Abstract
Successful pregnancy requires coordination of an array of signals and factors from multiple tissues. One such element, the liver receptor homolog-1 (Lrh-1, NR5A2), is an orphan nuclear receptor that regulates metabolism and hormone synthesis1. It is strongly expressed in granulosa cells of ovarian follicles and in the corpus luteum of rodents2 and humans. Germline ablation of the Lrh-1 gene in mice is embryo-lethal at gastrulation3. Depletion of Lrh-1 in the ovarian follicle demonstrates that it regulates genes required for both steroid synthesis and ovulation4. To study the effects of Lrh-1 on mouse gestation, we disrupted its expression in the corpus luteum, resulting in luteal insufficiency. Hormone replacement permitted embryo implantation but was followed by gestational failure with impaired endometrial decidualization, compromised placental formation, fetal growth retardation, and fetal death. Lrh-1 is expressed in the mouse and human endometrium. In a human model of primary culture of endometrial stromal cells, depletion of Lrh-1 by siRNA abrogated decidualization. These findings demonstrate that Lrh-1 is necessary for maintenance of the corpus luteum, for promotion of decidualization and for placental formation. It therefore plays multiple, indispensible roles in establishing and sustaining pregnancy.
To explore the role of Lrh-1 in gestation, we bred progesterone receptor (Pr)-Cre recombinase mice5 to mice with exons 3 and 4 of the Lrh-1 (Nr5a2) gene flanked by loxP sites (Nr5a2fl/fl)6. The resultant conditional knockout (cKO, genotype: Nr5a2fl/fl; PrCre/+) mice were uniformly infertile. Adult cKO females displayed increased frequency and duration of estrus, but mated regularly (Fig. S1a–b). Their ovaries contained corpora lutea, albeit smaller than in control littermates (CON, genotype: Nr5a2fl/fl), suggesting successful ovulation (Fig. 1a). Gonadotropin stimulation of immature CON and cKO mice indicated no impairment of ovulation in the cKO strain (Fig. S1c).
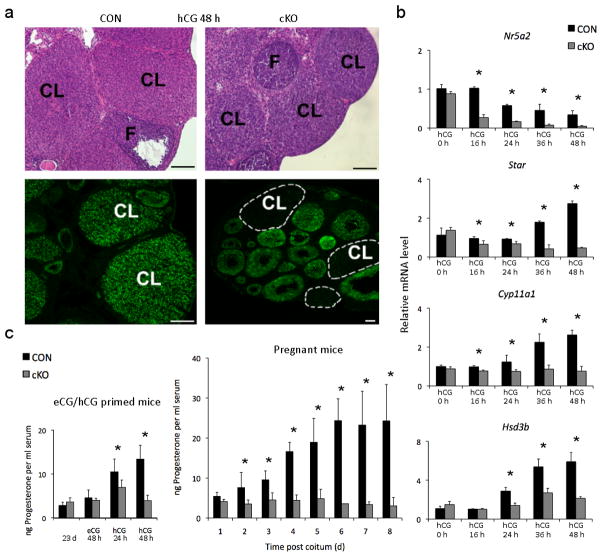
Figure 1. Deletion of Lrh-1 in the peri-ovulatory follicle via PrCre causes luteal insufficiency.
(a) Corpora lutea (CL) and follicles (F) in ovaries from CON (Nr5a2fl/fl) and cKO (Nr5a2fl/fl;PrCre/+) mice at 96 h after treatment with equine chorionic gonadotropin (eCG) to induce follicle development and 48 h after treatment with 5 IU human chorionic gonadotropin (hCG) to induce ovulation. Lower panels demonstrate ablation of the Lrh-1 signal in corpora lutea and its retention in follicles. Scale bars, 200 μm. (b) Abundance for Lrh-1 (Nr5a2) mRNA and the steroidogenic proteins Cyp11a1, Star and Hsd3b, determined by qPCR in granulosa cells aspirated prior to induction of ovulation with hCG (designated hCG 0h) and laser microdissected corpora lutea from CON and cKO mice at 0–48 h after hCG (n=3–10 mice per point) (c) Left panel: serum progesterone in immature CON and cKO mice prior to (23d), 48 h after gonadotropin induction of follicle development and 24 and 48 h after hCG. Right panel: peripheral progesterone concentrations in CON and cKO mice for the first eight days post copulation (dpc). Values are means ± SEM, n=5–10 mice, asterisks designate differences between cKO and CON animals at each time point (p<0.05).
In corpora lutea from CON mice isolated by laser microdissection4, the Lrh-1 transcript persisted through 72 h after the ovulatory stimulus (Fig. 1b, upper panel; Fig. S1d). Its abundance did not differ between granulosa cells of cKO and CON mice prior to the ovulatory stimulus, but it was extinguished in cKO corpora lutea by 48 h after treatment with human chorionic gonadotropin (hCG, Fig. 1b). Lrh-1 protein displayed time-dependent decline in cKO ovaries (Fig. 1a, lower panel; Fig. S1d). Abundance, Star, Cyp11a1 and Hsd3b, genes essential for steroidogenesis, did not differ between CON and cKO ovaries prior to the ovulatory stimulus, but was substantially reduced in cKO corpora lutea by 24 h (Fig. 1b), as were Ldlr and Scarb1, membrane receptors for importation of cholesterol substrate7 (Fig. S2a–b). Transcripts coding for steroidogenic factor-1 (Nr5a1) mRNA (Fig. S2c), a gene closely related to Lrh-1 and expressed in theca, granulosa and luteal cells2, did not differ between CON and cKO corpora lutea, nor did Cyp19a, Cyp17a1 and Areg. Other luteal factors in cKO mice, including Vegfa, Nos3, Pla2g4a and Ptgs2 were differentially expressed between CON and cKO corpora lutea in the hours following hCG treatment (Fig. S2d–g), while Vegfb was unaffected (Fig. S2h). RNA for 20α-hydroxysteroid dehydrogenase (Akr1c18), an enzyme involved in progesterone inactivation8, was higher in cKO relative to CON corpora lutea from 24h after the ovulatory stimulus (Fig. S2c). Reduced circulating progesterone confirmed compromised luteal function in immature cKO relative to CON mice from 24 h after the ovulatory stimulus; and in adult cKO mice from 48 h of mating, compared to progressive increases through early gestation in CON mice (Fig. 1c). Thus, in the absence of Lrh-1, luteal cells fail to synthesize normal amounts of progesterone, due to altered expression of genes associated with progesterone synthesis/metabolism.
To establish whether abnormality in fertilization or embryogenesis contributed to sterility of cKO mice, we collected eggs and embryos from oviducts and uteri of adult CON and cKO mice at intervals following mating (Fig. 2a). We found no differences between the yield of unfertilized eggs, zygotes or two-cell embryos between CON and cKO mice. On the fourth day post coitum (dpc 4), nearly all CON embryos recovered were blastocysts, while the majority in the cKO strain were morulae (Table S1). Timely progression of cKO embryos to blastocysts on dpc 4 was restored by implanting progesterone-releasing pellets on dpc 1 (cKO+P4, Table S1). Viability of embryos from cKO mice was confirmed by transplantation of zygotes on dpc 1 from cKO and CON mice into oviducts of CON mice, with no resultant difference in frequency of parturition nor in numbers of offspring born (Fig. 2b).
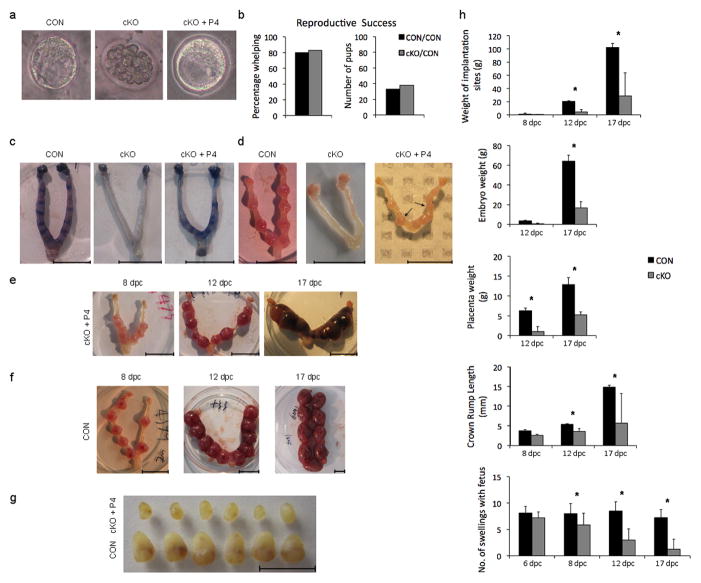
Figure 2. Implantation is compromised in cKO mice and gestation fails in progesterone supplemented cKO mice.
(a) Representative embryos recovered at dpc 4 from mated CON (left), cKO (middle) and progesterone treated cKO mice (cKO +P4 ). (b) Results of transfer of 15 zygotes per mouse from CON and cKO mice to oviducts of CON mice, with percentage whelping and number of pups per five recipients (c) Uterine horns from CON, cKO and CKO+P4 mice at dpc 5 following infusion of Evans blue dye to demonstrate incipient implantation sites (d) Representative uteri at dpc 8 from CON, cKO and CKO+P4 mice showing no implantation in cKO mice and abnormal postimplantation morphology, including variation in the size of implantation sites in cKO + P4 mice (arrows) (e) Representative uteri from cKO+P4 mice at dpc 8, 12 and 17. (f) Representative uteri from CON mice at dpc 8, 12 and 17. (g) Uterine swellings dissected at dpc 8 from CON (bottom) and cKO+P4 (top) mice. (h) Comparison of parameters of gestation in CON and cKO mice, including weight of implantation sites, embryo and placental weights, crown-rump length of embryos and number of uterine swellings per mouse; mean ± SEM; n=5–10 mice and asterisks indicate significant difference (P<0.05) between cKO and contemporary CON parameters at each time in gestation. Scale bars 10 mm.
While implantation failed in cKO mice, it was rescued by progesterone administration (Fig. 2c–d). Nevertheless, implanted embryos in cKO+P4 mice were improperly spaced at dpc 5 (Fig. 2d, right panel) and by dpc 8, there was embryo crowding (Fig. 2e, left panel) and variation in size of the uterine enlargements (Figs. 2d–e). By dpc 12, we saw crowding and further loss of uniformity in uterine swellings (Fig. 2e vs. 2f, middle panels). The mean weight of implantation sites, fetuses and placentas was lower in cKO than in CON mice by dpc 12 (Fig. 2h). By dpc 17, fetal-placental units were of markedly different sizes (Fig. 2e and f, right panel), and the number of sites with fetuses was fewer (Fig. 2h). Successful parturition occurred only rarely (1/22 cKO+P4 females).
Aberrant implantation in cKO+P4 mice raised the question of whether Lrh-1 is expressed in the uterus. We found Lrh-1 transcripts (Fig. 3a) and protein signal (Fig. 3b) in the epithelial and stromal compartments of the CON mouse endometrium through dpc 3-5. The signal persisted in the endometrial component of the placenta through gestation (Fig. S3a–b). In cKO mice, uterine Lrh-1 mRNA progressively declined from dpc 3 to nearly undetectable by dpc 5 (Fig. 3a). Lrh-1 protein was reduced in endometrial stroma of cKO relative to CON mice at dpc 4 and disappeared more slowly from the endometrial epithelium (Fig. 3c). It was not detected in the endometrial stroma at all sampling times during the aberrant pregnancies of P4 treated cKO mice (Fig S3c).
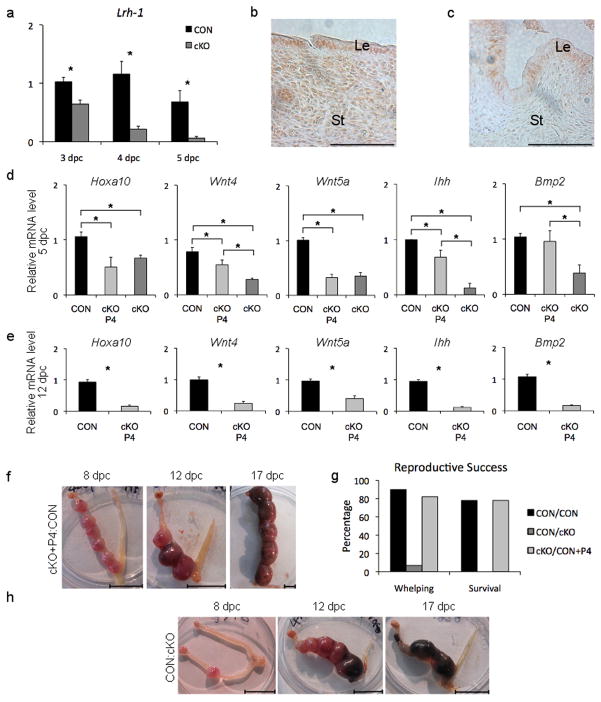
Figure 3. Lrh-1 is present in the endometrium of the mouse and its depletion results in failure of gestation.
(a) Transcript abundance of Lrh-1 (Nr5a2) in uteri of CON and cKO in mated mice from dpc 3 to dpc 5 showing depletion in the cKO uterus (b) Immunohistochemistry demonstrating nuclear localization of Lrh-1 in the CON mouse endometrium on dpc 4; (Le, luminal epithelium; St, stroma) (c) Immunohistochemistry demonstrating depletion of Lrh-1 in the cKO+P4 mouse endometrium on dpc 4 (d) Abundance of transcripts on dpc 5 for five genes in uteri from CON, cKO and cKO+P4 mice critical to decidualization and successful gestation (Hoxa10, Wnt4, Wnt5a, Ihh and Bmp2) (e) Abundance on dpc 12 of transcripts of the same subset of genes as in panel d critical to decidualization and successful gestation in uteri from CON and cKO+P4 mice. (f) Representative samples of normal gestation in one uterine horn at dpc 8, 12 and 17 following reciprocal transfer of a single ovary from a cKO mouse to bursa of ovariectomized CON mouse then supplemented with progesterone. (g) Gestational success following ovarian transplant of CON to CON, CON to cKO and cKO to CON progesterone-supplemented mice expressed in terms of percentage whelping and survival of pups. (h) Examples of pathological uteri at dpc 8, 12 and 17 found in mice in which a normal (CON) ovary was transferred to the bursa of an ovariectomized cKO mouse. Scale bars for panels b and c, 20 μm; for f and h, 10 mm. Asterisks indicate significant differences at P<0.05.
Examination of uteri on dpc 5 and implantation sites (sans fetus) at dpc 12 revealed that expression of genes essential for successful embryo implantation and placentation was compromised and not restored by progesterone replacement, including Hoxa10, Wnt4, Wnt5a, Ihh and Bmp2 (Fig. 3 d–e) and Ppard, Hbegf and Cyp19a1 (Fig. S4a–c)9,10. At dpc 5, transcript levels of a number of other genes tested were reduced in the cKO relative to the CON mouse, but were restored or modestly increased by progesterone, including Ptgs2, Pla2g4a, Vegfa, Pparg, Areg, Ki67 and Esr2 (Fig. S3d–j). Other implantation genes, Lif, Vegfb and Esr1 were not different between cKO and CON uteri. Multiple disruptions were not unexpected, as Lrh-1 targets numerous ovarian genes4. Thus, while implantation failure in cKO mice is due to luteal insufficiency, irregularities in expression of key endometrial genes persist in spite of progesterone replacement, resulting in aberrant gestation.
To further confirm pregnancy failure is of uterine provenance, single ovaries from a CON or cKO mice were reciprocally transferred to ovarian bursae of ovariectomized CON or cKO mice, that were then mated to fertile males. Mice receiving cKO ovaries, supplemented with progesterone to compensate for luteal deficiency, displayed progesterone in serum not different from those receiving a single CON ovary (43.5 ± 8.7 vs 33.5 ± 18.4 ng/ml). Unilateral implantation and pregnancy ensued in uteri of CON mice receiving either CON or cKO (+P4) ovaries (Fig. 3f) and viable offspring were born to all but a few (Fig. 3g). When CON ovaries were transplanted to cKO mice, implantation occurred, but gestation failed with a similar pathological signature similar to that seen in cKO+P4 mice mated to CON males, crowding, differential feto-placental size and fetal death (Fig. 3h). A single litter was born to a CON ovary/cKO uterus mouse, but no neonates survived to 12 h of life (Fig. 3g). Genotyping of dead fetuses and liveborn pups confirmed offspring were derived from the transplanted ovary. This trial clearly implicates uterine dysfunction as the genesis of gestational failure in cKO mice.
In the endothelial-chorial placenta, endometrial stromal cells differentiate into large, secretory and often polyploid cells, in a process known as decidualization11. Ovarian progesterone is a prerequisite for decidualization12. Both the embryo and artificial stimuli can induce a decidual reaction in mice on dpc 5 whereby a new gene expression pattern overwrites the stromal palimpsest, first by proliferation, then by terminal differential of stromal cells9. To further explore the role of Lrh-1 in uterine function, we induced artificial decidualization by intraluminal injection of oil13 into uteri of pseudopregnant CON and pseudopregnant cKO and cKO+P4 mice. The pronounced pseudopregnant deciduoma present in CON mice was lacking in the cKO uterus, and much reduced in spite of progesterone replacement (Fig. 4a–b). Likewise, robust stromal cell proliferation found in CON mice on dpc 4-5 was absent in cKO mice and only modestly compensated in cKO+P4 mice (Fig. S4a). Key genes required for decidualization including Hoxa-10, necessary for proliferation, and Wnt49,11,14 were underexpressed in the uterus at both dpc 5 and 12 (Fig. 3d–e), while Ptgs29,10 appeared to be expressed appropriately at the implantation site (Fig S5b–d).
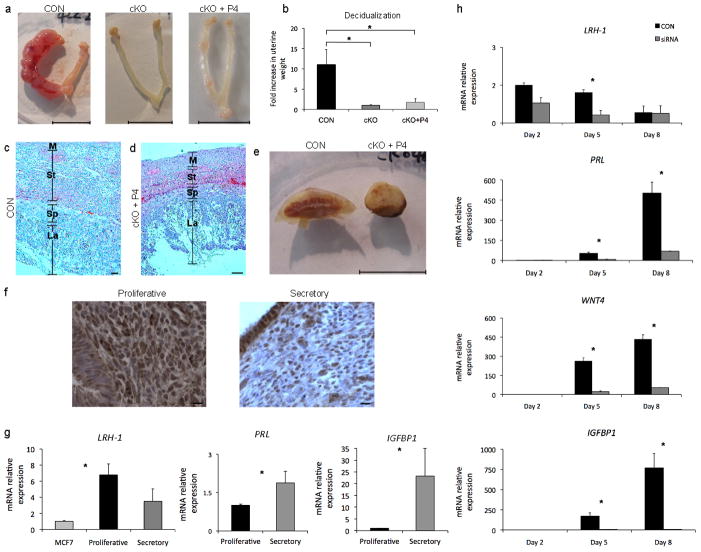
Figure 4. Lrh-1 is critical for decidualization of both the mouse and human endometrial stroma.
(a) Oil-induced deciduoma formation at dpc 8 in pseudopregnant CON mice, absent in both cKO and reduced in cKO+P4 mice, scale bars, 10 mm (b) Ratio of oil-treated to control uterine horn weights (Mean ± SEM) at dpc 8 following decidual stimulus at dpc 4 (c–d) Histological comparison of placental sections at dpc 12 from CON (c) and cKO+P4 (d) mice. (La, placental labyrinth; Sp, spongy zone; St, maternal stroma; M, myometrium). Scale bars, 100 μm. (e) Macroscopic comparison of placentas dissected from CON and cKO+P4 mice at dpc 17, scale bar, 10 mm. (f) Nuclear localization of Lrh-1 in the proliferative and secretory human endometrium, scale bar 10 μm (g) Abundance of transcripts for LRH-1(NR5A2) and the decidual markers PRL and IGFBP1 in human endometrium during the proliferative and secretory phases of the menstrual cycle. MCF-7 breast cancer cells were used as a positive control for LRH-1 transcript expression. (h) Quantitative PCR analysis demonstrating knockdown of Lrh-1 transcripts by siRNA (initiated on Day 0) in primary cultures of human endometrial stromal cells undergoing decidualization in vitro; and abundance of decidual marker genes LRH-1 (NR5A2), PRL, WNT4 and IGFBP1 in human stromal cells in vitro. Asterisks indicate significant differences (P<0.05) between control and siRNA knockdown at each sampling time.
Smaller embryos (Fig. 2g), smaller implantation sites and lower placental weight (Fig. 2h) indicated that placental formation was compromised in cKO+P4 mice. Placental depth in cKO mice was half that in CON mice at dpc 12, due to reductions in the maternal stroma, vascularization and the placental spongiotrophoblast layer (Fig. 4c–e). In contrast to lenticular CON placentas cKO+P4 placentas were markedly dome shaped (Fig. 4e). Circulating progesterone did not differ between CON and cKO+P4 mice through day 12 of gestation, but was marginally reduced at day 17 (Fig. S5e), presumably due to reductions in placental steroid synthetic components (Fig. S5f–g). Supplementation of cKO+P4 mice with a second progesterone pellet from dpc 10-17 doubled circulating concentrations (103.4 ± 7.1 ng/ml) over both CON and cKO mice bearing single implants, but did not result in liveborn mice.
We then explored whether LRH-1 was present in the human endometrium during the menstrual cycle. The LRH-1 transcript and nuclear signal was detected in both the proliferative and secretory phases of the menstrual cycle (Fig 4f–g), and the transcripts for markers of decidualization, PRL and IGFBP115 were found in the expected greater abundance during the secretory phase (Fig 4g). Proper decidualization is critical to successful gestation in humans16 and human uterine stromal fibroblasts undergo decidual reprogramming that shares multiple gene expression patterns with mouse decidualization12,17. We depleted LRH-1 mRNA by siRNA in primary culture of human endometrial stromal cells18 (Fig. 4h) thereby reducing abundance of decidual markers19,20, including PRL, and IGFBP1 (Fig. 4c), and KLF9, HAND2, and FOXO1a (Fig. S6a–c). Notably, siRNA reduction of LRH-1 mRNA abrogated the expression of WNT4 (Fig. 4h), an indispensible mediator of human decidualization21, and a gene disrupted in both early and late gestation (Fig. 3d–e) in cKO+P4 mice. A mechanistic link by which Lrh-1 drives decidualization in human stromal cells was then confirmed by chromatin immunoprecipitation (ChIP), demonstrating that WNT4 is a direct target of LRH-1 (Fig. S6d). The apparent reduction in LRH-1 mRNA as decidualization progresses in vivo (Fig. 4g) and the observed decline in vitro (Fig. 4h) are consistent with studies showing declining steroid receptor mRNA abundance that accompanies decidualization22, in spite of increased tissue sensitivity to the nuclear receptors. Waning expression attributed to their sumoylation22, a posttranslational modification that regulates the activity of LRH-123.
We then examined the dynamics of LRH-1 regulation of decidualization in a human endometrial stromal cell line (hESC)24 in vitro. Immunocytochemistry revealed nuclear expression of LRH-125 (Fig. S7a) and LRH-1 mRNA was upregulated a mean of 346 % during in vitro decidualization, as were PRL (427 %) and IGFBP1 (465 %) transcripts (Fig. S7b), further demonstrating the role of Lrh-1 in regulation human stromal cell decidualization. In contrast to the primary decidual cells, the LRH-1 RNA did not decline as decidualization progressed, presumably because the cells were immortalized24, and thus, not terminally differentiated.
Overall, these extensive findings demonstrate that maternal expression of Lrh-1 is required for two aspects of gestation, luteal function and placental formation. In the cKO mouse, correction of luteal deficiency by progesterone replacement rescues implantation and permits continuation of pregnancy, but placental development is compromised, resulting in fetal death, illustrating the critical role of Lrh-1 in the uterus. The effects are first manifest early during the peri-implantation period. Postimplantation gestational failure in progesterone-supplemented cKO mice is due to multiple causes. Lrh-1 deficiency in gestation results in a “ripple effect” whereby improperly executed pre- and peri-implantation events such as spacing and decidualization are propagated as placental anomalies through pregnancy14. Lrh-1 is further essential for human decidualization, which, in itself, is necessary for successful pregnancy. Depletion of Lrh-1 abrogates expression of genes known to be indispensible for differentiation of uterine stromal cells26,27. Among these is WNT421, that we show by ChIP as a target of Lrh-1, demonstrating a mechanism by which Lrh-1 regulates placental formation.
Methods
Mice and treatments
Animal experiments were approved by the Comité d’éthique d l’utilisation des animaux, Universite de Montréal. C57BL/6 background mutant mice were maintained on 14:10 h light: dark cycle, provided food and water ad libitum. The Nr5a2 floxed (Nr5a2fl/fl) mice, generated by J. Auwerx4 served as controls (CON). They were crossed with progesterone receptor Cre recombinase (PrCre/+) mice5 to produce Lrh-1 conditional knockout (cKO) mice. Immature (21-25 d) females were superovulated with 5 IU equine chorionic gonadotropin (Intervet Canada, Montreal) followed by 5 IU human chorionic gonadotropin (hCG, Intervet) 48 h later. Ovulated eggs from oviducts were enumerated 16 h after hCG injection. Ovaries were snap frozen and stored at −80 °C, or embedded in paraffin for histology. Vaginal smears were taken daily (09:00) to determine estrous stages. For breeding assays, eight cKO and eight CON 6-wk-old females were housed with reproductively proven C57BL/6J males for six months (two females per male), observed daily and parturition dates and litter sizes recorded. For analysis of gestation, adult (>60 days of age) female CON and cKO mice were bred. The day that copulatory plugs were found was designated day 1 post coitum (dpc 1). Animals were terminated at stages of gestation as dictated by experimental objectives. To establish frequency of mating, CON and cKO mice were housed with vasectomized males, and plugs, where present, were determined daily.
Vasectomies were performed by removal of 1 cm of vasa deferentia from males anesthetized by injection of Ketamine (100 mg/kg; Bioniche Animal Health, Bellevue, Canada) and Xylazine (10 mg/kg; Bayer Incorporation, Toronto, Canada). Sterility was confirmed by housing with fertile females and observing absence of sperm in copulatory plugs.
In cKO females mated with fertile males, a 2 cm length silastic implant (ID, 3.35 mm; OT, 4.65 mm; Dow Corning, Midland, MI., USA) containing progesterone (Sigma Chemical Co., St Louis, MO., USA) was implanted subcutaneously on dpc1 at 17:00. Implants were removed dpc 17 (17:00) to allow parturition. To test whether pregnant females required further P4, another implant was placed in some animals on dpc 10 (09:00) and both were removed at dpc 17 (17:00).
Deciduomas were induced in CON and cKO females mated to vasectomized males. On dpc 1, cKO females received progesterone implants. On the afternoon of dpc 4 (15:00), 50 μl sesame oil was infused intraluminally in one uterine horn. On dpc 8 (17:00), mice were killed and uterine weights of infused and noninfused horns were recorded13.
Incipient implantation sites on dpc 5 were identified by an intravenous injection of 1% (W/V) Evans Blue dye (100 μl, 1% in 1xPBS, Sigma). Injected mice were terminated 3 min later, and uteri removed for examination.
To investigate preimplantation development, mouse oviducts and uteri were flushed with PBS on dpc 1-4, and embryos recovered. After implantation, uteri of pregnant females were dissected at 15:00 on dpc 8, 12 and 17 and isolated implantation sites were weighed individually, then dissected to isolate embryos and placentas. Crown rump lengths were measured by Vernier caliper.
To assess quality of embryos, pseudopregnant recipients were generated by mating females with vasectomized CON males. Embryos on dpc 1 (09:00) were obtained from bred cKO or CON donors by flushing oviducts. Pseudopregnant recipients were anesthetized and zygotes (15/female) were transferred to oviducts on dpc 1. Recipients were observed once daily through time of expected parturition.
Ovary transplants were performed as described 28. Briefly, females were anesthetized, the ovarian fat pad with the ovary was exteriorized, the bursa opened, and ovaries removed. The excised ovaries were held briefly in M2 medium (Sigma) then grafted back into the bursal cavity of recipients. Following three-week recovery, the mice were mated with CON male mice. Samples from each gestation were genotyped to confirm that products of conception were derived from the transplanted ovary.
Human endometrial stromal cells: decidualization, transfection with siRNA and hormone treatment
Human uterine tissues were acquired from the National Institutes of Health, University of California at San Francisco Endometrial Tissue and DNA Bank approved by the UCSF Institutional Review Board, and collected and archived following informed consent29. For primary culture of stromal tissue, histologically normal endometrial samples from the proliferative phase were collected by biopsy from individuals of reproductive age (18–45) with normal menstrual cycles. The experimental protocol was approved by the Institutional Review Board of Baylor College of Medicine (Houston, TX). All subjects gave written informed consent. Samples were collected at room temperature and transported on ice in HBSS containing 1% antibiotic-antimycotic (Invitrogen, Carlsbad, CA., USA) and processed for the endometrial stromal cell isolation30. Tissue samples were washed with DMEM-F12 (Invitrogen) containing 1% antibiotic-antimycotic and minced to <1 mm3. Tissues were incubated for 1.5 h at 37°C in DMEM-F12 containing 0.25% (w/v) collagenase and 0.05% DNase I (Sigma). After enzymatic digestion, stromal cells were separated from epithelial aggregates using a 40 μm nylon cell strainer (BD-Biosciences, Durham, NC., USA). Filtrates were washed twice and plated in DMEM-F12 media containing 10% fetal bovine serum and 1% antibiotic-antimycotic. Cells were seeded in 6-well plates at a concentration of 1.5 × 105 cells/well, and cultured to confluence of approximately 50%, then transfected with four sequences of LRH-1 siRNA (ON-TARGET plus SMART Pool, L-003430-00) or with a pool of non- targeting sequences (ON-TARGET plus Non-Targeting Pool, D-001810-10-20, both from Dharmacon, Lafayette, CO., USA) using Lipofectamine 2000 (Invitrogen) according to manufacturer’s instructions. The day of cell plating and siRNA treatment was designated day 0. All decidualization experiments were conducted in opti-MEM media (Invitrogen) containing 2% charcoal-stripped fetal bovine serum and 1% antibiotic-antimycotic. On day 2, decidualization was induced by treatment with 1 μM medroxyprogesterone acetate, 10 nM E2, and 50 μM dibutyryl cAMP (all from Sigma-Aldrich) every 48 h31.
The human endometrial stromal cell line immortalized by retroviral transfection of human telomerase (ATCC CRL-4003) was cultured as described by Krikun et al.24. In vitro decidualization was induced when cells achieved 80–85 % confluence by incubation with 1 μM medroxyprogesterone acetate and 0.3 mM dibutryl cAMP for 7–9 days.
Chromatin Immunoprecipitation(ChIP)
Human endometrial stromal cells were seeded in 15 cm2 dishes, grown to approximately 75% confluency and decidualization induced. On day 3, 1% formaldehyde was added to crosslink chromatin and DNA. The ChIP was performed using the ChIP-IT® Express Chromatin Immunoprecipitation Kit (Active Motif, Carlsbad, CA., USA) according to manufacturer’s instructions. Shearing of chromatin was achieved by sonication and LRH-1 was immunoprecipitated (antibody SC-25389, Santa Cruz Biotechnology, Santa Cruz, CA., USA). Enrichment was evaluated by qPCR for ChIP using Sybr-Green technology (Applied Biosystems) using sequence specific primers (WNT4- F: TCCCAGCGGTCTGAAGGTAAAGAA, R: GGCTTTCTTTCCACCCATCACCTA; Neg- F: AGCATGCACGGCTATATGGACTCA, R: TGTGCAATGGCAAAGCCACAGA). Results were normalized to input.
Hormone Analysis
Mice were anesthetized using isofluorane (Baxter, Ontario, Canada) and blood collected by cardiac puncture. Progesterone and estradiol-17β levels were analyzed in a single radioimmunoassay7 for each. Intra-assay coefficients of variation were 7.76 % for progesterone and 4.66 % for estradiol-17β.
Laser Microdissection (LMD)
Frozen ovaries were sectioned at 25 μm onto polyethylene naphthalate (PEN) membrane-covered glass slides (PALM Microlaser Technologies, Bernried, Germany), stained with toluidine blue, dehydrated in 70% and 100% ethanol and warmed 30 min at 37 °C. Corpora lutea were dissected by LMD (Leica AS LMD, Germany), collected into extraction buffer from PicoPure RNA isolation kit (Arcturus, Mountainview, CA., USA).
Immunofluorescence and Immunohistochemistry
Ovaries were cryosectioned at 8 μm, fixed in acetone at −20°C for 10 min and blocked in IgG from the same animal species of the primary antibody for 1 h at room temperature. Cryosections were incubated with primary antibodies at 4 °C overnight, Lrh-1 (Santa Cruz SC-21132) and Cyp11a1 (Abcam Ltd. Cambridge, UK, ab78416-100) at dilutions of 1:100. For immunofluorescence, secondary antibodies were Alexa Fluor 594- or 488-conjugated IgG (Invitrogen-Molecular Probes, Carlsbad, CA., USA. dilution, 1:400). Slides were incubated with 4′, 6-diamidino-2-phenylindole (DAPI, Roche Applied Science, Mannheim, Germany) for 5 min at room temperature. Digital images were captured using an Olympus confocal microscope (Olympus FV1000, Tokyo, Japan) or a Leica fluorescence microscope (Mannheim, Germany). For immunohistochemistry of Lrh-1, paraffin sections of mouse uteri were incubated with the Santa Cruz antibody SC21132 and 1:100, paraffin sections of human uterine tissue incubated with R&D Systems PP-H325-00 mouse monoclonal antibodies at 1:50. Biotinylated secondary antibodies were used (Santa Cruz SC-2011, 1:200 dilution for mouse tissue, Abcam18293 for human tissue ) and sections were incubated with a diaminobenzidine (DAB)-peroxidase substrate (Santa Cruz) counterstained with hematoxylin, dehydrated, mounted, and digitally photographed.
EdU Incorporation Assay
Females (four of each genotype) mated to fertile CON males were injected with EdU (5-ethynyl-2′-deoxyuridine) (20 mg/kg) on the afternoon of dpc 4 (17:00). Uteri were harvested 2 h after injection. Incorporated EdU was detected using a Click-It imaging kit (Invitrogen) according to manufacturer’s instructions. EdU positive cells were counted at 40 X in four fields per section and four individual sections were quantified per specimen.
RNA Isolation and Real-Time Polymerase Chain Reaction (qPCR)
Total RNA was isolated from uteri or placentas using Trizol reagent (Invitrogen), from three or more animals, reverse transcribed with the SuperScript First-Strand Synthesis System (Invitrogen). For corpora lutea obtained by LMD, 1 μg RNA was used, while for uteri or placentas, 3 μg. Real-time PCR was performed in an ABI Prism 7300 instrument with SYBR green PCR master mix (Applied Biosystems) with general PCR conditions (3 min at 95°C, 40 cycles of 15 s at 95°C, 30 s at 60°C, and 30 s at 72°C) to amplify the product. Melting-curve analyses verified product identity. Samples were run in triplicate and were expressed relative to β-actin in the same sample. Data were normalized to a calibrator sample using ΔΔCt method with correction for amplification efficiency by LinRegPCR 11.0 software (Academic Medical Center, Amsterdam).
Statistical analyses
Data are represented as mean ± SEM; and all experiments were repeated at least three times. Data were analyzed using the Student’s t-test and one-way ANOVA followed by Tukey’s post hoc multiple-range test. Values were considered significantly different if P<0.05.
Supplementary Material
Acknowledgments
This study was funded by OPG 11018 from the Canadian Institutes of Health Research to BDM. CZ was supported by National Natural Science Foundation of China (31172040) and Shangdong Natural Science Foundation (ZR2011CM047). KS was supported by grants from the École Polytechniqe Fédérale de Lausanne, the Swiss National Science Foundation (SNF) and the Swiss Cancer League, JPL by NIH R01CA77530 and FJD by NIH U54 HD07495-31. MJL is supported by NIH 5T32HD007165. We are exceptionally grateful to V. Roussel for preparation of the figures, M. Dobias for hormone analyses, S. Ruiz Orduna for HESC experiments, J. Fenelon and K. Bertolin for aid with transcript studies, L. Lian for aid with oviduct transfer, X. Tang for statistical analyses and to L. Giudice for coordinating acquisition of human endometrium from the NIH-UCSF Endometrium Tissue Bank. CZ, RD, FJD and BDM planned mouse experiments, carried out by CZ and BDM. CZ, RD and BDM wrote the manuscript. FJD, EK and MJL planned and executed primary endometrial culture experiments and edited the manuscript, JS and JPL provided mouse models and edited the manuscript.
References
- 1.Lee YK, Moore DD. Liver receptor homolog-1, an emerging metabolic modulator. Front Biosci. 2008;13:5950–5958. doi: 10.2741/3128. 3128 [pii] [DOI] [PubMed] [Google Scholar]
- 2.Hinshelwood MM, et al. Expression of LRH-1 and SF-1 in the mouse ovary: localization in different cell types correlates with differing function. Mol Cell Endocrinol. 2003;207:39–45. doi: 10.1016/s0303-7207(03)00257-0. [DOI] [PubMed] [Google Scholar]
- 3.Labelle-Dumais C, Jacob-Wagner M, Pare JF, Belanger L, Dufort D. Nuclear receptor NR5A2 is required for proper primitive streak morphogenesis. Dev Dyn. 2006;235:3359–3369. doi: 10.1002/dvdy.20996. [DOI] [PubMed] [Google Scholar]
- 4.Duggavathi R, et al. Liver receptor homolog 1 is essential for ovulation. Genes Dev. 2008;22:1871–1876. doi: 10.1101/gad.472008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soyal SM, et al. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis. 2005;41:58–66. doi: 10.1002/gene.20098. [DOI] [PubMed] [Google Scholar]
- 6.Coste A, et al. LRH-1-mediated glucocorticoid synthesis in enterocytes protects against inflammatory bowel disease. Proc Natl Acad Sci U S A. 2007;104 doi: 10.1073/pnas.0702440104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miranda-Jimenez LM, Binelli M, Bertolin K, Pelletier RM, Murphy BD. Scavenger receptor-B1 and luteal function in mice. J Lipid Res. 2010;51:2362–2371. doi: 10.1194/jlr.M006973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi JH, Ishida M, Matsuwaki T, Yamanouchi K, Nishihara M. Involvement of 20alpha-hydroxysteroid dehydrogenase in the maintenance of pregnancy in mice. J Reprod Dev. 2008;54:408–412. doi: 10.1262/jrd.20045. [DOI] [PubMed] [Google Scholar]
- 9.Das SK. Regional development of uterine decidualization: molecular signaling by Hoxa-10. Mol Reprod Dev. 2010;77 doi: 10.1002/mrd.21133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang H, Xie H, Dey SK. Endocannabinoid signaling directs periimplantation events. AAPS J. 2006;8:E425–432. doi: 10.1007/BF02854916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Das SK. Cell cycle regulatory control for uterine stromal cell decidualization in implantation. Reproduction. 2009;137 doi: 10.1530/REP-08-0539. [DOI] [PubMed] [Google Scholar]
- 12.Ramathal CY, Bagchi IC, Taylor RN, Bagchi MK. Endometrial decidualization: of mice and men. Semin Reprod Med. 2010;28:17–26. doi: 10.1055/s-0029-1242989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim H, et al. Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell. 1997;91:197–208. doi: 10.1016/s0092-8674(00)80402-x. [DOI] [PubMed] [Google Scholar]
- 14.Cha J, Sun X, Dey SK. Mechanisms of implantation: Strategies for successful pregnancy. Nature Medicine. 2012;18:1754–1767. doi: 10.1038/nm.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunn CL, Kelly RW, Critchley HO. Decidualization of the human endometrial stromal cell: an enigmatic transformation. Reprod Biomed Online. 2003;7:151–161. doi: 10.1016/s1472-6483(10)61745-2. [DOI] [PubMed] [Google Scholar]
- 16.Cartwright JE, Fraser R, Leslie K, Wallace AE, James JL. Remodelling at the maternal-fetal interface: relevance to human pregnancy disorders. Reproduction. 2010;140:803–813. doi: 10.1530/REP-10-0294. [DOI] [PubMed] [Google Scholar]
- 17.Lynch VJ, Brayer K, Gellersen B, Wagner GP. HoxA-11 and FOXO1A cooperate to regulate decidual prolactin expression: towards inferring the core transcriptional regulators of decidual genes. PLoS One. 2009;4:e6845. doi: 10.1371/journal.pone.0006845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang M, Naidu D, Hearing P, Handwerger S, Tabibzadeh S. LEFTY, a member of the transforming growth factor-beta superfamily, inhibits uterine stromal cell differentiation: a novel autocrine role. Endocrinology. 2010;151:1320–1330. doi: 10.1210/en.2009-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buzzio OL, Lu Z, Miller CD, Unterman TG, Kim JJ. FOXO1A differentially regulates genes of decidualization. Endocrinology. 2006;147:3870–3876. doi: 10.1210/en.2006-0167. [DOI] [PubMed] [Google Scholar]
- 20.Huyen DV, Bany BM. Evidence for a conserved function of heart and neural crest derivatives expressed transcript 2 in mouse and human decidualization. Reproduction. 2011;142:353–368. doi: 10.1530/REP-11-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Q, et al. WNT4 Acts Downstream of BMP2 and Functions via beta-Catenin Signaling Pathway to Regulate Human Endometrial Stromal Cell Differentiation. Endocrinology. 2012 doi: 10.1210/en.2012-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cloke B, et al. The androgen and progesterone receptors regulate distinct gene networks and cellular functions in decidualizing endometrium. Endocrinology. 2008;149:4462–4474. doi: 10.1210/en.2008-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chalkiadaki A, Talianidis I. SUMO-dependent compartmentalization in promyelocytic leukemia protein nuclear bodies prevents the access of LRH-1 to chromatin. Mol Cell Biol. 2005;25:5095–5105. doi: 10.1128/MCB.25.12.5095-5105.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krikun G, et al. A novel immortalized human endometrial stromal cell line with normal progestational response. Endocrinology. 2004;145 doi: 10.1210/en.2003-1606. [DOI] [PubMed] [Google Scholar]
- 25.Yang FM, et al. Liver receptor homolog-1 localization in the nuclear body is regulated by sumoylation and cAMP signaling in rat granulosa cells. The FEBS journal. 2009;276:425–436. doi: 10.1111/j.1742-4658.2008.06785.x. [DOI] [PubMed] [Google Scholar]
- 26.Franco HL, et al. WNT4 is a key regulator of normal postnatal uterine development and progesterone signaling during embryo implantation and decidualization in the mouse. FASEB J. 2011;25:1176–1187. doi: 10.1096/fj.10-175349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Q, et al. Bone morphogenetic protein 2 functions via a conserved signaling pathway involving Wnt4 to regulate uterine decidualization in the mouse and the human. J Biol Chem. 2007;282 doi: 10.1074/jbc.M704723200. [DOI] [PubMed] [Google Scholar]
- 28.Shaw JM, Trounson AO. Ovarian tissue transplantation and cryopreservation. Application to maintenance and recovery of transgenic and inbred mouse lines. Methods Mol Biol. 2002;180:229–251. doi: 10.1385/1-59259-178-7:229. [DOI] [PubMed] [Google Scholar]
- 29.Sheldon E, et al. Biobanking human endometrial tissue and blood specimens: standard operating procedure and importance to reproductive biology research and diagnostic development. Fertil Steril. 2011;95:2120–2122. doi: 10.1016/j.fertnstert.2011.01.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Markoff E, Zeitler P, Peleg S, Handwerger S. Characterization of the synthesis and release of prolactin by an enriched fraction of human decidual cells. J Clin Endocrinol Metab. 1983;56:962–968. doi: 10.1210/jcem-56-5-962. [DOI] [PubMed] [Google Scholar]
- 31.Kessler CA, Schroeder JK, Brar AK, Handwerger S. Transcription factor ETS1 is critical for human uterine decidualization. Mol Hum Reprod. 2006;12:71–76. doi: 10.1093/molehr/gal008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.