Abstract
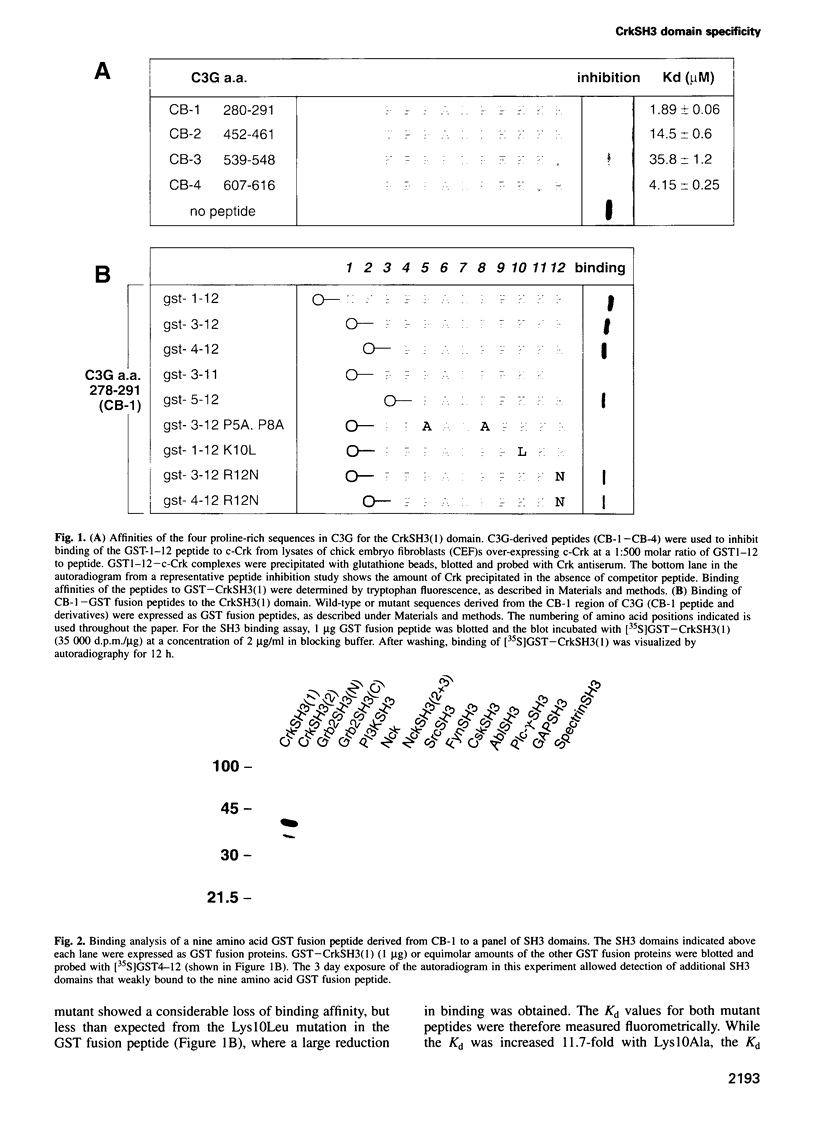
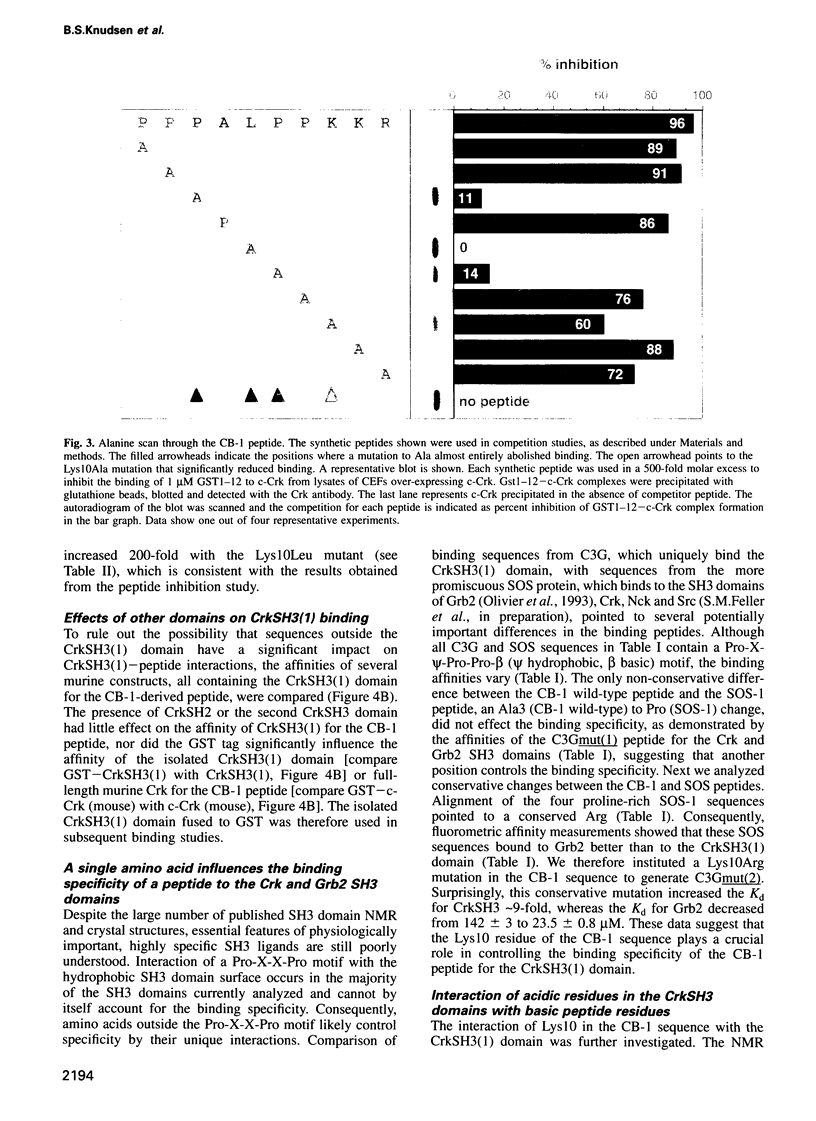
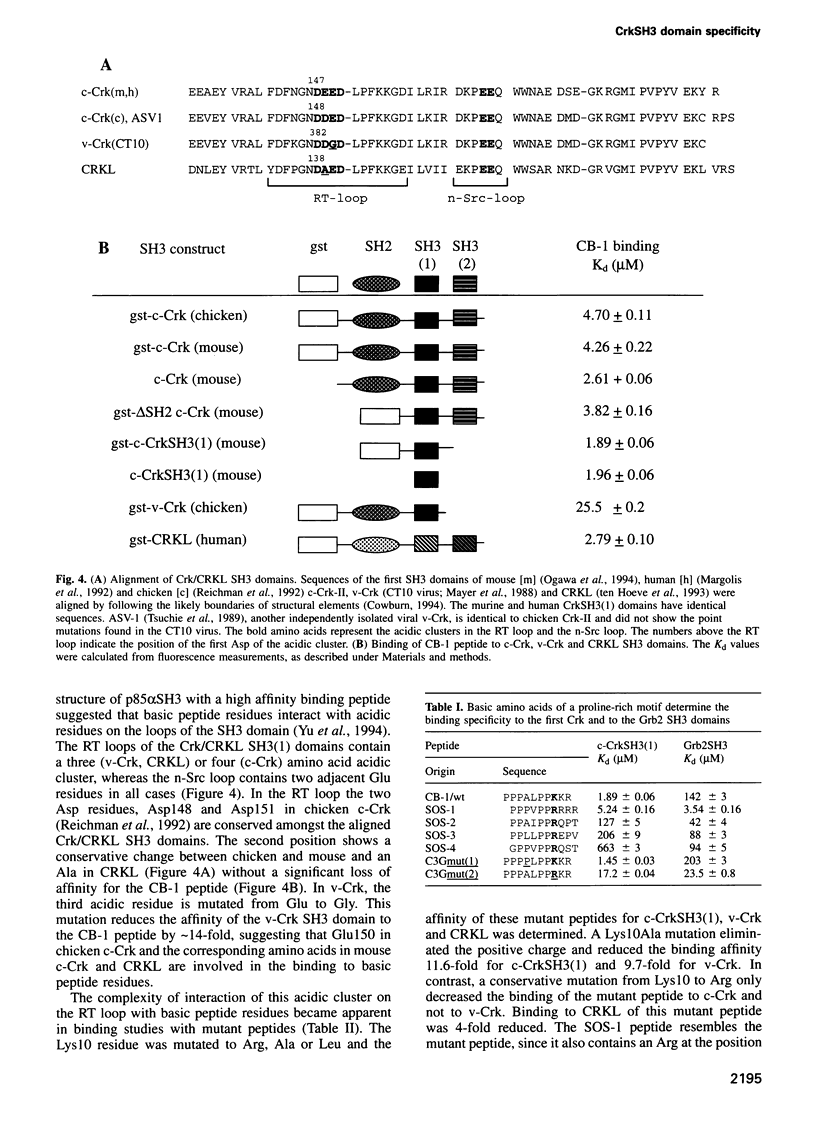
The specificity of SH3 domain complex formation plays an important role in determining signal transduction events. We have previously identified a highly specific interaction between the first CrkSH3 domain [CrkSH3(1)] and proline-rich sequences in the guanine nucleotide exchange factor C3G. A 10 amino acid peptide derived from the first proline-rich sequence (P3P4P5A6L7P8P9K10K11R12) bound with a Kd of 1.89 +/- 0.06 microM and fully retained the high affinity and unique selectivity for the CrkSH3(1) domain. Mutational analysis showed that P5, P8, L7 and K10 are critical for high affinity binding. A conservative mutation, K10R, significantly decreased the affinity for the CrkSH3(1) domain while increasing the affinity for Grb2. Comparative binding studies with the K10R and K10A mutant peptides to c-Crk and v-Crk further suggested that K10 binds via a charge-dependent and a charge-independent interaction to the RT loop of the CrkSH3(1) domain. Besides determining important structural features necessary for high affinity and specificity binding to the CrkSH3(1) domain, our results also demonstrate that a conservative mutation in a single amino acid can significantly alter the specificity of an SH3 binding peptide.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bar-Sagi D., Rotin D., Batzer A., Mandiyan V., Schlessinger J. SH3 domains direct cellular localization of signaling molecules. Cell. 1993 Jul 16;74(1):83–91. doi: 10.1016/0092-8674(93)90296-3. [DOI] [PubMed] [Google Scholar]
- Booker G. W., Gout I., Downing A. K., Driscoll P. C., Boyd J., Waterfield M. D., Campbell I. D. Solution structure and ligand-binding site of the SH3 domain of the p85 alpha subunit of phosphatidylinositol 3-kinase. Cell. 1993 May 21;73(4):813–822. doi: 10.1016/0092-8674(93)90259-s. [DOI] [PubMed] [Google Scholar]
- Chardin P., Camonis J. H., Gale N. W., van Aelst L., Schlessinger J., Wigler M. H., Bar-Sagi D. Human Sos1: a guanine nucleotide exchange factor for Ras that binds to GRB2. Science. 1993 May 28;260(5112):1338–1343. doi: 10.1126/science.8493579. [DOI] [PubMed] [Google Scholar]
- Cheadle C., Ivashchenko Y., South V., Searfoss G. H., French S., Howk R., Ricca G. A., Jaye M. Identification of a Src SH3 domain binding motif by screening a random phage display library. J Biol Chem. 1994 Sep 30;269(39):24034–24039. [PubMed] [Google Scholar]
- Cheng G., Ye Z. S., Baltimore D. Binding of Bruton's tyrosine kinase to Fyn, Lyn, or Hck through a Src homology 3 domain-mediated interaction. Proc Natl Acad Sci U S A. 1994 Aug 16;91(17):8152–8155. doi: 10.1073/pnas.91.17.8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti P., Mayer B. J., Thiel G., Baltimore D. Identification of a protein that binds to the SH3 region of Abl and is similar to Bcr and GAP-rho. Science. 1992 Aug 7;257(5071):803–806. doi: 10.1126/science.1379745. [DOI] [PubMed] [Google Scholar]
- Cowburn D. Helical encounter. Nat Struct Biol. 1994 Aug;1(8):489–491. doi: 10.1038/nsb0894-489. [DOI] [PubMed] [Google Scholar]
- Cussac D., Frech M., Chardin P. Binding of the Grb2 SH2 domain to phosphotyrosine motifs does not change the affinity of its SH3 domains for Sos proline-rich motifs. EMBO J. 1994 Sep 1;13(17):4011–4021. doi: 10.1002/j.1460-2075.1994.tb06717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feller S. M., Knudsen B., Hanafusa H. c-Abl kinase regulates the protein binding activity of c-Crk. EMBO J. 1994 May 15;13(10):2341–2351. doi: 10.1002/j.1460-2075.1994.tb06518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feller S. M., Ren R., Hanafusa H., Baltimore D. SH2 and SH3 domains as molecular adhesives: the interactions of Crk and Abl. Trends Biochem Sci. 1994 Nov;19(11):453–458. doi: 10.1016/0968-0004(94)90129-5. [DOI] [PubMed] [Google Scholar]
- Feng S., Chen J. K., Yu H., Simon J. A., Schreiber S. L. Two binding orientations for peptides to the Src SH3 domain: development of a general model for SH3-ligand interactions. Science. 1994 Nov 18;266(5188):1241–1247. doi: 10.1126/science.7526465. [DOI] [PubMed] [Google Scholar]
- Finan P., Shimizu Y., Gout I., Hsuan J., Truong O., Butcher C., Bennett P., Waterfield M. D., Kellie S. An SH3 domain and proline-rich sequence mediate an interaction between two components of the phagocyte NADPH oxidase complex. J Biol Chem. 1994 May 13;269(19):13752–13755. [PubMed] [Google Scholar]
- Gout I., Dhand R., Hiles I. D., Fry M. J., Panayotou G., Das P., Truong O., Totty N. F., Hsuan J., Booker G. W. The GTPase dynamin binds to and is activated by a subset of SH3 domains. Cell. 1993 Oct 8;75(1):25–36. [PubMed] [Google Scholar]
- Knudsen B. S., Feller S. M., Hanafusa H. Four proline-rich sequences of the guanine-nucleotide exchange factor C3G bind with unique specificity to the first Src homology 3 domain of Crk. J Biol Chem. 1994 Dec 30;269(52):32781–32787. [PubMed] [Google Scholar]
- Lim W. A., Richards F. M. Critical residues in an SH3 domain from Sem-5 suggest a mechanism for proline-rich peptide recognition. Nat Struct Biol. 1994 Apr;1(4):221–225. doi: 10.1038/nsb0494-221. [DOI] [PubMed] [Google Scholar]
- Lim W. A., Richards F. M., Fox R. O. Structural determinants of peptide-binding orientation and of sequence specificity in SH3 domains. Nature. 1994 Nov 24;372(6504):375–379. doi: 10.1038/372375a0. [DOI] [PubMed] [Google Scholar]
- Liu X., Marengere L. E., Koch C. A., Pawson T. The v-Src SH3 domain binds phosphatidylinositol 3'-kinase. Mol Cell Biol. 1993 Sep;13(9):5225–5232. doi: 10.1128/mcb.13.9.5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis B., Silvennoinen O., Comoglio F., Roonprapunt C., Skolnik E., Ullrich A., Schlessinger J. High-efficiency expression/cloning of epidermal growth factor-receptor-binding proteins with Src homology 2 domains. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):8894–8898. doi: 10.1073/pnas.89.19.8894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda M., Hashimoto Y., Muroya K., Hasegawa H., Kurata T., Tanaka S., Nakamura S., Hattori S. CRK protein binds to two guanine nucleotide-releasing proteins for the Ras family and modulates nerve growth factor-induced activation of Ras in PC12 cells. Mol Cell Biol. 1994 Aug;14(8):5495–5500. doi: 10.1128/mcb.14.8.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer B. J., Baltimore D. Mutagenic analysis of the roles of SH2 and SH3 domains in regulation of the Abl tyrosine kinase. Mol Cell Biol. 1994 May;14(5):2883–2894. doi: 10.1128/mcb.14.5.2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer B. J., Hamaguchi M., Hanafusa H. Characterization of p47gag-crk, a novel oncogene product with sequence similarity to a putative modulatory domain of protein-tyrosine kinases and phospholipase C. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 2):907–914. doi: 10.1101/sqb.1988.053.01.104. [DOI] [PubMed] [Google Scholar]
- Mayer B. J., Hanafusa H. Association of the v-crk oncogene product with phosphotyrosine-containing proteins and protein kinase activity. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2638–2642. doi: 10.1073/pnas.87.7.2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musacchio A., Saraste M., Wilmanns M. High-resolution crystal structures of tyrosine kinase SH3 domains complexed with proline-rich peptides. Nat Struct Biol. 1994 Aug;1(8):546–551. doi: 10.1038/nsb0894-546. [DOI] [PubMed] [Google Scholar]
- Musacchio A., Wilmanns M., Saraste M. Structure and function of the SH3 domain. Prog Biophys Mol Biol. 1994;61(3):283–297. doi: 10.1016/0079-6107(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Ogawa S., Toyoshima H., Kozutsumi H., Hagiwara K., Sakai R., Tanaka T., Hirano N., Mano H., Yazaki Y., Hirai H. The C-terminal SH3 domain of the mouse c-Crk protein negatively regulates tyrosine-phosphorylation of Crk associated p130 in rat 3Y1 cells. Oncogene. 1994 Jun;9(6):1669–1678. [PubMed] [Google Scholar]
- Olivier J. P., Raabe T., Henkemeyer M., Dickson B., Mbamalu G., Margolis B., Schlessinger J., Hafen E., Pawson T. A Drosophila SH2-SH3 adaptor protein implicated in coupling the sevenless tyrosine kinase to an activator of Ras guanine nucleotide exchange, Sos. Cell. 1993 Apr 9;73(1):179–191. doi: 10.1016/0092-8674(93)90170-u. [DOI] [PubMed] [Google Scholar]
- Pleiman C. M., Hertz W. M., Cambier J. C. Activation of phosphatidylinositol-3' kinase by Src-family kinase SH3 binding to the p85 subunit. Science. 1994 Mar 18;263(5153):1609–1612. doi: 10.1126/science.8128248. [DOI] [PubMed] [Google Scholar]
- Rawlings D. J., Saffran D. C., Tsukada S., Largaespada D. A., Grimaldi J. C., Cohen L., Mohr R. N., Bazan J. F., Howard M., Copeland N. G. Mutation of unique region of Bruton's tyrosine kinase in immunodeficient XID mice. Science. 1993 Jul 16;261(5119):358–361. doi: 10.1126/science.8332901. [DOI] [PubMed] [Google Scholar]
- Reichman C. T., Mayer B. J., Keshav S., Hanafusa H. The product of the cellular crk gene consists primarily of SH2 and SH3 regions. Cell Growth Differ. 1992 Jul;3(7):451–460. [PubMed] [Google Scholar]
- Ren R., Mayer B. J., Cicchetti P., Baltimore D. Identification of a ten-amino acid proline-rich SH3 binding site. Science. 1993 Feb 19;259(5098):1157–1161. doi: 10.1126/science.8438166. [DOI] [PubMed] [Google Scholar]
- Ren R., Ye Z. S., Baltimore D. Abl protein-tyrosine kinase selects the Crk adapter as a substrate using SH3-binding sites. Genes Dev. 1994 Apr 1;8(7):783–795. doi: 10.1101/gad.8.7.783. [DOI] [PubMed] [Google Scholar]
- Rotin D., Bar-Sagi D., O'Brodovich H., Merilainen J., Lehto V. P., Canessa C. M., Rossier B. C., Downey G. P. An SH3 binding region in the epithelial Na+ channel (alpha rENaC) mediates its localization at the apical membrane. EMBO J. 1994 Oct 3;13(19):4440–4450. doi: 10.1002/j.1460-2075.1994.tb06766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozakis-Adcock M., McGlade J., Mbamalu G., Pelicci G., Daly R., Li W., Batzer A., Thomas S., Brugge J., Pelicci P. G. Association of the Shc and Grb2/Sem5 SH2-containing proteins is implicated in activation of the Ras pathway by tyrosine kinases. Nature. 1992 Dec 17;360(6405):689–692. doi: 10.1038/360689a0. [DOI] [PubMed] [Google Scholar]
- Seedorf K., Kostka G., Lammers R., Bashkin P., Daly R., Burgess W. H., van der Bliek A. M., Schlessinger J., Ullrich A. Dynamin binds to SH3 domains of phospholipase C gamma and GRB-2. J Biol Chem. 1994 Jun 10;269(23):16009–16014. [PubMed] [Google Scholar]
- Sparks A. B., Quilliam L. A., Thorn J. M., Der C. J., Kay B. K. Identification and characterization of Src SH3 ligands from phage-displayed random peptide libraries. J Biol Chem. 1994 Sep 30;269(39):23853–23856. [PubMed] [Google Scholar]
- Tamemoto H., Kadowaki T., Tobe K., Yagi T., Sakura H., Hayakawa T., Terauchi Y., Ueki K., Kaburagi Y., Satoh S. Insulin resistance and growth retardation in mice lacking insulin receptor substrate-1. Nature. 1994 Nov 10;372(6502):182–186. doi: 10.1038/372182a0. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Morishita T., Hashimoto Y., Hattori S., Nakamura S., Shibuya M., Matuoka K., Takenawa T., Kurata T., Nagashima K. C3G, a guanine nucleotide-releasing protein expressed ubiquitously, binds to the Src homology 3 domains of CRK and GRB2/ASH proteins. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3443–3447. doi: 10.1073/pnas.91.8.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchie H., Chang C. H., Yoshida M., Vogt P. K. A newly isolated avian sarcoma virus, ASV-1, carries the crk oncogene. Oncogene. 1989 Nov;4(11):1281–1284. [PubMed] [Google Scholar]
- Williamson M. P. The structure and function of proline-rich regions in proteins. Biochem J. 1994 Jan 15;297(Pt 2):249–260. doi: 10.1042/bj2970249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Chen J. K., Feng S., Dalgarno D. C., Brauer A. W., Schreiber S. L. Structural basis for the binding of proline-rich peptides to SH3 domains. Cell. 1994 Mar 11;76(5):933–945. doi: 10.1016/0092-8674(94)90367-0. [DOI] [PubMed] [Google Scholar]
- ten Hoeve J., Morris C., Heisterkamp N., Groffen J. Isolation and chromosomal localization of CRKL, a human crk-like gene. Oncogene. 1993 Sep;8(9):2469–2474. [PubMed] [Google Scholar]






