Abstract
Background
Optimal vitamin D status is important for overall health and well-being, particularly in the elderly. Although vitamin D synthesis in the skin declines with age, exposure to sunlight still seems to help older-aged adults to achieve adequate serum 25-hydroxyvitamin D (25OHD) levels. Elderly people would therefore benefit from outdoor leisure activities, but the effects of different types of pastime on serum 25OHD levels have yet to be thoroughly investigated.
Aims
To assess the association of different pastimes with 25OHD deficiency in elderly subjects.
Methods
A sample of 2,349 community-dwelling elderly individuals (1,389 females and 960 males) enrolled in the Progetto Veneto Anziani was analyzed. Brisk walking, cycling, gardening and fishing were classed as outdoor activities, and dancing and gym workouts as indoor pastimes. Any activities undertaken for at least 1 hour/week during the previous month were considered as being practiced regularly. Logistic regression models were used to estimate the association between different pastimes and 25OHD deficiency.
Results
Serum 25OHD levels were significantly higher in individuals who engaged in outdoor pastimes (+25% in women, +27.7% in men) compared to those who did not. In particular, subjects regularly practicing gardening or cycling had higher serum 25OHD levels than those who did not, whereas 25OHD levels differed little between subjects who did or did not undertake indoor activities. Among the outdoor pastimes considered, logistic regression analysis confirmed a lower likelihood of vitamin D deficiency (25OHD<50 nmol/L) for cyclists (OR 0.51, 95% CI 0.37–0.69 in women; OR 0.50, 95% CI 0.29–0.87 in men) and gardeners (OR 0.62, 95% CI 0.47–0.83 in women; OR 0.46, 95% CI 0.26–0.80), but not for brisk walkers.
Conclusions
Regular cycling and gardening reduce the likelihood of inadequate vitamin D status in Caucasian elderly people, irrespective of their age, BMI and comorbidities, and of the season of the year.
Introduction
The role of vitamin D in bone health and muscle function has long been recognized, and an increasing body of evidence suggests that inadequate vitamin D levels may contribute not only to osteoporosis, but also to cardiovascular disorders, type 2 diabetes and cancer [1]–[3]. Vitamin D deficiency is a frequent finding among older adults, reaching a prevalence beyond 50% in various epidemiological studies [4], [5]. Several factors may account for this hypovitaminosis D epidemic among elderly people, such as a reduced dietary vitamin D intake or an increased vitamin D storage in adipose tissue [6], but the main culprit is thought to be the age-related decrease in 7-dehydrocholesterol (7-DHC) content in the epidermis, which means an impoverished substrate for vitamin D production [7]. Recommendations concerning exposure to sunlight have consequently been overshadowed by the prescription of oral supplements, which are generally the preferred method for preventing or correcting hypovitaminosis D. Despite the age-related decline in vitamin D production by the skin, there is some evidence that older adults can still maintain adequate serum vitamin D levels by taking advantage of exposure to ultraviolet light (UV) [8]. Since older adults have more leisure time and therefore more opportunities to spend time outdoors, promoting regular activities that involve their exposure to sunlight could contribute to preventing hypovitaminosis D.
To the best of our knowledge, few studies [9]–[11] have investigated the relationship between physical activity and vitamin D status in the elderly, and none have considered the effects of different outdoor pastimes on serum 25OHD levels. Identifying the activities associated with a lower probability of hypovitaminosis D in elderly subjects might have important implications for public health.
We hypothesized that, for older adults, regular outdoor leisure-time activities entailing exposure to sunlight, such as brisk walking, gardening, cycling, and so on, might not have the same effect on serum 25OHD levels. The aim of the present study was thus to assess the association of different regular physical activities with vitamin D deficiency in a population-based sample of healthy community-dwelling elderly subjects.
Methods
Ethics approval
The local Ethical Committees of Padua University and of the Local Health Units (ULSS) n. 15 and n. 18 of the Veneto Region approved the study protocol, and participants gave their written informed consent.
Data source and subjects
The data considered in this analysis were drawn from the Progetto Veneto Anziani (Pro.V.A.), an observational cohort study on the Italian population aged ≥65 years living in two geographical areas in the north-east of Italy (Camposampiero and Rovigo). The study population included 3,099 age- and sex-stratified Caucasian community-dwelling participants (1,245 men and 1,854 women) randomly selected between 1995 and 1997 using a multistage stratified method designed to keep the male-to-female ratio at 2:3 and to oversample the oldest age group. Sampling procedures and data collection methods have been described in detail elsewhere [12].
Participants were examined at city hospitals by trained physicians and nurses. Health status was ascertained by integrating information obtained from a physical examination and a review of the medical records. For the analyses conducted in the present study, participants lacking serum 25OHD test results (n = 272) and those in wheelchairs or unable to walk (n = 89), or with leg and/or arm amputations (n = 23) were excluded. Cases diagnosed with primitive hyperparathyroidism (n = 17) or moderate-to-severe renal failure (n = 11) (defined as a glomerular filtration rate <30 ml/minute) were also excluded. Participants' physical performance status and aerobic capacity were measured using the standardized 6-minute walking test (6MWT) [13], and those unable to walk at their usual pace for six minutes (n = 338) were excluded. The final sample consisted of 2,349 well-performing and self-sufficient individuals with a complete set of data on their physical activities and comorbidities.
Clinical and laboratory data
Information was collected on participants' formal education, smoking and regular exercising habits during an in-person interview. Educational level (the total number of years of school attended) was dichotomized as ≤5 versus >5 years of schooling. Smoking habits were classified as “never/former” (for at least a year in the past) versus “current” smokers. Physical activities were grouped into two categories, i.e. outdoor activities (brisk walking, cycling, gardening, and fishing), and indoor activities (dancing, exercising at the gym). Participants were asked to report how many hours a week they had spent on each of the above-mentioned pastimes in the previous month. An activity was considered regular if it had been practiced for more than 1 hour a week during the previous month.
Any medical conditions were identified by board-certified physicians involved in the study, who examined all the clinical information collected on each participant, including clinical history, self-reported symptoms (using standardized questionnaires), medical and hospital records, blood tests, and a physical examination. The prior major diseases considered were any of the following: cardiovascular diseases (CVD: congestive heart failure, angina and myocardial infarction, stroke, and peripheral artery disease), diabetes, chronic obstructive pulmonary diseases (COPD), cancer, neurodegenerative diseases (Parkinson's, dementia), osteoarthritis (hand/knee/hip osteoarthritis, hip fracture). Depression was assessed using the Geriatric Depression Scale [14], and a score ≥11 was indicative of depressive symptoms. Cognitive function was assessed by administering the 30-item Mini Mental State Examination [15]. Scores for the MMSE range from 0 to 30, and a score below 24 is indicative of cognitive impairment [16]. Disability was defined as the inability or need for assistance to perform one or more activities of daily living (ADL): bathing, dressing, eating, using the toilet, or transferring.
Venous blood samples were obtained after an overnight fast, centrifuged and stored at −80°C. Routine biochemical tests were performed at city hospitals, and 25OHD tests at the Padua University laboratory. Serum 25OHD levels were measured by radioimmunoassay (RIA kit; Buhlmann Lab., Basel, Switzerland) in the ethanol extract of serum by a radiocompetitive assay using a binding protein obtained from rachitic rat serum. The sensitivity range is reported to be 1.25–160 ng/ml, and the coefficient of variation 5.3% and 8.9% intra- and inter-assay, respectively.
Serum intact PTH levels were measured using a two site immunoradiometric assay kit (N-tact PTHSP; DiaSorin): the intra-assay and inter-assay coefficients of variation for PTH were 3.0% and 5.5%, respectively. Serum creatinine was measured using a standard creatinine Jaffé method (Roche Diagnostics, Germany) and glomerular filtration rate (GFR) was calculated with the MDRD formula.
Statistical analysis
Participants' characteristics were summarized using medians (first and third quartiles) for continuous variables, and counts and percentages for categorical variables. Medians and proportions were calculated for the following five age groups: 65–69 years, 70–74 years, 75–79 years, 80–84 years, ≥85 years. Given the known gender-related differences, all data analyses were stratified by sex. Differences in categorical variables were examined using the chi-square test, while the non-parametric Mann-Whitney test was used to check differences between medians of covariates by age group. In the whole sample and within each age group, the non-parametric test was also used to examine the differences in 25OHD median values by level of physical activity (dichotomized in ≥1 hour/week vs <1 hour/week).
Multivariate logistic regression models were used to examine the association between outdoor/indoor pastimes and the odds of vitamin D deficiency (defined as serum 25OHD levels <50 nmol/L). Known factors associated with 25OHD levels and/or physical functionality were examined for inclusion in the analyses and a multivariate model was obtained. Age, smoking habits (never/former vs current smoker), body mass index (BMI; calculated as the weight in kg/height in meters squared), season of the year (November–February vs March–October), cognitive impairment, depression, GFR, distance covered in the 6-minute walking test, CVD, neurodegenerative diseases, osteo-articular diseases, osteoporosis, cancer, diabetes, and COPD were all added as confounders in the model. The covariate-adjusted odds ratios (ORs) of vitamin D deficiency were obtained for each outdoor and indoor activity.
All analyses were performed using the SAS rel. 9.13 (Cary NC: SAS Institute). All statistical tests were two-tailed and statistical significance was assumed for a p-value <0.05.
Results
Participants' general characteristics
The sample consisted of 2,349 community-dwelling elderly subjects (960 men and 1,389 women). Their median age was 75 years (IQR 69–81.5) for the men and 74 (IQR 69–80) for the women. The median serum 25OHD levels were 95.0 nmol/L (IQR 61.6–133.5) in men and 59.0 nmol/L (IQR 38–88) in women. Vitamin D deficiency (25OHD <50 nmol/L) was more common among the females (34.2%), than among the males (11%); it was severe (25OHD <25 nmol/L) in 13% of the women and only 5% of the men. The proportions of participants regularly practicing at least one of the different outdoor and indoor leisure-time physical activities was generally higher for the men than for the women (84.1% vs 71.5%, p<0.0001, details not shown).
Tables 1 and 2 show the sample's general characteristics, as a whole and by age group, for men and women, respectively. In both genders, the median 25OHD levels tended to decline significantly from the youngest to the oldest individuals (p<0.0001). The proportions of subjects engaging in outdoor activities decreased with increasing age, with the exception of the brisk walkers, whose numbers did not drop significantly across age groups in the men (p = 0.26) or the women (p = 0.84). As for indoor activities, the proportion of men who danced and of women who attended the gym tended to decline (p = 0.14; p = 0.02, respectively).
Table 1. General characteristics in men (whole sample and by age group), expressed as medians (first and third quartiles) or percentages, as appropriate.
| Whole sample | Age group | p-value | |||||
| 65–69 years | 70–74 years | 75–79 years | 80–84 years | ≥85 years | |||
| (n = 960) | (n = 262) | (n = 208) | (n = 184) | (n = 132) | (n = 174) | ||
| Age (ys) | 75.0 (69.0–81.5) | 67.0 (66.0–68.0) | 72.0 (71.0–73.0) | 76.0 (75.0–78.0) | 81.5 (81.0–83.0) | 87.0 (86.0–89.0) | <0.0001 |
| 25OHD (nmol/L) | 95 (61.5–133.5) | 109.0 (77.0–144.0) | 104.5 (77.0–142.0) | 90.0 (60.0–139.5) | 90.5 (56.5–121.27) | 62.5 (46.0–99.0) | <0.0001 |
| BMI (kg/m2) | 26.67 (24.1–29.2) | 27.76 (25.02–30.30) | 27.37 (24.64–29.74) | 26.01 (24.12–28.10) | 25.96 (24.32–28.27) | 25.34 (22.26–28.56) | <0.0001 |
| 6MWD (m) | 369 (300–433) | 424.0 (363.0–469.0) | 392.0 (330.0–444.0) | 377.0 (320.0–429.0) | 329.0 (277.5–377.5) | 264.0 (173.0–338.0) | <0.0001 |
| COMORBIDITIES,% | |||||||
| Depression | 22.3 | 15.9 | 20.3 | 24.6 | 27.6 | 28.7 | 0.004 |
| Cognitive impairment | 33.2 | 12.6 | 22.7 | 31.5 | 43.5 | 71.3 | <0.0001 |
| Cardiovascular diseases | 26.7 | 14.6 | 27.0 | 23.9 | 41.7 | 36.4 | <0.0001 |
| Neurodegenerative diseases | 5.5 | 0.4 | 1.4 | 4.3 | 7.6 | 17.9 | <0.0001 |
| Osteo-articular diseases | 26.2 | 19.1 | 27.7 | 26.1 | 28.2 | 33.5 | 0.001 |
| Osteoporosis | 24.0 | 15.6 | 14.4 | 25.5 | 29.5 | 41.9 | <0.0001 |
| Any cancer | 8.7 | 6.9 | 6.2 | 10.9 | 7.6 | 13.2 | 0.47 |
| Diabetes | 8.0 | 9.1 | 7.2 | 10.3 | 7.6 | 5.2 | 0.27 |
| COPD | 15.2 | 10.3 | 12.5 | 12.5 | 18.2 | 26.4 | <0.0001 |
| OUTDOOR PHYSICAL ACTIVITY, % | |||||||
| Brisk walking | 39.7 | 41.5 | 33.5 | 45.5 | 38.3 | 39.3 | 0.26 |
| Cycling | 53.4 | 60.9 | 56.8 | 56.7 | 50.0 | 34.7 | <0.0001 |
| Gardening | 45.7 | 52.3 | 49.5 | 52.2 | 40.6 | 25.3 | <0.0001 |
| Fishing | 5.7 | 9.6 | 8.2 | 3.4 | 2.3 | 1.3 | 0.0008 |
| INDOOR PHYSICAL ACTIVITY, % | |||||||
| Dancing | 3.5 | 5.4 | 3.9 | 3.9 | 1.6 | 0.7 | 0.14 |
| Gym | 7.0 | 10.4 | 7.8 | 4.5 | 3.9 | 6.0 | 0.26 |
25(OH)D: 25-hydroxy-vitamin D; ys: years; BMI: body mass index; COPD: chronic obstructive pulmonary disease; 6MWD: six-minute walking distance.
Table 2. General characteristics in women (whole sample and by age group), expressed as medians (first and third quartiles) or percentages, as appropriate.
| Whole sample | Age groups | p-value | |||||
| 65–69 years | 70–74 years | 75–79 years | 80–84 years | ≥85 years | |||
| (n = 1389) | (n = 406) | (n = 325) | (n = 288) | (n = 210) | (n = 160) | ||
| Age (ys) | 74.0 (69.0–80.0) | 67.0 (66.0–68.0) | 72.0 (71.0–73.0) | 76.0 (75.0–77.0) | 82.0 (81.0–83.0) | 87.0 (86.0–89.0) | <0.0001 |
| 25OHD (nmol/L) | 59.0 (38.0–88.0) | 71.0 (49.0–100.0) | 66.0 (45.0–93.0) | 59.0 (38.5–82.5) | 47.5 (29.0–68.0) | 37.5 (25.0–56.5) | <0.0001 |
| BMI (kg/m2) | 27.73 (24.87–30.70) | 27.83 (25.19–31.03) | 27.94 (25.10–30.87) | 27.84 (24.90–30.52) | 27.82 (24.54–30.77) | 26.45 (23.32–29.56) | <0.0001 |
| 6MWD (m) | 313.0 (240.0–373.0) | 362.5 (313.0–408.0) | 323.0 (273.0–380.0) | 302.0 (240.0–360.0) | 261.0 (180.0–320.0) | 182.5 (124.5–249.0) | <0.0001 |
| COMORBIDITIES,% | |||||||
| Depression | 38.5 | 32.7 | 32.8 | 42.5 | 43.4 | 53.7 | <0.0001 |
| Cognitive impairment | 37.4 | 20.7 | 24.8 | 39.4 | 59.9 | 74.8 | <0.0001 |
| Cardiovascular diseases | 17.3 | 6.9 | 13.0 | 19.4 | 26.7 | 36.2 | <0.0001 |
| Neurodegenerative diseases | 5.5 | 0.2 | 1.5 | 4.2 | 8.6 | 25.0 | <0.0001 |
| Osteo-articular diseases | 38.3 | 27.3 | 39.4 | 41.2 | 49.8 | 44.7 | <0.0001 |
| Osteoporosis | 55.9 | 40.4 | 50.5 | 58.7 | 74.3 | 76.7 | <0.0001 |
| Any cancer | 6.9 | 7.1 | 5.5 | 8.0 | 7.1 | 6.9 | 0.98 |
| Diabetes | 9.9 | 7.4 | 9.5 | 11.9 | 12.9 | 9.4 | 0.06 |
| COPD | 5.3 | 4.4 | 5.2 | 4.9 | 5.7 | 7.5 | 0.64 |
| OUTDOOR PHYSICAL ACTIVITY, % | |||||||
| Brisk walking | 25.2 | 25.4 | 24.1 | 24.6 | 24.7 | 29.2 | 0.84 |
| Cycling | 35.8 | 51.2 | 46.6 | 32.0 | 14.8 | 3.6 | <0.0001 |
| Gardening | 38.6 | 44.3 | 45.6 | 37.4 | 32.7 | 17.5 | <0.0001 |
| Fishing | - | - | - | - | - | - | - |
| INDOOR PHYSICAL ACTIVITY, % | |||||||
| Dancing | 0.9 | 1.2 | 0.9 | 1.1 | 0.5 | 0 | 0.54 |
| Gym | 8.3 | 12.2 | 8.1 | 6.4 | 6.4 | 4.4 | 0.02 |
25(OH)D: 25-hydroxy-vitamin D; ys: years; BMI: body mass index; COPD: chronic obstructive pulmonary disease; 6MWD: six-minute walking distance.
25OHD and physical activity
In both genders, median serum 25OHD level was higher in subjects who engaged in outdoor pastimes compared to those who did not (for women: 68 nmol/L (95% CI 64–71; IQR 45–94) vs 51 nmol/L (95% CI 48.54; IQR 32–74) respectively, p < 0.0001; for men: 101 nmol/L (95% CI 98–107; IQR 71–144) vs 73 nmol/L (95% CI 65–83; IQR 45–107) respectively, p < 0.0001).
Tables 3 and 4 show the median levels of 25OHD in individuals who did and did not practice the various outdoor and indoor activities by gender and age class. In all age groups, the men and women who regularly spent time gardening or cycling had significantly higher median 25OHD serum levels than those seen in participants who did not engage in these outdoor activities.
Table 3. Serum 25OHD levels (nmol/L) by age group and physical activity in men.
| Age groups | |||||
| 65–69 years | 70–74 years | 75–79 years | 80–84 years | ≥85 years | |
| (n = 262) | (n = 208) | (n = 184) | (n = 132) | (n = 174) | |
| OUTDOOR PHYSICAL ACTIVITY | |||||
| Brisk walking <1 hour/week | 105.5 (76.0–142.0) | 106.0 (80.0–146.0) | 99.0 (69.0–145.0) | 95.0 (56.0–125.0) | 65.0 (42.0–104.0) |
| Brisk walking >1 hour/week | 110.0 (78.0–148.5) | 104.0 (77.0–138.0) | 89.0 (59.0–120.0) | 89.0 (58.0–125.0) | 57.0 (48.0–97.0) |
| Cycling <1 hour/week | 102.0 (66.0–134.0) | 99.0 (72.0–122.0) | 81.0 (53.0–110.0) | 75.5 (51.5–122.0) | 55.0 (37.0–96.0) |
| Cycling >1 hour/week | 111.0 (79.0–151.0) * | 114.0 (86.0–159.0) * | 105.0 (73.0–152.0) * | 96.5 (70.5–125.0) * | 82.5 (55.0–113.0) * |
| Gardening <1 hour/week | 103.5 (67.5–138.0) | 99.0 (723.5–127.5) | 86.0 (50.0–110.0) | 89.0 (54.5–115.0) | 57.0 (42.5–89.0) |
| Gardening >1 hour/week | 110.5 (80.5–151.5) * | 112.5 (82.0–158.0) ** | 110.0 (73.0–156.0) ** | 98.0 (70.0–134.5) * | 98.5 (50.0–148.0) ** |
| Fishing <1 hour/week | 106.0 (76.0–142.0) | 104.0 (77.0–136.0) | 90.5 (60.0–144.0) | 92.0 (57.0–125.0) | 60.0 (45,5–99.5) |
| Fishing >1 hour/week | 116.0 (96.0–159.0) * | 150.0 (94.0–186.0) * | 116.0 (79.0–136.0) | 75.0 (64.0–108.0) | 89.0 (12.0–166.0) |
| INDOOR PHYSICAL ACTIVITY | |||||
| Dancing <1 hour/week | 109.5 (77.0–144.0) | 102.5 (77.0–138.0) | 90.0 (59.0–144.0) | 91.5 (57.0–125.0) | 60 (45.0–100.0) |
| Dancing >1 hour/week | 98.5 (53.0–137.0) | 139.0 (119.0–208.0) * | 120.0 (80.0–136.0) | 84.0 (70.0–98.0) | 79.0 |
| Gym <1 hour/week | 108.0 (7.0–140.0) | 104.0 (77.0–146.0) | 90.5 (60.0–144.0) | 92.0 (57.0–128.0) | 62.0 (46.0–100.0) |
| Gym >1 hour/week | 145.0 (70.0–160.0) | 124.0 (82.0–140.5) | 93.5 (62.0–151.0) | 70.0 (51.0–91.0) | 51.0 (35.0–73.0) |
Comparison between age groups: * p<0.05; ** p<0.01.
Table 4. Serum 25OHD levels (nmol/L) by age group and physical activity in women.
| Age groups | |||||
| 65–69 years | 70–74 years | 75–79 years | 80–84 years | ≥85 years | |
| (n = 406) | (n = 325) | (n = 288) | (n = 210) | (n = 160) | |
| OUTDOOR PHYSICAL ACTIVITY | |||||
| Brisk walking <1 hour/week | 71.0 (49.0–100.0) | 68.0 (45.0–93.0) | 57.0 (37.0–79.0) | 46.5 (31.5–67.5) | 40.0 (28.0–57.0) |
| Brisk walking >1 hour/week | 73.0 (50.0–99.0) | 65.0 (40.0–91.0) | 66.0 (40.0–92.0) * | 53.0 (28.0–73.0) | 36.0 (30.0–71.5) |
| Cycling <1 hour/week | 66.0 (44.0–94.5) | 62.0 (40.0–86.0) | 55.0 (37.0–79.0) | 46.0 (28.0–65.5) | 38.0 (28.0–57.0) |
| Cycling >1 hour/week | 77.0 (54.0–105.0) * | 76.0 (52.0–102.0) ** | 65.5 (48.0–88.0) ** | 58.5 (42.0–90.0) * | 37.0 (35.0–72.0) |
| Gardening <1 hour/week | 68.0 (46.0–98.0) | 62.0 (41.0–88.0) | 55.0 (37.0–80.5) | 46.0 (28.0–65.5) | 37.0 (25.0–56.0) |
| Gardening >1 hour/week | 76.0 (53.0–103.0) * | 73.0 (47.0–97.0) * | 61.0 (45.0–85.0) * | 53.0 (33.0–82.0) ** | 45.5 (35.5–83.0) ** |
| INDOOR PHYSICAL ACTIVITY | |||||
| Dancing <1 hour/week | 71.0 (49.0–100.0) | 66.0 (45.0–93.0) | 58.5 (38.0–83.0) | 50.0 (31.0–69.0) | – |
| Dancing >1 hour/week | 80.0 (66.0–139.0) | 72.0 (43.0–84–0) | 65.0 (60.0–88.0) | 10.0 | – |
| Gym <1 hour/week | 70.0 (48.0–98.0) | 66.0 (45.0–92.0) | 59.0 (40.0–82.0) | 47.0 (30.0–69.0) | 38.0 (28.0–57.0) |
| Gym >1 hour/week | 86.0 (65.0–120.0) ** | 76.5 (51.0–115.0) | 40.0 (30.0–89.0) | 57.0 (36.0–66.0) | 35.0 (22.0–71.0) |
Comparison between age groups: * p<0.05; ** p<0.01.
The median 25OHD levels did not differ significantly between men who did or did not walk regularly, whereas for the women the difference in 25OHD serum levels was significant only for those aged 75–79. Men aged 65–75 years who went fishing regularly had significantly higher serum 25OHD levels than those who did not; none of the women engaged regularly in this activity.
Among the indoor pastimes considered, individuals who danced or attended the gym did not have significantly higher 25OHD levels than those who did not, except for men aged 70–74 years who danced regularly, and 65- to 74-year-old women who exercised at the gym. The small numbers of participants engaging in indoor activities (especially after splitting them into the five age groups considered) may have negatively affected the likelihood of a significant difference coming to light, however.
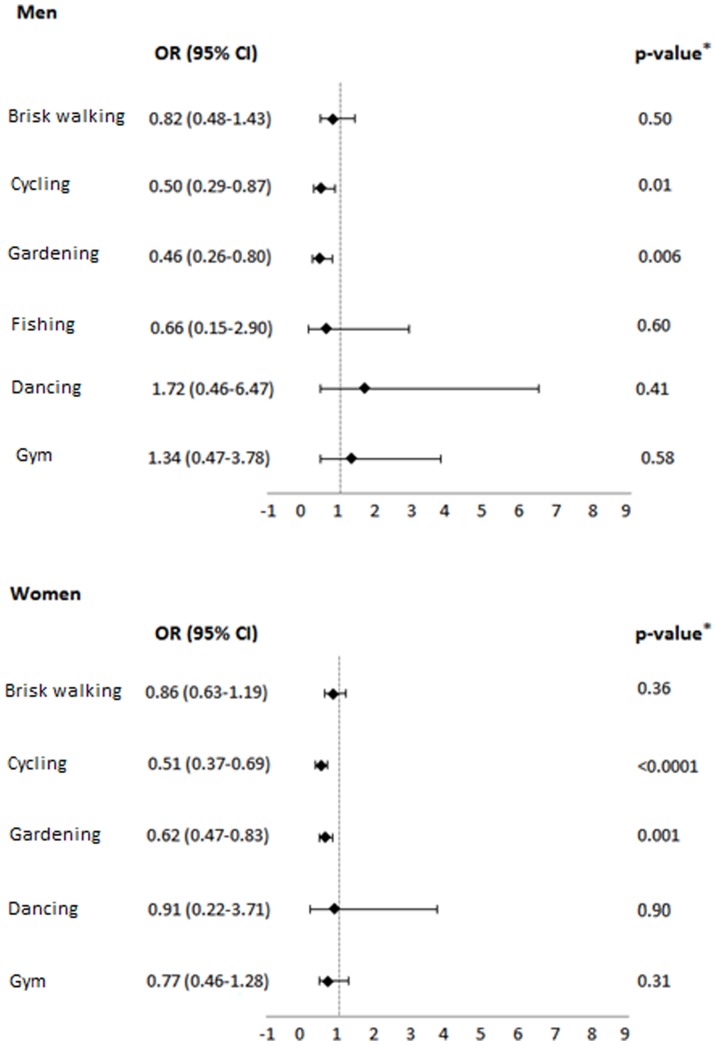
The covariate-adjusted odds ratios of vitamin D deficiency (defined as serum 25OHD levels <50 nmol/L) for each of the outdoor and indoor activities are shown in Figure 1. The indoor pastimes showed no significant association with low 25OHD levels. Among the outdoor activities, both men and women who were regularly cycling or gardening were less likely to be deficient in vitamin D (for cycling: OR 0.50, 95% CI 0.29–0.87 in men, and OR 0.51, 95% CI 0.37–0.69 in women; for gardening: OR 0.46, 95% CI 0.26–0.80 in men, and OR 0.62, 95% CI 0.47–0.83 in women). On the other hand, brisk walking and fishing were unassociated with any lower likelihood of hypovitaminosis D in either gender.
Figure 1. Results of logistic regression analysis for significant independent factors associated with vitamin D deficiency.
* Adjusted for age, body mass index, smoking habits, season, 6-minute walking distance, cognitive status, depression, cardiovascular diseases, neurodegenerative diseases, osteo-articular diseases, osteoporosis, cancer, diabetes, and chronic obstructive pulmonary disease.
Discussion
The present study on an ample sample of Caucasian elderly subjects living in north-east Italy (45°N) showed that different outdoor activities might not have the same effects in maintaining high vitamin D levels. Our findings suggest that outdoor pastimes involving a considerable exposure to sunlight, such as cycling and gardening - but not brisk walking - are associated with a lower probability of vitamin D deficiency in well-performing, community-dwelling elderly men and women.
In our sample, serum 25OHD concentrations were generally higher in men than in women, whose median level (59.0 nmol/L) was lower than the men's 25th percentile (61.5 nmol/L). This finding is consistent with a study by Maeda et al. [17], who analyzed 25OHD levels in three groups of elderly patients (nursing-home residents and community-dwelling elderly who did or did not get regular exercise), finding serum vitamin D concentrations higher in men than in women in all three groups. This might be because men have traditionally engaged more in outdoor activities, as confirmed by the higher proportion of men than women in our sample practicing some sort of physical activity. As van Dam et al. [18] pointed out, however, women's higher percentage of fat mass compared to men [19] may increase the former's vitamin D storage in adipose tissue and account for females' lower serum 25OHD concentrations.
Our findings indicate that serum 25OHD levels gradually decrease with age in both genders. So do people's tendencies to engage in outdoor physical activities as they get older, although this cannot be explained by physical disability (as reported in the Methods, participants unable to complete the standardized 6MWT were excluded and only well-performing subjects with no disabilities were considered in the present study). This picture might be due to some activities (e.g. gardening, cycling, dancing, fishing) requiring more strength, coordination and balance than is needed for brisk walking (an activity for which the participation rates remained fairly stable across age groups in both genders).
Concerning the relationship between physical activity and 25OHD levels, as was to be expected, we found no association between indoor activities and hypovitaminosis D. Although it would seem obvious that indoor physical activity does not modify 25OHD levels, some authors have suggested that physical activity per se may raise vitamin D levels [20], [21], possibly by means of a transient exercise-induced increase in PTH levels [22].
It is currently assumed that any outdoor activity exposing people to more or less sunlight should improve vitamin D status, but our findings suggest that this is not entirely so. Among the outdoor pastimes considered in our large population-based sample, only cycling and gardening (not brisk walking or fishing) were associated with a lower likelihood of having serum 25OHD concentrations <50 nmol/L. In the large cohort of elderly subjects from the National Health and Nutrition Examination Survey (NHANES) III, outdoor activities (walking, jogging and gardening) were found associated with higher levels of serum 25OHD; unfortunately these three activities were clustered together and no details were reported on the association between each of these activities and 25OHD serum levels [10]. There may be several reasons why not all outdoor activities have the same association with serum vitamin D levels. For instance, gardening is rarely only an occasional pastime, it usually demands a certain regularity, and it can keep someone occupied for hours on a daily basis. People who are gardening are also quite likely to wear light clothes and expose a greater body surface area to sunlight. The same can be said of cyclists, whereas brisk walkers may well be wearing more clothes and engaging in this activity early in the morning or in the evenings, when the UV radiation from the sun is feeble. Unfortunately the time of day when participants engaged in their pastimes was not recorded, and this is the main shortcoming of our study. Another limit of the present study lies in that the activities and the number of hours spent per week in the previous month were self-reported by participants during face-to face interviews, so the chances of an under- or over-reporting bias should be taken into account.
The main strengths of our study lie in its population-based design and large sample size, comprising a proportion of men and women representative of the general elderly population of north-east Italy. Another strength relates to the large number of confounders and diagnosed diseases investigated. The proportion of participants taking vitamin D supplements was less than 1% in our study sample as a whole, so the serum 25OHD levels identified in our study were hardly influenced by its oral supplementation. In addition, all participants in the present study were well-performing and in good health, as demonstrated by the results of the standardized 6-minute walking test, which is an indirect marker of aerobic capacity and exercise tolerance [13].
In conclusion, the present study demonstrates that outdoor physical activities are not all equally beneficial in terms of vitamin D status. Engaging for at least an hour a week in activities such as cycling or gardening seems to help reduce the likelihood of vitamin D deficiency in well-performing elderly people. Considering all the extraskeletal effects of vitamin D, spending time regularly in these activities (even only for a short time each week) could have other overall health benefits, as well as a positive effect on cardiovascular performance. Campaigns aiming to contain the hypovitaminosis D epidemic among older adults should not only recommend vitamin D supplementation, but also promote the benefits of this kind of outdoor pastime.
Funding Statement
This study was funded by Fondazione Cassa di Risparmio di Padova e Rovigo, University of Padova, Azienda Unità Locale Socio Sanitaria 15 and 18 of the Veneto region; by the Intramural Research Program of the National Institute on Aging, National Institutes of Health; and by Veneto Region Research Project 104/02 (GC). The funding institutes had no role in the design, methods, subject recruitment, data collection, analysis, or preparation of the manuscript or in the decision to submit the manuscript for publication.
References
- 1. Wang L, Manson JE, Song Y, Sesso HD (2010) Systematic review: Vitamin D and calcium supplementation in prevention of cardiovascular events. Ann Intern Med 152: 315–323. [DOI] [PubMed] [Google Scholar]
- 2. Lindqvist PG, Olsson H, Landin-Olsson M (2010) Are active sun exposure habits related to lowering risk of type 2 diabetes mellitus in women, a prospective cohort study? Diabetes Res Clin Pract 90: 109–114. [DOI] [PubMed] [Google Scholar]
- 3. Merlino LA, Curtis J, Mikuls TR, Cerhan JR, Criswell LA, et al. (2004) Vitamin D intake is inversely associated with rheumatoid arthritis: results from the Iowa Women's Health Study. Arthritis Rheum 50: 72–77. [DOI] [PubMed] [Google Scholar]
- 4. Robinson PJ, Bell RJ, Lanzafame A, Kirby C, Weekes A, et al. (2013) The prevalence of vitamin D deficiency and relationship with fracture risk in older women presenting in Australian general practice. Australas J Ageing 32: 177–183. [DOI] [PubMed] [Google Scholar]
- 5.Vernay M, Sponga M, Salanave B, Oléko A, Deschamps V, et al. (2012) Statut en vitamine D de la population adulte en France: l’Étude Nationale Nutrition Santé (ENNS, 2006–2007). In: Bulletin Epidémiologique Hebdomadaire n° 16–17/2012, pp. 189–194. Available: http://docireps971.canalblog.com/archives/2012/06/06/24433922.html. Accessed 9 December 2013.
- 6. Gallagher JC, Yalamanchili V, Smith LM (2013) The effect of vitamin D supplementation on serum 25(OH)D in thin and obese women. J Steroid Biochem Mol Biol 136: 195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. MacLaughlin J, Holick MF (1985) Aging decreases the capacity of human skin to produce vitamin D3. J Clin Invest 76: 1536–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chel VG, Ooms ME, Popp-Snijders C, Pavel S, Schothorst AA, et al. (1998) Ultraviolet irradiation corrects vitamin D deficiency and suppresses secondary hyperparathyroidism in the elderly. J Bone Miner Res 13: 1238–1242. [DOI] [PubMed] [Google Scholar]
- 9. Toffanello ED, Perissinotto E, Sergi G, Zambon S, Musacchio E, et al. (2012) Vitamin D and physical performance in elderly subjects: the Pro.V.A study. PLOS ONE 7: e34950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Scragg R, Camargo CA (2008) Frequency of leisure-time physical activity and serum 25-hydroxyvitamin D levels in the US population: results from the third National Health and Nutrition Examination Survey. Am J Epidemiology 168: 577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kluczynski MA, Lamonte MJ, Mares JA, Wactawski-Wende J, Smith AW, et al. (2011) Duration of physical activity and serum 25-hydroxyvitamin D status of postmenopausal women. Ann Epidemiol 21: 440–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Corti M-C, Guralnik JM, Sartori L, Baggio G, Manzato E, et al. (2002) The effect of cardiovascular and osteoarticular diseases on disability in older Italian men and women: rationale, design, and sample characteristics of the Progetto Veneto Anziani (Pro.V.A.) Study. J Am Geriatr Soc 50: 1535–1540. [DOI] [PubMed] [Google Scholar]
- 13. Guyatt GH, Sullivan MJ, Thompson PJ, Fallen EL, Pugsley SO, et al. (1985) The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J 132: 919–923. [PMC free article] [PubMed] [Google Scholar]
- 14.Parmelee PA, Lawton MP, Katz IR (1989) Psychological Assessment: A Journal of Consulting and Clinical Psychology 1, 331–338.
- 15. Folstein MF, Folstein SE, Mc Hugh PR (1975) “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: 189–198. [DOI] [PubMed] [Google Scholar]
- 16. Crum RM, Anthony JC, Bassett SS, Folstein MF (1993) Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA 269: 2386–2391. [PubMed] [Google Scholar]
- 17. Maeda SS, Saraiva GL, Kunii IS, Hayashi LF, Cendoroglo MS, et al. (2013) Factors affecting vitamin D status in different populations in the city of Sao Paulo, Brazil: the Sao Paulo vitamin D Evaluation Study (SPADES). BMC Endocr Disord 13: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van Dam RM, Snijder MB, Dekker JM, Stehouwer CD, Bouter LM, et al. (2007) Potentially modifiable determinants of vitamin D status in an older population in the Netherlands: the Hoorn Study. Am J Clin Nutr 85: 755–761. [DOI] [PubMed] [Google Scholar]
- 19. Coin A, Sergi G, Minicuci N, Giannini S, Barbiero E, et al. (2008) Fat-free mass and fat mass reference values by dual-energy X-ray absorptiometry (DEXA) in a 20-80 year-old Italian population. Clin Nutr 27: 87–94. [DOI] [PubMed] [Google Scholar]
- 20. Zittermann A, Sabatschus O, Jantzen S, Platen P, Danz A, et al. (2000) Exercise-trained young men have higher calcium absorption rates and plasma calcitriol levels compared with age-matched sedentary controls. Calcif Tissue Int 67: 215–219. [DOI] [PubMed] [Google Scholar]
- 21. Gómez J (2006) The role of insulin-like growth factor I components in the regulation of vitamin D. Curr Pharm Biotechnol. 7: 125–132. [DOI] [PubMed] [Google Scholar]
- 22. Barry DW, Kohrt WM (2007) Acute effects of 2 hours of moderate-intensity cycling on serum parathyroid hormone and calcium. Calcif Tissue Int 80: 359–365. [DOI] [PubMed] [Google Scholar]