E. A. Calcineurin acts in synergy with PMA to inactivate I kappa B/MAD3, an inhibitor of NF-kappa B. EMBO J. 1994 Feb 15;13(4):861–870. doi: 10.1002/j.1460-2075.1994.tb06329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franza B. R., Jr, Josephs S. F., Gilman M. Z., Ryan W., Clarkson B. Characterization of cellular proteins recognizing the HIV enhancer using a microscale DNA-affinity precipitation assay. 1987 Nov 26-Dec 2Nature. 330(6146):391–395. doi: 10.1038/330391a0. [DOI] [PubMed] [Google Scholar]
- Fraser J. D., Straus D., Weiss A. Signal transduction events leading to T-cell lymphokine gene expression. Immunol Today. 1993 Jul;14(7):357–362. doi: 10.1016/0167-5699(93)90236-E. [DOI] [PubMed] [Google Scholar]
- Ghosh P., Tan T. H., Rice N. R., Sica A., Young H. A. The interleukin 2 CD28-responsive complex contains at least three members of the NF kappa B family: c-Rel, p50, and p65. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1696–1700. doi: 10.1073/pnas.90.5.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S., Baltimore D. Activation in vitro of NF-kappa B by phosphorylation of its inhibitor I kappa B. Nature. 1990 Apr 12;344(6267):678–682. doi: 10.1038/344678a0. [DOI] [PubMed] [Google Scholar]
- Graves L. M., Bornfeldt K. E., Raines E. W., Potts B. C., Macdonald S. G., Ross R., Krebs E. G. Protein kinase A antagonizes platelet-derived growth factor-induced signaling by mitogen-activated protein kinase in human arterial smooth muscle cells. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10300–10304. doi: 10.1073/pnas.90.21.10300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilli M., Chiu J. J., Lenardo M. J. NF-kappa B and Rel: participants in a multiform transcriptional regulatory system. Int Rev Cytol. 1993;143:1–62. doi: 10.1016/s0074-7696(08)61873-2. [DOI] [PubMed] [Google Scholar]
- Hasler F., Bluestein H. G., Zvaifler N. J., Epstein L. B. Analysis of the defects responsible for the impaired regulation of EBV-induced B cell proliferation by rheumatoid arthritis lymphocytes. II. Role of monocytes and the increased sensitivity of rheumatoid arthritis lymphocytes to prostaglandin E. J Immunol. 1983 Aug;131(2):768–772. [PubMed] [Google Scholar]
- Heck S., Kullmann M., Gast A., Ponta H., Rahmsdorf H. J., Herrlich P., Cato A. C. A distinct modulating domain in glucocorticoid receptor monomers in the repression of activity of the transcription factor AP-1. EMBO J. 1994 Sep 1;13(17):4087–4095. doi: 10.1002/j.1460-2075.1994.tb06726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkel T., Machleidt T., Alkalay I., Krönke M., Ben-Neriah Y., Baeuerle P. A. Rapid proteolysis of I kappa B-alpha is necessary for activation of transcription factor NF-kappa B. Nature. 1993 Sep 9;365(6442):182–185. doi: 10.1038/365182a0. [DOI] [PubMed] [Google Scholar]
- Hentsch B., Mouzaki A., Pfeuffer I., Rungger D., Serfling E. The weak, fine-tuned binding of ubiquitous transcription factors to the Il-2 enhancer contributes to its T cell-restricted activity. Nucleic Acids Res. 1992 Jun 11;20(11):2657–2665. doi: 10.1093/nar/20.11.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hordijk P. L., Verlaan I., Jalink K., van Corven E. J., Moolenaar W. H. cAMP abrogates the p21ras-mitogen-activated protein kinase pathway in fibroblasts. J Biol Chem. 1994 Feb 4;269(5):3534–3538. [PubMed] [Google Scholar]
- Hoyos B., Ballard D. W., Böhnlein E., Siekevitz M., Greene W. C. Kappa B-specific DNA binding proteins: role in the regulation of human interleukin-2 gene expression. Science. 1989 Apr 28;244(4903):457–460. doi: 10.1126/science.2497518. [DOI] [PubMed] [Google Scholar]
- Häfner S., Adler H. S., Mischak H., Janosch P., Heidecker G., Wolfman A., Pippig S., Lohse M., Ueffing M., Kolch W. Mechanism of inhibition of Raf-1 by protein kinase A. Mol Cell Biol. 1994 Oct;14(10):6696–6703. doi: 10.1128/mcb.14.10.6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo M., Leevers S. J., Marshall C. J., Cantrell D. p21ras couples the T cell antigen receptor to extracellular signal-regulated kinase 2 in T lymphocytes. J Exp Med. 1993 Oct 1;178(4):1199–1208. doi: 10.1084/jem.178.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June C. H., Bluestone J. A., Nadler L. M., Thompson C. B. The B7 and CD28 receptor families. Immunol Today. 1994 Jul;15(7):321–331. doi: 10.1016/0167-5699(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Kammer G. M. The adenylate cyclase-cAMP-protein kinase A pathway and regulation of the immune response. Immunol Today. 1988 Jul-Aug;9(7-8):222–229. doi: 10.1016/0167-5699(88)91220-0. [DOI] [PubMed] [Google Scholar]
- Kang S. M., Tran A. C., Grilli M., Lenardo M. J. NF-kappa B subunit regulation in nontransformed CD4+ T lymphocytes. Science. 1992 Jun 5;256(5062):1452–1456. doi: 10.1126/science.1604322. [DOI] [PubMed] [Google Scholar]
- Kato J. Y., Matsuoka M., Polyak K., Massagué J., Sherr C. J. Cyclic AMP-induced G1 phase arrest mediated by an inhibitor (p27Kip1) of cyclin-dependent kinase 4 activation. Cell. 1994 Nov 4;79(3):487–496. doi: 10.1016/0092-8674(94)90257-7. [DOI] [PubMed] [Google Scholar]
- Kaye J., Porcelli S., Tite J., Jones B., Janeway C. A., Jr Both a monoclonal antibody and antisera specific for determinants unique to individual cloned helper T cell lines can substitute for antigen and antigen-presenting cells in the activation of T cells. J Exp Med. 1983 Sep 1;158(3):836–856. doi: 10.1084/jem.158.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr L. D., Inoue J., Davis N., Link E., Baeuerle P. A., Bose H. R., Jr, Verma I. M. The rel-associated pp40 protein prevents DNA binding of Rel and NF-kappa B: relationship with I kappa B beta and regulation by phosphorylation. Genes Dev. 1991 Aug;5(8):1464–1476. doi: 10.1101/gad.5.8.1464. [DOI] [PubMed] [Google Scholar]
- Kieran M., Blank V., Logeat F., Vandekerckhove J., Lottspeich F., Le Bail O., Urban M. B., Kourilsky P., Baeuerle P. A., Israël A. The DNA binding subunit of NF-kappa B is identical to factor KBF1 and homologous to the rel oncogene product. Cell. 1990 Sep 7;62(5):1007–1018. doi: 10.1016/0092-8674(90)90275-j. [DOI] [PubMed] [Google Scholar]
- Kincade P. W., Lee G., Watanabe T., Sun L., Scheid M. P. Antigens displayed on murine B lymphocyte precursors. J Immunol. 1981 Dec;127(6):2262–2268. [PubMed] [Google Scholar]
- Lederer J. A., Liou J. S., Todd M. D., Glimcher L. H., Lichtman A. H. Regulation of cytokine gene expression in T helper cell subsets. J Immunol. 1994 Jan 1;152(1):77–86. [PubMed] [Google Scholar]
- Lee H. J., Koyano-Nakagawa N., Naito Y., Nishida J., Arai N., Arai K., Yokota T. cAMP activates the IL-5 promoter synergistically with phorbol ester through the signaling pathway involving protein kinase A in mouse thymoma line EL-4. J Immunol. 1993 Dec 1;151(11):6135–6142. [PubMed] [Google Scholar]
- Lee J. H., Li Y. C., Doerre S., Sista P., Ballard D. W., Greene W. C., Franza B. R., Jr A member of the set of kappa B binding proteins, HIVEN86A, is a product of the human c-rel proto-oncogene. Oncogene. 1991 Apr;6(4):665–667. [PubMed] [Google Scholar]
- Lenardo M. J., Kuang A., Gifford A., Baltimore D. NF-kappa B protein purification from bovine spleen: nucleotide stimulation and binding site specificity. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8825–8829. doi: 10.1073/pnas.85.23.8825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo O., Foo M., Sachs D. H., Samelson L. E., Bluestone J. A. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1374–1378. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link E., Kerr L. D., Schreck R., Zabel U., Verma I., Baeuerle P. A. Purified I kappa B-beta is inactivated upon dephosphorylation. J Biol Chem. 1992 Jan 5;267(1):239–246. [PubMed] [Google Scholar]
- Linsley P. S., Ledbetter J. A. The role of the CD28 receptor during T cell responses to antigen. Annu Rev Immunol. 1993;11:191–212. doi: 10.1146/annurev.iy.11.040193.001203. [DOI] [PubMed] [Google Scholar]
- Liu J. FK506 and cyclosporin, molecular probes for studying intracellular signal transduction. Immunol Today. 1993 Jun;14(6):290–295. doi: 10.1016/0167-5699(93)90048-P. [DOI] [PubMed] [Google Scholar]
- Liu J., Farmer J. D., Jr, Lane W. S., Friedman J., Weissman I., Schreiber S. L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991 Aug 23;66(4):807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- Lohoff M., Matzner C., Röllinghoff M. Polyclonal B-cell stimulation by L3T4+ T cells in experimental leishmaniasis. Infect Immun. 1988 Aug;56(8):2120–2124. doi: 10.1128/iai.56.8.2120-2124.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohoff M., Schmitt E., Reske-Kunz A. B., Röllinghoff M. Different response of TH1 cells for stimulation with anti-CD3 antibodies. Eur J Immunol. 1990 Mar;20(3):653–658. doi: 10.1002/eji.1830200328. [DOI] [PubMed] [Google Scholar]
- Miyamoto S., Chiao P. J., Verma I. M. Enhanced I kappa B alpha degradation is responsible for constitutive NF-kappa B activity in mature murine B-cell lines. Mol Cell Biol. 1994 May;14(5):3276–3282. doi: 10.1128/mcb.14.5.3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molitor J. A., Walker W. H., Doerre S., Ballard D. W., Greene W. C. NF-kappa B: a family of inducible and differentially expressed enhancer-binding proteins in human T cells. Proc Natl Acad Sci U S A. 1990 Dec;87(24):10028–10032. doi: 10.1073/pnas.87.24.10028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosialos G., Hamer P., Capobianco A. J., Laursen R. A., Gilmore T. D. A protein kinase-A recognition sequence is structurally linked to transformation by p59v-rel and cytoplasmic retention of p68c-rel. Mol Cell Biol. 1991 Dec;11(12):5867–5877. doi: 10.1128/mcb.11.12.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz E., Zubiaga A. M., Merrow M., Sauter N. P., Huber B. T. Cholera toxin discriminates between T helper 1 and 2 cells in T cell receptor-mediated activation: role of cAMP in T cell proliferation. J Exp Med. 1990 Jul 1;172(1):95–103. doi: 10.1084/jem.172.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumann M., Scheidereit C. Activation of NF-kappa B in vivo is regulated by multiple phosphorylations. EMBO J. 1994 Oct 3;13(19):4597–4607. doi: 10.1002/j.1460-2075.1994.tb06781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M., Tsapos K., Scheppler J. A., Ross J., Franza B. R., Jr Identification of complex formation between two intracellular tyrosine kinase substrates: human c-Rel and the p105 precursor of p50 NF-kappa B. Oncogene. 1992 Nov;7(11):2095–2104. [PubMed] [Google Scholar]
- Niller H. H., Hennighausen L. Phytohemagglutinin-induced activity of cyclic AMP (cAMP) response elements from cytomegalovirus is reduced by cyclosporine and synergistically enhanced by cAMP. J Virol. 1990 May;64(5):2388–2391. doi: 10.1128/jvi.64.5.2388-2391.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak T. J., Rothenberg E. V. cAMP inhibits induction of interleukin 2 but not of interleukin 4 in T cells. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9353–9357. doi: 10.1073/pnas.87.23.9353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe S. J., Tamura J., Kincaid R. L., Tocci M. J., O'Neill E. A. FK-506- and CsA-sensitive activation of the interleukin-2 promoter by calcineurin. Nature. 1992 Jun 25;357(6380):692–694. doi: 10.1038/357692a0. [DOI] [PubMed] [Google Scholar]
- Owaki H., Varma R., Gillis B., Bruder J. T., Rapp U. R., Davis L. S., Geppert T. D. Raf-1 is required for T cell IL2 production. EMBO J. 1993 Nov;12(11):4367–4373. doi: 10.1002/j.1460-2075.1993.tb06121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. H., Levitt L. Overexpression of mitogen-activated protein kinase (ERK1) enhances T-cell cytokine gene expression: role of AP1, NF-AT, and NF-KB. Blood. 1993 Oct 15;82(8):2470–2477. [PubMed] [Google Scholar]
- Pessara U., Koch N. Tumor necrosis factor alpha regulates expression of the major histocompatibility complex class II-associated invariant chain by binding of an NF-kappa B-like factor to a promoter element. Mol Cell Biol. 1990 Aug;10(8):4146–4154. doi: 10.1128/mcb.10.8.4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeuffer I., Klein-Hessling S., Heinfling A., Chuvpilo S., Escher C., Brabletz T., Hentsch B., Schwarzenbach H., Matthias P., Serfling E. Octamer factors exert a dual effect on the IL-2 and IL-4 promoters. J Immunol. 1994 Dec 15;153(12):5572–5585. [PubMed] [Google Scholar]
- Phipps R. P., Stein S. H., Roper R. L. A new view of prostaglandin E regulation of the immune response. Immunol Today. 1991 Oct;12(10):349–352. doi: 10.1016/0167-5699(91)90064-Z. [DOI] [PubMed] [Google Scholar]
- Randak C., Brabletz T., Hergenröther M., Sobotta I., Serfling E. Cyclosporin A suppresses the expression of the interleukin 2 gene by inhibiting the binding of lymphocyte-specific factors to the IL-2 enhancer. EMBO J. 1990 Aug;9(8):2529–2536. doi: 10.1002/j.1460-2075.1990.tb07433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A. Signaling mechanisms in T cells. Crit Rev Immunol. 1991;10(6):495–519. [PubMed] [Google Scholar]
- Rappaport R. S., Dodge G. R. Prostaglandin E inhibits the production of human interleukin 2. J Exp Med. 1982 Mar 1;155(3):943–948. doi: 10.1084/jem.155.3.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd C. E., Janssen O., Cai Y. C., da Silva A. J., Raab M., Prasad K. V. Two-step TCR zeta/CD3-CD4 and CD28 signaling in T cells: SH2/SH3 domains, protein-tyrosine and lipid kinases. Immunol Today. 1994 May;15(5):225–234. doi: 10.1016/0167-5699(94)90248-8. [DOI] [PubMed] [Google Scholar]
- Russell M., Winitz S., Johnson G. L. Acetylcholine muscarinic m1 receptor regulation of cyclic AMP synthesis controls growth factor stimulation of Raf activity. Mol Cell Biol. 1994 Apr;14(4):2343–2351. doi: 10.1128/mcb.14.4.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg M., Butt E., Nolte C., Fischer L., Halbrügge M., Beltman J., Jahnsen T., Genieser H. G., Jastorff B., Walter U. Characterization of Sp-5,6-dichloro-1-beta-D-ribofuranosylbenzimidazole- 3',5'-monophosphorothioate (Sp-5,6-DCl-cBiMPS) as a potent and specific activator of cyclic-AMP-dependent protein kinase in cell extracts and intact cells. Biochem J. 1991 Oct 15;279(Pt 2):521–527. doi: 10.1042/bj2790521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt E., Van Brandwijk R., Van Snick J., Siebold B., Rüde E. TCGF III/P40 is produced by naive murine CD4+ T cells but is not a general T cell growth factor. Eur J Immunol. 1989 Nov;19(11):2167–2170. doi: 10.1002/eji.1830191130. [DOI] [PubMed] [Google Scholar]
- Schmitz M. L., Baeuerle P. A. The p65 subunit is responsible for the strong transcription activating potential of NF-kappa B. EMBO J. 1991 Dec;10(12):3805–3817. doi: 10.1002/j.1460-2075.1991.tb04950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber E., Matthias P., Müller M. M., Schaffner W. Rapid detection of octamer binding proteins with 'mini-extracts', prepared from a small number of cells. Nucleic Acids Res. 1989 Aug 11;17(15):6419–6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scribner J. D., Slaga T. J. Multiple effects of dexamethasone on protein synthesis and hyperplasia caused by a tumor promoter. Cancer Res. 1973 Mar;33(3):542–546. [PubMed] [Google Scholar]
- Seipel K., Georgiev O., Schaffner W. Different activation domains stimulate transcription from remote ('enhancer') and proximal ('promoter') positions. EMBO J. 1992 Dec;11(13):4961–4968. doi: 10.1002/j.1460-2075.1992.tb05603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986 Aug 29;46(5):705–716. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- Serfling E., Barthelmäs R., Pfeuffer I., Schenk B., Zarius S., Swoboda R., Mercurio F., Karin M. Ubiquitous and lymphocyte-specific factors are involved in the induction of the mouse interleukin 2 gene in T lymphocytes. EMBO J. 1989 Feb;8(2):465–473. doi: 10.1002/j.1460-2075.1989.tb03399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevetson B. R., Kong X., Lawrence J. C., Jr Increasing cAMP attenuates activation of mitogen-activated protein kinase. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10305–10309. doi: 10.1073/pnas.90.21.10305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya H., Yoneyama M., Taniguchi T. Involvement of a common transcription factor in the regulated expression of IL-2 and IL-2 receptor genes. Int Immunol. 1989;1(1):43–49. doi: 10.1093/intimm/1.1.43. [DOI] [PubMed] [Google Scholar]
- Sun S. C., Ganchi P. A., Ballard D. W., Greene W. C. NF-kappa B controls expression of inhibitor I kappa B alpha: evidence for an inducible autoregulatory pathway. Science. 1993 Mar 26;259(5103):1912–1915. doi: 10.1126/science.8096091. [DOI] [PubMed] [Google Scholar]
- Szabo S. J., Gold J. S., Murphy T. L., Murphy K. M. Identification of cis-acting regulatory elements controlling interleukin-4 gene expression in T cells: roles for NF-Y and NF-ATc. Mol Cell Biol. 1993 Aug;13(8):4793–4805. doi: 10.1128/mcb.13.8.4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhler M. D., McKnight G. S. Expression of cDNAs for two isoforms of the catalytic subunit of cAMP-dependent protein kinase. J Biol Chem. 1987 Nov 5;262(31):15202–15207. [PubMed] [Google Scholar]
- Vacca A., Felli M. P., Farina A. R., Martinotti S., Maroder M., Screpanti I., Meco D., Petrangeli E., Frati L., Gulino A. Glucocorticoid receptor-mediated suppression of the interleukin 2 gene expression through impairment of the cooperativity between nuclear factor of activated T cells and AP-1 enhancer elements. J Exp Med. 1992 Mar 1;175(3):637–646. doi: 10.1084/jem.175.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij C. L., Geerts M., Aarden L. A. Activation of interleukin-2 gene transcription via the T-cell surface molecule CD28 is mediated through an NF-kB-like response element. J Biol Chem. 1991 Aug 5;266(22):14179–14182. [PubMed] [Google Scholar]
- Whitehurst C. E., Boulton T. G., Cobb M. H., Geppert T. D. Extracellular signal-regulated kinases in T cells. Anti-CD3 and 4 beta-phorbol 12-myristate 13-acetate-induced phosphorylation and activation. J Immunol. 1992 May 15;148(10):3230–3237. [PubMed] [Google Scholar]
- Wu J., Dent P., Jelinek T., Wolfman A., Weber M. J., Sturgill T. W. Inhibition of the EGF-activated MAP kinase signaling pathway by adenosine 3',5'-monophosphate. Science. 1993 Nov 12;262(5136):1065–1069. doi: 10.1126/science.7694366. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]