Abstract
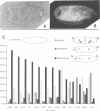
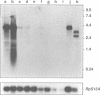
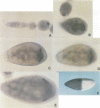
The Bicaudal-C (Bic-C) gene of Drosophila melanogaster is required for correct targeting of the migrating anterior follicle cells and for specifying anterior position. Females lacking any wild type copies of Bic-C produce only eggshells open at the anterior end, because of the failure of the columnar follicle cells to migrate in the correct position at the nurse cell--oocyte boundary. Embryos which develop from eggs produced in females with only one wild type copy of Bic-C show defects in anterior patterning and an abnormal persistence of oskar RNA in anterior regions. We cloned Bic-C and found that, in ovaries, Bic-C RNA is expressed only in germline cells. Bic-C RNA is localized to the oocyte in early oogenesis, and later concentrates at its anterior cortex. The Bic-C protein includes five KH domains similar to those found in the human fragile-X protein FMR1. Alteration of a highly conserved KH domain codon by mutation abrogates in vivo Bic-C function. These results suggest roles for the Bic-C protein in localizing RNAs and in intercellular signaling.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ajioka J. W., Smoller D. A., Jones R. W., Carulli J. P., Vellek A. E., Garza D., Link A. J., Duncan I. W., Hartl D. L. Drosophila genome project: one-hit coverage in yeast artificial chromosomes. Chromosoma. 1991 Sep;100(8):495–509. doi: 10.1007/BF00352200. [DOI] [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Ashburner M., Thompson P., Roote J., Lasko P. F., Grau Y., el Messal M., Roth S., Simpson P. The genetics of a small autosomal region of Drosophila melanogaster containing the structural gene for alcohol dehydrogenase. VII. Characterization of the region around the snail and cactus loci. Genetics. 1990 Nov;126(3):679–694. doi: 10.1093/genetics/126.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley C. T., Jr, Wilkinson K. D., Reines D., Warren S. T. FMR1 protein: conserved RNP family domains and selective RNA binding. Science. 1993 Oct 22;262(5133):563–566. doi: 10.1126/science.7692601. [DOI] [PubMed] [Google Scholar]
- Bardsley A., McDonald K., Boswell R. E. Distribution of tudor protein in the Drosophila embryo suggests separation of functions based on site of localization. Development. 1993 Sep;119(1):207–219. doi: 10.1242/dev.119.1.207. [DOI] [PubMed] [Google Scholar]
- Bender L. B., Kooh P. J., Muskavitch M. A. Complex function and expression of Delta during Drosophila oogenesis. Genetics. 1993 Apr;133(4):967–978. doi: 10.1093/genetics/133.4.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckanovich R. J., Posner J. B., Darnell R. B. Nova, the paraneoplastic Ri antigen, is homologous to an RNA-binding protein and is specifically expressed in the developing motor system. Neuron. 1993 Oct;11(4):657–672. doi: 10.1016/0896-6273(93)90077-5. [DOI] [PubMed] [Google Scholar]
- Christerson L. B., McKearin D. M. orb is required for anteroposterior and dorsoventral patterning during Drosophila oogenesis. Genes Dev. 1994 Mar 1;8(5):614–628. doi: 10.1101/gad.8.5.614. [DOI] [PubMed] [Google Scholar]
- Cruz-Alvarez M., Pellicer A. Cloning of a full-length complementary DNA for an Artemia salina glycine-rich protein. Structural relationship with RNA binding proteins. J Biol Chem. 1987 Oct 5;262(28):13377–13380. [PubMed] [Google Scholar]
- De Boulle K., Verkerk A. J., Reyniers E., Vits L., Hendrickx J., Van Roy B., Van den Bos F., de Graaff E., Oostra B. A., Willems P. J. A point mutation in the FMR-1 gene associated with fragile X mental retardation. Nat Genet. 1993 Jan;3(1):31–35. doi: 10.1038/ng0193-31. [DOI] [PubMed] [Google Scholar]
- Delahodde A., Becam A. M., Perea J., Jacq C. A yeast protein HX has homologies with the histone H2AF expressed in chicken embryo. Nucleic Acids Res. 1986 Nov 25;14(22):9213–9214. doi: 10.1093/nar/14.22.9213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D., Lipshitz H. D. Localized RNAs and their functions. Bioessays. 1993 Oct;15(10):651–658. doi: 10.1002/bies.950151004. [DOI] [PubMed] [Google Scholar]
- Duncan R., Bazar L., Michelotti G., Tomonaga T., Krutzsch H., Avigan M., Levens D. A sequence-specific, single-strand binding protein activates the far upstream element of c-myc and defines a new DNA-binding motif. Genes Dev. 1994 Feb 15;8(4):465–480. doi: 10.1101/gad.8.4.465. [DOI] [PubMed] [Google Scholar]
- Engebrecht J., Roeder G. S. MER1, a yeast gene required for chromosome pairing and genetic recombination, is induced in meiosis. Mol Cell Biol. 1990 May;10(5):2379–2389. doi: 10.1128/mcb.10.5.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ephrussi A., Dickinson L. K., Lehmann R. Oskar organizes the germ plasm and directs localization of the posterior determinant nanos. Cell. 1991 Jul 12;66(1):37–50. doi: 10.1016/0092-8674(91)90137-n. [DOI] [PubMed] [Google Scholar]
- Ephrussi A., Lehmann R. Induction of germ cell formation by oskar. Nature. 1992 Jul 30;358(6385):387–392. doi: 10.1038/358387a0. [DOI] [PubMed] [Google Scholar]
- Ferrandon D., Elphick L., Nüsslein-Volhard C., St Johnston D. Staufen protein associates with the 3'UTR of bicoid mRNA to form particles that move in a microtubule-dependent manner. Cell. 1994 Dec 30;79(7):1221–1232. doi: 10.1016/0092-8674(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Fumagalli S., Totty N. F., Hsuan J. J., Courtneidge S. A. A target for Src in mitosis. Nature. 1994 Apr 28;368(6474):871–874. doi: 10.1038/368871a0. [DOI] [PubMed] [Google Scholar]
- Gavis E. R., Lehmann R. Localization of nanos RNA controls embryonic polarity. Cell. 1992 Oct 16;71(2):301–313. doi: 10.1016/0092-8674(92)90358-j. [DOI] [PubMed] [Google Scholar]
- Gavis E. R., Lehmann R. Translational regulation of nanos by RNA localization. Nature. 1994 May 26;369(6478):315–318. doi: 10.1038/369315a0. [DOI] [PubMed] [Google Scholar]
- Gedeon A. K., Baker E., Robinson H., Partington M. W., Gross B., Manca A., Korn B., Poustka A., Yu S., Sutherland G. R. Fragile X syndrome without CCG amplification has an FMR1 deletion. Nat Genet. 1992 Aug;1(5):341–344. doi: 10.1038/ng0892-341. [DOI] [PubMed] [Google Scholar]
- Hay B., Jan L. Y., Jan Y. N. Localization of vasa, a component of Drosophila polar granules, in maternal-effect mutants that alter embryonic anteroposterior polarity. Development. 1990 Jun;109(2):425–433. doi: 10.1242/dev.109.2.425. [DOI] [PubMed] [Google Scholar]
- Kim-Ha J., Smith J. L., Macdonald P. M. oskar mRNA is localized to the posterior pole of the Drosophila oocyte. Cell. 1991 Jul 12;66(1):23–35. doi: 10.1016/0092-8674(91)90136-m. [DOI] [PubMed] [Google Scholar]
- Kim-Ha J., Webster P. J., Smith J. L., Macdonald P. M. Multiple RNA regulatory elements mediate distinct steps in localization of oskar mRNA. Development. 1993 Sep;119(1):169–178. doi: 10.1242/dev.119.1.169. [DOI] [PubMed] [Google Scholar]
- Klein P., Kanehisa M., DeLisi C. The detection and classification of membrane-spanning proteins. Biochim Biophys Acta. 1985 May 28;815(3):468–476. doi: 10.1016/0005-2736(85)90375-x. [DOI] [PubMed] [Google Scholar]
- Klämbt C., Glazer L., Shilo B. Z. breathless, a Drosophila FGF receptor homolog, is essential for migration of tracheal and specific midline glial cells. Genes Dev. 1992 Sep;6(9):1668–1678. doi: 10.1101/gad.6.9.1668. [DOI] [PubMed] [Google Scholar]
- Lantz V., Ambrosio L., Schedl P. The Drosophila orb gene is predicted to encode sex-specific germline RNA-binding proteins and has localized transcripts in ovaries and early embryos. Development. 1992 May;115(1):75–88. doi: 10.1242/dev.115.1.75. [DOI] [PubMed] [Google Scholar]
- Lantz V., Chang J. S., Horabin J. I., Bopp D., Schedl P. The Drosophila orb RNA-binding protein is required for the formation of the egg chamber and establishment of polarity. Genes Dev. 1994 Mar 1;8(5):598–613. doi: 10.1101/gad.8.5.598. [DOI] [PubMed] [Google Scholar]
- Lasko P. F., Ashburner M. Posterior localization of vasa protein correlates with, but is not sufficient for, pole cell development. Genes Dev. 1990 Jun;4(6):905–921. doi: 10.1101/gad.4.6.905. [DOI] [PubMed] [Google Scholar]
- Lavoie C., Tam R., Clark M., Lee H., Sonenberg N., Lasko P. Suppression of a temperature-sensitive cdc33 mutation of yeast by a multicopy plasmid expressing a Drosophila ribosomal protein. J Biol Chem. 1994 May 20;269(20):14625–14630. [PubMed] [Google Scholar]
- Lhoták V., Greer P., Letwin K., Pawson T. Characterization of elk, a brain-specific receptor tyrosine kinase. Mol Cell Biol. 1991 May;11(5):2496–2502. doi: 10.1128/mcb.11.5.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lhoták V., Pawson T. Biological and biochemical activities of a chimeric epidermal growth factor-Elk receptor tyrosine kinase. Mol Cell Biol. 1993 Nov;13(11):7071–7079. doi: 10.1128/mcb.13.11.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manseau L. J., Schüpbach T. cappuccino and spire: two unique maternal-effect loci required for both the anteroposterior and dorsoventral patterns of the Drosophila embryo. Genes Dev. 1989 Sep;3(9):1437–1452. doi: 10.1101/gad.3.9.1437. [DOI] [PubMed] [Google Scholar]
- Mohler J., Wieschaus E. F. Dominant maternal-effect mutations of Drosophila melanogaster causing the production of double-abdomen embryos. Genetics. 1986 Apr;112(4):803–822. doi: 10.1093/genetics/112.4.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell D. J., Rorth P., Spradling A. C. slow border cells, a locus required for a developmentally regulated cell migration during oogenesis, encodes Drosophila C/EBP. Cell. 1992 Oct 2;71(1):51–62. doi: 10.1016/0092-8674(92)90265-e. [DOI] [PubMed] [Google Scholar]
- Neuman-Silberberg F. S., Schüpbach T. The Drosophila dorsoventral patterning gene gurken produces a dorsally localized RNA and encodes a TGF alpha-like protein. Cell. 1993 Oct 8;75(1):165–174. [PubMed] [Google Scholar]
- Newmark P. A., Boswell R. E. The mago nashi locus encodes an essential product required for germ plasm assembly in Drosophila. Development. 1994 May;120(5):1303–1313. doi: 10.1242/dev.120.5.1303. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieretti M., Zhang F. P., Fu Y. H., Warren S. T., Oostra B. A., Caskey C. T., Nelson D. L. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell. 1991 Aug 23;66(4):817–822. doi: 10.1016/0092-8674(91)90125-i. [DOI] [PubMed] [Google Scholar]
- Ran B., Bopp R., Suter B. Null alleles reveal novel requirements for Bic-D during Drosophila oogenesis and zygotic development. Development. 1994 May;120(5):1233–1242. doi: 10.1242/dev.120.5.1233. [DOI] [PubMed] [Google Scholar]
- Reichman-Fried M., Dickson B., Hafen E., Shilo B. Z. Elucidation of the role of breathless, a Drosophila FGF receptor homolog, in tracheal cell migration. Genes Dev. 1994 Feb 15;8(4):428–439. doi: 10.1101/gad.8.4.428. [DOI] [PubMed] [Google Scholar]
- Ruohola-Baker H., Jan L. Y., Jan Y. N. The role of gene cassettes in axis formation during Drosophila oogenesis. Trends Genet. 1994 Mar;10(3):89–94. doi: 10.1016/0168-9525(94)90231-3. [DOI] [PubMed] [Google Scholar]
- Ruohola H., Bremer K. A., Baker D., Swedlow J. R., Jan L. Y., Jan Y. N. Role of neurogenic genes in establishment of follicle cell fate and oocyte polarity during oogenesis in Drosophila. Cell. 1991 Aug 9;66(3):433–449. doi: 10.1016/0092-8674(81)90008-8. [DOI] [PubMed] [Google Scholar]
- Sajjadi F. G., Pasquale E. B. Five novel avian Eph-related tyrosine kinases are differentially expressed. Oncogene. 1993 Jul;8(7):1807–1813. [PubMed] [Google Scholar]
- Schmidt C., Henkel B., Pöschl E., Zorbas H., Purschke W. G., Gloe T. R., Müller P. K. Complete cDNA sequence of chicken vigilin, a novel protein with amplified and evolutionary conserved domains. Eur J Biochem. 1992 Jun 15;206(3):625–634. doi: 10.1111/j.1432-1033.1992.tb16967.x. [DOI] [PubMed] [Google Scholar]
- Schüpbach T., Wieschaus E. Female sterile mutations on the second chromosome of Drosophila melanogaster. II. Mutations blocking oogenesis or altering egg morphology. Genetics. 1991 Dec;129(4):1119–1136. doi: 10.1093/genetics/129.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel V., Jongens T. A., Jan L. Y., Jan Y. N. pipsqueak, an early acting member of the posterior group of genes, affects vasa level and germ cell-somatic cell interaction in the developing egg chamber. Development. 1993 Dec;119(4):1187–1202. doi: 10.1242/dev.119.4.1187. [DOI] [PubMed] [Google Scholar]
- Siomi H., Choi M., Siomi M. C., Nussbaum R. L., Dreyfuss G. Essential role for KH domains in RNA binding: impaired RNA binding by a mutation in the KH domain of FMR1 that causes fragile X syndrome. Cell. 1994 Apr 8;77(1):33–39. doi: 10.1016/0092-8674(94)90232-1. [DOI] [PubMed] [Google Scholar]
- Siomi H., Matunis M. J., Michael W. M., Dreyfuss G. The pre-mRNA binding K protein contains a novel evolutionarily conserved motif. Nucleic Acids Res. 1993 Mar 11;21(5):1193–1198. doi: 10.1093/nar/21.5.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siomi H., Siomi M. C., Nussbaum R. L., Dreyfuss G. The protein product of the fragile X gene, FMR1, has characteristics of an RNA-binding protein. Cell. 1993 Jul 30;74(2):291–298. doi: 10.1016/0092-8674(93)90420-u. [DOI] [PubMed] [Google Scholar]
- Smith J. L., Wilson J. E., Macdonald P. M. Overexpression of oskar directs ectopic activation of nanos and presumptive pole cell formation in Drosophila embryos. Cell. 1992 Sep 4;70(5):849–859. doi: 10.1016/0092-8674(92)90318-7. [DOI] [PubMed] [Google Scholar]
- St Johnston D., Beuchle D., Nüsslein-Volhard C. Staufen, a gene required to localize maternal RNAs in the Drosophila egg. Cell. 1991 Jul 12;66(1):51–63. doi: 10.1016/0092-8674(91)90138-o. [DOI] [PubMed] [Google Scholar]
- St Johnston D., Brown N. H., Gall J. G., Jantsch M. A conserved double-stranded RNA-binding domain. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10979–10983. doi: 10.1073/pnas.89.22.10979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Johnston D., Driever W., Berleth T., Richstein S., Nüsslein-Volhard C. Multiple steps in the localization of bicoid RNA to the anterior pole of the Drosophila oocyte. Development. 1989;107 (Suppl):13–19. doi: 10.1242/dev.107.Supplement.13. [DOI] [PubMed] [Google Scholar]
- St Johnston R. D., Hoffmann F. M., Blackman R. K., Segal D., Grimaila R., Padgett R. W., Irick H. A., Gelbart W. M. Molecular organization of the decapentaplegic gene in Drosophila melanogaster. Genes Dev. 1990 Jul;4(7):1114–1127. doi: 10.1101/gad.4.7.1114. [DOI] [PubMed] [Google Scholar]
- Steward O., Banker G. A. Getting the message from the gene to the synapse: sorting and intracellular transport of RNA in neurons. Trends Neurosci. 1992 May;15(5):180–186. doi: 10.1016/0166-2236(92)90170-d. [DOI] [PubMed] [Google Scholar]
- Stroumbakis N. D., Li Z., Tolias P. P. RNA- and single-stranded DNA-binding (SSB) proteins expressed during Drosophila melanogaster oogenesis: a homolog of bacterial and eukaryotic mitochondrial SSBs. Gene. 1994 Jun 10;143(2):171–177. doi: 10.1016/0378-1119(94)90093-0. [DOI] [PubMed] [Google Scholar]
- Suter B., Steward R. Requirement for phosphorylation and localization of the Bicaudal-D protein in Drosophila oocyte differentiation. Cell. 1991 Nov 29;67(5):917–926. doi: 10.1016/0092-8674(91)90365-6. [DOI] [PubMed] [Google Scholar]
- Szabad J., Hoffmann G. Analysis of follicle-cell functions in Drosophila: the Fs(3)Apc mutation and the development of chorionic appendages. Dev Biol. 1989 Jan;131(1):1–10. doi: 10.1016/s0012-1606(89)80033-8. [DOI] [PubMed] [Google Scholar]
- Tamkun J. W., Deuring R., Scott M. P., Kissinger M., Pattatucci A. M., Kaufman T. C., Kennison J. A. brahma: a regulator of Drosophila homeotic genes structurally related to the yeast transcriptional activator SNF2/SWI2. Cell. 1992 Feb 7;68(3):561–572. doi: 10.1016/0092-8674(92)90191-e. [DOI] [PubMed] [Google Scholar]
- Tautz D., Pfeifle C. A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma. 1989 Aug;98(2):81–85. doi: 10.1007/BF00291041. [DOI] [PubMed] [Google Scholar]
- Taylor S. J., Shalloway D. An RNA-binding protein associated with Src through its SH2 and SH3 domains in mitosis. Nature. 1994 Apr 28;368(6474):867–871. doi: 10.1038/368867a0. [DOI] [PubMed] [Google Scholar]
- Trimble W. S., Cowan D. M., Scheller R. H. VAMP-1: a synaptic vesicle-associated integral membrane protein. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4538–4542. doi: 10.1073/pnas.85.12.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble W. S., Linial M., Scheller R. H. Cellular and molecular biology of the presynaptic nerve terminal. Annu Rev Neurosci. 1991;14:93–122. doi: 10.1146/annurev.ne.14.030191.000521. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Verkerk A. J., Pieretti M., Sutcliffe J. S., Fu Y. H., Kuhl D. P., Pizzuti A., Reiner O., Richards S., Victoria M. F., Zhang F. P. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991 May 31;65(5):905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- Wang C., Dickinson L. K., Lehmann R. Genetics of nanos localization in Drosophila. Dev Dyn. 1994 Feb;199(2):103–115. doi: 10.1002/aja.1001990204. [DOI] [PubMed] [Google Scholar]
- Wang C., Lehmann R. Nanos is the localized posterior determinant in Drosophila. Cell. 1991 Aug 23;66(4):637–647. doi: 10.1016/0092-8674(91)90110-k. [DOI] [PubMed] [Google Scholar]
- Wong G., Müller O., Clark R., Conroy L., Moran M. F., Polakis P., McCormick F. Molecular cloning and nucleic acid binding properties of the GAP-associated tyrosine phosphoprotein p62. Cell. 1992 May 1;69(3):551–558. doi: 10.1016/0092-8674(92)90455-l. [DOI] [PubMed] [Google Scholar]
- Wöhrle D., Kotzot D., Hirst M. C., Manca A., Korn B., Schmidt A., Barbi G., Rott H. D., Poustka A., Davies K. E. A microdeletion of less than 250 kb, including the proximal part of the FMR-I gene and the fragile-X site, in a male with the clinical phenotype of fragile-X syndrome. Am J Hum Genet. 1992 Aug;51(2):299–306. [PMC free article] [PubMed] [Google Scholar]
- Xu T., Caron L. A., Fehon R. G., Artavanis-Tsakonas S. The involvement of the Notch locus in Drosophila oogenesis. Development. 1992 Aug;115(4):913–922. doi: 10.1242/dev.115.4.913. [DOI] [PubMed] [Google Scholar]
- Yue L., Spradling A. C. hu-li tai shao, a gene required for ring canal formation during Drosophila oogenesis, encodes a homolog of adducin. Genes Dev. 1992 Dec;6(12B):2443–2454. doi: 10.1101/gad.6.12b.2443. [DOI] [PubMed] [Google Scholar]