Abstract
Background
Q fever is a common cause of febrile illness and community-acquired pneumonia in resource-limited settings. Coxiella burnetii, the causative pathogen, is transmitted among varied host species, but the epidemiology of the organism in Africa is poorly understood. We conducted a systematic review of C. burnetii epidemiology in Africa from a “One Health” perspective to synthesize the published data and identify knowledge gaps.
Methods/Principal Findings
We searched nine databases to identify articles relevant to four key aspects of C. burnetii epidemiology in human and animal populations in Africa: infection prevalence; disease incidence; transmission risk factors; and infection control efforts. We identified 929 unique articles, 100 of which remained after full-text review. Of these, 41 articles describing 51 studies qualified for data extraction. Animal seroprevalence studies revealed infection by C. burnetii (≤13%) among cattle except for studies in Western and Middle Africa (18–55%). Small ruminant seroprevalence ranged from 11–33%. Human seroprevalence was <8% with the exception of studies among children and in Egypt (10–32%). Close contact with camels and rural residence were associated with increased seropositivity among humans. C. burnetii infection has been associated with livestock abortion. In human cohort studies, Q fever accounted for 2–9% of febrile illness hospitalizations and 1–3% of infective endocarditis cases. We found no studies of disease incidence estimates or disease control efforts.
Conclusions/Significance
C. burnetii infection is detected in humans and in a wide range of animal species across Africa, but seroprevalence varies widely by species and location. Risk factors underlying this variability are poorly understood as is the role of C. burnetii in livestock abortion. Q fever consistently accounts for a notable proportion of undifferentiated human febrile illness and infective endocarditis in cohort studies, but incidence estimates are lacking. C. burnetii presents a real yet underappreciated threat to human and animal health throughout Africa.
Author Summary
Coxiella burnetii is a bacterium that can cause acute and chronic fever illness and pneumonia in humans. It is also a known cause of abortion in livestock species, and is principally transmitted to humans through contact with infected animal birth products. With growing awareness of the over-diagnosis and misclassification of malaria as the cause of fever illnesses in the tropics, including Africa, there is increased interest in the role of non-malarial causes of fever, such as C. burnetii. We performed a systematic review of the published literature on the epidemiology of C. burnetii in Africa to consolidate knowledge and identify knowledge gaps regarding the extent of this infection in humans and animals and the risk factors for infection transmission. Few studies on prevalence of infection in humans and animals used random sampling strategies, and among these only two studied linked human and animal populations. C. burnetii appears to be a common cause of severe fever illness in humans, but population-level incidence estimates are lacking. The differential risks for C. burnetii infection and potential control strategies within the various animal husbandry systems in Africa remain largely unexplored. We conclude that C. burnetii is an underappreciated threat to human and animal health throughout Africa.
Introduction
Coxiella burnetii, a zoonotic bacterial pathogen found worldwide except in New Zealand, is transmitted to humans through direct contact with milk, urine, feces, or semen from infected animals as well as inhalation of aerosolized particles from animal placentas, parturient fluids, aborted fetuses, and environmental dust [1]. While infection by C. burnetii in humans can be asymptomatic, symptomatic infection, known as Q fever, can present as an acute undifferentiated febrile illness with the possibility of focal manifestations, such as hepatitis and pneumonia. Acute disease can progress to chronic forms, such as endocarditis, in 0.5–2.0% of cases [2], [3], typically in individuals predisposed by valvular heart disease or immunodeficiency [4]. Q fever is also one of the infectious diseases that has been linked to chronic fatigue syndrome [5]. Infection by C. burnetii has been demonstrated in many animal species, but the principle reservoirs are thought to be sheep, goats, and cattle. In these livestock species infection is often asymptomatic but can cause abortion and reduce reproductive efficiency [1], [6].
Q fever has recently gained renewed attention after the largest-ever recorded outbreak which involved over 3,500 human cases in the Netherlands in 2007–2009 [7]. Recent studies in resource-limited settings have demonstrated C. burnetii as a common cause of febrile illness and community-acquired pneumonia [8]–[10]. Fever etiology research among hospitalized patients in northern Tanzania found Q fever was a more common cause of severe febrile illness than malaria [11], [12]. As control efforts have led to consistent decreases in malaria incidence throughout sub-Saharan Africa [13]–[17] the diagnosis, treatment, and control of non-malaria febrile illnesses, such as Q fever, are emerging as new public health priorities [12]. In addition to being sources for disease transmission to humans, C. burnetii infection in animals can decrease livestock productivity which can have socioeconomic and indirect health effects on humans, especially among livestock-keeping populations in resource-limited settings [18].
In light of recent findings establishing Q fever as an important cause of severe febrile illness in northern Tanzania [11], [12] and growing awareness of the potential economic impact of infection in animals, we systematically reviewed the existing literature on the epidemiology of C. burnetii infection among humans and animals in Africa. This survey aimed to consolidate knowledge and identify future research priorities for the following topics: the prevalence of C. burnetii infection in human and animal populations, including surveys of sera or shedding in body fluids; the incidence of disease due to C. burnetii in human and animal populations; risk factors for seropositivity or disease; and infection control efforts undertaken in Africa.
Methods
Search
Nine databases were searched with the search string described in Figure 1 including all countries in the 5 United Nations (UN) sub-regions of Africa [19]. These search terms were adapted to the particular language of each database, and for those databases that did not allow the combination of Boolean operators, (q fever) OR (Coxiella burnetii) was used. Two of the databases, CABI and EBSCO Global Health, were searched with the intention to include grey literature.
Figure 1. Databases and search terminology employed for systematic review of Coxiella burnetii epidemiology in Africa, all years.

Selection and data extraction
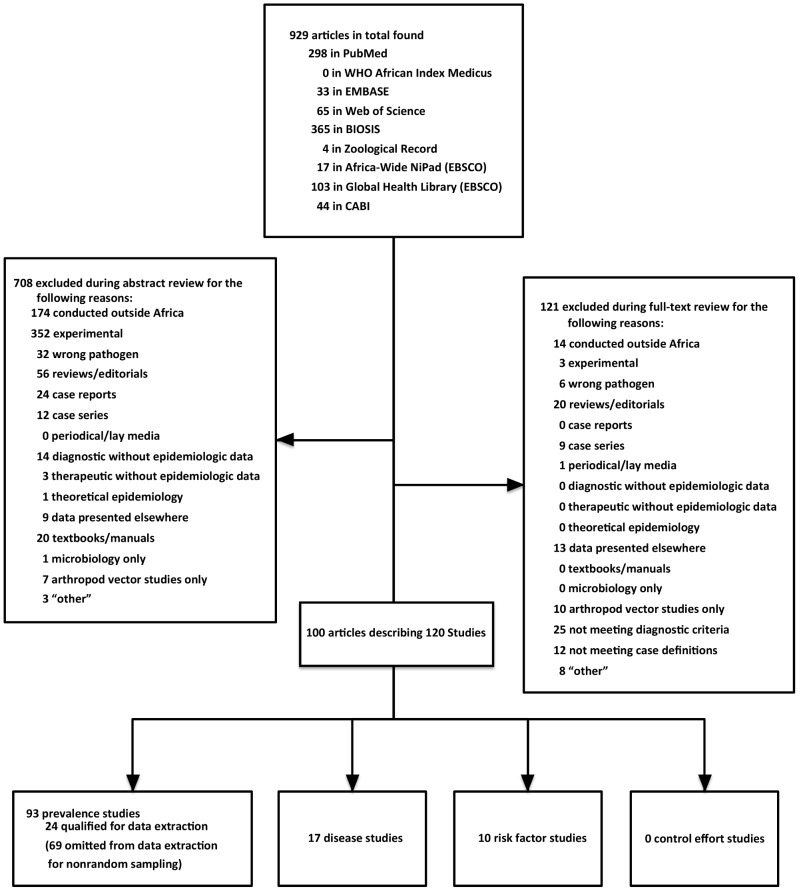
Citations for all years and in all languages were compiled and de-duplicated using EndNote (Thomson Reuters, New York, USA). Abstracts were independently reviewed by two investigators (SV and MPR) using combined language competency in English, French, Spanish, and Portuguese or Google Translate (Google, Mountain View, CA, USA) and included for full-text review upon meeting predetermined criteria ( Figure 2 ). Excluded abstracts described studies conducted outside Africa, basic science or immunology experiments, incorrect pathogens, reviews/editorials, case reports or case series, Q fever among returning travelers, periodical lay media content, diagnostic or therapeutic studies without epidemiologic data, theoretical epidemiology, duplicate data published elsewhere, textbooks/manuals, microbiologic studies without epidemiologic data, or arthropod sampling. The same criteria were applied during full-text review and to all grey literature containing sufficient information for adjudication. Cases of disagreement between the two investigators were resolved through independent review by a third investigator (JEBH).
Figure 2. Selection of eligible articles concerning Coxiella burnetii epidemiology in Africa.
Prevalence of Coxiella burnetii infection
Prevalence studies that presented evidence of current or prior infection with C. burnetii in humans and animals were included. We considered the following serologic tests and minimum antibody titer cut-offs for C. burnetii phase I and/or phase II antigen as acceptable evidence of infection in humans and animals based on expert consensus: complement fixation (CF) >1∶10 for animals [20] and ≥1∶4 for humans, microscopic agglutination test (MAT) ≥1∶4 for humans and animals, indirect fluorescent antibody (IFA) ≥1∶25 for animals and ≥1∶40 for humans, and ELISA validated against one of the above methods [20]–[23]. Capillary agglutination tests (CAT) were also accepted based on demonstrated correspondence to CF titers [23], [24]. The terms seropositive or seropositivity are used throughout to describe serologic reactions that met these titer cut-offs. For studies of pathogen shedding, confirmation of C. burnetii by nucleic acid detection, culture, or rodent inoculation was accepted. Studies of the prevalence of C. burnetii infection in humans and animals were classified by extent of study population characterization and sampling strategies, which were categorized as random (e.g., proportional, simple cluster, or simple random) or non-random. Prevalence studies that met these diagnostic criteria and used randomized sampling strategies qualified for data extraction. Prevalence studies with appropriate diagnostic criteria that used non-random sampling were included but did not undergo further data extraction.
Disease attributed to Coxiella burnetii
Studies reporting disease in animals due to C. burnetii were included if the reported cases met the World Organization for Animal Health (OIE) case definition for Q fever: abortion and/or stillbirth plus confirmed presence of the bacterial agent, accomplished by 1) isolation in culture; or 2) polymerase chain reaction (PCR), in situ hybridization, or immunohistochemistry of birth products or of associated vaginal discharge [20]. Evidence of C. burnetii on placental smears with stains deemed appropriate by OIE (Stamp, Ziehl-Neelsen, Gimenez, Giemsa or modified Koster) were considered presumptive for disease and included [20]. We also included seroprevalence studies of animals with a history of abortion, as these data, although not of confirmed cases, could yield information about potential associations between C. burnetii exposure and prevalence of animal abortion.
For studies of disease in humans, acute Q fever was defined according to the US Centers for Disease Control and Prevention (CDC) case definition: a compatible fever syndrome plus four-fold rise in antibody titers to Phase II antigen or detection in clinical specimens by PCR, immunohistochemistry (IHC), or culture [25]. Phase II antigen IFA antibody titer levels for IgG≥1∶200 and IgM≥1∶50 were included as cases based on the high positive predictive value of such results [26]. Studies reporting chronic disease in humans due to Q fever were included if the reported cases met the CDC case definition for confirmed chronic Q fever: culture-negative endocarditis or infected vascular aneurysm, chronic hepatitis, osteomyelitis, osteoarthritis, or pneumonitis with no other etiology plus IFA IgG antibody to C. burnetii phase I antigen ≥1∶800 or detection in clinical specimens by PCR, IHC, or cell-culture [25].
Risk factors for Coxiella burnetii infection or disease
Risk factor studies were evaluated using the same criteria for sampling design and case definitions that were applied to surveys of prevalence or disease, respectively. Risk factor analyses in prevalence studies were excluded if the prevalence study used a non-random sampling strategy.
Coxiella burnetii control efforts
Studies describing control efforts must have presented original data demonstrating the outcome of an intervention to decrease infection or disease incidence in human and/or animal populations.
Data extraction and analysis
For all qualifying studies, extracted data included study country, city or region, species, population census data when given, sample size, year of study, and diagnostic test as well as the number of seropositive or disease cases, risk factors, or control effort data where applicable. In the case of incomplete data or unclear methods, authors were contacted for further clarification when possible. Descriptive analyses of the extracted data were conducted. No quantitative meta-analysis was undertaken.
Results
A total of 1,662 citations were identified by the search conducted on December 3, 2012. After duplicates were removed, 929 articles remained ( Figure 2 ). Four of the six authors we contacted for further clarification responded to our inquiries. Ultimately, 100 articles describing 120 studies remained after full-text review. Forty-one articles describing 51 studies qualified for data extraction. The other 59 articles described 69 prevalence studies that did not qualify for data extraction due to non-random sampling methods (Table S1). Studies qualifying for data extraction were grouped into the following categories: 8 human and 13 animal seroprevalence; 3 animal milk shedding; 10 human disease; 7 animal abortion; and 7 human and 3 animal risk factor studies. These 51 studies spanned the years 1965–2012 and were conducted in 15 countries, mostly concentrated in the UN sub-regions of Northern, Western, and Middle Africa ( Table 1 & Figure 3 ).
Table 1. Studies of Coxiella burnetii infection prevalence and disease prevalence in humans and animals in Africa that qualified for data extraction.
| Sub-Region | Study Type | Country | Species (n) | Study Year(s) | Sample | Number Positive (%) | Ref |
| Northern | Prevalence | Egypt | Dogs (150) | 1998–1999 | serum | 34 (23%) | [37] |
| Egypt | Buffalo (45) | 2012* | serum | 0 (0%) | [27] | ||
| Cattle (54) | 7 (13%) | ||||||
| Goats (30) | 7 (23%) | ||||||
| Sheep (55) | 18 (33%) | ||||||
| Humans (92) | 15 (16%) | ||||||
| Egypt | Cattle (100) | 2009* | milk | 22 (22%) | [38] | ||
| Egypt | Humans (883) | 1991 | serum | 285 (32%) | [39] | ||
| Sudan | Goats (460) | 2010–2011 | serum | 109 (24%) | [36] | ||
| Disease | Morocco | Sheep prior abortion (115) and controls (156) | 1986–1987 | serum | 38 (33%) and 23 (15%) | [48] | |
| Tunisia | Goats & Sheep prior abortion (118) and Sheep controls (517) | 1997 | serum | 22 (12%) and 35 (7%) | [49] | ||
| Tunisia | Goats & Sheep abortion (72) | 2009* | birth products/vaginal fluids | 14 (19%)† | [45] | ||
| Tunisia | Human febrile illness (300) | 1993–1994 | serum | 5 (2%) | [54] | ||
| Tunisia | Human febrile illness (47) | 2004 | serum | 4 (9%) | [55] | ||
| Tunisia | Human endocarditis (98) | 1991–2000 | blood | 1 (1%) | [51] | ||
| Tunisia | Human endocarditis (33) | 2008 | serum | 1 (3%) | [52] | ||
| Algeria | Human endocarditis (61) | 2000–2003 | serum | 2 (3%) | [53] | ||
| Western | Prevalence | Nigeria | Cattle (306) Cattle (84) | 1983–1984 | serum milk | 169 (55%) 44 (52%) | [30] |
| Nigeria | Cattle (88) Cattle (169) | 1983–1984 | serum milk | 48 (55%) 41 (24%) | [31] | ||
| Senegal | Cattle (196) | 2007–2008 | serum | 7 (4%) | [29] | ||
| Ghana | Cattle (166) | 2012* | serum | 30 (18%) | [32] | ||
| Cote d'Ivoire | Humans (949) | 1965 | serum | 44 (5%) | [42] | ||
| Burkina Faso | Humans (1309) | 1975* | serum | 100 (8%) | [43] | ||
| Niger | Humans, children (177) | 1994 | serum | 17 (10%) | [40] | ||
| Ghana | Humans, children (219) | 2008* | serum | 37 (17%) | [41] | ||
| Disease | Niger | Goats prior abortion (75) and controls (47) | 1971–1972 | serum | 24 (32%) and 12 (29%) | [47] | |
| Burkina Faso | Human febrile illness (183) | 1995 | serum | 9 (5%) | [56] | ||
| Middle | Prevalence | Chad | Cattle (193) | 1985* | serum | 13 (7%) | [34] |
| Chad | Camels (142) | 1999–2000 | serum | 114 (80%) | [28] | ||
| Cattle (195) | serum | 8 (4%) | |||||
| Goats (134) | serum | 18 (13%) | |||||
| Sheep (142) | serum | 16 (11%) | |||||
| Humans (368) | serum | 4 (1%) | |||||
| Cameroon | Cattle (1377) | 2000 | serum | 431 (32%) | [33] | ||
| Disease | Cameroon | Cattle prior abortion (116) | 1968* | serum | 0 (0%) | [50] | |
| Cameroon | Cattle prior abortion (490) and controls (193) | 1985* | serum | 14 (3%) and 13 (7%) | [34] | ||
| Cameroon | Human pneumonia (110) | 1991–1992 | serum | 6 (6%) | [57] | ||
| Cameroon | Human pneumonia (65) | 1991–1993 | serum | 6 (9%) | [58] | ||
| Southern | Prevalence | South Africa | Cattle (8900) | 1985–1986 | serum | 692 (8%) | [35] |
| Disease | South Africa | Cattle (6 farms) and sheep (6 farms) abortions | 1972–1976 | fetal tissue | 12 (100%) farms+ | [46] | |
| South Africa | Human pneumonia (92) | 1987–1988 | serum | 0 (0%) | [59] | ||
| Eastern | Prevalence | Tanzania | Humans, pregnant females (150) | 1993 | serum | 7 (5%) | [44] |
| Disease | Tanzania | Human febrile illness (483) | 2007–2008 | serum | 24 (5%) | [11] |
*Year(s) of sample collection for study unavailable; year of publication included instead.
C. burnetii detection by impression smear microscopy. Positive results were not provided at an individual animal level, only at the level of investigated farms.
C. burnetii detection by polymerase chain reaction.
Figure 3. Locations of Coxiella burnetii infection prevalence and disease studies in African humans and animals which qualified for data extraction.
Prevalence of Coxiella burnetii infection
Two surveys employed a systematic sampling strategy to assess seroprevalence among linked human and animal populations. The first, from three governorates surrounding Cairo, Egypt, reported C. burnetii seropositivity in 13% of cattle, 23% of goats, 33% of sheep, 0% of buffalo, and 16% of humans in close contact with these animals [27]. The second survey, undertaken in Chad, found 80% of camels, 4% of cattle, 13% of goats, 11% of sheep, and 1% of humans in close contact were seropositive [28]. All other seroprevalence studies sampled only humans or only animal species ( Table 1 ).
Surveys of cattle demonstrated seroprevalence ranging from 4% in Dakar, Senegal [29], to 55% around the city of Zaria, Nigeria [30], [31]. Other studies reporting cattle seroprevalence within this range were conducted in coastal Ghana (18%) [32], Cameroon's Adamawa Region (32%) [33], southern Chad (7%) [34], and South Africa's Transvaal Province (8%) [35]. Goat seropositivity ranged from 13% in Chad [28] to 23% in Egypt [27] and 24% in 8 Sudanese states [36]. Surveys of sheep revealed seroprevalences that ranged from 11% in Chad [28] to 33% in Egypt [27]. In Upper Egypt, 23% of dog sera samples indicated prior C. burnetii infection [37].
In studies of pathogen shedding in bovine milk, C. burnetii nucleic acid was detected in 22% of raw milk samples in Upper Egypt [38]. In Zaria, Nigeria, C. burnetii shedding among individual cows was reported in 63% of milk samples from extensively managed cattle and 43% of samples from semi-intensively managed cattle [30], whereas the prevalence was 26% and 22%, respectively, at the same location one year later [31]. No studies of shedding in fluids other than milk in asymptomatic humans or animals were found by our search, and no human milk shedding studies qualified for data extraction.
Seroprevalence in humans ranged from 1% in Chad [28] to 32% in a Nile Delta village in Egypt [39]. In Niamey, Niger, 10% of children ages 1 month-5 years were seropositive [40], and in Ghana's rural Ashanti Region, 17% of two-year-olds were seropositive [41]. Other surveys reported human seroprevalence at 5% in rural western Côte d'Ivoire [42], 8% among nomads sampled in rural northern Burkina Faso [43], and 5% of pregnant women attending an antenatal clinic in Dar es Salaam, Tanzania [44].
Disease attributed to Coxiella burnetii
Of disease studies in humans or animals, none estimated disease incidence, and two studies [45], [46] of livestock abortions met OIE definitions for either presumptive or confirmed cases. The remaining 5 animal abortion studies were serological investigations in individuals with history of abortions [34], [47]–[50].
Of two surveys of cattle with abortions in northern Cameroon, one did not detect serological evidence of C. burnetii infection in any cattle [50], while 3% in the other study were seropositive, compared to 7% among a non-random selection of cattle without abortions [34]. In South Africa, C. burnetii was found by smear microscopy in aborted calf fetuses at all of six cattle farms sampled [46].
In the Maradi Region of Niger, 32% of goats with previous abortions were seropositive, compared to 29% of non-randomly selected goats without a history of abortion [47]. Sheep with a history of abortion in Rabat, Morocco, were more likely than those with normal births to be seropositive for C. burnetii, 33% versus 15% (p<0.01) [48]. In a survey conducted in five Tunisian governorates, 7% of sheep without past abortions were seropositive for C. burnetii compared to 12% of small ruminants with previous abortions [49]. Another Tunisian study found that 19% of small ruminants with a history of abortion had C. burnetii detected by PCR analysis of birth products or vaginal secretions [45], and in South Africa, the pathogen was found by smear microscopy in aborted lamb fetuses from all of six sheep farms sampled [46].
Human cohorts comprising individuals with infective endocarditis in Sousse and Sfax, Tunisia, as well as Algiers, Algeria, have demonstrated C. burnetii as the causative pathogen in 1–3% of cases [51]–[53]. Two studies of febrile patients in Sousse, Tunisia, serologically identified acute Q fever in 2% and 9% of hospital admissions [54], [55]. Q fever was responsible for 5% of patients with acute febrile illness hospitalized in Bobo-Dioulasso, Burkina Faso [56] as well as 3% of pediatric and 8% of adult admissions for severe febrile illness at two referral hospitals in the Kilimanjaro Region of northern Tanzania [11]. In two studies of patients admitted for community-acquired pneumonia in Yaoundé and Douala, Cameroon, 6% and 9% of persons aged >15 years had serologically-confirmed acute Q fever [57], [58]. In these studies, Q fever was the third most common etiologic agent of pneumonia, after Streptococcus pneumoniae and Mycoplasma pneumoniae. At a major hospital in Cape Town, South Africa, C. burnetii was not found to be the cause of any pneumonia cases in a 92-patient cohort [59].
Risk factors for Coxiella burnetii infection or disease
Among Nigerian cattle near Zaria, no difference in seropositivity was detected for cattle managed semi-intensively versus extensively [30], [31]. In Cameroon, positive associations were found between seropositivity and cattle aged >2 years, female animals, those seen grazing with buffalo, and those for which the owner's ethnic group was recorded as Mbororo or ‘other’ when compared to Fulbe [33], [60].
In the Egypt study linking human and animal populations, rural human residents were more likely to test seropositive than those in urban areas [27]. In the linked study from Chad, human Q fever serostatus did not correlate with the proportion of seropositive animals within respective nomadic camps, and camel breeders were at higher risk for Q fever seropositivity than cattle breeders [28]. In Ghana, children of illiterate mothers had a two-fold higher risk of seropositivity compared to those of literate mothers [41]. There was no association detected between C. burnetii seropositivity and HIV serostatus in pregnant Tanzanian women [44]. In the hospitalized patient cohort in northern Tanzania, there was no difference in prevalence of acute Q fever infection in HIV-infected compared to HIV-uninfected individuals [11], and all cases of community-acquired pneumonia in the surveys of hospitalized patients in Cameroon were in HIV uninfected individuals [57], [58]. No studies of risk factors for animal disease remained after quality assessment.
Coxiella burnetii control efforts
No disease control studies were found by our search.
Discussion
The serological data reviewed in this study reveal evidence of widespread C. burnetii infection in multiple species and multiple sites throughout Africa. However, despite evidence of the pervasiveness of this pathogen, we found only 17 studies that used appropriate case definitions to quantify disease due to C. burnetii in humans and animals. Risk factors for exposure in humans and animals have been identified in some settings, but apart from assessing for associations between HIV and Q fever, we found no other evaluation of the epidemiologic risk factors for acute disease in animals or humans. No descriptions of disease control programs appear in published literature. C. burnetii was first reported in Africa in 1947 [61], but since then, the quantity and quality of epidemiologic research for this pathogen has been limited. We identified no disease incidence estimates, and the majority of research undertaken has limited validity due to non-random sampling procedures. Further, only two investigations using random sampling procedures studied linked human and animal livestock populations.
The majority of animal prevalence studies surveyed cattle, sheep, and goats. Studies of herds in Northern Africa, the Sahel and South Africa's Transvaal Province suggest C. burnetii infection in multiple ruminant species. Seroprevalence of C. burnetii ≤13% is frequently demonstrated in cattle [27]–[29], [34], [35] and generally higher seroprevalence (11–33%) is often observed among small ruminants [27], [28], [36], [48]. However, this pattern was not observed in all studies [30]–[33]. Our review revealed wide variation in seroprevalence even in areas of close proximity such as Lake Chad, where seroprevalence in cattle ranged from 4% to 31% [28], [33]. This is consistent with findings of high regional variability within Europe [62] and highlights the importance of understanding risk factors which may operate at a local scale and may be subtle. For example, risk factor analysis revealed that cattle seropositivity differed by owner's ethnic group (Mbororo vs. Fulbe) in Cameroon, despite these groups' similar nomadic pastoralism [60]. Herds raised by unspecified ‘other ethnic groups’ had an even greater risk of infection, highlighting the need to further explore how different animal husbandry practices might modify infection risk for humans and animals. Further, the high seroprevalence observed in camels and the greater infection risk among Arab camel breeders compared to cattle breeders warrants further investigation of C. burnetii infection in camels and the potential risk factors related to camel husbandry practices [28].
Human seroprevalence was <8% [28], [42]–[44] with the exception of surveys in children and in the Nile Delta of Egypt [27], [39]–[41]. A recent survey in The Gambia found C. burnetii seroprevalence was highest in young children, although the reasons for this are still unclear [63]. In the only risk factor analysis among children found by our search, Ghanaian children with illiterate mothers were more likely to be seropositive than children with literate mothers, but no difference was attributable to other socioeconomic factors tested [41]. The study of Egyptians in close contact with animals reported a high overall seroprevalence (16%) with greater seropositivity among rural (22%) vs. urban (4%) residents [27]. In contrast, the only other linked human-animal survey studied nomads in Chad, and found a relatively low human seropositivity (1%), despite the high-risk behaviors of handling aborted animal materials and consuming raw cow's milk [28], which was shown to contain C. burnetii in 15–63% of samples from other African settings [30], [31], [38].
Only two studies [45], [46] used direct detection of C. burnetii in animal acute disease cases. The other studies investigating the potential role of C. burnetii as a cause of livestock abortions in Africa measured the seroprevalence in individuals with history of abortion as compared to individuals without history of abortion. Two cohort studies found no difference between C. burnetii seroprevalence in cattle with previous abortions compared to those without history of abortion [34], [50]. Similar studies in sheep and goats, by contrast, generally showed a higher C. burnetii seroprevalence in individuals with history of abortion compared to those without history of abortion [45], [47]–[49]. This serologic approach, however, has limited value for inferring causation in livestock abortion cases, which is compounded by non-random selection of control livestock without abortions in all but one study [30].
Q fever accounted for 2–9% of humans hospitalized for febrile illness in 3 different African sub-regions [11], [54]–[56]. Although Q fever was the third most common detected cause of community-acquired pneumonia at two of Cameroon's largest hospitals [57], [58], no cases of community-acquired pneumonia were attributed to C. burnetii after a year-long survey at a Cape Town hospital [59]. Interestingly, all of the Cameroonian patients were HIV-uninfected, and in the Tanzania severe febrile illness cohort there was no association between HIV serostatus and acute Q fever [11], [44]. The proportion of infective endocarditis cases in Africa attributed to C. burnetii was slightly lower than proportions found in European settings [64]. However, all endocarditis studies found by our review were conducted in Northern Africa [51]–[53], highlighting a key knowledge gap on the role of Q fever in endocarditis elsewhere on the continent.
We identified few studies that elucidated the epidemiologic risk factors for C. burnetii infection in humans and animals in Africa. This knowledge gap highlights the need for future studies that randomly sample linked human-animal populations in order to estimate seroprevalence and determine the dynamics of pathogen transmission. Such research requires large, representative samples as well as detailed surveys of herds and households from multiple locations, agricultural systems, and ethnic groups.
C. burnetii is clearly an important cause of human and animal disease in Africa, although illness and death have not been estimated at the population level. In animals, C. burnetii has been implicated as an etiologic agent of abortion in livestock from the most northern to the most southern reaches of the continent, but studies should include confirmatory microbiological and histological testing of abortion materials, descriptions of other disease sequelae, randomly sampled non-aborting controls, and tandem serological and bacterial shedding surveys to determine the rate of asymptomatic shedding. Estimates of economic losses due to decreases in milk production, fecundity, or birth weight are also needed. For humans, limited data suggest that Q fever frequently causes severe febrile illness in cohorts throughout Africa, yet no studies quantifying disease incidence, disability, or deaths at the population level exist. Further, aside from infective endocarditis studies, proportions and clinical features of conversion to chronic Q fever in African populations are absent.
Limitations
Superseded geographic or biological terminology may have caused us to inadvertently miss pertinent research. The already remote chances of communicating with authors of older manuscripts were complicated by the absence of electronic contact information. We excluded arthropod vector studies, but surveys of invertebrates and non-domestic animals may contribute to knowledge about C. burnetii transmission. Comparisons between studies and sub-regions were restricted by changes in diagnostic methods over time, frequently small sample sizes, and the low total number of studies. The low number of studies for each research question and the heterogeneity of these studies precluded a more extensive quantitative analysis of the epidemiology of C. burnetii in Africa.
Conclusions
To our knowledge, this is the first systematic review of the epidemiology of C. burnetii in Africa from a ‘One Health’ perspective. Taken together, these findings suggest: 1) exposure to C. burnetii is a common finding in many animal host species across Africa, but seroprevalence varies widely by species and location, and the risk factors underlying this variability are largely unknown; 2) C. burnetii has been implicated as a cause of livestock abortion and could be responsible for substantial economic burdens, but more rigorous studies are required to determine this and other sequelae of disease in animals; 3) risk factors for human exposure to Q fever are poorly understood, but a more detailed understanding of how human exposure in different communities is linked with animal infection patterns and animal husbandry practices is clearly needed; and 4) Q fever accounts for a notable proportion of undifferentiated human febrile illness and infective endocarditis but studies describing other acute or chronic disease manifestations are scarce. The picture is complex, but the existing literature suggests that C. burnetii is found across diverse settings in Africa and presents a real yet underappreciated threat to human and animal health throughout Africa.
Key Points
Over the past 60 years, scores of studies have measured the prevalence of past or current infection of C. burnetii in humans and animals in Africa, but we found only 24 studies that used systematic, random sampling strategies, and of these only two studied linked human and animal populations.
Our review identified only two studies of livestock abortion cases which sought to directly detect C. burnetii in animal birth products or in the female reproductive tract. Comprehensive studies of the etiology of livestock abortion in African contexts are required, and these studies should include C. burnetii in the battery of pathogens detection.
Data from human cohort studies conducted in diverse settings in Africa show that Q fever accounts for 2–9% of severe fever cases, but more generalizable estimates of disease burden, such as incidence of Q fever, are lacking.
Data on risk factors for C. burnetii transmission in Africa are limited, and further risk factor analyses oriented to the unique context of various African animal infection patterns and animal husbandry systems is warranted.
Key Publications in the Field
Blanc GM, L. A. and Maurica, A. (1947) Présence du virus de la ‘Q fever’ dans le Maroc méridional. Bull Acad Natl Med 131: 138–143.
Kaabia N, Rolain JM, Khalifa M, Ben Jazia E, Bahri F, et al. (2006) Serologic study of rickettsioses among acute febrile patients in central Tunisia. Ann N Y Acad Sci 1078: 176–179.
Nahed HG, Khaled AAM (2012) Seroprevalence of Coxiella burnetii antibodies among farm animals and human contacts in Egypt. J Am Sci 8: 619–621.
Schelling E, Diguimbaye C, Daoud S, Nicolet J, Boerlin P, et al. (2003) Brucellosis andQ-fever seroprevalences of nomadic pastoralists and their livestock in Chad. Prev Vet Med 61: 279–293.
Scolamacchia F, Handel IG, Fevre EM, Morgan KL, Tanya VN, et al. (2010) Serological patterns of brucellosis, leptospirosis and Q fever in Bos indicus cattle in Cameroon. PLoS One 5: e8623.
Supporting Information
Studies of Coxiella burnetii seroprevalence in humans and animals in Africa with limited validity due to non-random sampling methods. Note: The term ‘Domestic Animals’ was employed if >3 livestock or household animal species were investigated.
(DOC)
Acknowledgments
The authors would like to thank Megan von Isenburg, MSLS, Associate Director for Research and Education at Duke Medical Center Library for her assistance in developing the search strategy.
Funding Statement
This research was supported by the joint US National Institutes of Health (NIH) – National Science Foundation (NSF) Ecology of Infectious Disease program (R01TW009237) and the UK Biotechnology and Biological Sciences Research Council (BBSRC) (BB/J010367/1). SV and MPR receive funding support from the Fogarty Global Health Fellowship through the Fogarty International Center at the US National Institutes of Health (R25TW009343). JEBH and SC both receive funding support from the BBSRC (BB/J010367/1). SC also receives funding support from the BBSRC (BB/H009302/1 and BB/H00935/1), the World Health Organization (HQNTD1206296), and the Wellcome Trust (096400/Z/11/Z). EAR and JAC received support from the NIH Fogarty International Center AIDS International Training and Research Program (D43 PA-03-018) and the Duke Clinical Trials Unit and Clinical Research Sites (U01 AI069484). We acknowledge the Hubert-Yeargan Center for Global Health at Duke University and Kilimanjaro Christian Medical Centre for critical infrastructure support. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. US NIH:http://www.nih.gov US NSF: http://www.nsf.gov UK BBSRC: http://www.bbsrc.ac.uk.
References
- 1. Tissot-Dupont H, Raoult D (2008) Q fever. Infect Dis Clin North Am 22: 505–514, ix. [DOI] [PubMed] [Google Scholar]
- 2. Tissot-Dupont H, Vaillant V, Rey S, Raoult D (2007) Role of sex, age, previous valve lesion, and pregnancy in the clinical expression and outcome of Q fever after a large outbreak. Clin Infect Dis 44: 232–237. [DOI] [PubMed] [Google Scholar]
- 3. van der Hoek W, Versteeg B, Meekelenkamp JC, Renders NH, Leenders AC, et al. (2011) Follow-up of 686 patients with acute Q fever and detection of chronic infection. Clin Infect Dis 52: 1431–1436. [DOI] [PubMed] [Google Scholar]
- 4. Brouqui P, Dupont HT, Drancourt M, Berland Y, Etienne J, et al. (1993) Chronic Q fever. Ninety-two cases from France, including 27 cases without endocarditis. Arch Intern Med 153: 642–648. [DOI] [PubMed] [Google Scholar]
- 5. Ayres JG, Flint N, Smith EG, Tunnicliffe WS, Fletcher TJ, et al. (1998) Post-infection fatigue syndrome following Q fever. QJM 91: 105–123. [DOI] [PubMed] [Google Scholar]
- 6. Angelakis E, Raoult D (2010) Q Fever. Vet Microbiol 140: 297–309. [DOI] [PubMed] [Google Scholar]
- 7. van der Hoek W, Dijkstra F, Schimmer B, Schneeberger PM, Vellema P, et al. (2010) Q fever in the Netherlands: an update on the epidemiology and control measures. Euro Surveill 15: pii: 19520. [PubMed] [Google Scholar]
- 8. Epelboin L, Chesnais C, Boulle C, Drogoul AS, Raoult D, et al. (2012) Q fever pneumonia in French Guiana: prevalence, risk factors, and prognostic score. Clin Infect Dis 55: 67–74. [DOI] [PubMed] [Google Scholar]
- 9. Manock SR, Jacobsen KH, de Bravo NB, Russell KL, Negrete M, et al. (2009) Etiology of acute undifferentiated febrile illness in the Amazon basin of Ecuador. Am J Trop Med Hyg 81: 146–151. [PubMed] [Google Scholar]
- 10. Fiorillo SP, Diefenthal HC, Goodman PC, Ramadhani HO, Njau BN, et al. (2013) Chest radiography for predicting the cause of febrile illness among inpatients in Moshi, Tanzania. Clin Radiol 68: 1039–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Prabhu M, Nicholson WL, Roche AJ, Kersh GJ, Fitzpatrick KA, et al. (2011) Q fever, spotted fever group, and typhus group rickettsioses among hospitalized febrile patients in northern Tanzania. Clin Infect Dis 53: e8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Crump JA, Morrissey AB, Nicholson WL, Massung RF, Stoddard RA, et al. (2013) Etiology of Severe Non-malaria Febrile Illness in Northern Tanzania: A Prospective Cohort Study. PLoS Negl Trop Dis 7: e2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. O'Meara WP, Bejon P, Mwangi TW, Okiro EA, Peshu N, et al. (2008) Effect of a fall in malaria transmission on morbidity and mortality in Kilifi, Kenya. Lancet 372: 1555–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mmbando BP, Vestergaard LS, Kitua AY, Lemnge MM, Theander TG, et al. (2010) A progressive declining in the burden of malaria in north-eastern Tanzania. Malar J 9: 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schellenberg D, Menendez C, Aponte J, Guinovart C, Mshinda H, et al. (2004) The changing epidemiology of malaria in Ifakara Town, southern Tanzania. Trop Med Int Health 9: 68–76. [DOI] [PubMed] [Google Scholar]
- 16. Karema C, Aregawi MW, Rukundo A, Kabayiza A, Mulindahabi M, et al. (2012) Trends in malaria cases, hospital admissions and deaths following scale-up of anti-malarial interventions, 2000–2010, Rwanda. Malar J 11: 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Geiger C, Agustar HK, Compaore G, Coulibaly B, Sie A, et al. (2013) Declining malaria parasite prevalence and trends of asymptomatic parasitaemia in a seasonal transmission setting in North-Western Burkina Faso between 2000 and 2009–2012. Malar J 12: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Perry BD, Grace D, Sones K (2011) Current drivers and future directions of global livestock disease dynamics. Proceedings of the National Academy of Sciences of the United States of America doi:10.1073/pnas.1012953108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.United Nations Statistics Division. (2013) Composition of macro geographical (continental) regions, geographical sub-regions, and selected economic and other groupings. unstats.un.org/unsd/methods/m49/m49regin.htm. New York: United Nations Statistics Division. Accessed: 29 November 2012. [Google Scholar]
- 20.World Organisation for Animal Health (2012) Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, 7th Ed. Geneva: World Organisation for Animal Health (OIE). 292–303. [Google Scholar]
- 21. Fournier PE, Marrie TJ, Raoult D (1998) Diagnosis of Q fever. J Clin Microbiol 36: 1823–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Scrimgeour EM, Al-Ismaily SI, Rolain JM, Al-Dhahry SH, El-Khatim HS, et al. (2003) Q Fever in human and livestock populations in Oman. Ann N Y Acad Sci 990: 221–225. [DOI] [PubMed] [Google Scholar]
- 23. McQuiston JH, Childs JE (2002) Q fever in humans and animals in the United States. Vector Borne Zoonotic Dis 2: 179–191. [DOI] [PubMed] [Google Scholar]
- 24. Luoto L (1953) A capillary agglutination test for bovine Q fever. J Immunol 71: 226–231. [PubMed] [Google Scholar]
- 25.US Centers for Disease Control and Prevention. (2012) 2012 Nationally Notifiable Diseases and Conditions and Current Case Definitions. Atlanta, GA: US Centers for Disease Control and Prevention. [Google Scholar]
- 26. Dupont HT, Thirion X, Raoult D (1994) Q fever serology: cutoff determination for microimmunofluorescence. Clin Diagn Lab Immunol 1: 189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nahed HG, Khaled AAM (2012) Seroprevalence of Coxiella burnetii antibodies among farm animals and human contacts in Egypt. J Am Sci 8: 619–621. [Google Scholar]
- 28. Schelling E, Diguimbaye C, Daoud S, Nicolet J, Boerlin P, et al. (2003) Brucellosis and Q-fever seroprevalences of nomadic pastoralists and their livestock in Chad. Prev Vet Med 61: 279–293. [DOI] [PubMed] [Google Scholar]
- 29. Kamga-Waladjo AR, Gbati OB, Kone P, Lapo RA, Chatagnon G, et al. (2010) Seroprevalence of Neospora caninum antibodies and its consequences for reproductive parameters in dairy cows from Dakar-Senegal, West Africa. Trop Anim Health Prod 42: 953–959. [DOI] [PubMed] [Google Scholar]
- 30. Adesiyun AA, Jagun AG, Tekdek LB (1984) Coxiella burnetii antibodies in some Nigerian dairy cows and their suckling calves. Int J Zoonoses 11: 155–160. [PubMed] [Google Scholar]
- 31. Adesiyun AA, Jagun AG, Kwaga JK, Tekdek LB (1985) Shedding of Coxiella burnetii in milk by Nigerian dairy and dual purposes cows. Int J Zoonoses 12: 1–5. [PubMed] [Google Scholar]
- 32. Adu-Addai B, Koney EB, Addo P, Kaneene J, Mackenzie C, et al. (2012) Importance of infectious bovine reproductive diseases: an example from Ghana. Vet Rec 171: 47–47. [DOI] [PubMed] [Google Scholar]
- 33. Scolamacchia F, Handel IG, Fevre EM, Morgan KL, Tanya VN, et al. (2010) Serological patterns of brucellosis, leptospirosis and Q fever in Bos indicus cattle in Cameroon. PLoS One 5: e8623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Domenech J, Trap D, Gaumont R (1985) [Study of reproductive pathology in cattle in Central Africa: survey of chlamydiosis and Q fever]. Rev Elev Med Vet Pays Trop 38: 138–143. [PubMed] [Google Scholar]
- 35. Gummow B, Poerstamper N, Herr S (1987) The incidence of Coxiella burnetii antibodies in cattle in the Transvaal. Onderstepoort J Vet Res 54: 569–571. [PubMed] [Google Scholar]
- 36. Hussien MO, ElFahal AM, Enan KA, Taha KM, Mohammed MS, et al. (2012) Seroprevalence of Q fever in goats in the Sudan. Vet World 5: 394–397. [Google Scholar]
- 37. Amal SMS, Asmaa AAH, Ismail AA, Nasr SEM, Yasukazu M, et al. (2002) Prevalence of Coxiella burnetii infection among dogs and humans in upper Egypt. Assiut Vet Med J 47: 205–215. [Google Scholar]
- 38. Amin WF, Ahmed SO (2009) Detection of Coxiella burnetii in bovine milk samples using polymerase chain reaction. Assiut Vet Med J 55: 23–31. [Google Scholar]
- 39. Corwin A, Habib M, Watts D, Darwish M, Olson J, et al. (1993) Community-based prevalence profile of arboviral, rickettsial, and Hantaan-like viral antibody in the Nile River Delta of Egypt. Am J Trop Med Hyg 48: 776–783. [DOI] [PubMed] [Google Scholar]
- 40. Julvez J, Michault A, Kerdelhue C (1997) Serological study of rickettsioses in Niamey, Niger./Étude sérologique des rickettsioses a Niamey, Niger. Med Trop (Mars) 57: 153–156. [PubMed] [Google Scholar]
- 41. Kobbe R, Kramme S, Kreuels B, Adjei S, Kreuzberg C, et al. (2008) Q fever in young children, Ghana. Emerg Infect Dis 14: 344–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gidel R, Lefevre M, Athawet B (1966) Investigation on the epidemiology of rickettsiosis in a rural section of the Ivory Coast. Med Trop (Mars) 26: 649–661. [Google Scholar]
- 43. Gidel R, Athawet B (1975) [Serological survey of human brucellosis and rickettsial diseases in a group of a nomad population in the sahelian regions of Upper Volta]. Ann Soc Belg Med Trop 55: 77–83. [PubMed] [Google Scholar]
- 44. Anstey NM, Tissot Dupont H, Hahn CG, Mwaikambo ED, McDonald MI, et al. (1997) Seroepidemiology of Rickettsia typhi, spotted fever group rickettsiae, and Coxiella burnetti infection in pregnant women from urban Tanzania. Am J Trop Med Hyg 57: 187–189. [DOI] [PubMed] [Google Scholar]
- 45. Berri M, Rekiki A, Boumedine KS, Rodolakis A (2009) Simultaneous differential detection of Chlamydophila abortus, Chlamydophila pecorum and Coxiella burnetii from aborted ruminant's clinical samples using multiplex PCR. BMC Microbiol 9: 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schutte AP, Kurz J, Barnard BJ, Roux DJ (1976) Q fever in cattle and sheep in Southern Africa. A preliminary report. Onderstepoort J Vet Res 43: 129–132. [PubMed] [Google Scholar]
- 47. Haumesser JB, Poutrel B (1973) Rickettsiosis in the Niger. Epidemiological investigation carried out in the Maradi area. Rev Elev Med Vet Pays Trop 26: 293–298. [PubMed] [Google Scholar]
- 48. Benkirane A, Jabli N, Rodolakis A (1990) Frequency of abortion and seroprevalence of the principal diseases causing ovine infectious abortion in the area of Rabat (Morocco). Ann Rech Vet 21: 267–273. [PubMed] [Google Scholar]
- 49. Rekiki A, Thabti F, Dlissi I, Russo P, Sanchis R, et al. (2005) Seroprevalence survey of major infectious abortive diseases in small ruminants in Tunisia. Rev Med Vet (Toulouse) 156: 395–401. [Google Scholar]
- 50. Maurice Y, Fernagut R, Gerome R (1968) [Rickettsial diseases of North Cameroon; epidemiological study]. Rev Elev Med Vet Pays Trop 21: 341–349. [PubMed] [Google Scholar]
- 51. Omezzine-Letaief A, Alaoui FZ, Bahri F, Mahdhaoui A, Boughzela E, et al. (2004) Infectious endocarditis with negative blood cultures. Arch Mal Coeur Vaiss 97: 120–124. [PubMed] [Google Scholar]
- 52. Znazen A, Trabelsi I, Maaloul I, Gargouri S, Maazoun Y, et al. (2009) Investigation of blood culture negative endocarditis in a tertiary care centre in Tunisia. Int J Antimicrob Agents 33: S9.19303572 [Google Scholar]
- 53. Benslimani A, Fenollar F, Lepidi H, Raoult D (2005) Bacterial zoonoses and infective endocarditis, Algeria. Emerg Infect Dis 11: 216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. OmezzineLetaief A, Dupont HT, Bahri F, Ernez M, Raoult D, et al. (1997) Seroepidemiologic study among 300 febrile patients in a infectious disease hospital ward. Med Mal Infect 27: 663–666. [Google Scholar]
- 55. Kaabia N, Rolain JM, Khalifa M, Ben Jazia E, Bahri F, et al. (2006) Serologic study of rickettsioses among acute febrile patients in central Tunisia. Ann N Y Acad Sci 1078: 176–179. [DOI] [PubMed] [Google Scholar]
- 56. Ki-Zerbo GA, Tall F, Nagalo K, Ledru E, Durand G, et al. (2000) Rickettsiosis and Q fever in pyretic patients hospitalized at the Bobo- Dioulasso Hospital (Burkina Faso). Med Mal Infect 30: 270–274. [Google Scholar]
- 57. Koulla-Shiro S, Kuaban C, Belec L (1996) Acute community-acquired bacterial pneumonia in human immunodeficiency virus (HIV) infected and non-HIV-infected adult patients in Cameroon: Aetiology and outcome. Tuber Lung Dis 77: 47–51. [DOI] [PubMed] [Google Scholar]
- 58. Koulla-Shiro S, Kuaban C, Belec L (1997) Microbial etiology of acute community-acquired pneumonia in adult hospitalized patients in Yaounde-Cameroon. Clin Microbiol Infect 3: 180–186. [DOI] [PubMed] [Google Scholar]
- 59. Maartens G, Lewis SJ, de Goveia C, Bartie C, Roditi D, et al. (1994) ‘Atypical’ bacteria are a common cause of community-acquired pneumonia in hospitalised adults. S Afr Med J 84: 678–682. [PubMed] [Google Scholar]
- 60. Mazeri S, Scolamacchia F, Handel IG, Morgan KL, Tanya VN, et al. (2012) Risk factor analysis for antibodies to Brucella, Leptospira and C. burnetii among cattle in the Adamawa Region of Cameroon: a cross-sectional study. Trop Anim Health Prod 45: 617–623. [DOI] [PubMed] [Google Scholar]
- 61. Blanc GM, L A;, Maurica A (1947) Présence du virus de la ‘Q fever’ dans le Maroc méridional. Bull Acad Natl Med 131: 138–143. [Google Scholar]
- 62. Georgiev M, Afonso A, Neubauer H, Needham H, Thiery R, et al. (2013) Q fever in humans and farm animals in four European countries, 1982 to 2010. Euro Surveill 18: pii: 20407. [PubMed] [Google Scholar]
- 63. van der Hoek W, Sarge-Njie R, Herremans T, Chisnall T, Okebe J, et al. (2013) Short communication: prevalence of antibodies against Coxiella burnetii (Q fever) in children in The Gambia, West Africa. Trop Med Int Health 18: 850–853. [DOI] [PubMed] [Google Scholar]
- 64. Maurin M, Raoult D (1999) Q fever. Clin Microbiol Rev 12: 518–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Studies of Coxiella burnetii seroprevalence in humans and animals in Africa with limited validity due to non-random sampling methods. Note: The term ‘Domestic Animals’ was employed if >3 livestock or household animal species were investigated.
(DOC)