Abstract
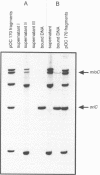
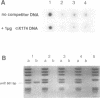
The 94 C-terminal amino acids of the initiator protein DnaA of Escherichia coli are required and sufficient for specific binding to the cognate DNA binding site. The binding domain contains two potential amphipathic alpha-helices and a third alpha-helix. It represents a new DNA binding motif so far not found in other DNA binding proteins. Temperature-sensitive mutations in the binding motif, dnaA204, dnaA205 and dnaA211, abolish DNA binding. In the solid-phase DNA binding assay, applicable to other DNA binding proteins, fusions of domains of DnaA protein to beta-galactosidase are reacted with biotinylated anti-beta-galactosidase antibody. These are coupled to streptavidin-coated magnetic beads. The DNA binding domain is able to selectively remove the DNA target (oriC) from the liquid phase. Alternatively, the DNA binding domain is fused to a peptide containing a target sequence which is naturally biotinylated in vivo in E.coli. This fusion protein can be coupled directly to streptavidin-coated magnetic beads. Homologies between DnaA protein and transcription factors of the NtrC family are discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beyersmann D., Messer W., Schlicht M. Mutants of Escherichia coli B-r defective in deoxyribonucleic acid initiation: dnaI, a new gene for replication. J Bacteriol. 1974 Jun;118(3):783–789. doi: 10.1128/jb.118.3.783-789.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramhill D., Kornberg A. Duplex opening by dnaA protein at novel sequences in initiation of replication at the origin of the E. coli chromosome. Cell. 1988 Mar 11;52(5):743–755. doi: 10.1016/0092-8674(88)90412-6. [DOI] [PubMed] [Google Scholar]
- Buhk H. J., Messer W. The replication origin region of Escherichia coli: nucleotide sequence and functional units. Gene. 1983 Oct;24(2-3):265–279. doi: 10.1016/0378-1119(83)90087-2. [DOI] [PubMed] [Google Scholar]
- Cronan J. E., Jr Biotination of proteins in vivo. A post-translational modification to label, purify, and study proteins. J Biol Chem. 1990 Jun 25;265(18):10327–10333. [PubMed] [Google Scholar]
- Crooke E., Thresher R., Hwang D. S., Griffith J., Kornberg A. Replicatively active complexes of DnaA protein and the Escherichia coli chromosomal origin observed in the electron microscope. J Mol Biol. 1993 Sep 5;233(1):16–24. doi: 10.1006/jmbi.1993.1481. [DOI] [PubMed] [Google Scholar]
- Dayhoff M. O., Barker W. C., Hunt L. T. Establishing homologies in protein sequences. Methods Enzymol. 1983;91:524–545. doi: 10.1016/s0076-6879(83)91049-2. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diederich L., Roth A., Messer W. A versatile plasmid vector system for the regulated expression of genes in Escherichia coli. Biotechniques. 1994 May;16(5):916–923. [PubMed] [Google Scholar]
- Ferré-D'Amaré A. R., Prendergast G. C., Ziff E. B., Burley S. K. Recognition by Max of its cognate DNA through a dimeric b/HLH/Z domain. Nature. 1993 May 6;363(6424):38–45. doi: 10.1038/363038a0. [DOI] [PubMed] [Google Scholar]
- Fujita M. Q., Yoshikawa H., Ogasawara N. Structure of the dnaA and DnaA-box region in the Mycoplasma capricolum chromosome: conservation and variations in the course of evolution. Gene. 1992 Jan 2;110(1):17–23. doi: 10.1016/0378-1119(92)90439-v. [DOI] [PubMed] [Google Scholar]
- Fuller R. S., Funnell B. E., Kornberg A. The dnaA protein complex with the E. coli chromosomal replication origin (oriC) and other DNA sites. Cell. 1984 Oct;38(3):889–900. doi: 10.1016/0092-8674(84)90284-8. [DOI] [PubMed] [Google Scholar]
- Funnell B. E., Baker T. A., Kornberg A. In vitro assembly of a prepriming complex at the origin of the Escherichia coli chromosome. J Biol Chem. 1987 Jul 25;262(21):10327–10334. [PubMed] [Google Scholar]
- Gille H., Messer W. Localized DNA melting and structural pertubations in the origin of replication, oriC, of Escherichia coli in vitro and in vivo. EMBO J. 1991 Jun;10(6):1579–1584. doi: 10.1002/j.1460-2075.1991.tb07678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen F. G., Koefoed S., Atlung T. Cloning and nucleotide sequence determination of twelve mutant dnaA genes of Escherichia coli. Mol Gen Genet. 1992 Jul;234(1):14–21. doi: 10.1007/BF00272340. [DOI] [PubMed] [Google Scholar]
- Koonin E. V. DnaC protein contains a modified ATP-binding motif and belongs to a novel family of ATPases including also DnaA. Nucleic Acids Res. 1992 Apr 25;20(8):1997–1997. doi: 10.1093/nar/20.8.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markmeyer P., Rühlmann A., Englisch U., Cramer F. The pAX plasmids: new gene-fusion vectors for sequencing, mutagenesis and expression of proteins in Escherichia coli. Gene. 1990 Sep 1;93(1):129–134. doi: 10.1016/0378-1119(90)90146-i. [DOI] [PubMed] [Google Scholar]
- Masai H., Nomura N., Arai K. The ABC-primosome. A novel priming system employing dnaA, dnaB, dnaC, and primase on a hairpin containing a dnaA box sequence. J Biol Chem. 1990 Sep 5;265(25):15134–15144. [PubMed] [Google Scholar]
- Messer W., Hartmann-Kühlein H., Langer U., Mahlow E., Roth A., Schaper S., Urmoneit B., Woelker B. The complex for replication initiation of Escherichia coli. Chromosoma. 1992;102(1 Suppl):S1–S6. doi: 10.1007/BF02451779. [DOI] [PubMed] [Google Scholar]
- Morett E., Segovia L. The sigma 54 bacterial enhancer-binding protein family: mechanism of action and phylogenetic relationship of their functional domains. J Bacteriol. 1993 Oct;175(19):6067–6074. doi: 10.1128/jb.175.19.6067-6074.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murre C., Bain G., van Dijk M. A., Engel I., Furnari B. A., Massari M. E., Matthews J. R., Quong M. W., Rivera R. R., Stuiver M. H. Structure and function of helix-loop-helix proteins. Biochim Biophys Acta. 1994 Jun 21;1218(2):129–135. doi: 10.1016/0167-4781(94)90001-9. [DOI] [PubMed] [Google Scholar]
- North A. K., Klose K. E., Stedman K. M., Kustu S. Prokaryotic enhancer-binding proteins reflect eukaryote-like modularity: the puzzle of nitrogen regulatory protein C. J Bacteriol. 1993 Jul;175(14):4267–4273. doi: 10.1128/jb.175.14.4267-4273.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada C. A., Marians K. J. Mechanism of DNA A protein-dependent pBR322 DNA replication. DNA A protein-mediated trans-strand loading of the DNA B protein at the origin of pBR322 DNA. J Biol Chem. 1991 Oct 5;266(28):18895–18906. [PubMed] [Google Scholar]
- Samols D., Thornton C. G., Murtif V. L., Kumar G. K., Haase F. C., Wood H. G. Evolutionary conservation among biotin enzymes. J Biol Chem. 1988 May 15;263(14):6461–6464. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer C., Messer W. DnaA protein/DNA interaction. Modulation of the recognition sequence. Mol Gen Genet. 1991 Apr;226(1-2):34–40. doi: 10.1007/BF00273584. [DOI] [PubMed] [Google Scholar]
- Schauzu M. A., Kücherer C., Kölling R., Messer W., Lother H. Transcripts within the replication origin, oriC, of Escherichia coli. Nucleic Acids Res. 1987 Mar 25;15(6):2479–2497. doi: 10.1093/nar/15.6.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seufert W., Messer W. DnaA protein binding to the plasmid origin region can substitute for primosome assembly during replication of pBR322 in vitro. Cell. 1987 Jan 16;48(1):73–78. doi: 10.1016/0092-8674(87)90357-6. [DOI] [PubMed] [Google Scholar]
- Skarstad K., Boye E. The initiator protein DnaA: evolution, properties and function. Biochim Biophys Acta. 1994 Mar 1;1217(2):111–130. doi: 10.1016/0167-4781(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Woelker B., Messer W. The structure of the initiation complex at the replication origin, oriC, of Escherichia coli. Nucleic Acids Res. 1993 Nov 11;21(22):5025–5033. doi: 10.1093/nar/21.22.5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa H., Ogasawara N. Structure and function of DnaA and the DnaA-box in eubacteria: evolutionary relationships of bacterial replication origins. Mol Microbiol. 1991 Nov;5(11):2589–2597. doi: 10.1111/j.1365-2958.1991.tb01967.x. [DOI] [PubMed] [Google Scholar]