Abstract
Background
Bariatric surgery is associated with improved cognition and it is possible that such improvements are found at extended follow-ups. We hypothesized that cognitive improvement would be maintained 3 years post-bariatric surgery.
Methods
Fifty bariatric patients were recruited from the Longitudinal Assessment of Bariatric Surgery parent project. Participants completed a computerized cognitive test battery to assess cognitive function at 12-weeks, 12-months, 24-months, and 36-months post-surgery.
Results
Repeated measures revealed main effects for attention, executive function, and memory. Attention improved up to 24-months and then slightly declined, though still fell within the average range at 36-months. Improvements in executive function reached its peak at 36-months post-surgery. Short-term improvements in memory were maintained at 36-months. No main effect emerged for language.
Conclusion
Bariatric surgery may lead to lasting improvements in cognition. Prospective studies with extended follow-ups (e.g., 10 years) should examine whether bariatric surgery can attenuate cognitive decline in severely obese persons.
Keywords: Bariatric surgery, obesity, cognitive function, weight loss
Introduction
Obesity has reached epidemic proportions, with up to 35.5% of adult men and 35.8% of adult women categorized as obese.1 It is well established that obesity leads to poor health (e.g., diabetes, hypertension, coronary artery disease)2 and outcomes (e.g., increased mortality and morbidity risk).3 Extant evidence also shows that obesity adversely affects the brain. For example, obesity has been linked with increased risk for neurological changes, including Alzheimer’s disease, vascular dementia, as well as brain atrophy.4,5 Indeed, obese persons also exhibit impairments on formal cognitive testing, including on tasks of attention, executive function, and memory.6,7
Bariatric surgery has become an increasingly popular and effective treatment option for weight loss among severely obese individuals.8,9 Bariatric surgery is associated with lower mortality and morbidity risk, decreased hospitalizations, and reduced need for medications.10–12 Recent work also shows that bariatric surgery is associated with cognitive improvement at 12 weeks and 24-months post-operatively, particularly in memory abilities.13,14
Despite these findings, the long-term impact of bariatric surgery on cognitive function remains poorly understood. This is unfortunate, as the acute improvements in cognitive function after surgery and the substantial weight loss may reduce the known cognitive decline in obese persons15 or even the risk of Alzheimer’s disease.4 The purpose of the current study was to determine the effects of bariatric surgery on cognitive function up to 3 years post-surgical intervention. Based on past studies, we hypothesized that bariatric surgery patients would exhibit improved cognitive function immediately following surgery and such improvements would be maintained at the long-term follow-up, including 36-months post-operatively. Exploratory analyses among a subsample also examined the hypothesis that cognitive benefits would last up to 4-years post-bariatric surgery.
Materials and Methods
Trial Design and Participants
A total of 50 consecutive bariatric patients were recruited into this multi-site prospective study examining the neurocognitive effects of bariatric surgery. All participants were part of the Longitudinal Assessment of Bariatric Surgery (LABS) parent project and were recruited from three LABS sites.16 Individuals participating in the parent project who were eligible for the current study were approached at the time of enrollment regarding this ancillary cognitive study. Greater than 80% of participants approached opted to enroll. For study inclusion, participants were required to be enrolled in LABS, between the ages of 20–70, and English-speaking. Exclusion criteria included history of neurological disorder or injury (e.g. dementia, stroke, seizures), moderate or severe head injury (defined as >10 minutes loss of consciousness),17 past or current history of severe psychiatric illness (e.g. schizophrenia, bipolar disorder), past or current history of alcohol or drug abuse (defined by DSM-IV criteria), history of a learning disorder or developmental disability (defined by DSM-IV criteria), or impaired sensory function that precluded cognitive testing (e.g. visual deficits preventing adequate perception of test stimuli) per participant report or examiner observation.
Medical history was obtained via medical record review from the LABS study as well as participant self-report. Within the sample, just 1 bariatric surgery patient underwent gastric banding procedure and thus no comparisons for type of surgery (Roux-en-Y gastric bypass vs. gastric banding) were conducted.
The present sample represents all individuals that have completed 36 months of follow-up data. Participants excluded as a result of attrition and/or subsequent missing data across time points were not different in terms of age (t(127) = .63, p = .53), baseline BMI (t(127) = .54, p = .59), or baseline cognitive function in attention (t(127) = .42, p = .68), executive function (t(127) = .14, p = .89), memory (t(127) = 1.14, p = .26), or language (t(127) = −.98, p = .33).
In addition, exploratory analyses also examined cognitive function 48-months post bariatric surgery. For these analyses, the sample size was reduced to 21 as a result of further participant attrition. Participants excluded as a result of incomplete data at 48-months did not differ in terms of age or baseline medical history of diabetes (χ2 (1, N = 50) = .49, p = .49) or sleep apnea (χ2 (1, N = 50) = 2.79, p = .10). However, participants excluded were more likely to have a diagnosis of hypertension at baseline (χ2 (1, N = 50) = 6.65, p = .01; 65.5% vs. 28.6%). See Table 1 for demographic and clinical characteristics.
Table 1.
Demographic and Medical Characteristics
| BASELINE CHARACTERISTICS | |
|---|---|
| N | 50 |
| Age, mean (SD) | 44.08 (10.76) |
| Female (%) | 92.0 |
| Body Mass Index, mean (SD) | 46.61 (5.27) |
| Hypertension (% yes) | 50.0 |
| Diabetes (% yes) | 24.0 |
| Sleep Apnea (% yes) | 32.0 |
| 36-MONTH FOLLOW-UP* | |
| Body Mass Index, mean (SD) N = 47 |
32.35 (6.57) |
| Hypertension (% yes) N = 44 |
26.0 |
| Diabetes (% yes) N = 44 |
18.0 |
| Sleep Apnea (% yes) N = 44 |
10.0 |
| 48-MONTH FOLLOW-UP | |
| Body Mass Index, mean (SD) N = 20 |
33.02 (6.27) |
| Hypertension (% yes) N = 19 |
19.0 |
| Diabetes (% yes) N = 19 |
19.0 |
| Sleep Apnea (% yes) N =19 |
4.8 |
Note.
Due to missing data at follow-up time-points sample sizes for 36-months and 48-months were reduced and the above values are based on complete data at the respective time point
Interventions and Clinical Follow-Up
All procedures were approved by the appropriate Institutional Review Boards prior to study onset. All participants provided written informed consent prior to study involvement. Bariatric surgery participants completed a series of self-report instruments and a computerized cognitive test battery at baseline (within 30 days prior to surgery), 12 weeks (± 5 days), 12 months (± 30 days), 24 months (± 30 days), 36 months (± 30 days), and 48 months (± 30 days) following surgery. Medical records were reviewed by research staff to corroborate and supplement self-report.
Outcomes
The Integneuro cognitive test battery assesses estimated premorbid intellectual abilities as well as performance in multiple cognitive domains (e.g., attention, executive Function, verbal memory) and can be completed in 45–60 minutes. It has excellent psychometric properties18,19 and has been shown to be sensitive to the effects of obesity in past work.20 Specific tests were categorized into attention, executive function, memory, and language domains and included:
Attention and Executive Function
Digit Span
This test assesses basic auditory attention and working memory. Participants are presented with a series of digits on the touch-screen, separated by a one-second interval. The subject is then immediately asked to enter the digits on a numeric keypad on the touch-screen. The number of digits in each sequence is gradually increased from 3 to 9, with two sequences at each level. The participants complete these same procedures in a backward sequence. Total digit span for both forwards and backwards served as the dependent variable.
Switching of Attention
This test is a computerized adaptation of the Trail Making Test and consists of two parts.21 Participants are asked to touch a series of 25 numbers in ascending order as quickly as possible. An array of 13 numbers (1–13) and 12 letters (A-L) is presented. Participants are asked to touch numbers and letters alternately in ascending order. The first part of this test assesses attention and psychomotor speed and the second part assesses executive function.
Verbal Interference
This task taps the ability to inhibit automatic and irrelevant responses and is similar to the Stroop Color Word Test.22 Participants are presented with colored words one at a time. Below each colored word is a response pad with the four possible words displayed in black and in fixed format. In the first part, the subject is required to the name of each word as quickly as possible, assessing attention. In the second part, the subject is required to name to the color of each word as quickly as possible, assessing executive function.
Maze Task
This task is a computerized adaptation of the Austin Maze23 and assesses executive function. Participants are presented with a grid (8×8 matrix) of circles and asked to identify the hidden path through the grid. Distinct auditory and visual cues are presented for correct and incorrect responses. The trial ends when the subject completed the maze twice without error or after 10 minutes has elapsed.
Memory
Verbal List-learning
Participants are read a list of 12 words a total of 4 times and asked to recall as many words as possible following each trial. Following presentation and recall of a distraction list, participants are asked to recall words from the original list. After a 20-minute filled delay, participants are again asked to recall target words. Finally, a recognition trial comprised of target words and foils is completed. Total learning, Long Delay Free Recall, and Recognition of these verbal list items were used to assess memory.
Language
Animal and Letter Fluency
This test asks individuals to generate words beginning with a given letter of the alphabet for 60 seconds. A different letter is used for each of the three trials. Finally, participants were then asked to generate as many animals as possible within 60 seconds.
Data Analyses
To facilitate clinical interpretation, all neuropsychological measures were transformed to T-scores (a distribution with a mean of 50 and standard deviation of 10) using normative data correcting for age, gender, and premorbid intelligence. Composite scores were computed for attention, executive function, memory, and language that consisted of the mean of the T-scores of neuropsychological measures within each cognitive domain. Consistent with clinical convention, a T-score ≤ 35 (1.5 SD below the mean) was reflective of cognitive impairment.
Repeated measures analysis of variance (ANOVA) was performed to determine change in cognitive function following bariatric surgery. Specifically, analyses examined changes in attention, executive function, memory, and language across the following time points: baseline, 12-weeks, 12-months, 24-months, and 36-months. Separate exploratory analyses were then conducted to examine cognitive function in each domain from baseline to 48-months post-bariatric surgery. These analyses were conducted separately because of reduced sample size due to participant attrition from 36- to 48-months post-bariatric surgery (see Participant section of the Methods). Repeated measures and bivariate correlation analyses also examined the possible effects of weight regain on cognitive function.
Results
Baseline and 36-Month BMI and Comorbid Medical Status
Based on conventional BMI categories, the current sample of bariatric surgery patients were classified as very severely obese at baseline Mean BMI = 46.61 (SD = 5.27)). However, the average BMI reduced to 32.35 (SD = 6.57) (i.e., moderately obese) at 36-months, which represents a significant decline (Λ = .13, F(1, 46) = 300.06, p < .001). A similar pattern emerged with comorbid medical status. Specifically, relative to baseline, significantly fewer participants had hypertension (χ2 (df = 1) = 4.49, p = .03) or diabetes (χ2 (df = 1) = 14.55, p < .001). See Table 1.
Baseline and 36-month Post-Operative Cognitive Function in Bariatric Surgery Patients
At baseline, when compared to normative data, cognitive test performance in the bariatric surgery patients fell within the low average range for memory and in the average range for all other cognitive domains. See Table 2. Comparison of individual test results reveals that bariatric surgery patients had clinically meaningful impairments cognitive function at baseline. When using a T-score cutoff of 35, 12.0% and 8.0% of patients showed impairments in memory and executive function. Impairments in attention and language were less common (4.0% of patients for both domains).
Table 2.
Neuropsychological Test Performance (T-scores) Following Bariatric Surgery (N = 50)
| Baseline M(SD) | 12-weeks M(SD) | 12-months M(SD) | 24-months M(SD) | 36-months M(SD) | 48-months* M(SD) | |
|---|---|---|---|---|---|---|
| Attention/Executive Function | ||||||
| Digit Span Total | 49.30(8.41) | 50.63(9.78) | 53.01(10.76) | 55.56(11.15) | 51.15(10.46) | 53.47(12.41) |
| SOA-A | 55.17(14.12) | 58.83(14.39) | 61.65(16.39) | 59.76(13.31) | 52.54(12.99) | 58.53(10.65) |
| SOA-B | 52.28 (17.80) | 58.42(12.69) | 57.68(15.09) | 58.80(13.44 | 58.48(13.02) | 61.90(11.43) |
| Verbal Interference Word | 51.98(11.45) | 54.10(12.95) | 56.09(15.31) | 59.15(11.42) | 45.75(19.10) | 50.41(11.50) |
| Verbal Interference Color/Word | 52.78 (11.84) | 60.98(13.81) | 60.54(12.38) | 64.60(11.21) | 65.49(11.06) | 67.81(12.79) |
| Maze Errors | 50.51(12.86) | 56.32(12.83) | 54.96(11.58) | 53.52(11.04) | 55.68(13.37) | 58.55(14.19) |
| Memory | ||||||
| Total Learning | 43.19(14.07) | 47.09(14.94) | 51.86(14.05) | 49.28(15.42) | 46.37(16.22) | 55.24(12.62) |
| LDFR | 46.78(11.80) | 50.59(13.40) | 56.21(10.08) | 51.72(12.87) | 51.49(13.98) | 56.80(12.77) |
| Recognition | 42.12(10.57) | 53.06(10.10) | 51.65(8.67) | 50.76(9.26) | 54.14(9.68) | 53.53(7.69) |
| Language | ||||||
| Verbal Fluency | 45.45(10.43) | 45.99(9.79) | 47.02(10.27) | 49.71(9.92) | 48.41(9.33) | 47.55(9.23) |
| Animal Fluency | 50.26(10.83) | 50.33(10.82) | 51.09(11.91) | 50.74(11.53) | 50.18(12.48) | 52.86(14.61) |
Note. Averages were based on complete data for each time point.
Abbreviations—SOA-A = Switching of Attention Number; SOA-B = Switching of Attention Letter Number; LDFR = Long Delay Free Recall
N = 21
At 36-month post-operative follow up, bariatric surgery patients cognitive test performance for all domains of cognitive function fell within the average range and prevalence of impairment was also less prevalent.
Cognitive Function Post Bariatric Surgery
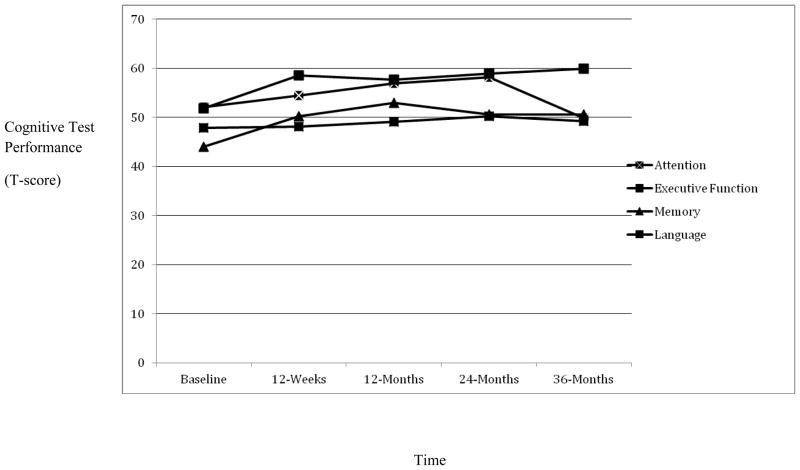
Figure 1 shows the trajectory of cognitive function for each domain from baseline to 36 months post-bariatric surgery. Repeated measures ANOVA examined changes in attention, executive function, memory, and language following bariatric surgery at 12-weeks, 12-months, 24-months, and 36-months. See Table 3. There was a significant main effect for attention across the five time points (Λ = .32, F(4, 46) = 24.15, p < .001). As shown in Figure 1, attention generally improved up until 24-months and then declined between 24-months and 36-months post bariatric surgery (Λ = .40, F(1, 49) = 72.38, p < .001). Consistent with this pattern, follow-up analyses showed significant improvements in attention from baseline to 12-weeks (Λ = .85, F(1, 49) = 8.47, p < .01), 12-months (Λ = .69, F(1, 49) = 22.20, p < .001) and to 24-months (Λ = .60, F(1, 49) = 33.37, p < .001).
Figure 1. Cognitive Function 36-months Post-Bariatric Surgery (N = 50).
Note. Higher scores on the y-axis are reflective of better cognitive function. X-axis reflects baseline through 36-month post-bariatric surgery time points.
Table 3.
Cognitive Function for Bariatric Surgery Patients 36-Months post-operatively (T-score Mean (SD))
| Attention | Executive FuntionMemory | Language | ||
|---|---|---|---|---|
| Time Point | ||||
| Baseline | 52.15(7.90) | 51.86(10.24) | 44.03(10.42) | 47.85(9.27) |
| 12-weeks | 54.52(8.48) | 58.58(10.60) | 50.25(10.40) | 48.16(8.37) |
| 12-months | 56.92(10.04) | 57.73(10.55) | 52.95(8.29) | 49.06(9.62) |
| 24-months | 58.16(9.34) | 58.98(10.04) | 50.59(10.71) | 50.23(8.97) |
| 36-months | 49.81(10.99) | 59.88(9.80) | 50.67(11.62) | 49.49(9.47) |
| F | 24.15** | 16.75** | 15.16** | 2.32 |
| Partial eta | .68 | .59 | .57 | .17 |
Note.
p < .001
For executive function, there was also a significant main effect for time (Λ = .41, F(4, 46) = 16.75, p < .001). The most significant improvements in executive function was found 12-weeks post bariatric surgery (Λ = .56, F(1, 49) = 38.60, p < .001), and after a slight non-significant decline from 12-weeks to 12-months (Λ = .98, F(1, 49) = .91, p = .35), executive function continued to improve until it reached its peak at 36-months post-operatively (see Figure 1). Of note, executive function significantly improved from baseline to each of the distant time points [baseline to 12-months (Λ = .65, F(1, 49) = 26.76, p < .001); baseline to 24-months (Λ = .53, F(1, 49) = 44.09, p < .001); and baseline to 36-months (Λ = .45, F(1, 49) = 60.89, p < .001).
For memory, there was a significant main effect across the five time points (Λ = .57, F(4, 46) = 15.16, p < .001). Examination of Figure 1 shows that memory gradually improved up until 12-months and then remained largely unchanged from 12-months to 36-months post-bariatric surgery. Follow-up analyses showed significant improvements in memory from baseline to each of the distant time points [12-weeks (Λ = .62, F(1, 49) = 30.31, p < .001), 12-months (Λ = .44, F(1, 49) = 63.40, p < .001), 24-months (Λ = .72, F(1, 49) = 19.32, p < .001), 36-months (Λ = .68, F(1, 49) = 23.56, p < .001)].
No significant main effect emerged for language (Λ = .83, F(4, 46) = 2.32, p = .07).
48-month Post Operative Cognitive Function
Exploratory analyses for cognitive test performance at 48-months post bariatric surgery showed that attention, memory and language functioning fell within the average range while executive function fell within the high average to above average range. Repeated measures ANOVAs showed significant improvements from baseline to 48-months post bariatric surgery in the following domains: executive function (Λ = .49, F(1, 20) = 20.58, p < .001; baseline executive function M (SD) = 53.84 (10.88) vs. executive function at 48-months M (SD) = 62.75 (10.32)) and memory (Λ = .39, F(1, 20) = 31.60, p < .001; baseline memory M (SD) = 46.60 (5.54) vs. memory at 48-months M (SD) = 55.19 (8.11). No such pattern emerged for attention (Λ = .99, F(1, 20) = .29, p = .60; baseline attention M(SD) = 55.02 (6.61 vs. attention at 48-months M (SD) = 54.14 (8.41) or language (Λ = .99, F(1, 49) = .14, p = .71; baseline language M (SD) = 49.44 (11.41 vs. language at 48-months M (SD) = 50.20 (10.94).
Weight Regain and Cognitive Function
Participants exhibited significant declines in BMI from baseline to 36-months post-bariatric surgery (F(1,46) = 300.06, p < .001). However, significant weight regain was observed between 24-months and 36-months post-operatively (F(1,46) = 14.42, p < .001; BMI M (SD) = 31.07 (6.67) versus 32.25 (6.66)). Specifically, 74.5% of participants exhibited an increase in BMI during this time period, with an average increase of 1.27 ± 2.30 (range from −5.16 to 8.15, 17.0% with a >3.0 BMI unit increase). Participants who exhibited weight regain from 24- to 36-months post-operatively also demonstrated significant declines in attention (F(1,34) = 67.24, p < .001). No changes were noted for any other of the cognitive domains (p > .05). Bivariate correlations also revealed a trend for increase in BMI from 24- to 36-months following surgery and poorer 36-month performance in attention (r(47) = −.27, p = .069). No such pattern emerged for the other cognitive domains (p > .10).
Discussion
The current study hypothesized that bariatric surgery patients would demonstrate postoperative improvements in cognitive function that would be maintained at a 36-month follow-up. Our findings support this hypothesis and extend past work by demonstrating the long-term cognitive benefits of bariatric surgery. Specifically, the current findings demonstrate that improvements in memory are maintained at 36-months post-operatively and further indicate that executive function improves up to this time point. Exploratory analyses at longer follow-up visits also yielded promising results, indicating that these cognitive benefits may be maintained up until 48-months. These findings continue to support the notion that cognitive dysfunction related to obesity may be at least partially reversible following bariatric surgery.
The implications of these findings are noteworthy in light of the extant literature that demonstrates midlife obesity as a risk factor for Alzheimer’s disease and vascular dementia.24–26 Given that the current sample of participants were in mid-life at study onset, and demonstrated high rates of impairment in memory and executive function, they may have been at increased risk for future dementia. However, following surgery, performance across multiple domains of cognitive function improved suggesting that bariatric surgery may reduce risk for Alzheimer’s disease in obese individuals. Future research should clarify this possibility by examining the cognitive benefits of bariatric surgery at very extended follow-ups (e.g., 10 years), particularly given the many surgical patients that re-gain weight over time.27
The mechanisms for improved cognition following bariatric surgery are not well understood. Although rates of medical co-morbidities (i.e., hypertension, diabetes) decreased in the current sample, previous research has shown that the cognitive benefits of bariatric surgery largely occur independent of changes in medical condition status.14 Studies using direct measurements of possible mechanisms (e.g., plasma concentrations of HbAlC, ambulatory blood pressure) are much needed to elucidate whether more subtle changes are associated with cognitive improvement. Future research should also examine other potential mechanisms of change, including factors closely linked with obesity and known to impair cognitive function (e.g., cerebral blood flow, vascular function).
Interestingly, we found that weight regain was associated with reduced attention from 24-months to 36-months post-operatively. Although the average amount of regain was relatively small (1.27 ± 2.30 BMI units), such findings highlight the importance of maintaining weight loss in bariatric surgery patients to prevent increased risk for cognitive decline. The mechanisms for this association likely involve the return of comorbid medical conditions that adversely impact cognitive function (e.g., hypertension, diabetes, sleep apnea) or even from the independent effects of adiposity.28–31 Future research is much needed to investigate factors that promote maintenance of weight loss and clarify the differential effects of weight regain on neurocognitive outcomes.
The current findings are limited in several ways. First, the lack of a control group of obese participants limits the generalizability of these findings. For instance, it is possible that practice effects may have contributed to post-operative cognitive improvements in the current sample. However, several factors argue against practice effects as being a key contributor. First, past work that utilizes obese controls demonstrates improved cognitive function at 12-weeks and 24-months post-bariatric surgery,13,14 suggesting this pattern is likely to continue at extended follow-ups. In addition, the long-term intervals between test administrations also diminish practice effects as a confounding variable. Nonetheless, future randomized case-controlled studies are needed to confirm the current findings and fully elucidate whether bariatric surgery can attenuate cognitive decline in severely obese individuals. In addition, the mechanisms for the cognitive benefits of bariatric surgery are not well understood and prospective studies that implement labwork and neuroimaging are much needed. Similarly, research has shown that cognition also improves following behavioral weight loss interventions32 and future work is needed to clarify the differential effects of behavioral and surgical weight loss on the brain.
In brief summary, this study replicates past work demonstrating improvements in cognitive function following bariatric surgery and further indicates that post-operative cognitive benefits may be maintained up to 3 years post-operatively. Future research is needed to elucidate the mechanisms for these improvements and examine whether these benefits are maintained over extended periods of time.
Acknowledgments
Data collection supported by DK075119. Manuscript supported in part by HL089311.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 2.Must A, Spadano J, Coakley E, et al. The disease burden associated with overweight and obesity. JAMA. 2006;295:1549–1556. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 3.Haslam DW, James WP. Obesity. Lancet. 2005;366:1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 4.Fitzpatrick A, Kuller LH, Lopez O, et al. Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Arch Neurol. 2009;66:336–42. doi: 10.1001/archneurol.2008.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gunstad J, Paul RH, Cohen RA, et al. Relationship between body mass index and brain volume in healthy adults. Int J Neurosci. 2008;118:1582–1593. doi: 10.1080/00207450701392282. [DOI] [PubMed] [Google Scholar]
- 6.Cournot M, Marqui JC, Ansiau D, et al. Relation between body mass index and cognitive function in healthy middle-aged men and women. Neurology. 2006;67:1208–1214. doi: 10.1212/01.wnl.0000238082.13860.50. [DOI] [PubMed] [Google Scholar]
- 7.Gunstad J, Lhotsky A, Wendell CR, et al. Longitudinal examination of obesity and cognitive function: results from the Baltimore Longitudinal Study of Aging. Neuroepidemiology. 2010;34:222–29. doi: 10.1159/000297742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shinogle J, Owings M, Kozak L. Gastric bypass as treatment for obesity: trends, characteristics, and complications. Obes Res. 2005;13:2202–2209. doi: 10.1038/oby.2005.273. [DOI] [PubMed] [Google Scholar]
- 9.Mann T, Tomiyama AJ, Westling E, et al. Medicares search for effective obesity treatments: diets are not the answer. Am Psychol. 2007;62:220–223. doi: 10.1037/0003-066X.62.3.220. [DOI] [PubMed] [Google Scholar]
- 10.Potteiger CE, Paragi PR, Inverso, et al. Bariatric surgery: shedding the monetary weight of prescription costs in the managed care arena. Obes Surg. 2004;14:725–730. doi: 10.1381/0960892041590999. [DOI] [PubMed] [Google Scholar]
- 11.Sampalis J, Liberman M, Auger S, Christou NV. The impact of weight reduction surgery on health care costs in morbidly obese patients. Obes Surg. 2004;14:939–947. doi: 10.1381/0960892041719662. [DOI] [PubMed] [Google Scholar]
- 12.Masoomi H, Nguyen NT, Stamos MJ, Smith BR. Overview of outcomes of laparoscopic and open roux-en-Y gastric bypass in the United States. Surg Technol Int. 2012 epub ahead of print. [PubMed] [Google Scholar]
- 13.Gunstad J, Strain G, Devlin MJ, et al. Improved memory function 12 weeks after bariatric surgery. Surg Obes Relat Dis. 2011;7:465–72. doi: 10.1016/j.soard.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alosco ML, Spitznagel MB, Strain G, et al. Improved memory function two years after bariatric surgery. Obesity. doi: 10.1002/oby.20494. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeki Al, Hazzouri A, Haan MN, et al. Central obesity, leptin and cognitive decline: the Sacramento Area Latino Study on Aging. Dement Geriatr Cogn Disor. 2012;33:400–409. doi: 10.1159/000339957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belle SH, Berk PD, Courcoulas AP, et al. Safety and efficacy of bariatric surgery: Longitudinal Assessment of Bariatric Surgery. Surg Obes Rel Dis. 2007;3:116–26. doi: 10.1016/j.soard.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alexander M. Mild traumatic brain injury: Pathophysiology, natural history, and clinical management. Neurol. 1999;45:1253–60. doi: 10.1212/wnl.45.7.1253. [DOI] [PubMed] [Google Scholar]
- 18.Paul RH, Lawrence J, Williams, et al. Preliminary validity of “integneuro”” a new computerized battery of neurocognitive tests. Int J Neurosci. 2005;115:1549–1567. doi: 10.1080/00207450590957890. [DOI] [PubMed] [Google Scholar]
- 19.Williams LM, Simms E, Clark CR, et al. The test-retest reliability of a standardized neurocognitive and neurophysiological test battery: “neuromarker”. Int J Neurosci. 2005;115:1605–1630. doi: 10.1080/00207450590958475. [DOI] [PubMed] [Google Scholar]
- 20.Gunstad J, Paul RH, Cohen RA, et al. Elevated body mass index is associated with executive dysfunction in otherwise healthy adults. Compr Psychiatry. 2007;48:57–61. doi: 10.1016/j.comppsych.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Reitan R. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Motor Skills. 1958;8:271–6. [Google Scholar]
- 22.Golden C. Stroop color and word task: a manual for clinical and experimental uses. Wood Dale: Stoeling; 1978. [Google Scholar]
- 23.Walsh K. Understanding Brain Damage – A Primer of Neuropsychological Evaluation. Melbourne: Churchill Livingstone; 1985. [Google Scholar]
- 24.Hassing LB, Dahl AK, Thorvaldsson V, et al. Overweight in midlife and risk of dementia: a 40-year follow-up study. Int J Obes. 2009;33:893–98. doi: 10.1038/ijo.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whitmer RA, Gustafson DR, Barrett-Connor E, et al. Central obesity and increased risk of dementia more than three decades later. Neurology. 2008 Sep 30;71:1057–64. doi: 10.1212/01.wnl.0000306313.89165.ef. [DOI] [PubMed] [Google Scholar]
- 26.Kivipelto M, Ngandu T, Fratiglioni L, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 2005;62:1556–60. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- 27.Christou NV, Look D, MacLean LD. Weight gain after short- and long-limb gastric bypass in patients followed for longer than 10 years. Ann Surg. 2006;244:734–40. doi: 10.1097/01.sla.0000217592.04061.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papademetriou V. Hypertension and cognition function: blood pressure regulation and cognitive function: a review of the literature. Geriatrics. 2005;60:20–2. [PubMed] [Google Scholar]
- 29.Awad N, Gagnon M, Messier C. The relationship between impaired glucose tolerance, type 2 diabetes, and cognitive function. J Clin Exp Neuropsychol. 2004;26:1044–80. doi: 10.1080/13803390490514875. [DOI] [PubMed] [Google Scholar]
- 30.Aloia M, Arnedt J, Davis J, et al. Neuropsychological sequelae of obstructive sleep apnea-hypopnea syndrome: a critical review. J Int Neuropsychol Soc. 2004;10:772– 85. doi: 10.1017/S1355617704105134. [DOI] [PubMed] [Google Scholar]
- 31.Butner KL, Nikols-Richardson SM, Clark SF, et al. A review of weight loss following Roux-en-Y gastric bypass vs restrictive bariatric surgery: impact on adiponectin and insul. Obes Surg. 2010;20:559–568. doi: 10.1007/s11695-010-0089-z. [DOI] [PubMed] [Google Scholar]
- 32.Smith PJ, Blumenthal JA, Babyak MA, et al. Effects of the dietary approaches to stop hypertension diet, exercise and caloric restriction on neurocognition in overweight adults with high blood pressure. Hypertension. 2010;55:1331–8. doi: 10.1161/HYPERTENSIONAHA.109.146795. [DOI] [PMC free article] [PubMed] [Google Scholar]