Abstract
Background
Thoracolumbar vertebral metastasis (TVM) affects a large number of cancer patients. However, safe and effective palliative care remains controversial. The aim of the present study was to investigate the safety and efficacy of minimally invasive image-guided radiofrequency ablation (RFA) with percutaneous kyphoplasty (PKP) for TVM treatment.
Material/Methods
A retrospective study of 26 patients (mean age: 59.31±11.62 years) was conducted, including 38 vertebral metastases at T11, T12, L1, L2, L3, L4, L5, and S1 with abundant blood vessels. Patients underwent RFA with PKP (4–6 min, 95±5°C, 150 W, effective electrode area of 1.5–2.0 cm) under general anesthesia from February 2005 to January 2009. Electrodes were inserted into the lesions and pre- and post-operative visual analog scale (VAS) scores and X-rays were collected on day 3, week 1, and months 1, 3, and 6. Tumor recurrence and pain level were also evaluated. Safety assessment was conducted based on complications and adverse events. The mean follow-up time was 8.4±2.1 months.
Results
A mean of 2.69±0.93 ablation was performed per patient. The ablation procedure required a mean of 15.08±4.64 min, while the injection of bone cement required a mean of 6.73±0.83 min, for a mean total operating time of 47.77±7.13 min. Postoperative VAS scores were significantly lower on day 3, week 1, and months 1, 3, and 6 (P<0.01), without any complications or tumor recurrence.
Conclusions
Image-guided RFA with PKP was safe and effective for TVM treatment when used with careful consideration of bone cement volume/viscosity, injection location, and temperature.
MeSH Keywords: Catheter Ablation, Neoplasm Metastasis - therapy, Spinal Neoplasms
Background
More than two-thirds of all cancer patients will develop bone metastases [1], and thoracolumbar spinal vertebrae are more commonly affected than any other bone [2]. In the United States alone, more than 350,000 cases of spinal metastasis are reported each year due to prostate, breast, kidney, lung, and thyroid cancers [3,4]. After invading the spinal bone, growth factors that stimulate osteoblastic and osteolytic activities are up-regulated, resulting in a cycle of bone destruction and local cancer cell proliferation that can cause symptomatic pain, mechanic instability, neurological dysfunction, sphincter control failure, hypercalcemia, and pathological fractures [1]. Furthermore, recent improvements in cancer therapy have improved survival in many cancer types, and spinal metastasis occurrence is expected to rise dramatically over the next decade with this increased life expectancy [5]. Thus, there is an urgent need for improved treatments for spinal metastases.
Recent radiosurgical techniques improved primary and adjunctive treatment of spinal metastases [6], but no technique has yet been demonstrated to dramatically improve life expectancy in these patients [7]; however, skillful medical management and advanced interventions have improved their quality of life and reduced postoperative morbidity [8]. These improvements are largely due to the wide modern implementation of palliative and conservative intervention strategies, including radiation therapy, chemotherapy, and hormonal interventions [8]. Notably, these treatments promote affected vertebra ossification, thus alleviating spinal compression and reducing the occurrence of pathological fractures [9]. Conventionally, most practitioners think that laminectomy is the only approach for spinal surgery of cancer metastases, but many minimally invasive techniques for spinal surgery have recently become available, including endoscopy, kyphoplasty or vertebroplasty, and stereotactic radiosurgery [10,11]. While each technique is extensively reported alone, few clinical studies have documented the efficacy and safety of combinations of these methods for treating spinal metastases.
Real-time image-guided radiofrequency ablation (RFA) is a minimally invasive treatment recently developed for treating vertebral tumors, using image-guided positioning of an electrode in the lesion to kill malignant cells percutaneously with minimal damage to adjacent healthy tissues [12]. However, RFA does not alleviate nerve function or prevent pathologic fracture [12]. Consequently, RFA has recently been combined with percutaneous vertebroplasty (PVP), which involves direct injection of bone cement into the vertebra under fluoroscopic guidance, thus reconstructing the mechanical stability of the vertebral body and additionally removing remaining malignant cell or damaged nerve endings during the heated hardening process [13,14]. However, the potentially serious complications that can be caused by extravasation of cement into foraminal, epidural, and dural spaces or paravertebral veins has limited its wide implementation [15]. Percutaneous kyphoplasty (PKP) using balloon dilatation can be used to reduce the rate of cement leakage, making these procedures safer and more effective [16].
Thus, it is hypothetically feasible to combine RFA and PKP to alleviate symptoms from vertebral tumors and to restore vertebral height with reduced risk [17,18]. The aim of the present retrospective study was to review the efficacy and safety of this procedure in clinical thoracolumbar vertebral metastatic patients treated at a single center. These findings may provide a basis for further study of combined RFA and PKP for spinal metastases.
Material and Methods
Study design
We performed a retrospective study of 26 patients (M: F, 12: 14; mean age 59.31±11.62 years, range 32–75 years) was conducted, including 38 vertebral metastases at T11, T12, L1, L2, L3, L4, L5, and S1 (n=4, 7, 5, 8, 2, 7, 2, and 3, respectively), all with abundant blood vessels. Patients underwent image-guided RFA with PKP under general anesthesia from February 2005 to January 2009 at the Department of orthopedics, Shanghai 10th People’s Hospital, Tongji University School of Medicine. The study protocol was approved by the Ethics Committee of Tongji University (China), and all patients provided written informed consent for participation.
Patients
Inclusion criteria were: 1) diagnosed with unresectable malignant vertebral metastasis according to the criteria provided by Tokuhashi [19]; 2) sought treatment for untreatable pain that severely affected quality of life; 3) metastatic lesion caused by pathologic fractures on X-ray images; and 4) symptoms that were not effectively alleviated by routine chemotherapy, radiation therapy, or other treatments.
Exclusion criteria were: 1) unable to tolerate surgery; 2) allergy to required anesthetics or antibiotics; 3) signs of infection at the surgical site or localized skin infection; or 4) vertebral fracture lines extending or crossing the vertebral posterior margin, vertebral bone destruction or incompletion, osteoblastic tumors, or spinal cord or nerve compression.
Preoperative preparation
Patients underwent electrocardiography (ECG), bleeding and clotting assessment, spinal anteroposterior, 3-dimensional (3D) vertebra reconstruction, lateral X-ray imaging, and scanning with computed tomography (CT), magnetic resonance imaging (MRI), and systemic radionuclide bone scan. Patients aged >65 years additionally completed heart and lung function assessments. Tumor location, size, and proximity to adjacent tissues/organs were examined, and anatomical examination of the pedicle was conducted. Appropriate electrode type, puncture site, and needle insertion angle were selected using these parameters, as previously described [20], and based on the operator’s judgment. In case of abnormal, large, multiple, or irregularly shaped bone metastases, multiple needle punctures were required to reach the ablation range sufficient to achieve an effective 2-puncture radius overlap of 30–50%, as previously described [21]. Preoperative digital subtraction angiography (DSA) and local vascular gel foam embolization were performed when necessary.
Tumor biopsy
Tumor biopsy was performed in all patients. Patients were placed in the prone position under general anesthesia. The skin around the incision was disinfected with Anerdian (Shanghai Likang Disinfectant Hi-tech Co., Shanghai, China). Unilateral or bilateral transpedicular needle insertion was conducted using CT or C-arm X-ray equipment for visualization (operative field diameter=30 cm). Using this path, a bone biopsy needle (Cook Medical, Bloomington, IN, USA) was inserted. Lesion biopsy specimens were sampled and pathologically examined using routine methods.
Surgical intervention
Intravenous steroids were administered to all patients before surgery to prevent anaphylactic reactions to bone cement. Immediately following biopsy, an appropriately sized ablation electrode was inserted along the same path as the biopsy needle. The size of the electrode was selected according to the desired effect diameter (2.0, 2.5, or 4.0 cm). The cathode plate was affixed to the posterolateral lower limb and fully opened. Ablation was conducted continuously for 4–6 minutes at 95±5°C and 150 W, generating an effective diameter of 1.5–2.0 cm. The ablation electrode was inserted into the lesion center or adjusted for multiple ablation. The electrode was then slowly removed, and thermocoagulation was performed on the percutaneous puncture to prevent bleeding. The procedure was repeated, if necessary, based on lesion scope and size. When possible, ablation was extended 0.5–1.0 cm outside the tumor boundary, avoiding nerves, blood vessels, and vital organs (Figure 1A, 1B).
Figure 1.
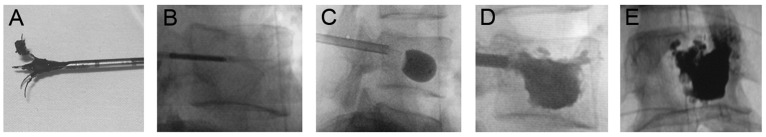
RFA and PVP procedure. (A) RFA umbrella electrode; (B) vertebral lesions treated with RFA; (C) vertebral formation by balloon; (D) bone cement injected into T11; (E) bone cement injected into L1.
A unilateral or bilateral balloon was implanted and inflated with a dedicated balloon pressurizer (Medtronic, Fridley, MN, USA) (Figure 1C). Excessive dilation was avoided to prevent vertebral re-fracture and balloon rupture. A 3–5 ml (~1.5 ml inside tube) volume of bone cement (Tianjin Synthetic Material research Institute, Tianjin, China) was slowly pushed into the vertebral cavity, filling it completely while avoiding leakage (Figure 1D, 1E). Then, the injector and cannula were removed, avoiding cement tail formation. The incision in the percutaneous puncture site was sutured using 1 to 2 Ethicon sutures (Johnson & Johnson, New Brunswick, NJ, USA).
Postsurgical care
Immediately after surgery, antibiotic treatments (cefradine IV, 2 g/12 h for 3 days) were administered for 3 days to prevent infection, and glucocorticoids (dexamethasone IV, 10 mg/day for 3 days, 5 mg/day on the fourth day) were administered as needed to prevent swelling. Local pain was treated with non-steroidal anti-inflammatory drugs (NSAIDs) or narcotics, including indomethacin (100 mg) suppository and bucinnazine (100 mg) intramuscular injection. Six h after disappearance of the anesthetic effect, a bolus dose of non-steroidal anti-inflammatory drug (celecoxib 200 mg or fenbid 300 mg) was administered to ease pain at the puncture site.
Follow-up
Patients were followed up for a minimum of 3 months, and up to 18 months. Clinical assessments were conducted postoperatively at day 3, week 1, and months 1, 3, and 6. At each examination, visual analogue scales (VAS) scores were self-reported and recorded on a 0–10 point scale to indicate pain. Outcomes of tumor recurrence and pain levels were examined at each follow-up visit.
Efficacy assessments
Stability outcomes were assessed by postsurgical X-ray to measure vertebral height.
Safety assessments
Complications and adverse events (AEs) at each follow-up visit were used to assess safety.
Statistical analysis
SPSS 11.0 (SPSS, Inc., Chicago, IL, USA) was used for all data analysis. Pre- and postoperative VAS scores were compared using paired t-tests. P-values <0.05 were considered significant (P<0.05).
Results
Patient demographic, clinical and surgical characteristics
The number of men and women (12:14) was similar. Patients were aged 35–75 years (mean of 59.31±11.62). All patients completed a minimum of 3 months of follow-up (mean of 8.31±3.87 months). A total of 12 (46.2%) patients received analgesics and no significant differences were observed in patients who did or did not received analgesics (P>0.05). Most patients (12/26, 46.2%) had 2 lesions, 13 (50.0%) patients had only 1 lesion, and only 1 (3.8%) patient had 3 lesions. The average number of lesions was 1.68±0.56 per patient. Spinal metastatic lesions were secondary to prostate cancer (5/26, 19.2%), breast cancer (6/26, 23.1%), lung cancer (3/26, 11.5%), sacrum cancer (2/26, 7.7%), kidney cancer (2/26, 7.7%), lymphoma (2/26, 7.7%), esophageal cancer (1/26, 3.9%), adenocarcinoma (1/26, 3.9%), liver cancer (2/26, 7.7%), mesenchymal cancer (1/26, 3.9%), and thyroid cancer (1/26, 3.9%) (Table 1).
Table 1.
Patient general information and preoperative and postoperative VAS score.
| Patient | Age | Gender | Primary cancer | Follow-up (months) | Lesion no. | Location | Preoperative VAS | Postoperative VAS | Analgesic | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3d | 1w | 1m | 3m | 6m | |||||||||
| 1 | 62 | M | Prostate | 18 | 3 | L2, L4 | 6 | 5 | 2 | 2 | 2 | 3 | U |
| 2 | 54 | M | Liver | 6 | 2 | T11, T12 | 7 | 6 | 3 | 3 | 2 | 3 | U |
| 3 | 43 | F | Lymphoma | 3 | 2 | L1 | 9 | 7 | 4 | 4 | 3 | 2 | N |
| 4 | 41 | F | Mesenchymal malignant tumor | 4 | 2 | L1 | 6 | 6 | 2 | 3 | 2 | 3 | N |
| 5 | 70 | M | Prostate | 9 | 2 | L2 | 9 | 8 | 4 | 3 | 3 | 2 | U |
| 6 | 37 | M | Sacrum | 8 | 2 | L4, S1 | 7 | 7 | 2 | 2 | 2 | 3 | N |
| 7 | 75 | M | Sacrum | 7 | 2 | L5, s1 | 8 | 8 | 3 | 3 | 2 | 2 | U |
| 8 | 32 | F | Breast | 6 | 2 | L4, L5 | 7 | 8 | 2 | 2 | 2 | 3 | U |
| 9 | 64 | F | Breast | 8 | 2 | T11, L1 | 7 | 7 | 5 | 4 | 3 | 2 | U |
| 10 | 62 | F | Lung | 7 | 1 | T12, L2 | 8 | 6 | 5 | 3 | 4 | 3 | N |
| 11 | 57 | M | Prostate | 5 | 1 | L2 | 8 | 8 | 4 | 5 | 3 | 4 | U |
| 12 | 68 | F | Thyroid | 14 | 2 | T11 | 8 | 7 | 5 | 3 | 3 | 4 | N |
| 13 | 69 | M | Prostate | 13 | 1 | L4 | 7 | 5 | 5 | 3 | 2 | 2 | U |
| 14 | 46 | F | Breast | 12 | 1 | L3 | 8 | 6 | 7 | 4 | 4 | 3 | N |
| 15 | 58 | F | Breast | 10 | 1 | T12 | 6 | 5 | 4 | 3 | 2 | 2 | U |
| 16 | 59 | F | Esophagus | 8 | 2 | L2, L3 | 6 | 7 | 6 | 5 | 3 | 4 | U |
| 17 | 63 | M | Adenocarcinoma | 13 | 2 | L4 | 7 | 6 | 6 | 5 | 2 | 2 | U |
| 18 | 58 | M | Kidney | 7 | 1 | S1 | 8 | 7 | 5 | 4 | 4 | 4 | N |
| 19 | 65 | F | Kidney | 5 | 2 | L1, L2 | 8 | 8 | 6 | 3 | 4 | 3 | U |
| 20 | 64 | F | Breast | 7 | 2 | T12 | 9 | 5 | 6 | 3 | 2 | 2 | N |
| 21 | 55 | F | Lymphoma | 4 | 2 | L4 | 7 | 6 | 5 | 5 | 3 | 3 | N |
| 22 | 80 | M | Prostate | 9 | 1 | T12 | 8 | 7 | 6 | 4 | 4 | 2 | N |
| 23 | 57 | M | Liver | 5 | 2 | L2, T12 | 10 | 8 | 5 | 5 | 2 | 3 | N |
| 24 | 69 | M | Lung | 6 | 1 | L4 | 8 | 6 | 6 | 4 | 3 | 5 | N |
| 25 | 70 | F | Lung | 6 | 2 | T11, L2 | 8 | 7 | 6 | 4 | 2 | 3 | U |
| 26 | 64 | F | Breast | 16 | 1 | T12, L1 | 10 | 6 | 7 | 5 | 4 | 5 | N |
| Total | 59.31± 11.62 | 12:14* | 8.31 ±3.87 | 1.68 ±0.56 | 7.69± 1.12 | 6.62± 1.02 | 4.65± 1.55 | 3.62± 0.98 | 2.77± 0.82 | 2.96± 0.92 | 12:14* | ||
D – day; w – week; m – month; N – not used; U – used.
M: F or U: N.
A mean of 2.69±0.93 (2~5) ablation was performed per patient. The ablation procedure required a mean of 15.08±4.64 (10~25) min, while the injection of bone cement required a mean of 6.73±0.83 (5~8) min, for a mean total operating time of 47.77±7.13 (36~63) min. No balloon leakage was observed in any patient.
Postoperative efficacy outcomes
RFA with PKP treatment was successful in all patients (n=26), and no need for secondary surgery was reported at any time point during follow-up. Minor pain was reported during postsurgical week 1, which was generally effectively alleviated by oral analgesics. No pain or other symptoms were reported after week 1. Changes in patient X-rays before and after treatment indicated improved postoperative vertebral height in all patients, indicating good clinical efficacy (Figures 2 and 3). No recurrence was reported.
Figure 2.
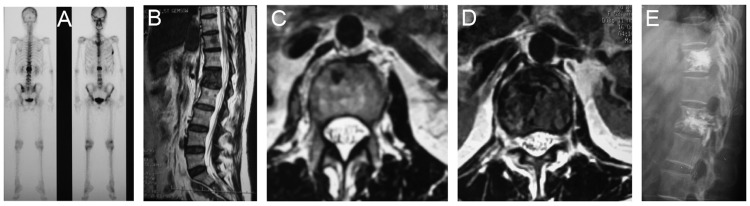
T11 and L1 metastasis from breast cancer before and after RFA+PKP treatment. (A) ECT scan; (B) sagittal MRI; (C) T11 axial MRI; (D) L1 axial MRI; (E) X-ray showing the vertebrae after RFA+PKP, more precisely the bone cement in the inferior articular process of the vertebra.
Figure 3.
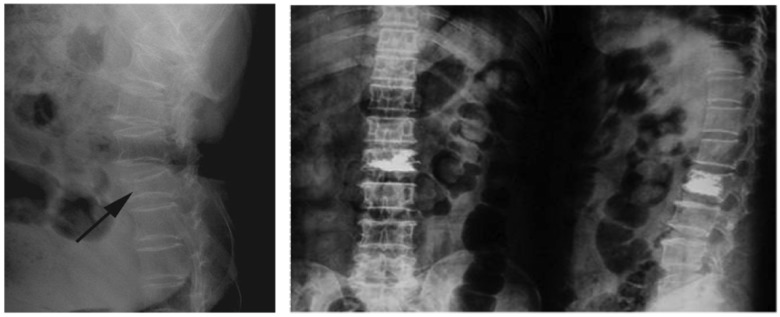
Imaging of thoracolumbar metastatic tumors representing changes in vertebral height before and after treatment.
Postoperative safety outcomes
No treatment-related complications or adverse events occurred during or after the intervention. No symptomatic nerve damage or deaths were reported.
VAS scores indicate clinical efficacy
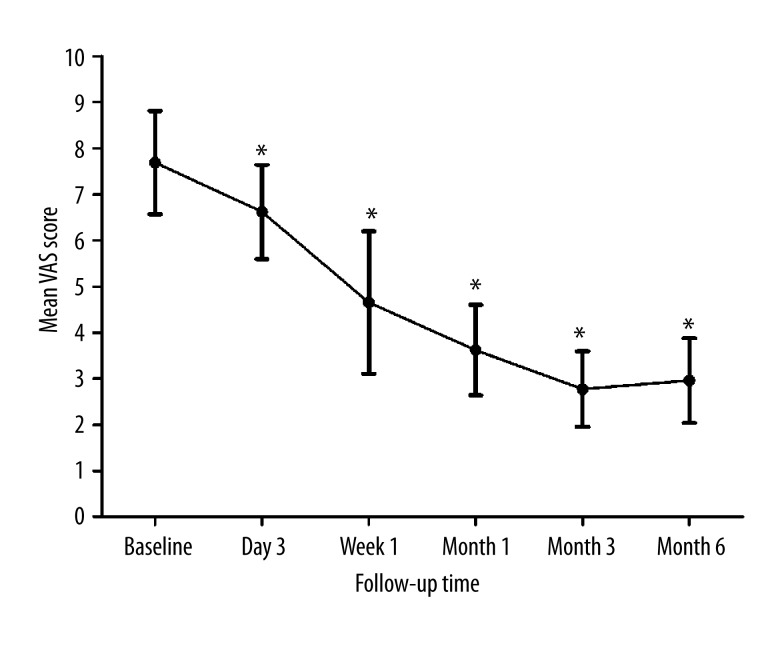
Mean VAS scores at baseline, 3 days, at 1 week, and at months 1, 3, and 6 were 7.69±1.12, 6.62±1.02, 4.65±1.55, 3.62±0.98, 2.77±0.82, and 2.96±0.92, respectively (Table 1, Figure 4). The highest postoperative VAS scores were observed at day 3, reaching as high as 10. The maximum VAS score by 1 week was 7, and no VAS score higher than 5 was reported at any subsequent times. Compared to the preoperative mean VAS score (7.69±1.12), VAS score was significantly decreased at all time points (P<0.01) (Figure 4), suggesting the relief of symptoms and effectiveness of the treatment of RFA combined with PKP.
Figure 4.
Changes in mean preoperative and postoperative VAS. * P<0.01 vs. baseline.
Discussion
The aim of the present retrospective study was to review the efficacy and safety of RFA with PKP in clinical thoracolumbar vertebral metastatic patients treated at a single center. An assessment of 26 patients with vertebral metastases treated with RFA with PKP revealed that this treatment approach had high efficacy and good safety. No major adverse events or complications were reported, and no recurrence or secondary surgical intervention occurred during follow-up. Furthermore, pain peaked at 3 days after RFA with PKP intervention, and was then relieved because no VAS score above 5 was reported by 6 months. All pain was effectively controlled using medication only. This preliminary study indicates that RFA with PKP is a feasible minimally invasive spinal approach for treatment of thoracolumbar vertebral metastasis patients, thus meriting further exploration for spinal metastases treatment.
In theory, RFA with PKP may be associated with similar complication risks as are other conventional vertebrae cementation techniques, which pose a real risk of serious complications and even death due to bone cement leakage [15]. Zhao et al. [22] suggested that a careful selection of patients, appropriate bone cement viscosity, proper bone cement amounts, and precise location of bone cement injections all contribute to successful operations. These parameters may account for the wide variation in success rates of PKP techniques among different studies [23], and appropriate selection of these parameters in the current study may have contributed to the good overall results. Thus, further study will be required to determine the most appropriate parameters and patient eligibility criteria for wide implementation of RFA with PKP.
The integrity of the vertebral posterior cortex provides an important safeguard to prevent spinal cord injury [23]. Implementation of RFA with PKP has been controversial due to the fear of adverse effects of spinal bone cement extraversion into this space [15]. Nakatsuka et al. [24] reported that RFA treatment of vertebral metastases produced notable complications in almost one-quarter of patients, including nerve damage and sciatica, and high postoperative pain. Furthermore, this study also reported that RFA could result in incomplete paraplegia and nerve root irritation immediately following surgery, though all symptoms were alleviated by rehabilitation therapy [24]. Conversely, the outcomes reported in the present study are much more positive. To achieve these good results, careful attention was paid to the positioning of the needle, with an optimal placement of no closer than 1 cm from any important nerves or blood vessels [25]. Additionally, it has been suggested that temperature ranges should be carefully controlled at 42°C, never exceeding 43°C [26]. Notably, this strategy allows the protective posterior cortex to form a natural thermal insulator, crucial for the protection of the structure around the vertebral body [27]. Thus, pending further large-scale investigation, we recommend these parameters.
In addition, no balloon leakage was observed in the present study, unlike previous studies. Garfin et al. [28] reported that a major complication of PKP procedures in spinal metastases was cement leakage from the balloon, compressing surrounding blood vessels, nerves, and vital organs, and they reported a balloon leakage rate of 5–8% with 2–3% of affected patients requiring additional decompression surgery. Thus, the present study employed a technique in which balloon inflation, bone cement injection volume, and procedural timing was carefully controlled during surgery to prevent posterior cortex injury to the vertebral body. Previously, it has also been reported that CT guidance could significantly improve PKP outcomes and reduce complications, including cement leakage [29]. Thus, the present study used these best-practice techniques, demonstrating that current limitations of PKP surgery may be due to technical failures by the operator rather than inappropriateness of the PKP intervention per se. In fact, it is possible that many more patients could be eligible for minimally invasive PKP intervention, as well as those who are currently treated with more invasive surgical alternatives, necessitating better standardisation of PKP and interventionist training.
In the present study, RFA with PKP rapidly and effectively alleviated the pain associated with vertebral compression due to spinal metastases in a variety of cancer types. However, no study has assessed the combination of these 2 procedures, making comparisons with previous studies difficult. Nevertheless, these findings are consistent with a report by Dupuy et al. [27] indicating the effectiveness of RFA treatment for easing pain in bone metastases patients. Additionally, a recent clinical study by Callstrom et al. [30] confirmed that RFA treatment was safe for patients with severe pain caused by bone metastases, and that it was able to significantly alleviate pain. While RFA is effective for treating local lesions in other areas of the body, greater consideration of the specific biological characteristics of the tumor are required for treating spinal metastasis to ensure maximum safety and efficacy. A previous meta-analysis of PKP studies showed that PKP was associated with reduced pain and improved functional outcomes; balloon PKP also improved vertebral height loss and spinal deformity [31]. Furthermore, the benefits of PKP were demonstrated to be superior to classical nonsurgical treatments of vertebral metastases [32]. As for classical surgical approaches such as radical resection, no studies have compared resection, PKP, and RFA in vertebral metastasis patients. However, some studies showed promising results of tumor curettage followed by stabilization using bone grafting or methylmethacrylate [33,34]. This approach provided outcomes that were comparable with the present studies, but postoperative complications were more common.
RFA intervention and RFA with PKP treatments improve the quality of life by alleviating symptoms of vertebral compression (palliative treatment), but do not actually cure cancer in the spine [35,36]. Additionally, the positive findings of this relatively small study may be attributable to other factors, such as patient selection and operator experience, which are difficult to quantify. Furthermore, it is unknown if metastases from different primary tumor types may affect the response to treatments. Therefore, future studies should focus on only 1 tumor type. Thus, further large-scale clinical studies will be required to confirm these findings. However, these results do provide a positive first step toward improving palliative care for spinal metastasis patients.
Conclusions
The present study demonstrated that RFA with PKP using CT-guided and balloon-inflated surgical spaces facilitated bone cement filling with no complications in 26 patients with thoracolumbar vertebral spinal metastases. Thus, RFA with PKP with the appropriate parameters of bone cement viscosity, needle positioning, bone cement volume, and temperature could alleviate the pain caused by tumors and restore vertebral height and stability. Thus, we recommend that this approach could be considered for treatment of spinal metastases, although further standardization of surgical methods and patient eligibility criteria are required to ensure a safe and effective treatment.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
Source of support: Departmental sources
References
- 1.Maccauro G, Spinelli MS, Mauro S, et al. Physiopathology of spine metastasis. Int J Surg Oncol. 2011;2011:107969. doi: 10.1155/2011/107969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abeloff MD, Armitage JO, Niederhuber JE, et al. Abeloff’s clinical oncology. Churchill Livingstone/Elsevier; Philadelphia: 2008. [Google Scholar]
- 3.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–93. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 4.Piccioli A, Capanna R. Linee Guida SIOT. 2008. Il Trattamento delle Metastasi Ossee. [in Italian] [Google Scholar]
- 5.Weber MH, Burch S, Buckley J, et al. Instability and impending instability of the thoracolumbar spine in patients with spinal metastases: a systematic review. Int J Oncol. 2011;38:5–12. [PubMed] [Google Scholar]
- 6.Sheehan JP, Shaffrey CI, Schlesinger D, et al. Radiosurgery in the treatment of spinal metastases: tumor control, survival, and quality of life after helical tomotherapy. Neurosurgery. 2009;65:1052–61. doi: 10.1227/01.NEU.0000359315.20268.73. discussion 61–62. [DOI] [PubMed] [Google Scholar]
- 7.Tse V, Berman SA. Spinal Metastasis Treatment and Management: Medical Care. MedScape. 2013 [Google Scholar]
- 8.Lee BH, Kim TH, Chong HS, et al. Prognostic factor analysis in patients with metastatic spine disease depending on surgery and conservative treatment: review of 577 cases. Ann Surg Oncol. 2013;20:40–46. doi: 10.1245/s10434-012-2644-4. [DOI] [PubMed] [Google Scholar]
- 9.Rasulova N, Lyubshin V, Djalalov F, et al. Strategy for bone metastases treatment in patients with impending cord compression or vertebral fractures: a pilot study. World J Nucl Med. 2011;10:14–19. doi: 10.4103/1450-1147.82114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klimo P, Jr, Schmidt MH. Surgical management of spinal metastases. Oncologist. 2004;9:188–96. doi: 10.1634/theoncologist.9-2-188. [DOI] [PubMed] [Google Scholar]
- 11.Yimin Y, Zhiwei R, Wei M, Jha R. Current status of percutaneous vertebroplasty and percutaneous kyphoplasty – a review. Med Sci Monit. 2014;20:826–36. doi: 10.12659/MSM.889479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goetz MP, Callstrom MR, Charboneau JW, et al. Percutaneous image-guided radiofrequency ablation of painful metastases involving bone: a multicenter study. J Clin Oncol. 2004;22:300–6. doi: 10.1200/JCO.2004.03.097. [DOI] [PubMed] [Google Scholar]
- 13.Togawa D, Lewandrowsky KU, McLain RF. Cancer in the Spine: Current Clinical Oncology. Springer; 2006. The pathophysiology of spinal metastases; pp. 17–23. [Google Scholar]
- 14.Mercadante S. Malignant bone pain: pathophysiology and treatment. Pain. 1997;69:1–18. doi: 10.1016/s0304-3959(96)03267-8. [DOI] [PubMed] [Google Scholar]
- 15.Al-Nakshabandi NA. Percutaneous vertebroplasty complications. Ann Saudi Med. 2011;31:294–97. doi: 10.4103/0256-4947.81542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ren H, Shen Y, Zhang YZ, et al. Correlative factor analysis on the complications resulting from cement leakage after percutaneous kyphoplasty in the treatment of osteoporotic vertebral compression fracture. J Spinal Disord Tech. 2010;23:e9–15. doi: 10.1097/BSD.0b013e3181c0cc94. [DOI] [PubMed] [Google Scholar]
- 17.Mendel E, Bourekas E, Gerszten P, Golan JD. Percutaneous techniques in the treatment of spine tumors: what are the diagnostic and therapeutic indications and outcomes? Spine (Phila Pa 1976) 2009;34:S93–100. doi: 10.1097/BRS.0b013e3181b77895. [DOI] [PubMed] [Google Scholar]
- 18.Masala S, Manenti G, Roselli M, et al. Percutaneous combined therapy for painful sternal metastases: a radiofrequency thermal ablation (RFTA) and cementoplasty protocol. Anticancer Res. 2007;27:4259–62. [PubMed] [Google Scholar]
- 19.Tokuhashi Y, Matsuzaki H, Oda H, et al. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976) 2005;30:2186–91. doi: 10.1097/01.brs.0000180401.06919.a5. [DOI] [PubMed] [Google Scholar]
- 20.Hanaoka T, Suyama K, Taguchi A, et al. Shifting of puncture site in the fossa ovalis during radiofrequency catheter ablation: intracardiac echocardiography-guided transseptal left heart catheterization. Jpn Heart J. 2003;44:673–80. doi: 10.1536/jhj.44.673. [DOI] [PubMed] [Google Scholar]
- 21.Jankharia B, Burute N. Percutaneous radiofrequency ablation for osteoid osteoma: How we do it. Indian J Radiol Imaging. 2009;19:36–42. doi: 10.4103/0971-3026.44523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao L, Wang L, Wang G, et al. Prevention and treatment of bone cement leakage in percutaneous kyphoplasty for osteoporotic vertebral body compression fracture [Chinese] Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2009;23:404–7. [PubMed] [Google Scholar]
- 23.Denaro V, Longo UG, Maffulli N, Denaro L. Vertebroplasty and kyphoplasty. Clin Cases Miner Bone Metab. 2009;6:125–30. [PMC free article] [PubMed] [Google Scholar]
- 24.Nakatsuka A, Yamakado K, Maeda M, et al. Radiofrequency ablation combined with bone cement injection for the treatment of bone malignancies. J Vasc Interv Radiol. 2004;15:707–12. doi: 10.1097/01.rvi.0000133507.40193.e4. [DOI] [PubMed] [Google Scholar]
- 25.Rosenthal DI, Hornicek FJ, Torriani M, et al. Osteoid osteoma: percutaneous treatment with radiofrequency energy. Radiology. 2003;229:171–75. doi: 10.1148/radiol.2291021053. [DOI] [PubMed] [Google Scholar]
- 26.Mannion RJ, Woolf CJ. Pain mechanisms and management: a central perspective. Clin J Pain. 2000;16:S144–56. doi: 10.1097/00002508-200009001-00006. [DOI] [PubMed] [Google Scholar]
- 27.Dupuy DE, Hong R, Oliver B, Goldberg SN. Radiofrequency ablation of spinal tumors: temperature distribution in the spinal canal. Am J Roentgenol. 2000;175:1263–66. doi: 10.2214/ajr.175.5.1751263. [DOI] [PubMed] [Google Scholar]
- 28.Garfin SR, Yuan HA, Reiley MA. New technologies in spine: kyphoplasty and vertebroplasty for the treatment of painful osteoporotic compression fractures. Spine (Phila Pa 1976) 2001;26:1511–15. doi: 10.1097/00007632-200107150-00002. [DOI] [PubMed] [Google Scholar]
- 29.Pizzoli AL, Brivio LR, Caudana R, Vittorini E. Percutaneous CT-guided vertebroplasty in the management of osteoporotic fractures and dorsolumbar metastases. Orthop Clin North Am. 2009;40:449–58. vii. doi: 10.1016/j.ocl.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Callstrom MR, Charboneau JW, Goetz MP, et al. Painful metastases involving bone: feasibility of percutaneous CT- and US-guided radio-frequency ablation. Radiology. 2002;224:87–97. doi: 10.1148/radiol.2241011613. [DOI] [PubMed] [Google Scholar]
- 31.Bouza C, Lopez-Cuadrado T, Cediel P, et al. Balloon kyphoplasty in malignant spinal fractures: a systematic review and meta-analysis. BMC Palliat Care. 2009;8:12. doi: 10.1186/1472-684X-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berenson J, Pflugmacher R, Jarzem P, et al. Balloon kyphoplasty versus non-surgical fracture management for treatment of painful vertebral body compression fractures in patients with cancer: a multicentre, randomised controlled trial. Lancet Oncol. 2011;12:225–35. doi: 10.1016/S1470-2045(11)70008-0. [DOI] [PubMed] [Google Scholar]
- 33.Sundaresan N, Sachdev VP, Holland JF, et al. Surgical treatment of spinal cord compression from epidural metastasis. J Clin Oncol. 1995;13:2330–35. doi: 10.1200/JCO.1995.13.9.2330. [DOI] [PubMed] [Google Scholar]
- 34.Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366:643–48. doi: 10.1016/S0140-6736(05)66954-1. [DOI] [PubMed] [Google Scholar]
- 35.Gronemeyer DH, Schirp S, Gevargez A. Image-guided radiofrequency ablation of spinal tumors: preliminary experience with an expandable array electrode. Cancer J. 2002;8:33–39. doi: 10.1097/00130404-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Callstrom MR, Charboneau JW, Goetz MP, et al. Image-guided ablation of painful metastatic bone tumors: a new and effective approach to a difficult problem. Skeletal Radiol. 2006;35:1–15. doi: 10.1007/s00256-005-0003-2. [DOI] [PubMed] [Google Scholar]