Abstract
Background
This experiment was performed to compare the effects of Phenytoin (PHT) and Hypericin (HP) cream on healing of burn wounds in rats.
Material/Methods
Twenty rats were divided into 3 groups and second-degree burn wounds were created. The burn wounds in the first, second, and third groups were covered twice daily with PHT cream, HP cream, and saline (control), respectively. At the end of days 3, 7, 14, and 21, full-thickness skin biopsies were done for histopathologic and immunohistochemical analyses.
Results
Histopathologic evaluations at the 14th day showed that re-epithelialization scores were greater in the HP group than the PHT group, but on day 21, re-epithelialization scores were higher in the PHT group than the HP group. Collagen content on days 3 and 14 in the PHT group was found to be higher than in the HP group. Well-vascularized granulation tissue on day 7 in the PHT group was higher than in other groups. HP and PHT groups had a significant increase in VEGF and TGF-β expression in burn wound healing area compared to the control group on all days.
Conclusions
Topical application of HP can promote re-epithelialization in burn wounds to shorten the wound healing time for superficial burns. Phenytoin, on the other hand, contributes to healing by increasing vascularized granulation tissue and collagen synthesis through re-epithelialization. The increased VEGF and TGF-β expression following PHT and HP treatment strongly indicate that PHT and HP treatment promotes VEGF and TGF-β production and action in the burn wound area.
MeSH Keywords: Receptors, VEGF, TGF-β Receptor, Burn, Hypericum perforatums, Phenytoin
Background
Second-degree or partial thickness burns are restricted to the superficial dermis, but may also involve the reticular dermis layer; these kinds of burns produce blisters, severe pain, and redness [1]. The main causes of second-degree burns are scalds from hot water and liquids [2]. Burn wound healing consists of systematic processes that repair the damaged tissue partially or completely. Today, therapy for burn wounds mainly focuses on therapeutic products [3]. These products include various pharmacological and herbal agents [4]. In recent years, the consumption of herbal products has increased dramatically, and Hypericum perforatum-derived products make this presently one of the most consumed medicinal plants in the world [5,6]. Hypericum perforatum (family: Hypericaceae), more commonly known as ‘St. John’s wort’, has anti-inflammatory, antibacterial, antimicrobial, antiviral, antidepressant, and antitumor effects and wound-healing properties [7–9]. The active components of Hypericum perforatum are the naphthodianthrone, hypericin, and the phloroglucin derivative, hyperforin [9]. Hypericin (HP), the photodynamic active plant pigment, is the most the active component of Hypericum perforatum, which is responsible from the wound healing [7,10].
Phenytoin (PHT) is one of the pharmacological agents that have been used for their antiepileptic activity and also to promote burn wound healing. PHT increases neovascularization, myofibroblast and fibroblast proliferation, collagen production and deposition, extracellular matrix production, and growth factors and their mediators’ activity. Additionally, PHT reduces edema, wound exudate, and bacterial load. The effect of topical PHT in the healing of decubitus ulcers, traumatic ulcers, and a variety of chronic non-healing ulcers and burns in clinical and experimental studies have been reported [11,12].
Growth factors have been shown to play multiple and critical roles in wound healing processes [13,14]. Transforming growth factor-β (TGF-β) is a pleiotropic cytokine that participates in the maintenance of epidermal homeostasis. The main sources of TGF-β production in wound healing are platelets, monocytes/macrophages, and fibroblasts [15]. It is important in re-epithelialization, inflammation, angiogenesis, granulation tissue formation, wound contraction, and connective tissue regeneration [16]. Vascular endothelial growth factor (VEGF) is a member of the platelet-derived growth factor family and is a specific mitogen for vascular endothelial cells, fibroblasts, and epithelial cells. The most important role of VEGF is to stimulate angiogenesis by increasing vascular permeability and endothelial cell proliferation, and to induce degradation of basement membrane and endothelial cell migration in wound healing [17].
The present study aimed to compare the efficacy of topical treatment with HP and PHT on second-degree burn wound healing with ultrastructural and histopathological evaluations and also to determine the possible effects of PHT and HP treatment on VEGF TGF-β expression with immunohistochemical analysis in a Wistar rat model.
Material and Methods
Animals
The study protocol was approved by the Animal Ethics Committee of Kahramanmaras Sutcu Imam University (number 2011/4) and adhered to the National Institutes of Health Guidelines for the Use of Experimental Animals. Twenty male Wistar rats (200–220 g) were housed in individual cages in a temperature-controlled room with alternating 12-h light–dark cycles, and acclimatized for 1 week before the study commenced. Animals were allowed free access to water and rat chow. Food was withheld for 12 h before the operation, with water provided ad libitum during this period. Each rat was weighed and anesthetized with ketamine (50 mg/kg) intramuscularly. Then, the dorsum was shaved and second-degree burns were inflicted on an area of the dorsal region. Burns were generated with a special metallic device, made of stainless steel, which was cone-shaped, with a diameter of 1 cm, and equipped with a control thermometer. The burn injury was produced by laying a boiling water-heated metal stick on the skin for 10 sec. We used 20 animals that were divided into 3 groups: control, PHT treatment, and HP treatment groups; these were composed of 4, 8, and 8 animals each, respectively.
Treatment protocols
PHT Group Lesions: Burn wounds in Group I lesions were treated with PHT. For the preparation of 3% PHT cream, 3 g of phenytoin powder (D4505 Sigma 5,5-Diphenylhydantoin sodium salt ≥99%, Sigma Aldrich) was incorporated with 100 g of simple ointment base. Then, it was applied over the wound surface of Group 1 rats.
HP Group Lesions: Burn wounds in Group II lesions were treated with HP. For the preparation of 0.25% HP cream, 25 mg of dry Hypericin (H26425, Alfa Aesar) was added to 10 g of simple ointment base and was stirred until a homogeneous mixture was obtained. Then, it was applied over the wound surface of Group 2 rats.
Control (C) Group Lesions: Burn wounds in Group III lesions were given saline. Treatment was started in all groups on the first day after burn injury and cream was applied twice daily (8:00 AM and 8:00 PM) for 21 days. The cream was spread directly onto the burn wound, and the burn wound area was covered completely. The cream (PHT/HP) stuck to the burn wound, and no extra coverage was needed to confirm the presence of the gel on the wound. At the end of 21st day, the rats were anesthetized with 100 mg/kg ketamine i.m. and tissue samples were removed.
Histopathological study
Immuno-histopathologic and electron microscopic follow-up procedures were used for the skin samples taken from each group on the 3rd, 7th, 14th and 21st days. All biopsy specimens were 3-mm punched. Biopsies are divided into 2 halves for ultrastructural studies and histopathological examinations. The biopsy specimens were fixed in 10% formaldehyde, embedded in paraffin, and then sectioned and stained using Hematoxylin-Eosin (H&E) and Masson’s trichrome staining techniques. The histopathologic examination was done under an Olympus optical microscope and was performed by 2 pathologists blinded to the experiment groups. Inflammation, re-epithelialization, neovascularization, occurrence of granulation tissue, and collagen accumulation were evaluated in these specimens according to the scoring system used for histopathologic examination, as previously described [18]. The scoring is made as follows: Inflammation: acute (polymorphonuclear leukocytes), chronic (lymphocytes), and mixed. During this evaluation, inflammatory cells less than 25% were mild, 25–50% were moderate, and more than 50% were intense presence. Re-epithelialization covers of 1–25% mild, 25–50% moderate, 50–100% intense presence of the wound. Granulation tissue, characterized by the presence of fibroblasts, myofibroblasts, and neovascularization, and amount of collagen fiber (according to Masson’s trichrome staining, intensity was decided according to density of blue color on light microscopy). These parameters were evaluated as − = absent; + = mild; ++ = moderate, and +++ = intense presence.
Ultrastructural changes in the epidermal regeneration were examined using a transmission electron microscope (TEM 911 Carl Zeiss). For histological examination, all tissues were fixed in a phosphate-buffered solution containing 2.5% glutaraldehyde (Agar Scientific LTD, Cambridge, UK) for 3 h; then they were post-fixed in 1% osmium tetraoxide (OsO4) (Taab, England, UK) and dehydrated in a series of graded alcohols (25%, 50%, 75%, 95%, and absolute alcohol). After passing through propylene oxide, the specimens were embedded in Araldyte CY 212 (Agar Scientific LTD, Cambridge, UK), DDSA (2-dodecenyl succinic anhydride) (Agar Scientific LTD, Cambridge, UK), BDMA (benzyldimethyl amine) (Agar Scientific LTD, Cambridge, UK) and dibutylpytalate (Agar Scientific LTD, Cambridge, UK), and polymerized at 56°C in an incubator for 48 h. Semi-thin sections were cut and stained with toluidine blue and examined with a light microscope (Nikon, Tokyo, Japan). Ultra-thin sections including the skin were cut and stained with uranyl acetate (Taab, England, UK) and lead citrate (Fluka Steinheim, Switzerland). These sections were examined with a LEO 906E transmission electron microscope (TEM) (Oberkochen, Germany).
Immunohistochemical study
Tissue sections (3 μm) were prepared from formalin-fixed, paraffin-embedded tissue. After that, the slides were deparaffinized with no antigen retrieval step. The endogenous peroxidase activity was blocked by incubation with 0.3% H2O2 for 10 min (Novocastra, UK). The sections were blocked with 10% normal goat serum. Mouse monoclonal antibodies recognizing human TGF-β (1:150 dilution, clone TGFβ17, Novocastra, UK) and rabbit polyclonal anti-humanVEGF (1:100 dilution, clone FFPE, Biogenex, USA) were applied as primary antibodies at room temperature for 30 min. The slides were incubated with a secondary antibody (rabbit-anti-mouse IgG, Novocastra, UK) for 8 min at room temperature, DAB Chromogen (Novocastra, UK) and counter-stained with hematoxylin for 5 min (Novocastra, UK), and then were cleared and mounted. The areas of highest protein expression evident at low-power scanning were taken for analysis. Staining was considered negative only after careful examination of the entire tissue section under high power. A semi-quantitative grading system was used to compare immunohistochemical staining intensities. Each biopsy field of staining was graded with the following scale: − (no staining), 1+ (weak staining), 2+ (moderate staining), and 3+ (strong staining) for TGF-β and VEGF [19].
Statistical analysis
All statistical analyses were done using “SPSS 12.0 for Windows”. All data were categorical variables, so the chi-squared test was used to compare the groups. P< 0.05 was accepted a statistically significant.
Results
Histopathological and immunohistochemical findings
On the 3rd day, the burn wounds progressed to necrotic eschars, and there were no measurable differences among the groups. Coagulation necrosis was seen throughout the entire thickness of the epidermis and in the superficial part of the dermis and adnexa. Re-epithelialization was not started in any of the groups. Acute inflammation was seen in all groups and it was more common in the control group than in the PHT and HP groups, but it was not statistically significant. New capillary formation (neovascularization) was observed in all groups, at a higher level in the PHT and HP groups than in the control group, but it was not statistically significant. More collagenation was seen in the PHT group than in the control and HP groups (P=0.00). However, granulation tissue was not observed in any of the groups. According to immunohistochemical examination, TGF-β values of the HP group were significantly greater than in the PHT and control groups, and VEGF values of the PHT group were significantly greater than in the HP and control groups (P<0.05). The comparison of histopathologic and immunohistochemical parameters at day 3 between experimental groups is presented in Table 1.
Table 1.
Comparison of all response between experimental groups at day 3, 7, 14 and 21 of study.
| PHT group | HP group | Control group | P | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| − | + | ++ | +++ | − | + | ++ | +++ | − | + | ++ | +++ | ||
|
3rd
day Re-epithelialization (%) |
100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 1.000 |
| Vascularization (%) | 12.5 | 87.5 | 0 | 0 | 0 | 100 | 0 | 0 | 50 | 50 | 0 | 0 | 0.071 |
| Granulation tissue (%) | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 1.000 |
| Collagen content (%) | 0 | 0 | 37.5 | 62.5 | 100 | 0 | 0 | 0 | 0 | 100 | 0 | 0 | 0.000* |
| VEGF (%) | 0 | 0 | 75 | 25 | 0 | 25 | 75 | 0 | 0 | 100 | 0 | 0 | 0.005* |
| TGF-β1 (%) | 0 | 75 | 25 | 0 | 0 | 25 | 75 | 0 | 100 | 0 | 0 | 0 | 0.000* |
|
7th
day Re-epithelialization (%) |
75 | 25 | 0 | 0 | 71.4 | 28.6 | 0 | 0 | 75 | 25 | 0 | 0 | 0.402 |
| Vascularization (%) | 12.5 | 0 | 75 | 12.5 | 57.1 | 0 | 42.9 | 0 | 0 | 50 | 50 | 0 | 0.032* |
| Granulation tissue (%) | 25 | 75 | 0 | 0 | 71.4 | 28.6 | 0 | 0 | 25 | 75 | 0 | 0 | 0.142 |
| Collagen content (%) | 12.5 | 0 | 0 | 87.5 | 0 | 28.6 | 0 | 71.4 | 0 | 0 | 0 | 100 | 0.280 |
| VEGF (%) | 25 | 0 | 50 | 25 | 57.1 | 0 | 0 | 42.9 | 0 | 0 | 100 | 0 | 0.028* |
| TGF-β1 (%) | 25 | 12.5 | 50 | 12.5 | 57.1 | 0 | 0 | 42.9 | 0 | 0 | 100 | 0 | 0.041* |
|
14th
day Re-epithelialization (%) |
25 | 0 | 0 | 75 | 14.3 | 14.3 | 0 | 71.4 | 75 | 25 | 0 | 0 | 0.098 |
| Vascularization (%) | 0 | 0 | 0 | 100 | 0 | 0 | 28.6 | 71.4 | 0 | 0 | 0 | 100 | 0.147 |
| Granulation tissue (%) | 12.5 | 0 | 87.5 | 0 | 28.6 | 28.6 | 42.9 | 0 | 0 | 0 | 100 | 0 | 0.176 |
| Collagen content (%) | 0 | 0 | 0 | 100 | 0 | 85.7 | 14.3 | 0 | 0 | 100 | 0 | 0 | 0.000* |
| VEGF (%) | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 100 | 0 | 0.000* |
| TGF-β1 (%) | 0 | 0 | 0 | 100 | 0 | 0 | 28.6 | 71.4 | 0 | 0 | 100 | 0 | 0.002* |
|
21st
day Re-epithelialization (%) |
37.5 | 0 | 0 | 62.5 | 57.1 | 0 | 0 | 42.9 | 75 | 0 | 0 | 25 | 0.451 |
| Vascularization (%) | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 100 | 1.000 |
| Granulation tissue (%) | 0 | 0 | 100 | 0 | 28.6 | 0 | 71.4 | 0 | 0 | 25 | 75 | 0 | 0.108 |
| Collagen content (%) | 0 | 87.5 | 12.5 | 0 | 0 | 100 | 0 | 0 | 0 | 25 | 75 | 0 | 0.000* |
| VEGF (%) | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 100 | 1.000 |
| TGF-β1 (%) | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 100 | 0 | 0.000* |
Statistically significant.
On the 7th day, new epithelia were visible at the infundibulum of the pilous folliculi for all groups. The severity of inflammation was lowest in the PHT group (P=0.03). There were massive and progressively increased areas of angiogenic capillaries in the burn wound bed area. Increased neovascularization was observed in the PHT group compared to the HP and control groups (Figure 1A1, PHT group; 1B1, HP group, and 1C1 Control group) (P=0.03). More collagenation was seen in all groups (Figure 1A2, 1B2, 1C2). The wound bed area was completely occupied by immature granulation tissue in all groups. Granulation tissue in the HP group was limited to burn wound bed area, while it extended up to the upper layers of the dermis in the PHT group. TGF-β and VEGF immunoreactivities were stronger in treatment groups compared to the control group (Figure 1A3, 1B3, 1C3 and 1A4, 1B4, 1C4) (P=0.02, P=0.04). The comparison of histopathologic and immunohistochemical parameters at day 7 between experimental groups is presented in Table 1.
Figure 1.
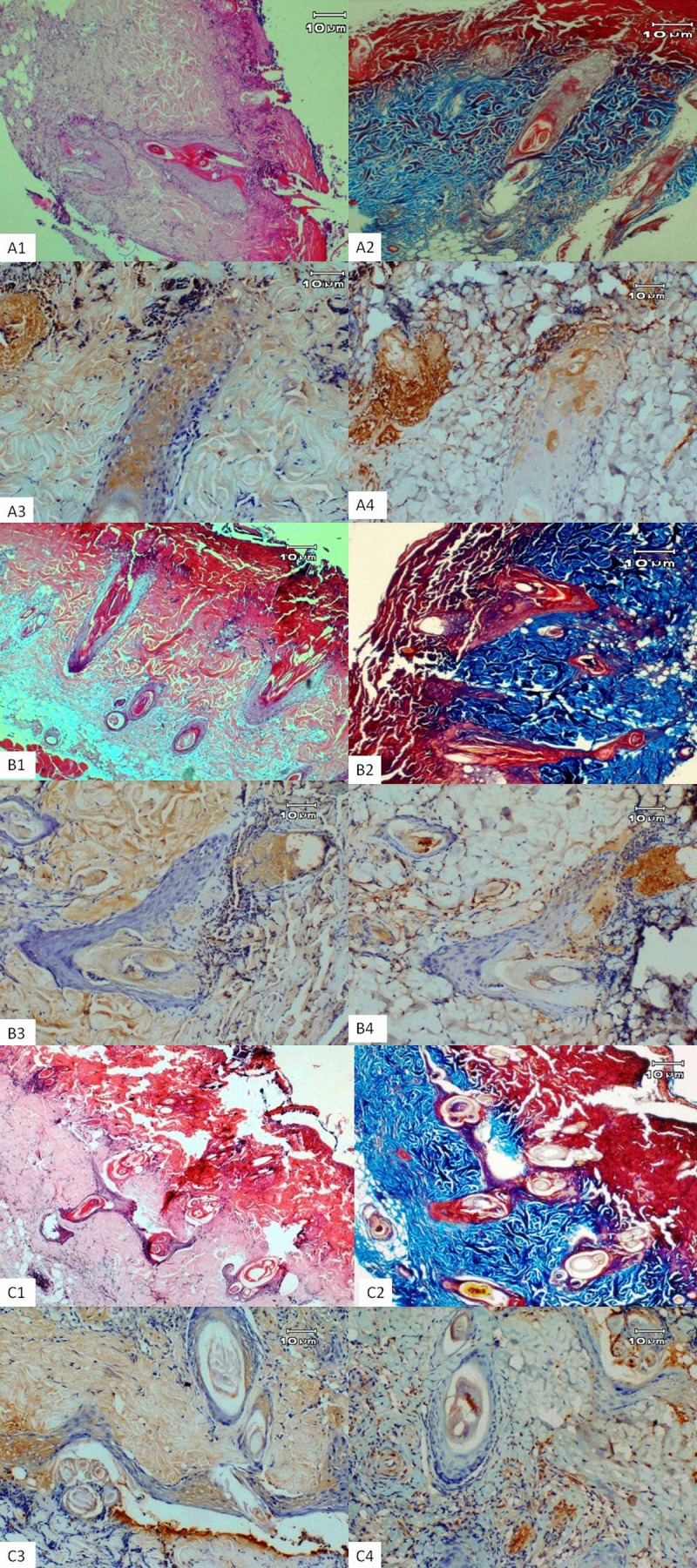
The microscopic image of the burnt skin wounds on day 7: PHT group (A); HP group (B); Control group (C), H&E stain: (A1, B1 and C1) ×40. Masson’s trichrome stain: (A2, B2 and C2) ×40. Immunohistochemical staining of TGF-β and VEGF, respectively (A3–A4, B3–B4, C3–C4) ×100. New epithelium showing at the infundibulum of the pilous folliculi for all groups
On the 14th day, the epidermal layer was seen with 3–4 layers of granulomatous cells in the PHT and HP groups. Hair follicles and adnexa improved to a greater extent in the HP group compared to the PHT group. Re-epithelialization scores were significantly greater in the PHT and HP groups than in the control group (Figure 2A1, PHT group; 2B1, HP group, and 2C1 Control group) (P=0.04).
Figure 2.
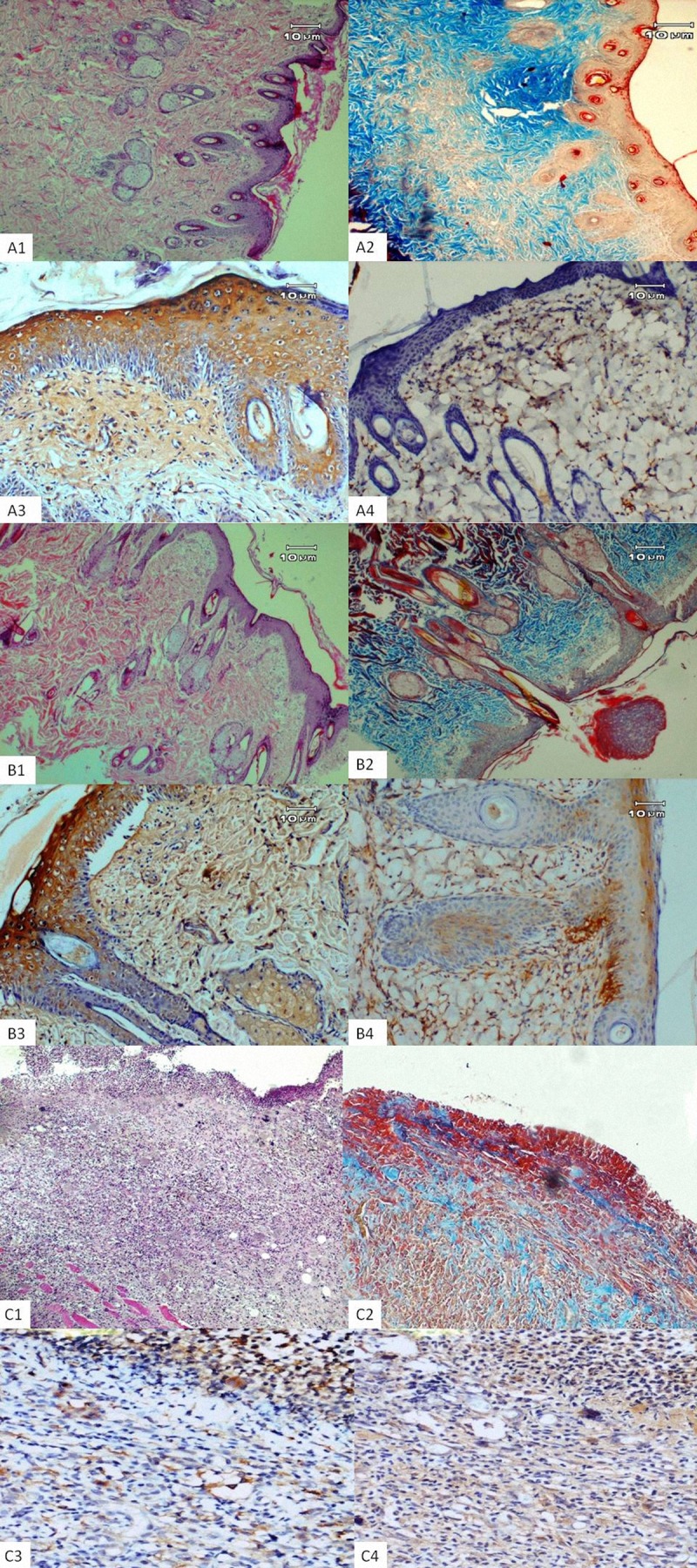
The microscopic image of the burnt skin wounds on day 14: PHT group (A); HP group (B); Control group (C), H&E stain: (A1, B1 and C1) ×40. Masson’s trichrome stain: (A2, B2 and C2) ×40. Immunohistochemical staining of TGF-β and VEGF, respectively (A3–A4, B3–B4, C3–C4) ×100. Epidermal layer and hair follicles, adnexa are seen in PHT and HP groups. However, at the wound surface, granulation tissue and abundant inflammatory infiltrate persist for the control group.
According to day 14 TEM microscopic findings of the PHT treatment group, the basement membrane was composed of an osmiophilic band showing some degree of undulation and sporadic fibrin deposits with irregular shape. The nuclei in the basal cells had slight edema, with a regular nuclear membrane and diffuse chromatin. The intercellular edema was pronounced in the basal cell layer, indicating that the desmosome protrusions were cut off. In the upper layer of the stratum spinosum, the intercellular edema was highly characteristic, and the cell processes appeared to be almost normal. Stratum granulosum was observed to contain Odland bodies. Nearer the surface, the cells were flattened and contained stretched tonofilament bundles (Figure 3A, 3B). In the HP treatment group, the stratum spinosum in the cell boundaries could be distinguished properly and desmosomal adhesions and tonofilaments were denser compared to the PHT group; the stratum spinosum and squamous cells in the superficial layer had less intracellular fluid compared to those in the PHT group. The stratum granulosum of the nucleus was regular and cytoplasmic with small spherical keratohyalin granules (Figure 3C). The severity of inflammation was lowest in the PHT group compared to other groups, as on day 7 (P=0.01). There was no significant difference in neovascularization scores between the groups. Collagen content of he PHT group was significantly higher than other groups (Figure 2A2, 2B2, 2C2) (P=0.00). The wound bed area was completely occupied by mature granulation tissue in all groups. The granulation tissue score was lower in HP and PHT groups compared to the control group. Again, VEGF and TGF-β immunoreactivities were stronger in the treatment groups than in the control group (Figure 2A3, 2B3, 2C3 and 2A4, 2B4, 2C4) (P=0.00). The comparison of histopathologic and immunohistochemical parameters at day 14 among experimental groups is presented in Table 1.
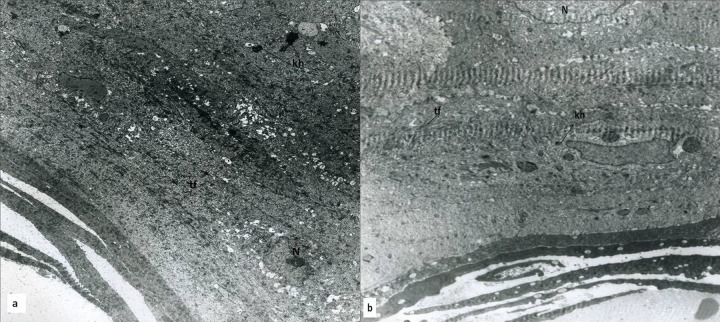
Figure 3.

(A) PHT group at day 14 of the treatment, the basement membrane of epidermis in a 14-day-old wound composed of an osmophilic band showing some degree of undulation and intercellular light edema (is), sporadic fibrin (f) depositions of irregular shape (original magnification ×2156). (B) In the upper layer of the stratum granulosum showing Odland bodies (OB) and tonofibril-poor cytoplasm (tf) (N: Nucleus, original magnification ×1670). (C) HP group at day 14 of the treatment, this micrograph shows that stratum spinosum in the cell boundaries could be distinguished properly and desmosomal adhesions (hd) and bundles of tonofilaments (tf), which tend to be aggregated in parallel strands, and less intracellular fluid, are more uniform compared to Figure 3B (original magnification ×1670).
On the 21st day, the epidermal layer and dermis were more mature in the treatment groups. The control group exhibited a wide area of ulcerations containing fibrinous exudates and inflammatory cells, a mild degree of inflammation, and neovascularization with congestions in the dermis, which indicates that the healing had not been completed. Re-epithelialization scores were greater in the PHT group than in the HP and control groups, but the results were not statistically significant (Figure 4A1, PHT group; 4B1, HP group, and 4C1 Control group). According to day 21 TEM microscopic findings of the PHT (Figure 5A) and HP (Figure 5B) treatment groups, the basal cells and the basement membrane had a normal consistency. The stratum spinosum layer had intracellular edema and desmosomal attachments were normal in appearance. The line of squamous cells was of regular appearance. The stratum granulosum also had observable keratohyalin granules, which were found to be the closest to normal skin. Intracellular fluid was reduced considerably compared to day 14. Adhesion units and tonofilaments were also close to normal.
Figure 4.
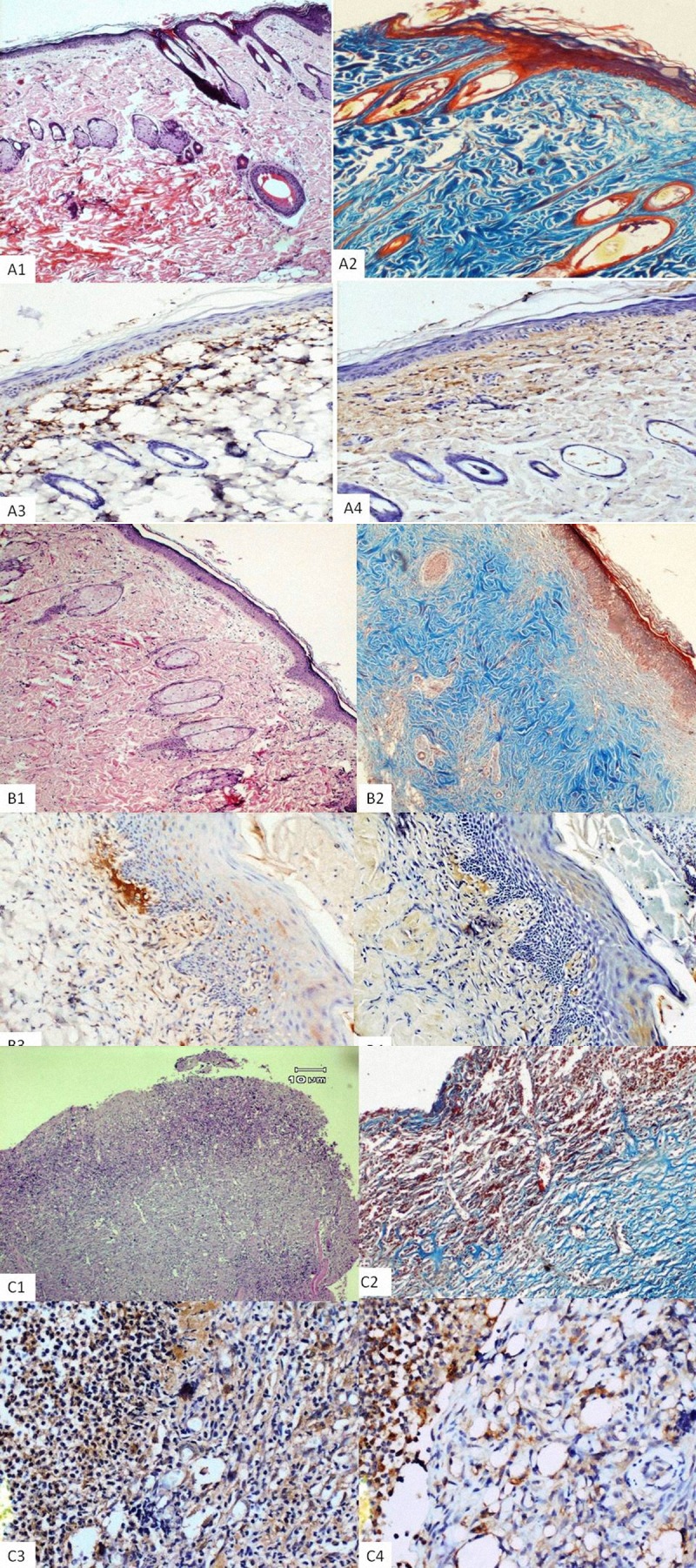
The microscopic image of the burnt skin wounds, on day 21: PHT group (A); HP group (B); control group (C), H&E stain: (A1, B1, and C1) ×40. Masson’s trichrome stain: (A2, B2 and C2) ×40. Immunohistochemical staining of TGF-β and VEGF, respectively (A3–4, B3–4, C3–C4) ×100. A relatively well-shuffled granulation tissue and inflammatory infiltrate of the control group at day 21 of treatment can be seen.
Figure 5.
(A) On day 21 after PHT treatment, in the corneum cells there are nuclear residues (n) and remaining keratohyalin granules (kh) (original magnification ×2156). (B) On day 21 after HP treatment, the lines of squamous cells were of regular appearance (original magnification ×1670).
Chronic inflammation was lower in the PHT group than in the other groups and acute-chronic inflammation was only observed in the control group (P=0.00). Neovascularization scores of all groups were higher. Collagen content of treatment groups was significantly lower than in the control group (Figure 4A2, 4B2, 4C2) (P=0.00). TGF-β immunoreactivities were stronger in the treatment group than in the control group (Figure 4A3, 4B3, 4C3) (P=0.00), but VEGF immunoreactivities were similar in all groups (Figure 4A4, 4B4, 4C4).
The comparison of histopathologic and immunohistochemical parameters on day 21 between experimental groups is presented in Table 1.
Discussion
Therapy for second-degree burn wounds today mainly focuses on various therapeutic agents [3]. It has been known that HP and PHT promote burn wound healing, but which one is more effective on wound healing has not been studied until now. This study seems to be the first published research dealing with the comparison of HP and PHT effects on burn wound healing.
HP is known to be effective in wound healing [17]. Our results also showed that higher hypericin content has a positive effect on second degree burn wound healing. Rao et al. reported that when using topical Hypericum perforatum and Calendula (another wound-healing herb) in treatment of incision wounds, re-epithelialization occurred after 15 days with HP treatment and by 16.5 days with Calendula treatment [20]. Meena et al. investigated the burn healing effect of PHT wounds based on pathological analyses; they reported a re-epithelialization score of 13.66±0.12 days, compared to 19.00±0.68 with standard silver sulphodiazine and 22.66±0.66 days in the control group [21]. In our study, obvious microscopic re-epithelialization was observed in all groups on day 7. On day 14, rapid regeneration keratohyalin granules and Odland bodies in the HP group was recorded. It was more successfully formed in the superficial layer of the epidermis in the HP group compared to the PHT group. The stage of epidermal repair was approximately 2 days earlier in the HP group than in the PHT group. Histopathological evaluation was not statistically significant in the scores of re-epithelialization. Our results could support the idea that HP is more potent for superficial burn wound treatment, as reported elsewhere [3]. On day 21 of our study, we showed with light microscopy that the re-epithelialization process in animals treated with PHT was a little better than in those that received HP. However, in the ultrastructural evaluation, regenerated epithelium was similar to normal epithelium in morphology compared to that in control group epithelium.
A number of clinical studies have indicated that PHT decreases the bacterial load of wounds [12]. Topical phenytoin was reported to eliminate Staphylococcus aureus, Escherichia coli, Klebsiella spp., and Pseudomonas spp. from wounds within 7–10 days [23]. The findings of Suntar et al. show that Hypericum perforatum cream formulation had bactericidal and candicidal activities [8]. According to the literature, HP has antiviral rather than antibacterial efficiency [24]. In our study, we found infection in the majority of control group subjects, but not in the treatment group subjects.
The formation of well-vascularized granulation tissue in the wound bed is a prerequisite for wound healing. Granulation tissue provides a substratum for epidermal cells to migrate and cover the wound, but the progressive proliferation of fibroblasts and continued accumulation of collagen is also seen. Collagen is one of the most dominant extracellular matrix proteins in granulation tissue. It assists the wound and plays an important role in homeostasis [13,25]. A number of clinical trials have provided evidence of an increased rate of repair with reduced edema and inflammation and the enhanced formation of well-vascularized granulation tissue following the topical application of PHT to cutaneous wounds [26]. In a rat model study of skin wound healing by Habibipour et al., PHT-treated wounds showed a significant increase in collagen deposition and neovascularization and accelerated healing of both open and closed wound [27]. Suntar et al. reported that HP had no effect on the proliferation capacity of fibroblasts and new vessel formation [7]. In our study, collagen content was significantly higher on days 3 and 14 in the PHT group than in others, and more blood vessels were seen in the granulation tissue of the PHT group compared to the HP and control groups on day 7. Ultrastructurally, epidermal edema was more common in the PHT group compared to the HP group. It may be concluded that edema in the PHT group may be related to vascular leakage due to higher rates of angiogenesis [22]. This result indicates that PHT is probably a rich source of the angiogenesis modulators that are commonly required for wound healing. We have mentioned the anti-inflammatory effect of PHT above. In our study, inflammation scores on days 7, 14, and 21 were lower in the PHT group compared to the HP and control groups. We believe that this anti-inflammatory effect contributes to the recovery effect of PHT.
VEGF and TGF-β factors have been suggested to be the most important of the growth factor families involved in the wound healing process [28]. Our data revealed that HP and PHT groups had a significant increase in VEGF and TGF-β expression in the burn wound healing area compared to the control group on all days. Therefore, it could be proposed that both VEGF and TGF-β could cause those tissue alterations by promoting HP and PHT on the burn wound healing effect, which enhanced the epithelialization, fibroblast proliferation, collagen deposition, and angiogenesis. TGF-β has been shown to have important implications in all phases of wound healing. The primary effect of TGF-β on wound re-epithelialization is well known [29]. Additionally, TGF-β has been shown in animal models to stimulate the deposition of collagen and fibrosis development [30]. Tredget et al. suggested that the overexpression of TGF-β1 speeds the rate of wound closure in partial-thickness wounds by promoting keratinocyte migration. However, the overexpression of TGF-β1 on full-thickness wounds slows the rate of wound re-epithelialization [31]. Our data revealed that TGF-β expression was increased significantly in the HP group compared to the PHT group on day 3. The earliest and highest levels of TGF-β protein expression may be involved in the mechanism of HP improving the re-epithelialization wound healing of burns. This is indirectly supported by the TGF-β enhanced proliferation and migration of keratinocytes, fibroblasts, and endothelial cells in HP-treated wounds. On days 7, 14, and 21, TGF-β expression was observed in the newly-formed collagen areas and inflammatory granulation tissue in the PHT group. This may support the hypothesis that increased collagen was mediated by TGF-β in the PHT treatment group. Swamy et al. showed that the mechanism of action of PHT in wound healing in vivo has also been mediated partly via PDGF and TGF-β receptors [11].
Since VEGF is a highly mitogenic and angiogenic factor, the histopathologic findings demonstrating the increased number of blood vessels following PHT treatment were consistent with the increased expression of VEGF. On day 3, VEGF was significantly increased in the PHT group, which strongly indicates that PHT treatment promoted VEGF production and action in the wound regions. The added effect of increased TGF-β on subsequent days indicates that these 2 factors contributed to the wound-healing activity of PHT in a correlated manner. In a study by Turan et al. on wound healing, immunohistochemical expression of VGF was higher in the rats treated with PHT, which is consistent with the findings of our study [23].
Conclusions
In this experimental study, we compared the effects of HP and PHT on the burn wound healing process. Delayed burn wound healing was observed histopathologically following the use of saline. Topical application of HP can promote re-epithelialization in burn wounds and shortens the wound healing time of superficial burns. PHT, on the other hand, contributes to healing by increasing vascularized granulation tissue and collagen synthesis through re-epithelialization. This indicates that PHT may be more effective than HP in the treatment of deep wounds with tissue defects. VEGF and TGF-β could cause those tissue alterations by promoting the PHT wound healing effect to a greater extent than HP. Additional studies will be aimed towards better understanding of the mechanism of action of HP and PHT on burn wound healing.
Acknowledgements
The authors would like to thank to pharmacist Levent Akgul for preparing the cream formulations.
Footnotes
Source of support: This study was supported by a research grant from Kahramanmaras Sutcu Imam University (2011/5-3M)
References
- 1.Monstrey S, Hoeksema H, Verbelen J, et al. Assessment of burn depth and burn wound healing potential. Burns. 2008;34:761–69. doi: 10.1016/j.burns.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Değim Z, Çelebi N, Alemdaroğlu C, et al. Evaluation of chitosan gel containing liposome-loaded epidermal growth factor on burn wound healing. Int Wound J. 2011;8:343–54. doi: 10.1111/j.1742-481X.2011.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.dos Tavares Pereira DS, Lima-Ribeiro MH, de Pontes-Filho NT, et al. Development of animal model for studying deep second-degree thermal burns. J Biomed Biotechnol. 2012;2012:460841. doi: 10.1155/2012/460841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laforet K, Woodbury GM, Sibbald RG. Wound bed preparation and complementary and alternative medicine. Adv Skin Wound Care. 2011;24:226–36. doi: 10.1097/01.ASW.0000397896.10380.5a. [DOI] [PubMed] [Google Scholar]
- 5.Wills RB, Bone K, Morgan M. Herbal products: active constituents, modes of action and quality control. Nutr Res Rev. 2000;13:47–77. doi: 10.1079/095442200108729007. [DOI] [PubMed] [Google Scholar]
- 6.Saddiqe Z, Naeem I, Maimoona A. A review of the antibacterial activity of Hypericum perforatum L. J Ethnopharmacol. 2010;131:511–21. doi: 10.1016/j.jep.2010.07.034. [DOI] [PubMed] [Google Scholar]
- 7.Süntar IP, Akkol EK, Yilmazer D, et al. Investigations on the in vivo wound healing potential of Hypericum perforatum L. J Ethnopharmacol. 2010;127:468–77. doi: 10.1016/j.jep.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Süntar I, Akkol EK, Keleş H, et al. A novel wound healing ointment: a formulation of Hypericum perforatum oil and sage and oregano essential oils based on traditional Turkish knowledge. J Ethnopharmacol. 2011;134:89–96. doi: 10.1016/j.jep.2010.11.061. [DOI] [PubMed] [Google Scholar]
- 9.Schempp CM, Winghofer B, Lüdtke R, et al. Topical application of St John’s wort (Hypericum perforatum L) and of its metabolite hyperforin inhibits the allostimulatory capacity of epidermal cells. Br J Dermatol. 2000;142:979–84. doi: 10.1046/j.1365-2133.2000.03482.x. [DOI] [PubMed] [Google Scholar]
- 10.Linde K. St. John’s wort – an overview. Forsch Komplementmed. 2009;16:146–45. doi: 10.1159/000209290. [DOI] [PubMed] [Google Scholar]
- 11.Swamy SM, Tan P, Zhu YZ, et al. Role of phenytoin in wound healing: microarray analysis of early transcriptional responses in human dermal fibroblasts. Biochem Biophys Res Commun. 2004;314:661–66. doi: 10.1016/j.bbrc.2003.12.146. [DOI] [PubMed] [Google Scholar]
- 12.Shaw J, Hughes CM, Lagan KM, et al. The use of topical phenytoin in wound healing: a systematic review. Br J Dermatol. 2007;157:997–1004. doi: 10.1111/j.1365-2133.2007.08160.x. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Chen J, Kirsner R. Pathophysiology of acute wound healing. Clin Dermatol. 2007;25:9–18. doi: 10.1016/j.clindermatol.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Barrientos S, Stojadinovic O, Golinko MS, et al. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 15.Faler BJ, Macsata RA, Plummer D, et al. Transforming growth factor-β and wound healing. Perspect Vasc Surg Endovasc Ther. 2006;18:55–62. doi: 10.1177/153100350601800123. [DOI] [PubMed] [Google Scholar]
- 16.Kane CJM, Hebda PA, Mansbridge JN, Hanawalt PC. Direct evidence for spatial and temporal regulation of transforming growth factor-beta1 expression during cutaneous wound healing. J Cell Physiol. 1991;148:157–73. doi: 10.1002/jcp.1041480119. [DOI] [PubMed] [Google Scholar]
- 17.Bao P, Kodra A, Tomic-Canic M, et al. The role of vascular endothelial growth factor in wound healing. J Surg Res. 2009;153:347–58. doi: 10.1016/j.jss.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oliveira PC, Pinheiro AL, de Castro IC, et al. Evaluation of the effects of polarized light (λ400–200 nm) on the healing of third-degree burns in induced diabetic and non-diabetic rats. Photomed Laser. 2011;29:619–25. doi: 10.1089/pho.2010.2914. [DOI] [PubMed] [Google Scholar]
- 19.Ermertcan AT, Inan S, Ozturkcan S, et al. Comparison of the effects of collagenase and extract of Centella asiatica in an experimental model of wound healing: an immunohistochemical and histopathological study. Wound Repair Regen. 2008;16:674–81. doi: 10.1111/j.1524-475X.2008.00417.x. [DOI] [PubMed] [Google Scholar]
- 20.Rao SG, Udupa AL, Rao G, et al. Wound healing activity of Calendula officinalis and Hypericum: two homeopathic drugs promoting wound healing in rats. Fitoterapia. 1991;62:508–10. [Google Scholar]
- 21.Meena K, Mohan AV, Sharath B, et al. Effect of topical phenytoin on burn wound healing in rats. Indian J Exp Biol. 2011;49:56–59. [PubMed] [Google Scholar]
- 22.Eriksson G. Regeneration of epidermis after second degree burns. A light and electron microscopic study on clinical burns. Scand J Plast Reconstr Surg. 1972;6:83–92. doi: 10.3109/02844317209036706. [DOI] [PubMed] [Google Scholar]
- 23.Turan M, Saraydýn SU, Bulut HE, et al. Do vascular endothelial growth factor and basic fibroblast growth factor promote phenytoin’s wound healing effect in rat? An immunohistochemical and histopathologic study. Dermatol Surg. 2004;30:1303–9. doi: 10.1111/j.1524-4725.2004.30404.x. [DOI] [PubMed] [Google Scholar]
- 24.Panossian AG, Gabrielian E, Manvelian V, et al. Immunosuppressive effects of hypericin on stimulated human leukocytes: inhibition of the arachidonic acid release, leukotriene B4 and interleukin-1 alpha production, and activation of nitric oxide formation. Phytomedicine. 1996;3:19–28. doi: 10.1016/S0944-7113(96)80005-5. [DOI] [PubMed] [Google Scholar]
- 25.Broughton G, II, Janis JE, Attinger CE. Wound healing: an overview. Plast Reconstr Surg. 2006;117:1e-S–32e-S. doi: 10.1097/01.prs.0000222562.60260.f9. [DOI] [PubMed] [Google Scholar]
- 26.Masgrau-peya E, Lacour M, Salomon D. Topical phenytoin accelerates healing in epidermolysis bullosa simplex. Dermatology. 1995;190:254. doi: 10.1159/000246708. [DOI] [PubMed] [Google Scholar]
- 27.Habibipour S, Oswald TM, Zhang F, et al. Effect of sodium diphenylhydantoin on skin wound healing in rats. Plast Reconstr Surg. 2003;112:1620–27. doi: 10.1097/01.PRS.0000086773.96319.DA. [DOI] [PubMed] [Google Scholar]
- 28.Kane CJM, Hebda PA, Mansbridge JN, Hanawalt PC. Direct evidence for spatial and temporal regulation of transforming growth factor-beta1 expression during cutaneous wound healing. J Cell Physiol. 1991;148:157–73. doi: 10.1002/jcp.1041480119. [DOI] [PubMed] [Google Scholar]
- 29.Faler BJ, Macsata RA, Plummer D, et al. Transforming growth factor-beta and wound healing. Perspect Vasc Surg Endovasc Ther. 2006;18:55–62. doi: 10.1177/153100350601800123. [DOI] [PubMed] [Google Scholar]
- 30.Lynch SE, Colvin RB, Antoniades HN. Growth factors in wound healing. Single and synergistic effects on partial thickness porcine skin wounds. J Clin Invest. 1989;84:640–46. doi: 10.1172/JCI114210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tredget EB, Demare J, Chandran G, et al. Transforming growth factor-beta and its effect on re-epithelialization of partial-thickness ear wounds in transgenic mice. Wound Repair Regen. 2005;13:61–67. doi: 10.1111/j.1067-1927.2005.130108.x. [DOI] [PubMed] [Google Scholar]