Abstract
Although commonly known as a highly toxic chemical, cyanide is also an essential reagent for many industrial processes in areas such as mining, electroplating, and synthetic fiber production. The “heavy” use of cyanide in these industries, along with its necessary transportation, increases the possibility of human exposure. Because the onset of cyanide toxicity is fast, a rapid, sensitive, and accurate method for the diagnosis of cyanide exposure is necessary. Therefore, a field sensor for the diagnosis of cyanide exposure was developed based on the reaction of naphthalene dialdehyde, taurine, and cyanide, yielding a fluorescent β-isoindole. An integrated cyanide capture “apparatus”, consisting of sample and cyanide capture chambers, allowed rapid separation of cyanide from blood samples. Rabbit whole blood was added to the sample chamber, acidified, and the HCN gas evolved was actively transferred through a stainless steel channel to the capture chamber containing a basic solution of naphthalene dialdehyde (NDA) and taurine. The overall analysis time (including the addition of the sample) was <3 min, the linear range was 3.13–200 μM, and the limit of detection was 0.78 μM. None of the potential interferents investigated (NaHS, NH4OH, NaSCN, and human serum albumin) produced a signal that could be interpreted as a false positive or a false negative for cyanide exposure. Most importantly, the sensor was 100% accurate in diagnosing cyanide poisoning for acutely exposed rabbits.
Cyanide (HCN or CN–, inclusively represented as CN) is commonly known as a poison and a chemical warfare agent (CWA). However, the industrial need for CN in many chemical processes, such as mineral extraction, electroplating, and the fabrication of synthetic fibers,1 drives cyanide production for industrial use to over 1.1 million tons per year.2 Therefore, industrial use of mass quantities of cyanide, with its associated transportation through highly populated areas, drastically increases the risk of exposure. Cyanide exposure may also occur through diet, smoke inhalation (fire or cigarette smoke), or exposure from illicit use.3,4 Illicit use can be targeted at a single individual (i.e., poisoning), a small group of targeted individuals (e.g., mass suicides), or a large group of people (e.g., terrorist attacks). Some of the more recent incidents of illicit cyanide use are the Tylenol Poisonings in 1982,5 the use of cyanide-gas-producing devices in Tokyo subway and railway station restrooms in 1995,6 ingestion of cyanide tablets by Michael Marin upon receipt of a guilty verdict for arson in June 2012,7 and the death of Urooj Khan, a lottery winner, in Chicago in July 2012.8 Another illicit, relatively little-known, use of cyanide is to stun exotic fish for easy capture, with an estimated 90% of the exotic fish originating from the Philippines captured in this manner.9
Whether the route of cyanide exposure is accidental or deliberate, the mechanism of cyanide toxicity is similar. Cyanide causes cellular death by blocking adenosine triphosphate (ATP) production through the binding of cytochrome c oxidase.10 The onset of cyanide toxicity is rapid, and toxic levels in blood can be observed at concentrations of approximately 19 μM11,12 while death can be observed at concentrations as low as 115 μM.12,13 Although CN is highly toxic, it is endogenously present in animals due to normal amino acid metabolism, dietary intake, and tobacco consumption.3,14 Because CN and its major metabolites, thiocyanate (SCN–) and 2-aminothiazoline-4-carboxylic acid (ATCA), have each been used as markers for cyanide exposure in biofluids,14−16 endogenous concentrations may complicate the diagnosis of cyanide exposure if not fully understood. Table 1 lists the ranges of endogenous concentrations of CN and its major metabolites, each of which are highly variable.
Table 1. Endogenous Levels of Cyanide Thiocyanate and ATCA in the Blood of Smokers and Non-Smokers.
Thiocyanate is the most common indirect marker of cyanide exposure because it is the major metabolite of cyanide, accounting for 80% of cyanide metabolism.18 ATCA has only recently been suggested for use as a biomarker, but it accounts for up to 20% of cyanide metabolism, with an increase in ATCA production as cyanide dose increases.3,19 Although SCN– is a valuable marker of cyanide exposure, metabolism of cyanide to thiocyanate is enzymatically rate limited20 and maximum thiocyanate concentrations can lag maximum cyanide concentrations by approximately 20 min to 6 h.21 Although ATCA mirrors the behavior of cyanide,19 its concentration in plasma has been found to be relatively low, necessitating an extremely sensitive diagnostic analysis.
Because of the rapid onset of toxic effects from cyanide poisoning and the difficulty in developing a rapid and sensitive analysis for ATCA, the most appropriate target for diagnosis of acute cyanide exposure is the direct analysis of cyanide as soon after exposure as possible. Although the detection of cyanide may be accomplished by several methods, including chromatography, mass spectrometry, fluorescence, and chemiluminescence,22 five recent methods for cyanide analysis from biological matrices have been proposed (Table 2) that focus on rapid analysis and/or portable technology. Three of these methods are based on a change in the absorbance of cobinamide (hydroxoaquocobinamide23,24 or hydroxocyanocobinamide25) in the presence of cyanide. The remaining two are based on fluorescence detection of cyanide upon its interaction with copper(II) cubic mesoporous graphitic carbon nitride (Cu2+-c-mpg-C3N4)26 or 1-(4′-nitrophenyl) benzimidazolium.27 Additionally, there have been a number of fluorometric and colorimetric probes developed in recent years for cyanide analysis,28 but these probes have yet to be integrated into sensor technology. Table 2 lists the analysis time and limits of detection (LODs) for the proposed sensors, along with potential issues associated with each technology. Although some of the listed CN detection techniques have LODs reaching concentrations into the nanomolar range, endogenous levels of CN in humans range from 0.02 to 10 μM (see Table 1). Furthermore, the toxic effects of CN appear at blood concentrations around 19 μM.12 Therefore, an LOD of 3 μM or less, as achieved by each technology listed in Table 2, is likely sufficient for diagnosis of CN exposure (i.e., an LOD of 3 μM is typically associated with a lower limit of quantification of around 10 μM). Considering this, the other characteristics listed in Table 2 are likely more important in comparing these diagnostic technologies. For the techniques proposed, large sample volumes (1 mL),23 interference from hydrogen sulfide,23,24 long analysis times,26,27 and unconfirmed ability to diagnose CN exposure,26,27 limit their application for diagnosis.
Table 2. Comparison of Recently Proposed Rapid Analysis Methods and/or Portable Technologies for the Diagnosis of Cyanide Exposure.
| investigators | core technology | sample prep method | analysis time (min) | LODa (μM) | notes |
|---|---|---|---|---|---|
| Ma et al., 201123 | hydroxoaquocobinamide | microdiffusion | ∼2 | 0.5b,c | H2S is an interferent. |
| Ma and Dasgupta, 201024 | hydroxoaquocobinamide | microdiffusion | ∼1.5 | 0.030b | H2S is an interferent, and the NaOH mobile phase is necessary.d |
| Tian et al., 201325 | hydroxocyanocobinamide | microdiffusion | <4 | 2.2b,c | Potential interferents were not evaluated, but H2S likely interferes. |
| Lee et al., 201226 | turn on fluorescence Cu2+–c-mpg-C3N4e | Isolate serum. Follow on sample prep not described. | 40f | 0.080g | The analysis time reported (10 min) likely did not include the time needed to clot blood and separate the serum.d,h |
| Kumar et al., 201327 | fluorescence of 1-(4′-nitrophenyl)benzimidazolium | Isolate serum then add HEPES buffer and DMSO solution. | 31f | 0.030g | The analysis time reported (<60 s) did not include the time needed to clot blood and separate serum.d,h |
LOD, limit of detection.
The listed LODs are for rabbit whole blood.
These techniques were verified using CN exposed rabbits.
Method not verified in an animal model.
c-mpg-C3N4 is cubic mesoporous graphitic carbon nitride.
Thirty minutes was added to the reported analysis time to account for the estimated time neseccary to clot blood and separate serum from blood.
The listed LODs are for human blood serum.
The sample preparation to obtain serum from blood requires extra equipment.
Considering limitations of the currently proposed rapid/portable CN detection techniques, there is a critical need for a rapid point-of-care diagnostic to confirm cyanide exposure and inform the administration of antidotes. The objective of this study was to develop a rapid and sensitive sensor for the accurate diagnosis of acute, toxic cyanide exposure.
Experimental Section
Materials
All materials used were HPLC grade unless otherwise indicated. Sodium hydroxide, sulfuric acid, sodium cyanide, KH2PO4, K2HPO4, and NH4OH were purchased from Fisher Scientific (Hanover Park, IL). 2,3-Naphthalene dialdehyde (NDA) was obtained from TCI America (Portland, OR). Taurine (2-aminoethane sulfonic acid) and NaBO2·4H2O were purchased from Alfa Aesar (Ward Hill, MA). NaSCN was purchased from Acros Organics (Morris Plains, NJ). Human serum albumin (HSA) and NaHS were purchased from Sigma-Aldrich (St. Louis, MO).
Phosphate (0.1 M)/borate (0.05 M) buffer and stock solutions of sodium hydroxide (1 M), sulfuric acid (1 M), and NaSCN (1 mM) were prepared in deionized water. Sodium cyanide standards and NaHS were obtained by dilution from 1.8 mM and 1 M stock solutions, respectively, with 10 mM NaOH. NH4OH was prepared by diluting the original aqueous solution (29% by weight or 14.5 M) to 30 μM in deionized water. The NDA (2 mM) stock solution was prepared in phosphate/borate buffer and 40% methanol. A taurine (50 mM) solution was prepared in phosphate/borate buffer. A standard HSA solution was obtained by dissolving 3.3 mg of HSA per mL of deionized water.
Biological Samples
Rabbit whole blood samples were obtained from two sources: (1) nonsterile whole blood with 2.5% EDTA from young rabbits was purchased from Pel-Freeze Biologicals (Rogers, AR) and (2) whole blood from cyanide exposed, New Zealand White rabbits (Oryctologus cuniculus, male, 3.5–4.5 kg) was obtained from the University of California, Irvine. Rabbits (n = 6) were administered lethal doses of 6.8 mM NaCN in 0.9% NaCl (1 mL/min continuous intravenous infusion) and blood was drawn prior to and 15, 25, and 35 min following the initiation of cyanide infusion. The blood samples were placed in EDTA tubes to prevent coagulation, frozen, and shipped on ice (overnight) to South Dakota State University for analysis of cyanide. Upon receipt, the blood was stored at −80 °C until cyanide analysis was performed.
All rabbits were cared for in compliance with the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health.29 All studies involving rabbits were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC).
Fluorometric Analysis of Cyanide
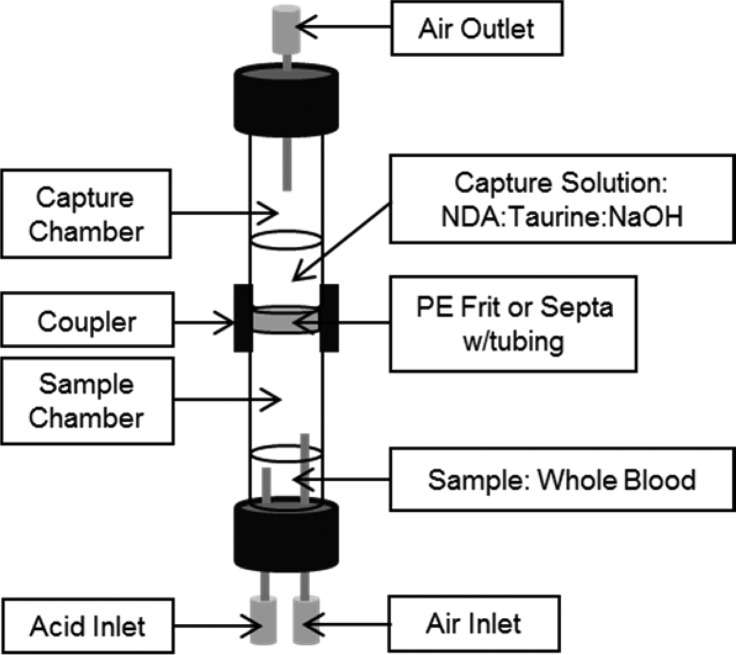
Microdiffusion was used to prepare cyanide for analysis. The microdiffusion of CN was accomplished via a stacked cyanide capture apparatus. A schematic of the stacked cyanide capture apparatus can be seen in Figure 1 with a lower chamber, called the sample chamber, used to contain cyanide standards, swabs, whole blood samples, or other sample matrices, and an upper chamber called the capture chamber, containing a capture solution of 0.5 mM NDA:12.5 mM taurine:0.1 M NaOH (1:1:1 by volume). These two chambers [8 (i.d.) × 50 mm long] were separated by a hydrophobic 10 micron porous polyethylene (PE) frit or a 1.5 mm thick silicone septum pierced with a 2 mm long, 28 gauge forward flow tube. The frit/septum was sandwiched between the sample and capture chamber using a 1.8 cm long piece of threaded (13 × 425) PVC tubing with a 1.6 cm external diameter as a coupler. A needle at the top of the capture chamber served as an outlet for the carrier gas. The sample chamber septum was pierced with two inlet needles (at the bottom of Figure 1), one for the injection of acid and one for introduction of air. Attempts to combine the acid and air introduction failed due to large viscosity fluctuation between the solution and the air, resulting in difficulty in controlling the rate of air flow through the chamber.
Figure 1.
Schematic of the stacked cyanide capture apparatus.
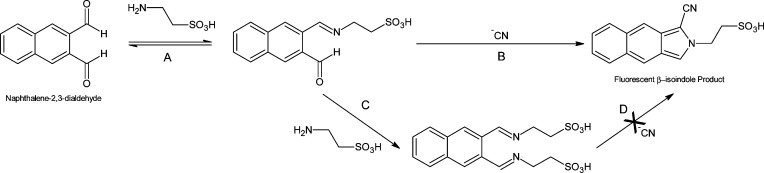
For the separation of CN from the biological matrix, the sample was placed in the sample chamber and acidified with sulfuric acid (300 μL of 1 M) and air (20 mL for the PE frit, 20 and 50 mL for the silicone septum were evaluated) was forced over the sample headspace to a capture solution where HCN gas was trapped in the capture chamber using strong base to convert HCN to nonvolatile CN–. The captured CN– was then reacted with NDA and taurine in the capture solution, resulting in a fluorescent β-isoindole product (Figure 2, Scheme A → B).30 The cyanide capture apparatus fit within the detector chamber so that the portion of the capture chamber containing the capture solution was in alignment with the LED and a photodiode or optic fiber connected to a spectrophotometric detector. Sample analysis time, beginning at sample introduction through fluorescence detection, was less than 3 min. During this study, the cyanide capture apparatus was cleaned with deionized water between analyses, but washing the air and acid inlets and the air outlet (Figure 1) was unnecessary [i.e., no carryover was observed except when using the PE frit and high concentrations of cyanide (>500 μM), likely due to HCN partitioning into the PE material; note that the PE material was not used for the majority of the study]. Although the cyanide capture apparatus was reused in this study, it could easily be designed to be disposable, eliminating the need for washing and the potential for carryover.
Figure 2.
The proposed reaction schemes for the possible reactions of NDA, taurine, and cyanide. Pathways A → B and A → C both yield H2O as a byproduct.
Fluorometric analysis was performed using one of two configurations. Fluorometric Configuration 1 (FC1) utilized a 420 nm light emitting diode (LED, TT Electronics, Weybridge, Surrey, KT13 9XB, England) positioned at a 90° angle from a 400–650 nm light sensitive photodiode (Avago Technologies, Ft. Collins, CO) and produced digital signals ranging from 0 to 218. Fluorometric Configuration 2 (FC2) consisted of a 410 nm high-powered LED (LED Engin, Inc., San Jose, CA) irradiated through a focusing lens and directed toward the sample. A second focusing lens positioned 90° from the irradiation path was used to direct the fluorescent light to a 600 μm optical fiber connected to a USB2000+ spectrophotometric detector. The signal at 500 nm was used to quantify the amount of cyanide in the sample. The focusing lenses, the optical fiber, and the spectrophotometric detector were purchased from Ocean Optics (Dunedin, FL). Since the detection limit for FC1 was not within the biologically relevant range desired, fluorometric analysis was performed using FC2, unless otherwise noted.
The field sensor dimensions were 15 × 20 × 30 cm (l × w × h). Housed within the sensor was an acid reservoir, cyanide capture apparatus chamber, a USB2000+ spectrophotometer (connected to a laptop computer), a valve switching mechanism, a 1 mL syringe with a 30 mm stroke linear actuator, and a 50 mL syringe with a 100 mm stroke linear actuator (each linear actuator served as a syringe pump). NDA and taurine were stored separately and added to the capture chamber prior to analysis.
Reagent Stability
The stability of capture solution reagents was an important factor pertaining to the field portability of the sensor. Three different capture solution storage scenarios were investigated. In scenario 1, all the capture solution reagents (NDA, taurine, and NaOH) were mixed together and stored as one solution. In scenario 2, NDA and taurine were mixed and stored as one solution, while the NaOH solution was added at the time of analysis. In scenario 3, all the capture solution reagents were stored individually and mixed at the time of analysis. All solutions were stored in amber vials at room temperature for the duration of the stability study and cyanide analysis was accomplished using FC1. A cyanide stock solution (200 μM) was analyzed for all scenarios. The stock solutions for scenarios 1 and 2 were analyzed from 0 to 60 min, with samples for scenario 3 analyzed up to 70 days.
Analysis of Possible Interferents
Potentially interfering compounds, NH4OH (30 μM), NaSCN (0.5 mM), HSA (3.3 mg/mL), and NaHS (110 μM), were evaluated alone (for false positive evaluation) and spiked with 20 μM NaCN (for false negative evaluation). The compounds of interest were evaluated at concentrations likely found in biological matrices during cyanide poisoning (i.e., the naturally occurring concentration of NH4OH as ammonia gas,31 thiocyanate in excess of the highest levels seen in smokers,16,32 the amount of HSA present in blood,31 and the highest concentration of H2S found in the blood of sulfide poisoning fatalities33). Multiple mixtures of the interferent solutions were used to evaluate additive effects to include (1) equal parts NaHS and NH4OH solutions, (2) equal parts NaSCN and NH4OH solutions, (3) equal parts NaSCN, NaHS, NH4OH, and HSA solutions, (4) equal parts NaSCN and HSA solutions, and (5) equal parts of NaHS, NH4OH, and HSA solutions.
Analysis of Cyanide from Rabbit Whole Blood
The analysis of cyanide from rabbit whole blood was optimized to include sample volume (50 and 100 μL), acid injection volume (200–500 μL), and acid concentration (0.25 to 2 M). Once the optimum conditions were determined, a calibration curve was created with 0.25 to 200 μM cyanide spiked rabbit blood calibrators analyzed in triplicate. Prior to each analysis, a 10 μM quality control (QC) standard (cyanide spiked rabbit whole blood) was analyzed to represent the concentration threshold, above which, a subject was said to be “exposed”. This concentration was chosen because it is the highest cyanide concentration that has been previously observed in the blood of human smokers.1 Cyanide exposed rabbit blood samples (from U. C., Irvine) were analyzed in triplicate for time points 0 (baseline), 15, 25, and 35 min. As a measure to verify the performance of the sensor, the rabbit blood samples were also analyzed using the LC–MS/MS analysis method for cyanide described by Bhandari et al. 2013.34
Data Analysis
The limit of detection (LOD) was determined as the analyte concentration that produced a signal-to-noise ratio (S/N) of 3, with the noise measured as the standard deviation of the blank. The lower limit of quantification (LLOQ) was defined as the analyte concentration that produced a S/N of at least 10, a measured concentration, calculated from the calibration curve, that was within 20% of the nominal concentration as a measure of accuracy, and a percent relative standard deviation (%RSD) of ≤20% as a measure of precision. For inclusion of calibrators in the linear range of the sensor, replicate calibration standards were required to produce a precision of ≤20% RSD and accuracy of 100 ± 20%. The upper limit of quantification (ULOQ) was defined as the highest analyte concentration that produced a measured concentration, that was within 20% of the nominal concentration as a measure of accuracy with a precision of ≤20% RSD. It should be noted that the spectrophotometer limited the maximum signal to ∼63,000 cps, which limited the ULOQ for both aqueous and blood samples. All quantitative analytical values (i.e., concentration, mean, standard deviation, etc.) were calculated using Microsoft Office Excel 2010 (Redmond, WA).
Caution
Cyanide is toxic and hazardous to humans at blood concentrations of ∼20 μM.12 HCN is produced from aqueous cyanide containing solutions near or below a pH of 9.2. Therefore, all aqueous cyanide standards were prepared in 10 mM NaOH and handled in a well-ventilated hood. HCN gas was produced during the acidification process in the sample chamber and then captured and derivatized in a basic solution containing NDA and taurine in the capture chamber. The fluorescent β-isoindole product was disposed of with organic waste. The proper use of personal protective equipment (i.e., gloves, lab coat, etc.), laboratory equipment (i.e., ventilation hood), and proper waste disposal must be followed to prevent the possibility of exposure.
Results and Discussion
Development of a Cyanide Sample Preparation Apparatus
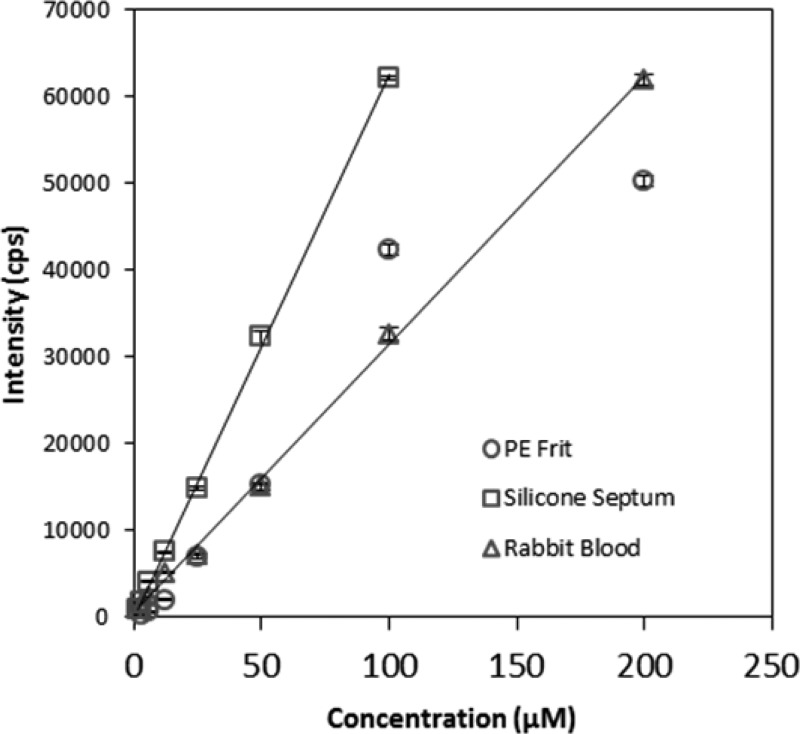
Two barrier materials were evaluated to separate the sample and capture chambers of the cyanide microdiffusion apparatus (Figure 1): (1) a silicone septa with stainless steel tubing and (2) a 10 micron porous PE frit. Figure 3 shows the calibration curves achieved with each barrier material. When the PE frit was used as the barrier, the signals observed for the lowest and highest concentrations tested were nonlinearly related to the cyanide concentration, and attempts to describe the calibration data with a linear fit produced nonzero intercepts. The PE frit produced an LOD of 3.13 μM and a linear range of 25–100 μM. When using the silicone septa, linear behavior was produced throughout the calibration range. When a low volume of air (20 mL) was used to carry HCN from the sample to the capture chamber, the linear range was 1.5–200 μM with a detection limit of 0.5 μM (data not shown). When using 50 mL of air, the LOD decreased to 0.25 μM, the sensitivity increased 2.4× (from 263 to 626 μM–1), and the linear range changed to 1.5–100 μM. (Note: with this larger air volume, the 200 μM cyanide standard produced a signal that saturated the detector, resulting in the reduced upper limit of quantification.) Linear least-squares treatment of the calibration data for both air volumes resulted in correlation coefficients of 0.999. The linear behavior of the silicon septa compared to the nonlinear behavior of the PE frit was likely due to nonequilibrium partitioning of HCN into the PE.
Figure 3.
Calibration curves obtained for the PE frit (20 mL of air), the silicone septa with forward flow tubing (50 mL of air shown), and rabbit whole blood (50 mL of air). Aqueous standards were used for the PE frit (○) and the silicone septa (□). A silicone septa, with forward flow tubing as the chamber separation material, was used for analysis of rabbit whole blood (△). Error bars represent standard deviation.
Aside from the analytical performance of the two barrier materials, foaming of the sample was a major practical issue. If heavy foaming occurred, it forced the capture solution out of the air outlet (Figure 1) and required lower flow rates and longer analysis times to ensure conservation of the capture solution. Separation of the sample and capture chamber with a polyethylene frit produced very small bubbles, resulting in heavy sample foaming due to minimal surface tension stress lengthening the time needed for the bubbles to burst. To avoid the loss of capture solution, the time necessary to deliver the air through the sample and capture chamber significantly increased. Conversely, when air was forced through the silicone septa, relatively large bubbles with uniform size and shape were produced, which significantly limited foaming and allowed a faster flow of air from the sample to the capture chamber. Moreover, the larger bubbles did not appear to hinder the transfer of HCN to the capture solution. Therefore, the silicone septum was preferred both practically and analytically.
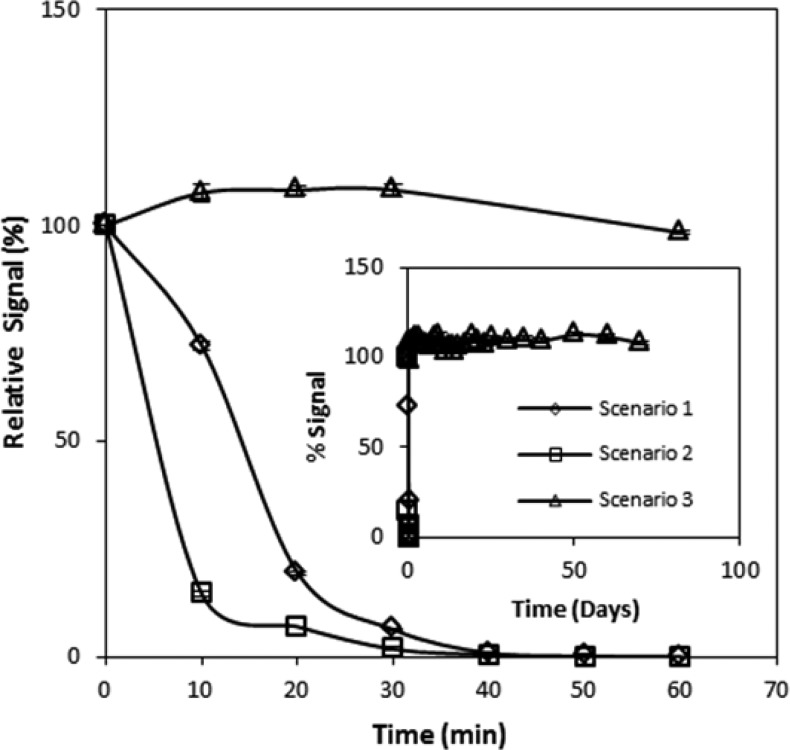
Reagent Stability
Reagent stability is crucial when developing a portable sensor, especially in locations where there is a lack of refrigeration and/or climate control. Figure 4 shows that under the storage conditions where the NDA and taurine were stored together (scenarios 1 and 2), extremely unstable mixtures resulted. Conversly, NDA and taurine stored separately (scenario 3) resulted in stable reagents for all time periods tested. The behavior of scenarios 1 and 2 was similar, where the initial fluorescent signal rapidly decreased until the fluorescence was essentially eliminated by 40 min. Visually, the solutions for scenarios 1 and 2 were initially clear but quickly became faintly yellow and orange, respectively, each also containing a small amount of black precipitate. Over time, these solutions became darker until the black precipitate pervaded. This color change was a visual indication that NDA and taurine were likely reacting together, potentially yielding an NDA–ditaurine complex (see Figure 2, pathway A → C → D). Since taurine was in excess, it is likely that by 40 min, the main component of the solutions was an NDA–ditaurine complex, which was incapable of producing the fluorescent β-isoindole product.
Figure 4.
Assessment of the short- and long-term stability of the capture solution reagents. The long-term stability of the reagents (up to 70 days) is presented in the inset. Error bars represent standard deviation.
Although more stringent storage conditions should be tested (e.g., larger variations in temperature to account for extremes the sensor may encounter), the stability of the capture solution reagents, when stored separately, is encouraging for use in a cyanide field sensor. In accordance with Figure 4, the reagents would not need special storage conditions when stored separately (i.e., the only special storage condition was the use of amber bottles). It should be noted that the day-to-day variations observed from storage scenario 3 were likely due to fluctuations in the temperature of the room and the electrical current produced from the sensor’s power source (i.e., dual 9 V batteries) and were not reflective of variability in the chemical or sample preparation strategies associated with the analysis.
Analysis of Possible Interferents
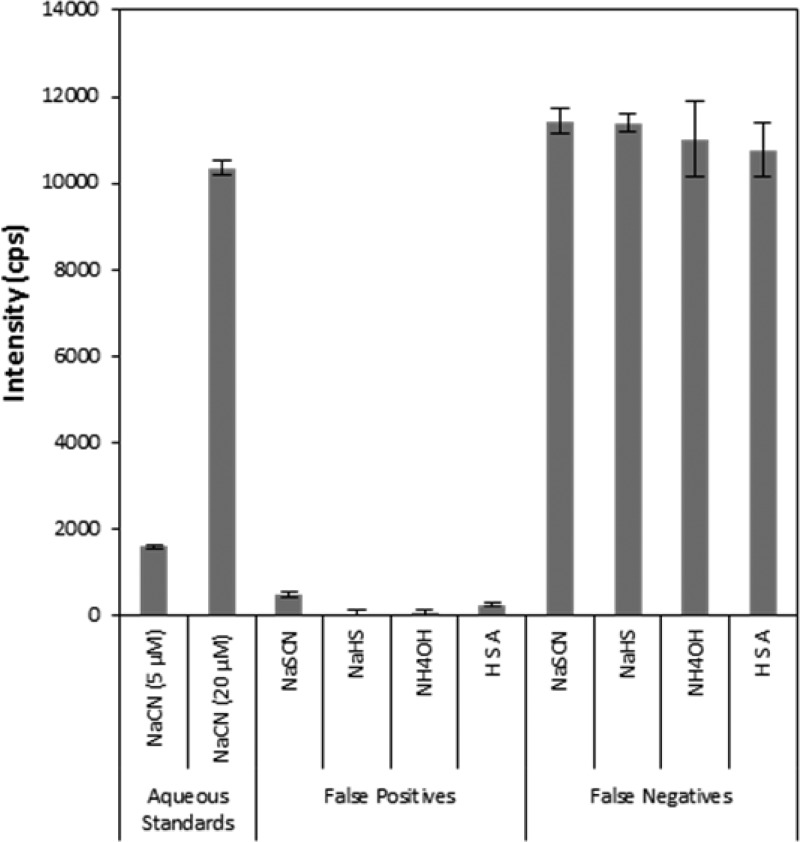
The evaluation of potential interferents was undertaken to assess the possibility of false positive or negative diagnosis of cyanide poisoning from common components of blood. Figure 5 shows that none of the compounds investigated produced false positive signals (i.e., above the 5 μM cyanide standard) and that all individually tested samples containing 20 μM NaCN produced signals within ±10% of the standard (i.e., no false negatives were observed). Similarly, none of the mixtures tested produced signals that could be interpreted as false positives or negatives (data not shown). The specificity of the current sensor is encouraging, considering H2S has been noted as a potential interferent for other methods of cyanide analysis. For example, the EPA ion chromatography method notes H2S (evolved when NaHS is acidified) as an interferent, masking the presence of cyanide35 and the cobinamide-based cyanide detection methods by Ma and Dasgupta also note H2S as a potential interferent.23,24
Figure 5.
Assessment of potential interferents to the sensor technology present in cyanide spiked blood. Error bars represent standard deviation.
Samples containing thiocyanate and HSA did produce a slightly elevated signal compared to the aqueous blank, but below the 5 μM NaCN aqueous standard. In 1971, Chung and Wood36 showed that thiocyanate produced cyanide under acidic conditions in the presence of hydrogen peroxide as an oxidizing agent. Since the sample chamber was under acidic conditions, oxygen bubbled through the sample chamber likely acted as an oxidizing agent, causing a small amount of cyanide to form. For HSA, the elevated fluorescence may have been due to the release of cyanide from cyanide–HSA adducts under acidic conditions.37
Analysis of Cyanide from Rabbit Whole Blood
The analysis of cyanide from whole blood required modification of the method used for aqueous solutions, with 100 μL of sample and 300 μL of 1.5 M H2SO4 found to be optimum conditions for the microdiffusion of cyanide. Because the surface-area-to-volume ratio of the sample chamber limited the amount of HCN gas evolved, lower volumes of acid were used and the concentration of acid became very important, with higher concentrations increasing the amount of HCN evolved. The linear range for cyanide quantification in whole blood was found to be 3.13–200 μM with a detection limit of 0.78 μM, a slope of 310 μM–1, and a correlation coefficient of 0.999 (Figure 3). Even with optimization, the recovery of cyanide was low (39% and 34% for 5 and 75 μM QC standards, respectively). The inefficient recovery of cyanide was likely caused upon addition to whole blood by its rapid transformation to volatile HCN gas at pH values below its pKa of 9.2,14 enzyme-catalyzed conversion to SCN– in the presence of a sulfur donor,3 and binding to blood components, including hemoglobin (Hb), methemoglobin (metHb), and albumin.3
Diagnosis of Cyanide Exposure in Rabbits
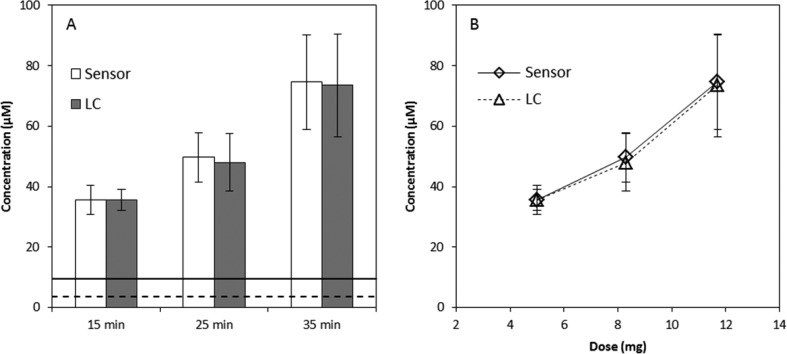
The described sensor was used to verify cyanide exposure in rabbits (Figure 6). Rabbit blood drawn prior to exposure produced a small amount of fluorescence due to endogenous cyanide concentrations,19,38 but it was below the LOD. Rabbit blood drawn at 15, 25, and 35 min into the infusion period produced cyanide concentrations of 35.6 ± 4.8, 49.7 ± 8.2, and 74.6 ± 15.6 μM, respectively, as measured by the sensor. These concentrations deviated by less than 3.5% of the concentrations found by LC–MS/MS (Figure 6A). Similar to observations of Bhandari et al.,19 blood cyanide concentrations exhibited a linear response to increasing doses of cyanide (Figure 6B). Each rabbit that could be considered “exposed” (i.e., CN concentration levels above 10 μM) was correctly diagnosed from the analysis of whole blood by the sensor. Moreover, each sample was analyzed for exposure in under 3 min, and triplicate analysis of individual rabbits produced measured cyanide concentrations with a %RSD of ≤12% for all time points. The interanimal variability observed was expected due to varying physiological characteristics of individual rabbits (e.g., animal size, levels of rhodanese present, etc.). Overall, the sensor was 100% accurate in diagnosing cyanide poisoning for acutely exposed rabbits.
Figure 6.
(A) Comparison of the cyanide concentrations found in the whole blood of cyanide exposed rabbits at 15, 25, and 35 min into the infusion period (5, 8.3, and 11.7 mg NaCN exposure, respectively). The dashed line represents the LLOQ (3.12 μM) and the solid line represents 10 μM cyanide, the threshold considered “cyanide exposure” for this study. Standard deviation values (±3 s) for the lines were not presented because they were negligible compared to the scale of the x axis. Note that for the 15 min time point n = 3 because three animals did not have blood drawn at that time interval. (B) Dose–response curves for three different doses of NaCN (5, 8.3, and 11.7 mg) intravenously administered to rabbits. Error bars represent standard deviation.
Conclusions
A rapid and sensitive cyanide field sensor was developed based on the detection of a fluorescent β-isoindole product produced by the reaction of NDA, taurine, and cyanide. The optimized sensor consists of a cyanide capture apparatus with two chambers separated by silicone septa punctured with small bore stainless steel tubing. This configuration produced a linear range of 1.5–100 μM with a detection limit of 0.25 μM for aqueous cyanide and a linear range of 3.13–200 μM with a detection limit of 0.78 μM for rabbit whole blood. None of the potential interferents produced a signal that could be considered a false positive or negative for cyanide exposure, and the excellent storage stability of the capture solution reagents make the described cyanide sensor highly applicable to field use. Compared to the rapid and/or portable sensors shown in Table 2, the described sensor has a rapid analysis time, a biologically relevant detection limit, and no known interferents. Although the analysis time for this sensor is short, rapid diagnosis of cyanide may be limited by the collection of blood (i.e., a finger prick with a lancet and collection of venous blood by trained personnel would require a significant amount of time). Studies are underway to link the salivary concentrations of cyanide with cyanide exposure, eliminating the need for invasive and potentially lengthy blood collection.
The performance of the sensor, most importantly the 100% accurate and rapid (<3 min) diagnosis of cyanide exposure in rabbits, is promising for the development of a highly robust field-portable sensor for the accurate diagnosis of cyanide exposure. Further sensor development, specifically focused on more rapid analysis and miniaturization, is currently underway.
Acknowledgments
The research was supported by the CounterACT Program, National Institutes of Health Office of the Director, and the National Institute of Allergy and Infectious Diseases, Interagency Agreement Numbers Y1-OD-0690-01/A-120-B.P2010-01, Y1-OD-1561-01/A120-B.P2011-01, AOD12060-001-00000/A120-B.P2012-01 and the USAMRICD under the auspices of the U.S. Army Research Office of Scientific Services Program Contract W911NF-11-D-0001 administered by Battelle (Delivery order 0079, Contract TCN 11077), USAMRMC W81XWH-12-2-0098, and NIH U54 NS079201. We gratefully acknowledge funding from the Oak Ridge Institute for Science and Education (ORISE). We thank the National Science Foundation Major Research Instrumentation Program (Grant CHE-0922816) for funding the AB SCIEX QTRAP 5500 LC–MS/MS. The LC–MS/MS instrumentation was housed in the South Dakota State University Campus Mass Spectrometry Facility, which was supported by the National Science Foundation/EPSCoR Grant 0091948 and the State of South Dakota. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of the Army, the National Institutes of Health, the National Science Foundation, or the Department of Defense. The authors declare that there are no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
References
- Logue B. A.; Hinkens D. M.; Baskin S. I.; Rockwood G. A. Crit. Rev. Anal. Chem. 2010, 40, 122–147. [Google Scholar]
- Mudder T. I.; Botz M. M. Eur. J. Miner. Process. Environ. Prot. 2004, 4, 62–74. [Google Scholar]
- Baskin S.; Brewer T. In Medical Aspects of Chemical and Biological Warfare; Sidell F.; Takafuji E.; Franz D., Eds.; Office of the Surgeon General, Department of the Army: Falls Church, VA, 1997, pp 271–286; [Google Scholar]; Baskin S. I.; Kelly J. B.; Maliner B. I.; Rockwood G. A.; Zoltani C. K. In Medical Aspects of Chemical Warfare; Tuorinsky S. D., Ed.; Office of the Surgeon General, Department of the Army: Falls Church, VA, 2008, pp 371–410. [Google Scholar]
- Moriya F.; Hashimoto Y. J. Forensic Sci. 2001, 46, 1421–5. [PubMed] [Google Scholar]
- Douglas J. E.; Olshaker M.. The Anatomy Of Motive: The FBI’s Legendary Mindhunter Explores The Key To Understanding And Catching Violent Criminals; Scribner: New York City, 1999. [Google Scholar]
- Okumuru T.; Ninomiya N.; Ohta M. Prehosp. Disaster Med. 2003, 18, 189–192. [DOI] [PubMed] [Google Scholar]
- Davenport P. Michael Marin, Ex-Wall Street Trader, Took Cyanide after Guilty Arson Verdict. Huffington Post (www.huffingtonpost.com), July 27, 2012. [Google Scholar]
- Keyser J. Urooj Khan, Chicago Lottery Winner’s Cyanide Death Under Investigation. Huffington Post (www.huffingtonpost.com), January 8, 2013. [Google Scholar]
- McManus J. W.; Reyes R. B.; Nanola C. L. J. Environ. Manage. 1997, 21, 69–78. [DOI] [PubMed] [Google Scholar]; Barber C. V.; Pratt R. V. Environment 1998, 40, 5–34. [Google Scholar]; Wabritz C.; Taylor M.; Green E.; Razak T., From Ocean to Aquarium: Cambridge, U.K., 2003. [Google Scholar]
- Cooper C. E.; Brown G. C. J. Bioenerg. Biomembr. 2008, 40, 533–539. [DOI] [PubMed] [Google Scholar]
- Cherian M. A.; Richmond I. J. Clin. Pathol. 2000, 53, 794–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toxicology Profile for Cyanide. Agency for Toxic Substances and Disease Registry; U.S. Department of Health and Human Services: Washington, D.C., 2006.
- Laforge M.; Gourlain H.; Fompeydie D.; Buneaux F.; Borron S. W.; Galliot-Guilley M. J. Toxicol., Clin. Toxicol. 1999, 37, 337–340. [DOI] [PubMed] [Google Scholar]
- Logue B. A.; Kirschten N. P.; Petrikovics I.; Moser M. A.; Rockwood G. A.; Baskin S. I. J. Chromatogr., B: Anal. Technol. Biomed. Life Sci. 2005, 819, 237–44. [DOI] [PubMed] [Google Scholar]
- Jackson R.; Petrikovics I.; Laic E. P. C.; Yu J. C. C. Anal. Methods 2010, 2, 552–557. [Google Scholar]
- Vinnakota C. V.; Peetha N. S.; Perrizo M. G.; Ferris D. G.; Oda R. P.; Rockwood G. A.; Logue B. A. Biomarkers 2012, 17, 625–633. [DOI] [PubMed] [Google Scholar]
- Minakata K.; Nozawa H.; Gonmori K.; Yamagishi I.; Suzuki M.; Hasegawa K.; Watanabe K.; Suzuki O. Anal. Bioanal. Chem. 2011, 400, 1945–1951. [DOI] [PubMed] [Google Scholar]
- Baskin S. I.; Petrikovics I.; Platoff G. E.; Rockwood G. A.; Logue B. A. Toxicol. Mech. Methods 2006, 16, 339–45. [DOI] [PubMed] [Google Scholar]; Baskin S. I.; Petrikovics I.; Kurche J. S.; Nicholson J. D.; Logue B. A.; Maliner B.; Rockwood G. A. In Pharmacological Perspectives of Toxic Chemicals and Their Antidotes; Flora S. J. S., Romano J. A. Jr., Baskin S. I., Sekhar K., Eds.; Narosa Publishing House: New Delhi, India, 2004, p 105; [Google Scholar]; Baskin S. I.; Isom G. E. In Comprehensive Toxicology; Sipes I. G., McQueen C. A., Gandolfi A. J., Eds.; Elsevier Science: New York, NY, 1997, pp 477–488. [Google Scholar]
- Bhandari R. K.; Oda R. P.; Petrikovics I.; Thompson D. E.; Brenner M.; Mohan S. B.; Bebarta V. S.; Rockwood G. A.; Logue B. A., J. Anal. Toxicol. 2014, in press. [DOI] [PMC free article] [PubMed]
- Sylvester D. M.; Hayton W. L.; Morgan R. L.; Way J. L. Toxicol. Appl. Pharmacol. 1983, 69, 265–271. [DOI] [PubMed] [Google Scholar]
- Sousa A. B.; Manzano H.; Soto-Blanco B.; Gorniak S. L. Arch. Toxicol. 2003, 77, 330–334. [DOI] [PubMed] [Google Scholar]
- Ma J.; Dasgupta P. K. Anal. Chim. Acta 2010, 673, 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J.; Ohira S.-I.; Mishra S. K.; Puanngam M.; Dasgupta P. K.; Mahon S. B.; Brenner M.; Blackledge W.; Boss G. R. Anal. Chem. 2011, 83, 4319–4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J.; Dasgupta P. K.; Blackledge W.; Boss G. R. Anal. Chem. 2010, 82, 6244–6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y.; Dasgupta P. K.; Mahon S. B.; Ma J.; Brenner M.; Wang Jian-Hua; Boss G. R. Anal. Chim. Acta 2013, 768, 129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E. Z.; Lee S. U.; Heo N.-S.; Stucky G. D.; Jun Y.-S.; Hong W. H. Chem. Commun. 2012, 48, 3942–3944. [DOI] [PubMed] [Google Scholar]
- Kumar S.; Singh P.; Hundal G.; Hunda M. S.; Kumar S. Chem. Commun. 2013, 49, 2667–2669. [DOI] [PubMed] [Google Scholar]
- Niu H.-T.; Su D.; Jiang X.; Yang W.; Yin Z.; Hea J.; Cheng J.-P. Org. Biomol. Chem. 2008, 6, 3038–3040. [DOI] [PubMed] [Google Scholar]; Niu H.-T.; Jiang X.; He J.; Cheng J.-P. Tetrahedron Lett. 2008, 49, 6521–6524. [Google Scholar]; Lv X.; Liu J.; Liu Y.; Zhao Y.; Chen M.; Wanga P.; Guo W. Org. Biomol. Chem. 2011, 9, 4954–4958. [DOI] [PubMed] [Google Scholar]; Li H.; Li B.; Jin L.-Y.; Kan Y.; Yin B. Tetrahedron. 2011, 67, 7348–7353. [Google Scholar]; Kumari N.; Jha S.; Bhattacharya S. J. Org. Chem. 2011, 76, 8215–8222. [DOI] [PubMed] [Google Scholar]
- Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academic Press: Washington, D.C., 2011. [Google Scholar]
- Carlson R. G.; Srinivasachar K.; Givens R. S.; Matuszewski B. K. J. Org. Chem. 1986, 51, 3978–3983. [Google Scholar]; Sano A.; Takezawa M.; Takitani S. Anal. Chim. Acta 1989, 225, 351–358. [Google Scholar]
- Diem K.Documenta Geigy: Scientific Tables, 7th ed.; Wiley: Basel, Switzerland, 1970. [Google Scholar]
- Tsuge K.; Kataoka M.; Seto Y. J. Health Sci. 2000, 46, 343–350. [Google Scholar]
- Air Quality Guidelines; Theakston F., Ed.; WHO Regional Office for Europe: Copenhagen, Denmark, 2000. [Google Scholar]
- Bhandari R. K.; Manandhar E.; Oda R. P.; Rockwood G. A.; Logue B. A.. Anal. Bioanal. Chem., 2014, in press. [DOI] [PubMed]
- Other Test Method 29, Sampling and Analysis for Hydrogen Cyanide Emissions from Stationary Sources; U. S. Environmental Protection Agency: Atlanta, GA, 2011.
- Chung J.; Wood J. L. J. Biol. Chem. 1971, 246, 555–560. [PubMed] [Google Scholar]
- Fasco M. J.; Hauer C. R. III; Stack R. F.; O’Hehir C.; Barr J. R.; Eadon G. A. Chem. Res. Toxicol. 2007, 20, 677–684. [DOI] [PubMed] [Google Scholar]
- Lundqulst P.; Rosllng H.; Sorbo B. Clin. Chem. 1985, 31, 591–595. [PubMed] [Google Scholar]; Takeda S.; Inada Y.; Tomaru T.; Ikeda T.; Tashiro N.; Morimoto F.; Shibata F. Masui 1990, 39, 701–707. [PubMed] [Google Scholar]