Abstract
Objectives
Long-term risk stratification in patients presenting with acute coronary syndromes (ACS) is possible by measuring cardiac troponin (cTn). The present study examined whether PAPP-A measured in an emergency department (ED) chest pain population in association with conventional and novel high sensitivity cTn (hs-cTnI) assays can predict long-term mortality.
Methods
In 320 patients with cTn measurements the earliest heparinized plasma PAPP-A concentration after presentation was used for risk stratification for death by Kaplan–Meier and Cox analyses. Subgroup analyses using the earliest PAPP-A concentrations were also performed in a cohort of subjects with presentation cTnI ≤99th percentile but with significantly changing cardiac troponin concentrations as measured by the AccuTnI assay and the hs-cTnI assay (n=45 and 120 subjects, respectively).
Results
Subjects with PAPP-A concentrations in the highest tertile were at higher risk for death (HR>2.00; p≤0.05 at 2 years) even after adjusting for cTnI at presentation. In the cohort with cTnI≤99th percentile but with changing hs-cTnI concentrations, subjects in the top PAPP-A tertile had a higher probability for death (p=0.02).
Conclusion
Early measurement of PAPP-A may identify chest pain patients at higher risk for long-term death. Additional prospective ACS studies are required to fully elucidate PAPP-A’s role.
Keywords: Pregnancy associated plasma protein A, Cardiac troponin, Death, Emergency department, High sensitivity
Introduction
Acute coronary syndromes (ACS) including unstable angina, non-ST and ST-elevation myocardial infarction are the leading causes of mortality and morbidity worldwide [1,2]. Despite sophisticated imaging technology and biochemical markers to assist in the diagnosis and monitoring of ACS patients, there still are patients who are inaccurately diagnosed in the emergency department (ED) and others whose risk of adverse events following presentation is underestimated [3–7]. The use of sensitive troponin assays has helped with better diagnosis [8], but additional biomarkers would be especially useful to identify patients at long-term risk after presentation. A few acute-care markers such as cardiac troponin (cTn), natriuretic peptides and C-reactive protein (CRP), have been shown to have longer-term predictive value in patients with ACS [7,9–16] but there are additional patients at risk who are not identified, perhaps in part because none of these biomarkers provides information about atherosclerotic plaque instability. A sensitive and specific early biomarker of atherosclerotic plaque instability would assist in risk stratification. Vulnerable coronary atherosclerotic plaques have been reported to contain pregnancy associated plasma protein-A (PAPP-A) [17,18]. Since these lesions appear to precede the development of myocardial ischemia and necrosis, PAPP-A could serve as a useful marker both for early diagnosis of ACS and to define those without acute events who are at subsequent risk for events [18,19]. In the present study, we used a new high-sensitivity PAPP-A (hsPAPP-A) assay to assess both the analytical and clinical utility of PAPP-A in an emergency department chest pain population characterized with a conventional sensitive cTnI assay and new research high-sensitivity cTnI (hs-cTnI) assay.
Methods and materials
Study population and laboratory analyses
The study population has been previously described [14,15,20–22]. Briefly, in 1996 after research ethics approval, 458 patients representing 500 separate patient presentations to the ED were enrolled in a Cardiac Markers study. The only inclusion criterion was the assessment by the triage staff that the patient had symptoms suggestive of cardiac ischemia; there were no exclusion criteria. Heparin plasma samples were collected at presentation and at scheduled intervals measured from the time of pain onset: hourly until 6 h after onset, then at 9, 12, 24, and 48 h or until the patient was discharged, declined further participation, or was removed from the study by those responsible for his/her care. Specimens were stored until 2003 when cTnI (AccuTnI™ Beckman Coulter) was measured in all available specimens.
In 2007 all remaining heparin plasma specimens (stored at −80 °C) with sufficient volume were thawed a second time and measured first by a highly sensitive research cardiac troponin I assay (hs-cTnI with a limit of detection of 2.06 ng/L, Beckman Coulter) [22] then with a hsPAPP-A ELISA (DSL-90-27600; Beckman Coulter) that measures uncomplexed PAPP-A and the PAPP-A/proMBP complex in equimolar concentration, but not proMBP nor other cross reactants such as PAPP-A2, MMP-9 and MMP-12 (both serum and heparin plasma are suitable sample types for this assay). The manufacturer has determined the lowest detectable level of PAPP-A (mean signal of 16 replicates of the zero calibrator plus two standard deviations) with 95% confidence to be 0.184 mIU/L. However, for this analysis we did not confirm the lowest detectable level, rather for any concentrations below the lowest calibrator (0.50 mIU/L), 0.49 mIU/L was assigned to these values for statistical purposes (n=51 or 5% of 1081 results). There were 1081 heparin plasma specimens from 357 patient presentations (320 unique subjects) that were measured with the PAPP-A assay; 70% of these specimens had sufficient volume for duplicate PAPP-A measurements in which case the average concentration was used. In cell culture systems, animal models and patients with STEMI it has been observed that treatment with heparin elevates circulating PAPP-A concentrations [23,24], and in our population 70 of the 320 subjects (22%) received heparin at some time during their hospital stay. To mitigate the potentially confounding effects of heparin on PAPP-A levels we used the earliest available specimen for outcome analysis. One third of the subjects had a PAPP-A measured after the 1st cardiac troponin (n =107; median (IQR) time after 1st specimen=2 (1–6) hours), however only 19 of these subjects received heparin. The total imprecision (CV; coefficient of variation, n=38) for the hsPAPP-A assay during the study at two quality control levels provided with the assay (4.82 and 12.67 mIU/L) was 14.2% and 10.6%, respectively; however, for lower concentrations, the manufacturer states a total imprecision of 9.9% at a PAPP-A concentration of 1.73 mIU/L. This is consistent with the duplicate CVs from our population (median concentration (IQR)=1.66 (1.03–3.26); median CV (IQR)=7% (3–14).
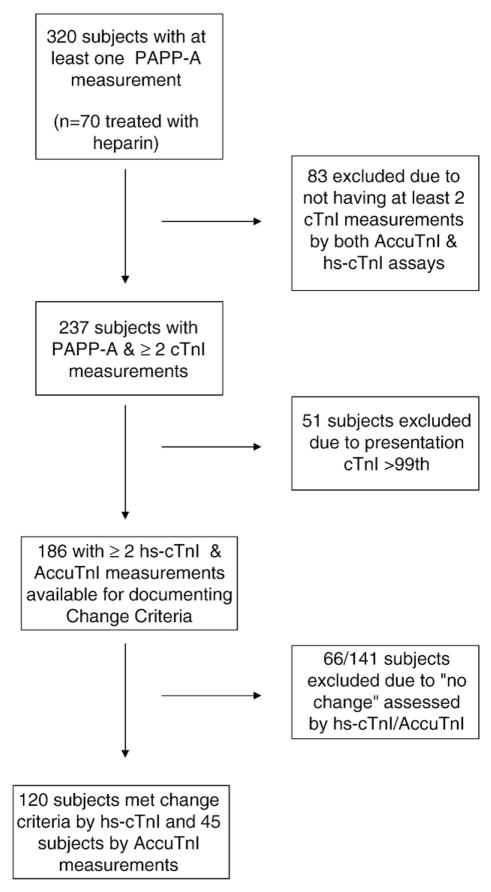
A cohort of subjects (186 from 320) was identified who had both AccuTnI and hs-cTnI measurements performed on multiple specimens (n=570 specimens with both cTnI measurements), and for whom the presentation AccuTnI was ≤99th percentile (≤0.04 μg/L), and for whom a concentration change in cTnI was observed, either for AccuTnI (n=45) or for hs-cTnI (n=120), respectively (see Fig. 1). Briefly, an analytically significant concentration change was noted if the difference between the highest and the lowest cTn concentrations observed within a subject was >3 SD or >20%, consistent with the 2007 MI definition and our previous work [2,22]. The earliest PAPP-A concentration was used for risk stratification in this cohort as well.
Fig. 1.
Flow diagram for the selection of subjects for the health outcome analyses.
Health outcomes and statistical analysis
After research ethics board approval, mortality outcomes were obtained via linkage to the Registered Persons Data Base (RPDB) [14,15]. The outcomes were captured as events post-presentation (i.e., either during the index hospitalization or afterwards). Between-group comparisons of central tendency (means, medians) were based on one-way analysis of variance (ANOVA), Mann Whitney and the Kruskal–Wallis tests. The nonparametric Spearman correlation coefficient was determined for PAPP-A, cTnI (AccuTnI) and hs-cTnI. The Pearson Chi-square test statistic was used to compare proportions.
The time to death was assessed by tertile analysis of the earliest PAPP-A value by Kaplan–Meier survival curves for up to 2 years and 10 years, with differences between groups determined by the log rank test. In keeping with our previous analyses the covariates and cutoffs chosen for the Cox proportional hazard models were the same [14,15]. Specifically, tertiles 2,3 were compared to tertile 1 (referent) in various models: model 1 adjusted for age and sex; model 2 adjusted for age, sex, and presentation AccuTnI ≥0.02 μg/L (i.e., detectable cardiac troponin) with the hazard ratios (HR) derived by partial likelihood estimation with the significance of the association based on the Wald Chi-square statistic. All statistical analyses were performed using SAS and Graphpad Prism with a p-value≤0.05 considered statistically significant.
Results
The median age was 64 years for the study population (n =320 with 60% men; see Table 1). The values for PAPP-A appear to manifest a rise during the first 9 h after pain onset but there is a considerable overlap in PAPP-A ranges between the different time points (Fig. 2A). There was no difference in the initial PAPP-A concentrations in those who received (n=70) and did not receive heparin (n=250) during their hospital stay (median (IQR) PAPP-A=1.22 (0.80–1.99) vs. 1.20 (0.79–1.97) mIU/L; p=0.68), however the peak PAPP-A was significantly higher in those who received heparin vs. no heparin (median (IQR) PAPP-A =4.03 (1.95–9.84) vs. 1.56 (0.95–2.96) mIU/L; p<0.01). In those who did not receive heparin, there was a significant yet weak correlation between the earliest PAPP-A and cTn (r<0.15; p<0.05) (Table 2). Assessing the baseline characteristics across the PAPP-A tertiles only sex and hs-cTnI were different among the groups (see Table 3 and Fig. 2B for PAPP-A concentrations).
Table 1.
Study cohort characteristics.
| Demographics | Total (n=320) |
|---|---|
| Males (%) | 192 (60%) |
| Median age (IQR) | 64 (51–74) |
| 1996 AMI diagnosis | 54 (16.9%) |
| # of PAPP-A measurements | |
| Median (IQR) | 2 (1–5) |
| Earliest PAPP-A concentration (mIU/L) | |
| Median (IQR) | 1.21 (0.80–1.97) |
| Time from pain onset for Early PAPP-A | |
| Median hours (IQR) | 4 (2–9) |
| Earliest cTnI concentration (3g/L) | |
| Median (IQR) | 0.01 (0.01–0.04) |
| Earliest hs-cTnI concentration (ng/L) | |
| Median (IQR) | 10.8 (5.0–54.7) |
| Death within 2 years | 50 (16%) |
| Death within 10 years | 141 (44%) |
Fig. 2.
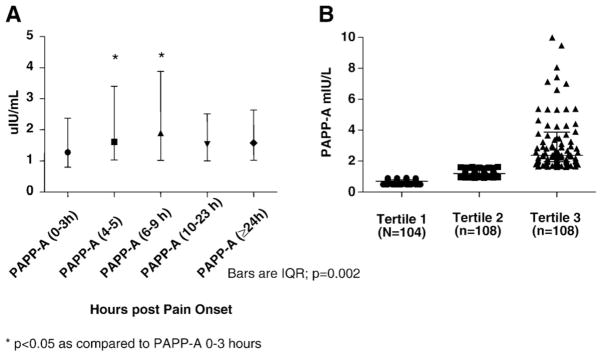
Serial time profile of PAPP-A after onset of pain (1081 specimens: 0–3 h n=228; 4–5 h n=227; 6–9 h n=262; 10–23 h n=180;≥24 h n=184) (A). Dot plot of presentation PAPP-A concentrations in each tertile (note 9 data points are >10 mIU/L in tertile 3 and are not included in this graph) (B).
Table 2.
Spearman correlation of PAPP-A with other biomarkers in overall population and in non-heparin subgroup.
| Biomarker | Overall population
|
No heparin (n =250)
|
||
|---|---|---|---|---|
| PAPP-A r | p-value | PAPP-A r | p-value | |
| Early cTnI | 0.08 | 0.18 | 0.14 | 0.02 |
| Early hs-cTnI | 0.09 | 0.09 | 0.13 | 0.04 |
Table 3.
Characteristics of group based on PAPP-A tertiles.
| Variable | Tertile 1 (n=104) (<0.92 mIU/L) median (IQR): 0.70 (0.52–0.79) | Tertile 2 (n=108) (0.92–1.62 mIU/L) median (IQR): 1.19 (1.07–1.40) | Tertile 3 (n=108) (N1.62 mIU/L) median (IQR): 2.37 (1.96–3.87) | p-value |
|---|---|---|---|---|
| Sex | ||||
| Male | 49 | 74 | 69 | <0.01 |
| Female | 55 | 34 | 39 | |
| History of MI/HF | ||||
| Yes | 36 | 36 | 42 | 0.67 |
| No | 68 | 72 | 66 | |
| ASA | ||||
| Yes | 27 | 28 | 21 | 0.43 |
| No | 77 | 80 | 87 | |
| Heparin treatment | ||||
| 23 | 21 | 26 | 0.68 | |
| 81 | 87 | 82 | ||
| 1996 MI diagnosis | ||||
| Yes | 12 | 17 | 25 | 0.07 |
| No | 92 | 91 | 83 | |
| Time from onset for earliest PAPP-A measurement | ||||
| Median hours (IQR) | 4 (2–9) | 4 (2–8) | 4 (2–8) | 0.42 |
| Earliest cTnI | ||||
| Median μg/L (IQR) | 0.01 (0.00–0.03) | 0.01 (0.00–0.04) | 0.01 (0.00–0.06) | 0.40 |
| Peak cTnI | ||||
| Median μg/L (IQR) | 0.02 (0.01–0.12) | 0.02 (0.01–0.14) | 0.03 (0.01–0.41) | 0.24 |
| Earliest hs-cTnI | ||||
| Median ng/L (IQR) | 8.11 (4.86–41.4) | 9.64 (4.34–38.3) | 19.5 (6.04–87.1) | 0.04 |
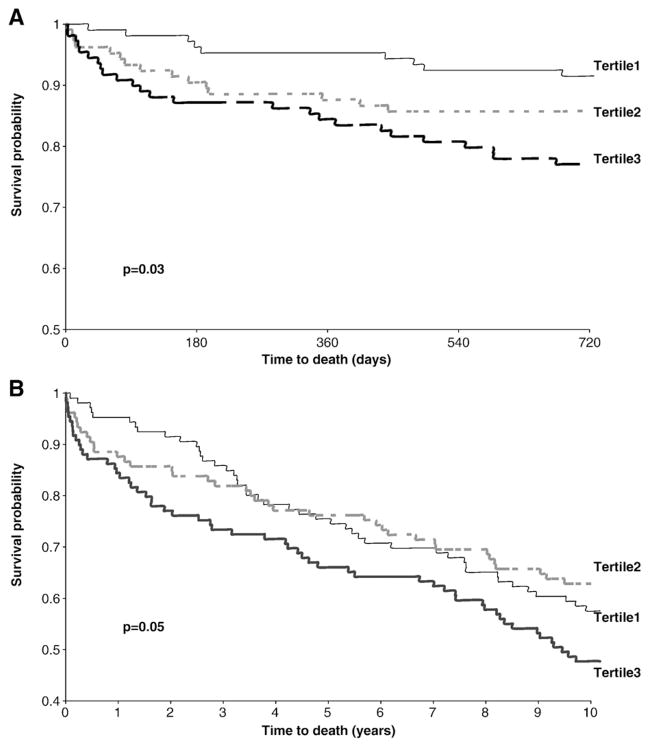
Kaplan–Meier analysis by tertiles with the earliest available PAPP-A measurement (i.e., at baseline) demonstrated that the higher PAPP-A concentrations were associated with a higher probability of death within 10 years (Figs. 3A,B). At 2 years, Cox proportional hazard analyses based on the tertiles, indicated that those subjects with PAPP-A concentrations in the upper third were at significantly higher risk for death, even after adjusting for age, sex, and baseline cTnI, all previously shown to enhance the predictive accuracy of these markers (Table 4).
Fig. 3.
Survival curves up to 2 years based on tertile analysis with baseline PAPP-A. (Tertile 1: PAPP-A<0.92; Tertile 2: PAPP-A 0.92–1.62; Tertile 3: PAPP-A>1.62 mIU/L) (A). Long-term survival, 10 years after presentation (B).
Table 4.
Proportional hazard model of time to death 2 years after ED presentation.
| Model | Tertile | Hazard ratio relative to tertile 1 | 95% CI | p-value |
|---|---|---|---|---|
| Crude | 2 | 1.90 | 0.84–4.30 | 0.123 |
| 3 | 2.96 | 1.38–6.35 | 0.005 | |
| Model 1 | 2 | 1.75 | 0.77–3.98 | 0.182 |
| 3 | 2.23 | 1.04–4.80 | 0.040 | |
| Model 2 | 2 | 1.82 | 0.80–4.14 | 0.154 |
| 3 | 2.15 | 1.00–4.63 | 0.050 |
Model 1: adjusted for age and sex.
Model 2: adjusted for age, sex, presentation cTnI ≥0.02 μg/L.
In the 186 subject cohort without elevated presentation AccuTnI (i.e., cTnI≤99th), for whom all available specimens were re-analyzed with the high sensitivity research hs-cTnI assay, 120 individuals had an analytically significant concentration change by the hs-cTnI assay and 45 had a change with the AccuTnI assay. Kaplan–Meier analysis of tertile PAPP-A baseline concentrations for two-year survival was not significant in subjects with the changing pattern manifested by the AccuTnI assay (p=0.47); however, PAPP-A was a significant marker for survival in the subjects with changes detectable by the hs-cTnI assay (p=0.02) (Fig. 4). However, PAPP-A was not useful in patients without a changing hs-cTnI pattern (p=0.90). Furthermore, after removing subjects who received heparin from the 186 cohort (n=46), those who had a changing hs-cTnI concentration (n=89) had a higher PAPP-A concentration as compared to those who had no change (n=51) (peak PAPP-A=1.88 mIU/L (1.06–3.66) vs. 1.29 (0.86–2.27); p=0.03).
Fig. 4.
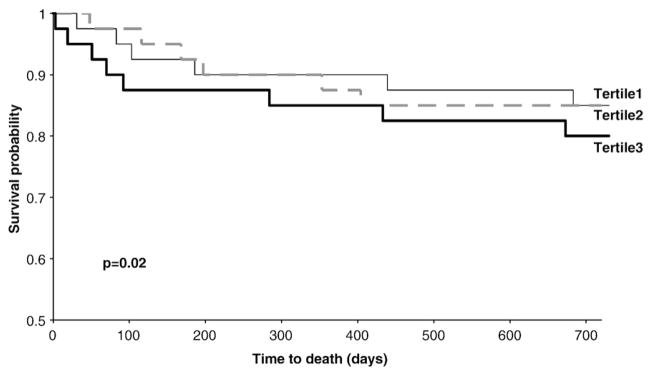
Two-year survival curves in the group of subjects (n=120) with changing hs-cTnI concentrations, stratified by baseline PAPP-A tertiles.
Discussion
PAPP-A is a 200 kDa metalloproteinase that circulates in distinct forms during pregnancy and ACS [25]. During pregnancy, PAPP-A circulates as a heterodimer with the pro-form of eosinophil major basic protein (proMBP). Bound proMBP acts as a protease inhibitor [19,26]. PAPP-A is found in unstable plaques, however the physiological role of PAPP-A in the atherosclerotic plaque is not clear. One hypothesis is that PAPP-A secretion is a repair mechanism involved in plaque stabilization. PAPP-A is secreted by vascular smooth muscle cells and cleaves insulin-like growth factor binding protein-4 (IGFBP-4) in an IGF dependent manner to amplify local bioactive IGFs. IGFs stimulate cell proliferation and differentiation resulting in tissue salvage and repair [27,28]. Conversely, it has been proposed that the metalloproteinase action of PAPP-A causes degradation of the extracellular matrix and may also mediate inflammatory events in atherogenesis thereby contributing to plaque destabilization [29,30]. Further examination of the role of PAPP-A in plaque rupture is required to elucidate its function. Regardless of the cause, previous studies have indicated that PAPP-A is a strong independent predictor of short-term (i.e., ≤6 months) cardiovascular events in patients with acute chest pain or ACS, even in those without cardiac troponin elevations [31,32]. However, those studies used higher cardiac troponin cutoffs (WHO cutoffs or 10% CV concentration cutoffs) than recommended by contemporary guidelines.
The present study extends previous reports by investigating an ACS population characterized with a novel high sensitivity cTnI assay [22], and longer range outcomes. The previous data have demonstrated that PAPP-A is a strong predictor of short-term (≤6 months) adverse events in patients with ACS [31,32]. Our data are similar and show the utility of an elevated PAPP-A as both an intermediate and long-term risk factor. These data also support the recent publication that PAPP-A is a predictor of long-term (median 9 years) all-cause mortality in patients with chronic stable angina pectoris [33], and thus provides indirect support for the notion that decreasing PAPP-A concentrations may be of therapeutic benefit. A recent study [34], demonstrated that high dose atorvastatin significantly decreased PAPP-A concentrations 1 month after an ACS event, and this observation raises the question of whether therapies targeting a reduction in PAPP-A might provide benefits over those targeting reduction in CRP levels after ACS [35,36]. Conversely, treatment with heparin during an acute event may increase plasma PAPP-A levels which may mask or alter the underlying pathophysiology [23,24]. Fortunately, during the time when this cohort was studied initially, heparin was not administered nearly as aggressively as it is today. However, we tried to avoid this confounding effect of heparin on PAPP-A concentrations by using the earliest specimen available. In the subgroup that did receive heparin therapy the peak PAPP-A concentration was significantly higher as compared to those that did not, lending support to these previous reports [23,24].
An intriguing finding was that PAPP-A measurement in those with acutely evolving myocardial injury (as characterized by the hs-cTnI concentration change) can identify those at increased probability for death. It also suggests that when a high sensitivity cTnI assay is employed to define elevations, it enriches the population being evaluated with patients potentially at risk. Once issues are addressed such as biological variability, and with it the optimal values to use to define significant changes, this sort of approach may be even more potent. Better pre hoc stratification may be very helpful for many biomarkers intended for risk stratification post events and might further amplify the importance of new high sensitivity troponin approaches. Conversely, it may make prognostication in those without cTn elevations far less common since a larger percentage will likely be without significant coronary artery disease. It may be for that reason that PAPP-A was not prognostic in those without changing hs-cTnI values; however the small number of subjects with no change in hs-cTnI (n=66) in this cohort precludes a definitive statement in this regard.
There are several limitations to our study. First, the prevalence of statin use was not documented in our study population, yet this would be expected to be small considering that the utility rate was low back in 1996. Second, the utility of measuring PAPP-A in conjugation with NT-proBNP/BNP and other cardiovascular biomarkers needs to be addressed as measurement of the natriuretic peptides has a documented role in prognostication for patients presenting with ACS. Third, the hsPAPP-A assay utilized here measures uncomplexed PAPP-A and the PAPP-A/proMBP complex in equimolar concentration, yet it appears that only uncomplexed PAPP-A is released during ACS, thus future assays only measuring the uncomplexed version may provide additional benefit. Fourth, the clinical use of heparin may increase the PAPP-A concentrations and thus could mask the underlying pathophysiological release of PAPP-A. We have tried to reduce this effect by using the earliest specimen in our analysis, however, this issue needs to be addressed by properly conducted studies assessing this time-dependent release of PAPP-A by heparin therapy. Thus it is imperative that prospective studies are undertaken in ACS populations with specimens obtained prior to heparin administration, as well as measuring all available in use clinical laboratory cardiac biomarkers (i.e., CRP, NT-proBNP/BNP, cTn) in these studies before the role of PAPP-A can be conclusively determined. Finally, a reference interval has not been established for the hsPAPP-A assay nor do we have data on the long-term effect of storage on PAPP-A concentrations.
Future studies are required not only for the validation of PAPP-A in risk stratification but will also serve to optimize both the collection time and cut-off concentrations for PAPP-A in the management of patients with ACS.
Acknowledgments
This study was funded by CIHR. Reagents were provided as an unrestricted grant by Beckman Coulter. Special thanks to Dr. Ed Young for input on heparin therapy and the Clinical Research and Clinical Trials Laboratory, Hamilton for performing the biomarker measurements.
References
- 1.Morrow DA, Cannon CP, Jesse RL, Newby LK, Ravkilde J, Storrow AB, et al. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Clin Chem. 2007;53:552–74. doi: 10.1373/clinchem.2006.084194. [DOI] [PubMed] [Google Scholar]
- 2.Thygesen K, Alpert JS, White HD. Joint ESC/AACF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. J Am Coll Cardiol. 2007;50:2173–95. doi: 10.1016/j.jacc.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Lee TH, Rouan GW, Weisberg MC, Brand DA, Acampora D, Stasiulewicz C, et al. Clinical characteristics and natural history of patients with acute myocardial infarction sent home from the emergency room. Am J Cardiol. 1987;60:219–24. doi: 10.1016/0002-9149(87)90217-7. [DOI] [PubMed] [Google Scholar]
- 4.Pope JH, Aufderheide TP, Ruthazer R, Woolard RH, Feldman JA, Beshansky JR, et al. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med. 2000;342:1163–70. doi: 10.1056/NEJM200004203421603. [DOI] [PubMed] [Google Scholar]
- 5.Worster A, Devereaux PJ, Heels-Ansdell D, Guyatt GH, Opie J, Mookadam F, et al. Capability of ischemia-modified albumin to predict serious outcomes in the short term among patients with potential acute coronary syndrome. CMAJ. 2005;172:1685–90. doi: 10.1503/cmaj.045194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaul P, Chang WC, Westerhout CM, Graham MM, Armstrong PW. Differences in admission rates and outcomes between men and women presenting to emergency departments with coronary syndromes. CMAJ. 2007;177:1193–9. doi: 10.1503/cmaj.060711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonaca MP, Morrow DA. Defining a role for novel biomarkers in acute coronary syndromes. Clin Chem. 2008;54:1424–31. doi: 10.1373/clinchem.2008.105387. [DOI] [PubMed] [Google Scholar]
- 8.MacRae AR, Kavsak PA, Lustig V, Bhargava R, Vandersluis R, Palomaki GE, et al. Assessing the requirement for the 6-hour interval between specimens in the American Heart Association classification of myocardial infarction in epidemiology and clinical research studies. Clin Chem. 2006;52:812–8. doi: 10.1373/clinchem.2005.059550. [DOI] [PubMed] [Google Scholar]
- 9.Sabatine MS, Morrow DA, de Lemos JA, Gibson CM, Murphy SA, Rifai N, et al. Multimarker approach to risk stratification in non-ST elevation acute coronary syndromes: simultaneous assessment of troponin I, C-reactive protein, and B-type natriuretic peptide. Circulation. 2002;105:170–3. doi: 10.1161/01.cir.0000015464.18023.0a. [DOI] [PubMed] [Google Scholar]
- 10.Wiviott SD, Cannon CP, Morrow DA, Murphy SA, Gibson CM, McCabe CH, et al. Differential expression of cardiac biomarkers by gender in patients with unstable angina/non-ST-elevation myocardial infarction: a TACTICS-TIMI 18 substudy. Cicrculation. 2004;109:580–6. doi: 10.1161/01.CIR.0000109491.66226.26. [DOI] [PubMed] [Google Scholar]
- 11.Bodi V, Sanchis J, Llacer A, Facila L, Nunex J, Pellicer M, et al. Multimarker risk strategy for predicting 1-month and 1-year major events in non-ST-elevation acute coronary syndromes. Am Heart J. 2005;149:268–74. doi: 10.1016/j.ahj.2004.05.053. [DOI] [PubMed] [Google Scholar]
- 12.James SK, Lindahl B, Timmer JR, Ottervanger JP, Siegbahn A, Stridsberg M, et al. Usefulness of biomarkers for predicting long-term mortality in patients with diabetes mellitus and non-ST-elevation acute coronary syndromes (a GUSTO IV substudy) Am J Cardiol. 2006:167–72. doi: 10.1016/j.amjcard.2005.08.036. [DOI] [PubMed] [Google Scholar]
- 13.Apple FS, Pearce LA, Chungn A, Ler R, Murakami MM. Multiple biomarker use for detection of adverse events in patients presenting with symptoms suggestive of acute coronary syndrome. Clin Chem. 2007;53:874–81. doi: 10.1373/clinchem.2006.080192. [DOI] [PubMed] [Google Scholar]
- 14.Kavsak PA, Newman AM, Lustig V, MacRae AR, Palomaki GE, Ko DT, et al. Long-term health outcomes associated with detectable troponin I concentrations. Clin Chem. 2007;53:220–7. doi: 10.1373/clinchem.2006.076885. [DOI] [PubMed] [Google Scholar]
- 15.Kavsak PA, MacRae AR, Newman AM, Lustig V, Palomaki GE, Ko DT, et al. Elevated C-reactive protein in acute coronary syndrome presentation is an independent predictor of long-term mortality and heart failure. Clin Biochem. 2007;40:326–9. doi: 10.1016/j.clinbiochem.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 16.Kavsak PA, Ko DT, Newman AM, Lustig V, Palomaki GE, MacRae AR, et al. Vascular versus myocardial dysfunction in acute coronary syndrome: are the adhesion molecules as powerful as NT-proBNP for long-term risk stratification? Clin Biochem. 2008;41:436–9. doi: 10.1016/j.clinbiochem.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Apple FS, Wu AH, Mair J, Ravkilde J, Panteghini M, Tate J, et al. Future biomarkers for detection of ischemia and risk stratification in acute coronary syndrome. Clin Chem. 2005;51:810–24. doi: 10.1373/clinchem.2004.046292. [DOI] [PubMed] [Google Scholar]
- 18.Bayes-Genis A, Conover CA, Overgaard MT, Bailey KR, Christiansen M, Holmes DR, et al. Pregnancy-associated plasma protein A as a marker of acute coronary syndromes. N Engl J Med. 2001;345:1022–9. doi: 10.1056/NEJMoa003147. [DOI] [PubMed] [Google Scholar]
- 19.Qin QP, Wittfooth S, Pettersson K. Measurement and clinical significance of circulating PAPP-A in ACS patients. Clin Chim Acta. 2007;380:59–67. doi: 10.1016/j.cca.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 20.Kavsak PA, MacRae AR, Lustig V, Bhargava R, Vandersluis R, Palomaki G, et al. The impact of the ESC/ACC redefinition of myocardial infarction and new sensitive troponin assays on the frequency of acute myocardial infarction. Am Heart J. 2006;152:118–25. doi: 10.1016/j.ahj.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 21.Kavsak PA, MacRae AR, Palomaki GE, Newman AM, Ko DT, Lustig V, et al. Health outcomes categorized by current and previous definitions of acute myocardial infarction in an unselected cohort of troponin-naïve emergency department patients. Clin Chem. 2006;52:2028–35. doi: 10.1373/clinchem.2006.073403. [DOI] [PubMed] [Google Scholar]
- 22.Kavsak PA, MacRae AR, Yerna MJ, Jaffe AS. Analytical and clinical utility of a next generation, highly sensitive cardiac troponin I assay for early detection of myocardial injury. Clin Chem. 2009;55:573–7. doi: 10.1373/clinchem.2008.116020. [DOI] [PubMed] [Google Scholar]
- 23.Laursen LS, Overgaard MT, Weyer K, Boldt HB, Ebbesen P, Christiansen M, et al. Cell surface targeting of pregnancy-associated plasma protein A proteolytic activity. J Biol Chem. 2002;277:47225–34. doi: 10.1074/jbc.M209155200. [DOI] [PubMed] [Google Scholar]
- 24.Terkelsen CJ, Oxvig C, Borgaard BL, Glerup S, Poulsen TS, Lassen JF, et al. Temporal course of pregnancy-associated plasma protein-A in angioplasty-treated ST-elevation myocardial infarction patients and potential significance of concomitant heparin administration. Am J Cardiol. 2009;103:29–35. doi: 10.1016/j.amjcard.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 25.Qin QP, Kokkala S, Lund J, Tamm N, Voipio-Pulkki LM, Pettersson K. Molecular distinction of circulating pregnancy-associated plasma protein A in myocardial infarction and pregnancy. Clin Chem. 2005;51:75–83. doi: 10.1373/clinchem.2004.036467. [DOI] [PubMed] [Google Scholar]
- 26.Consuegra-Sanchez L, Fredericks S, Kaski JC. Pregnancy-associated plasma protein-A (PAPP-A) and cardiovascular risk. Atherosclerosis. 2008 Aug 12; doi: 10.1016/j.atherosclerosis.2008.07.042. [Electronic publication ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Conti E, Carrozza C, Capoluongo E, Volpe M, Crea F, Zuppi C, et al. Insulin-like growth factor-1 as a vascular protective factor. Circulation. 2004;110:2260–5. doi: 10.1161/01.CIR.0000144309.87183.FB. [DOI] [PubMed] [Google Scholar]
- 28.Crea F, Andreotti F. Pregnancy associated plasma protein-A and coronary atherosclerosis: marker, friend, or foe? Eur Heart J. 2005;26:2075–6. doi: 10.1093/eurheartj/ehi475. [DOI] [PubMed] [Google Scholar]
- 29.Cosin-Sales J, Christiansen M, Kaminski P, Oxvig C, Overgaard MT, Cole D, et al. Pregnancy-associated plasma protein A and its endogenous inhibitor, the proform of eosinophil major basic protein (proMBP), are related to complex stenosis morphology in patients with stable angina pectoris. Circulation. 2004;109:1724–8. doi: 10.1161/01.CIR.0000124716.67921.D2. [DOI] [PubMed] [Google Scholar]
- 30.Cosin-Sales J, Kaski JC, Christiansen M, Kaminski P, Oxvig C, Overgaard MT, et al. Relationship among pregnancy associated plasma protein-A levels, clinical characteristics, and coronary artery disease extent in patients with chronic stable angina pectoris. Eur Heart J. 2005;26:2093–8. doi: 10.1093/eurheartj/ehi433. [DOI] [PubMed] [Google Scholar]
- 31.Lund J, Qin QP, Ilva T, Pettersson K, Voipio-Pulkki LM, Porela P, et al. Circulating Pregnancy-associated plasma protein A predicts outcome in patients with acute coronary syndrome but no troponin I elevation. Circulation. 2003;108:1924–6. doi: 10.1161/01.CIR.0000096054.18485.07. [DOI] [PubMed] [Google Scholar]
- 32.Heeschen C, Dimmeler S, Hamm CW, Fichtlscherer S, Simoons ML, Zeiher AM. Pregnancy-associated plasma protein-A levels in patients with acute coronary syndromes: comparison with markers of systemic inflammation, platelet activation, and myocardial necrosis. J Am Coll Cardiol. 2005;45:229–37. doi: 10.1016/j.jacc.2004.09.060. [DOI] [PubMed] [Google Scholar]
- 33.Consuegra-Sanchez L, Petrovic I, Cosin-Sales J, Holt DW, Christiansen M, Kaski JC. Prognostic value of circulating pregnancy-associated plasma protein-A (PAPP-A) and proform of eosinophil major basic protein (pro-MBP) levels in patients with chronic stable angina pectoris. Clin Chim Acta. 2008;391:18–23. doi: 10.1016/j.cca.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 34.Miedema MD, Conover CA, MacDonald H, Harrington SC, Oberg D, Wilson D, et al. Pregnancy associated plasma protein-A elevation in patients with acute coronary syndrome and subsequent atorvastatin therapy. Am J Cardiol. 2008;1001:35–9. doi: 10.1016/j.amjcard.2007.07.045. [DOI] [PubMed] [Google Scholar]
- 35.Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–8. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- 36.Morrow DA, de Lemons JA, Sabatine MS, Wiviott SD, Blazing MA, Shui A, et al. Clinical relevance of C-reactive protein during follow-up of patients with acute coronary syndromes in the Aggrastat-to-Zocor Trial. Circulation. 2006;114:281–8. doi: 10.1161/CIRCULATIONAHA.106.628909. [DOI] [PubMed] [Google Scholar]