Abstract
The molecular components which mediate cytokine signaling from the cell membrane to the nucleus were studied. Upon the interaction of cytokines with their receptors, members of the janus kinase (Jak) family of cytoplasmic protein tyrosine kinases and of the signal transducers and activators of transcription (Stat) family of transcription factors are activated through tyrosine phosphorylation. It has been suggested that the Stat proteins are substrates of the Jak protein tyrosine kinases. MGF-Stat5 is a member of the Stat family which has been found to confer the prolactin response. MGF-Stat5 can be phosphorylated and activated in its DNA binding activity by Jak2. The activation of MGF-Stat5 is not restricted to prolactin. Erythropoietin (EPO) and growth hormone (GH) stimulate the DNA binding activity of MGF-Stat5 in COS cells transfected with vectors encoding EPO receptor and MGF-Stat5 or vectors encoding GH receptor and MGF-Stat5. The activation of DNA binding by prolactin, EPO and GH requires the phosphorylation of tyrosine residue 694 of MGF-Stat5. The transcriptional induction of a beta-casein promoter luciferase construct in transiently transfected COS cells is specific for the prolactin activation of MGF-Stat5; it is not observed in EPO- and GH-treated cells. In the UT7 human hematopoietic cell line, EPO and granulocyte-macrophage colony stimulating factor activate the DNA binding activity of a factor closely related to MGF-Stat5 with respect to its immunological reactivity, DNA binding specificity and molecular weight. These results suggest that MGF-Stat5 regulates physiological processes in mammary epithelial cells, as well as in hematopoietic cells.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


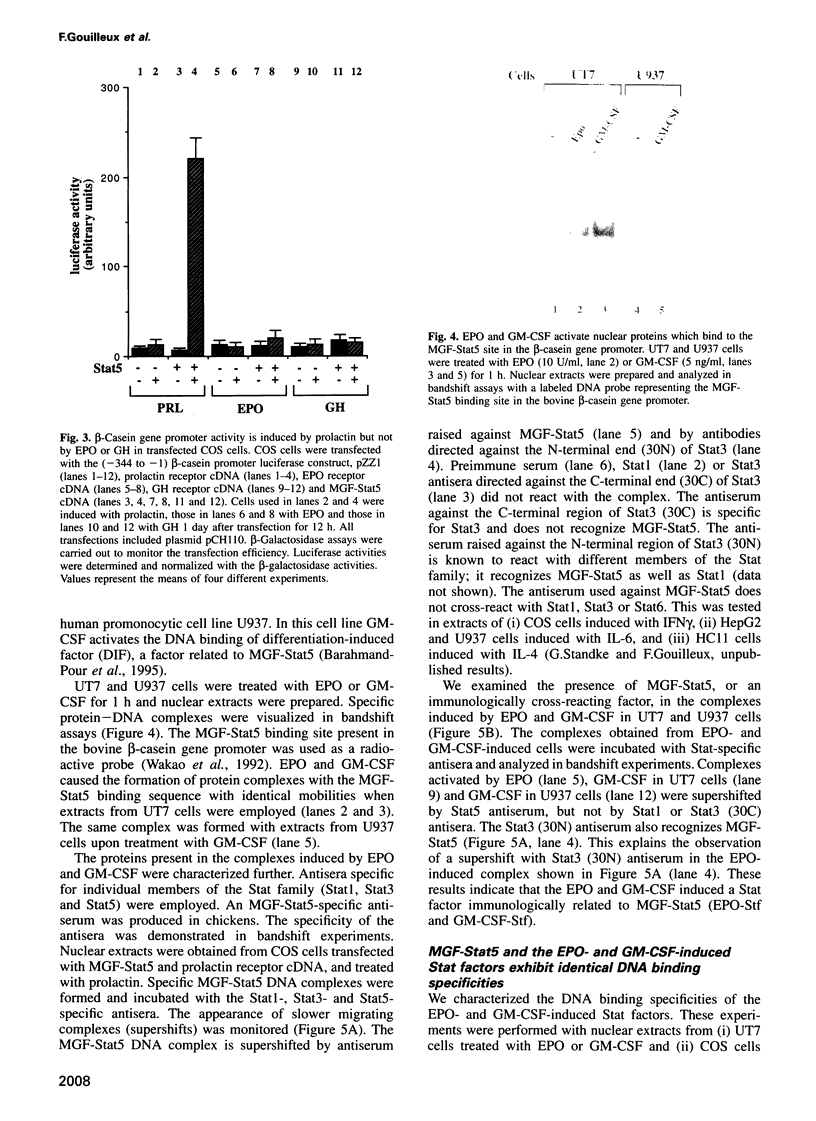




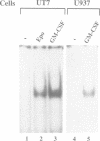
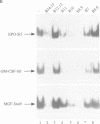
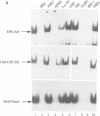
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akira S., Nishio Y., Inoue M., Wang X. J., Wei S., Matsusaka T., Yoshida K., Sudo T., Naruto M., Kishimoto T. Molecular cloning of APRF, a novel IFN-stimulated gene factor 3 p91-related transcription factor involved in the gp130-mediated signaling pathway. Cell. 1994 Apr 8;77(1):63–71. doi: 10.1016/0092-8674(94)90235-6. [DOI] [PubMed] [Google Scholar]
- Argetsinger L. S., Campbell G. S., Yang X., Witthuhn B. A., Silvennoinen O., Ihle J. N., Carter-Su C. Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase. Cell. 1993 Jul 30;74(2):237–244. doi: 10.1016/0092-8674(93)90415-m. [DOI] [PubMed] [Google Scholar]
- Barahmand-pour F., Meinke A., Eilers A., Gouilleux F., Groner B., Decker T. Colony-stimulating factors and interferon-gamma activate a protein related to MGF-Stat 5 to cause formation of the differentiation-induced factor in myeloid cells. FEBS Lett. 1995 Feb 20;360(1):29–33. doi: 10.1016/0014-5793(95)00072-h. [DOI] [PubMed] [Google Scholar]
- D'Andrea A. D., Lodish H. F., Wong G. G. Expression cloning of the murine erythropoietin receptor. Cell. 1989 Apr 21;57(2):277–285. doi: 10.1016/0092-8674(89)90965-3. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr, Kerr I. M., Stark G. R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994 Jun 3;264(5164):1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- Doppler W., Groner B., Ball R. K. Prolactin and glucocorticoid hormones synergistically induce expression of transfected rat beta-casein gene promoter constructs in a mammary epithelial cell line. Proc Natl Acad Sci U S A. 1989 Jan;86(1):104–108. doi: 10.1073/pnas.86.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusanter-Fourt I., Muller O., Ziemiecki A., Mayeux P., Drucker B., Djiane J., Wilks A., Harpur A. G., Fischer S., Gisselbrecht S. Identification of JAK protein tyrosine kinases as signaling molecules for prolactin. Functional analysis of prolactin receptor and prolactin-erythropoietin receptor chimera expressed in lymphoid cells. EMBO J. 1994 Jun 1;13(11):2583–2591. doi: 10.1002/j.1460-2075.1994.tb06548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilers A., Baccarini M., Horn F., Hipskind R. A., Schindler C., Decker T. A factor induced by differentiation signals in cells of the macrophage lineage binds to the gamma interferon activation site. Mol Cell Biol. 1994 Feb;14(2):1364–1373. doi: 10.1128/mcb.14.2.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finbloom D. S., Petricoin E. F., 3rd, Hackett R. H., David M., Feldman G. M., Igarashi K., Fibach E., Weber M. J., Thorner M. O., Silva C. M. Growth hormone and erythropoietin differentially activate DNA-binding proteins by tyrosine phosphorylation. Mol Cell Biol. 1994 Mar;14(3):2113–2118. doi: 10.1128/mcb.14.3.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X. Y., Schindler C., Improta T., Aebersold R., Darnell J. E., Jr The proteins of ISGF-3, the interferon alpha-induced transcriptional activator, define a gene family involved in signal transduction. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7840–7843. doi: 10.1073/pnas.89.16.7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaddy-Kurten D., Richards J. S. Regulation of alpha 2-macroglobulin by luteinizing hormone and prolactin during cell differentiation in the rat ovary. Mol Endocrinol. 1991 Sep;5(9):1280–1291. doi: 10.1210/mend-5-9-1280. [DOI] [PubMed] [Google Scholar]
- Gouilleux F., Sola B., Couette B., Richard-Foy H. Cooperation between structural elements in hormono-regulated transcription from the mouse mammary tumor virus promoter. Nucleic Acids Res. 1991 Apr 11;19(7):1563–1569. doi: 10.1093/nar/19.7.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouilleux F., Wakao H., Mundt M., Groner B. Prolactin induces phosphorylation of Tyr694 of Stat5 (MGF), a prerequisite for DNA binding and induction of transcription. EMBO J. 1994 Sep 15;13(18):4361–4369. doi: 10.1002/j.1460-2075.1994.tb06756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y., Jayatilak P. G., Parmer T. G., Gauldie J., Fey G. H., Gibori G. Alpha 2-macroglobulin expression in the mesometrial decidua and its regulation by decidual luteotropin and prolactin. Endocrinology. 1992 Sep;131(3):1321–1328. doi: 10.1210/endo.131.3.1380439. [DOI] [PubMed] [Google Scholar]
- Gupta S., Campbell D., Dérijard B., Davis R. J. Transcription factor ATF2 regulation by the JNK signal transduction pathway. Science. 1995 Jan 20;267(5196):389–393. doi: 10.1126/science.7824938. [DOI] [PubMed] [Google Scholar]
- Hermine O., Mayeux P., Titeux M., Mitjavila M. T., Casadevall N., Guichard J., Komatsu N., Suda T., Miura Y., Vainchenker W. Granulocyte-macrophage colony-stimulating factor and erythropoietin act competitively to induce two different programs of differentiation in the human pluripotent cell line UT-7. Blood. 1992 Dec 15;80(12):3060–3069. [PubMed] [Google Scholar]
- Hou J., Schindler U., Henzel W. J., Ho T. C., Brasseur M., McKnight S. L. An interleukin-4-induced transcription factor: IL-4 Stat. Science. 1994 Sep 16;265(5179):1701–1706. doi: 10.1126/science.8085155. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Witthuhn B. A., Quelle F. W., Yamamoto K., Thierfelder W. E., Kreider B., Silvennoinen O. Signaling by the cytokine receptor superfamily: JAKs and STATs. Trends Biochem Sci. 1994 May;19(5):222–227. doi: 10.1016/0968-0004(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Johnston J. A., Kawamura M., Kirken R. A., Chen Y. Q., Blake T. B., Shibuya K., Ortaldo J. R., McVicar D. W., O'Shea J. J. Phosphorylation and activation of the Jak-3 Janus kinase in response to interleukin-2. Nature. 1994 Jul 14;370(6485):151–153. doi: 10.1038/370151a0. [DOI] [PubMed] [Google Scholar]
- Larner A. C., David M., Feldman G. M., Igarashi K., Hackett R. H., Webb D. S., Sweitzer S. M., Petricoin E. F., 3rd, Finbloom D. S. Tyrosine phosphorylation of DNA binding proteins by multiple cytokines. Science. 1993 Sep 24;261(5129):1730–1733. doi: 10.1126/science.8378773. [DOI] [PubMed] [Google Scholar]
- Mathews L. S., Enberg B., Norstedt G. Regulation of rat growth hormone receptor gene expression. J Biol Chem. 1989 Jun 15;264(17):9905–9910. [PubMed] [Google Scholar]
- Miyajima A., Hara T., Kitamura T. Common subunits of cytokine receptors and the functional redundancy of cytokines. Trends Biochem Sci. 1992 Oct;17(10):378–382. doi: 10.1016/0968-0004(92)90004-s. [DOI] [PubMed] [Google Scholar]
- Müller M., Briscoe J., Laxton C., Guschin D., Ziemiecki A., Silvennoinen O., Harpur A. G., Barbieri G., Witthuhn B. A., Schindler C. The protein tyrosine kinase JAK1 complements defects in interferon-alpha/beta and -gamma signal transduction. Nature. 1993 Nov 11;366(6451):129–135. doi: 10.1038/366129a0. [DOI] [PubMed] [Google Scholar]
- Narazaki M., Witthuhn B. A., Yoshida K., Silvennoinen O., Yasukawa K., Ihle J. N., Kishimoto T., Taga T. Activation of JAK2 kinase mediated by the interleukin 6 signal transducer gp130. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2285–2289. doi: 10.1073/pnas.91.6.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse R. N., Feinman R., Shuai K., Darnell J. E., Jr, Ravetch J. V. Interferon gamma-induced transcription of the high-affinity Fc receptor for IgG requires assembly of a complex that includes the 91-kDa subunit of transcription factor ISGF3. Proc Natl Acad Sci U S A. 1993 May 1;90(9):4314–4318. doi: 10.1073/pnas.90.9.4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quelle F. W., Sato N., Witthuhn B. A., Inhorn R. C., Eder M., Miyajima A., Griffin J. D., Ihle J. N. JAK2 associates with the beta c chain of the receptor for granulocyte-macrophage colony-stimulating factor, and its activation requires the membrane-proximal region. Mol Cell Biol. 1994 Jul;14(7):4335–4341. doi: 10.1128/mcb.14.7.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler C., Fu X. Y., Improta T., Aebersold R., Darnell J. E., Jr Proteins of transcription factor ISGF-3: one gene encodes the 91-and 84-kDa ISGF-3 proteins that are activated by interferon alpha. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7836–7839. doi: 10.1073/pnas.89.16.7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler C., Shuai K., Prezioso V. R., Darnell J. E., Jr Interferon-dependent tyrosine phosphorylation of a latent cytoplasmic transcription factor. Science. 1992 Aug 7;257(5071):809–813. doi: 10.1126/science.1496401. [DOI] [PubMed] [Google Scholar]
- Schmitt-Ney M., Doppler W., Ball R. K., Groner B. Beta-casein gene promoter activity is regulated by the hormone-mediated relief of transcriptional repression and a mammary-gland-specific nuclear factor. Mol Cell Biol. 1991 Jul;11(7):3745–3755. doi: 10.1128/mcb.11.7.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt-Ney M., Happ B., Ball R. K., Groner B. Developmental and environmental regulation of a mammary gland-specific nuclear factor essential for transcription of the gene encoding beta-casein. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):3130–3134. doi: 10.1073/pnas.89.7.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuai K., Schindler C., Prezioso V. R., Darnell J. E., Jr Activation of transcription by IFN-gamma: tyrosine phosphorylation of a 91-kD DNA binding protein. Science. 1992 Dec 11;258(5089):1808–1812. doi: 10.1126/science.1281555. [DOI] [PubMed] [Google Scholar]
- Silvennoinen O., Witthuhn B. A., Quelle F. W., Cleveland J. L., Yi T., Ihle J. N. Structure of the murine Jak2 protein-tyrosine kinase and its role in interleukin 3 signal transduction. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8429–8433. doi: 10.1073/pnas.90.18.8429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standke G. J., Meier V. S., Groner B. Mammary gland factor activated by prolactin on mammary epithelial cells and acute-phase response factor activated by interleukin-6 in liver cells share DNA binding and transactivation potential. Mol Endocrinol. 1994 Apr;8(4):469–477. doi: 10.1210/mend.8.4.7519723. [DOI] [PubMed] [Google Scholar]
- Velazquez L., Fellous M., Stark G. R., Pellegrini S. A protein tyrosine kinase in the interferon alpha/beta signaling pathway. Cell. 1992 Jul 24;70(2):313–322. doi: 10.1016/0092-8674(92)90105-l. [DOI] [PubMed] [Google Scholar]
- Wakao H., Gouilleux F., Groner B. Mammary gland factor (MGF) is a novel member of the cytokine regulated transcription factor gene family and confers the prolactin response. EMBO J. 1994 May 1;13(9):2182–2191. doi: 10.1002/j.1460-2075.1994.tb06495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakao H., Schmitt-Ney M., Groner B. Mammary gland-specific nuclear factor is present in lactating rodent and bovine mammary tissue and composed of a single polypeptide of 89 kDa. J Biol Chem. 1992 Aug 15;267(23):16365–16370. [PubMed] [Google Scholar]
- Watling D., Guschin D., Müller M., Silvennoinen O., Witthuhn B. A., Quelle F. W., Rogers N. C., Schindler C., Stark G. R., Ihle J. N. Complementation by the protein tyrosine kinase JAK2 of a mutant cell line defective in the interferon-gamma signal transduction pathway. Nature. 1993 Nov 11;366(6451):166–170. doi: 10.1038/366166a0. [DOI] [PubMed] [Google Scholar]
- White M. F., Kahn C. R. The insulin signaling system. J Biol Chem. 1994 Jan 7;269(1):1–4. [PubMed] [Google Scholar]
- Wilks A. F., Harpur A. G. Cytokine signal transduction and the JAK family of protein tyrosine kinases. Bioessays. 1994 May;16(5):313–320. doi: 10.1002/bies.950160505. [DOI] [PubMed] [Google Scholar]
- Witthuhn B. A., Quelle F. W., Silvennoinen O., Yi T., Tang B., Miura O., Ihle J. N. JAK2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin. Cell. 1993 Jul 30;74(2):227–236. doi: 10.1016/0092-8674(93)90414-l. [DOI] [PubMed] [Google Scholar]
- Witthuhn B. A., Silvennoinen O., Miura O., Lai K. S., Cwik C., Liu E. T., Ihle J. N. Involvement of the Jak-3 Janus kinase in signalling by interleukins 2 and 4 in lymphoid and myeloid cells. Nature. 1994 Jul 14;370(6485):153–157. doi: 10.1038/370153a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Quelle F. W., Thierfelder W. E., Kreider B. L., Gilbert D. J., Jenkins N. A., Copeland N. G., Silvennoinen O., Ihle J. N. Stat4, a novel gamma interferon activation site-binding protein expressed in early myeloid differentiation. Mol Cell Biol. 1994 Jul;14(7):4342–4349. doi: 10.1128/mcb.14.7.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura M., Oka T. Isolation and structural analysis of the mouse beta-casein gene. Gene. 1989 May 30;78(2):267–275. doi: 10.1016/0378-1119(89)90229-1. [DOI] [PubMed] [Google Scholar]
- Yu-Lee L. Y., Hrachovy J. A., Stevens A. M., Schwarz L. A. Interferon-regulatory factor 1 is an immediate-early gene under transcriptional regulation by prolactin in Nb2 T cells. Mol Cell Biol. 1990 Jun;10(6):3087–3094. doi: 10.1128/mcb.10.6.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J., Wegenka U. M., Lütticken C., Buschmann J., Decker T., Schindler C., Heinrich P. C., Horn F. The signalling pathways of interleukin-6 and gamma interferon converge by the activation of different transcription factors which bind to common responsive DNA elements. Mol Cell Biol. 1994 Mar;14(3):1657–1668. doi: 10.1128/mcb.14.3.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z., Wen Z., Darnell J. E., Jr Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994 Apr 1;264(5155):95–98. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]