Abstract
OBJECTIVE
Staphylococcus aureus is a cause of community- and healthcare-acquired infections and is associated with substantial morbidity, mortality, and costs. Vancomycin minimum inhibitory concentrations (MICs) among S. aureus have increased, and reduced vancomycin susceptibility (RVS) may be associated with treatment failure. We aimed to identify clinical risk factors for RVS in S. aureus bacteremia.
DESIGN
Case-control.
SETTING
Academic tertiary care medical center and affiliated urban community hospital.
PATIENTS
Cases were patients with RVS S. aureus isolates (defined as vancomycin E-test MIC >1.0 μg/mL). Controls were patients with non-RVS S. aureus isolates.
RESULTS
Of 392 subjects, 134 (34.2%) had RVS. Fifty-eight of 202 patients (28.7%) with methicillin-susceptible S. aureus (MSSA) isolates had RVS, and 76 of 190 patients (40.0%) with methicillin-resistant S. aureus (MRSA) isolates had RVS (P =.02). In unadjusted analyses, prior vancomycin use was associated with RVS (odds ratio [OR], 2.08; 95% confidence interval [CI], 1.00–4.32; P =.046). In stratified analyses, there was significant effect modification by methicillin susceptibility on the association between vancomycin use and RVS (P = .04). In multivariable analyses, after hospital of admission and prior levofloxacin use were controlled for, the association between vancomycin use and RVS was significant for patients with MSSA infection (adjusted OR, 4.02; 95% CI, 1.11–14.50) but not MRSA infection (adjusted OR, 0.87; 95% CI, 0.36–2.13).
CONCLUSIONS
A substantial proportion of patients with S. aureus bacteremia had RVS. The association between prior vancomycin use and RVS was significant for patients with MSSA infection but not MRSA infection, suggesting a complex relationship between the clinical and molecular epidemiology of RVS in S. aureus.
Staphylococcus aureus, especially methicillin-resistant S. aureus (MRSA), is a significant cause of community- and health-care-acquired infections and is associated with substantial morbidity, mortality, length of hospital stay, and healthcare costs.1 For decades, vancomycin has been the mainstay of therapy for infections due to MRSA as well as infections due to methicillin-susceptible S. aureus (MSSA) when patients cannot tolerate beta-lactam antibiotics.2 However, delayed microbiological responses and therapeutic failures with vancomycin are well recognized.3
Recently it has been observed in some studies that minimum inhibitory concentrations (MICs) for vancomycin among both MRSA and MSSA have increased over time, especially when the E-test method is used for MIC determination.4,5 Several studies suggest that this reduced vancomycin susceptibility (RVS) may be associated with vancomycin treatment failures in cases of S. aureus infection, especially bacteremia.6–12 Limited in vitro studies also suggest that RVS also may be associated with resistance to other alternative antibiotics such as daptomycin.13,14
Given these concerns, it is critical that risk factors for RVS in S. aureus infection be clearly identified so that effective strategies to limit RVS may be developed. Few studies have attempted to elucidate epidemiological characteristics of RVS in S. aureus bacteremia10,15,16 and have been limited by small sample size,15,16 enrollment of patients over prolonged study periods during which clinical risk factors and patterns of antibiotic use could have changed,10 limited use of multivariable regression analysis,10,15,16 and exclusion of MSSA infections.10,15,16 We conducted the current study to identify clinical risk factors for bloodstream infections with S. aureus (both MRSA and MSSA) with RVS, with the specific hypothesis that prior vancomycin use is a significant risk factor for RVS.
METHODS
This study was conducted at 2 hospitals within the University of Pennsylvania Health System in Philadelphia, Pennsylvania: the Hospital of the University of Pennsylvania (HUP), a 725-bed academic tertiary care medical center, and Penn Presbyterian Medical Center (PPMC), a 344-bed urban community hospital. The study was reviewed and approved by the institutional review board of the University of Pennsylvania.
Study subjects were identified through records of the clinical microbiology laboratory, which processes and cultures all specimens obtained from patients at HUP and PPMC. All adult patients who had an inpatient blood culture positive for S. aureus during the period from December 1, 2007, through May 31, 2009, were eligible for inclusion. Each subject could be included only once, using the first S. aureus–positive blood culture identified during the study period.
Bacterial identification and susceptibility testing were performed and interpreted according to standard methods,17–20 using a semiautomated system (Vitek2, Biomerieux) or disk diffusion method. In addition, all isolates were tested by supplemental methods, using the E-test and microbroth dilution susceptibility methods. Vancomycin and daptomycin MICs were determined by E-test, using Mueller-Hinton agar (BBL, BD Diagnostic Systems)21 and, for comparison, microbroth dilution method.19 The microbroth dilution susceptibility panel was custom manufactured by Trek Diagnostic Systems and included half dilutions of vancomycin. The presence of glycopeptide heteroresistance was screened for by the macroE-test method, using E-test GRD vancomycin/teicoplanin strips with brain heart infusion agar (BBL)21 and by plating on brain heart infusion agar containing vancomycin;22 all screen-positive isolates were confirmed by population analysis, using a spiral plater (Advanced Instruments) and plating on brain heart infusion agar (BBL) containing vancomycin.23,24 RVS was primarily defined as a vancomycin E-test MIC greater than 1.0 μg/mL.25 In secondary analyses, RVS was defined as a vancomycin broth dilution MIC greater than 0.5 μg/mL.9
To assess the relationship between RVS and prior vancomycin use, we conducted a case-control study. All patients with RVS were considered case patients, and all subjects without RVS were considered control patients. All eligible control patients were included. Prior vancomycin use was defined as receipt of 1 or more doses of vancomycin during the same hospitalization up to 30 days prior to infection.
Other potential risk factors and confounders for RVS were ascertained through the use of a comprehensive clinical and administrative database, which has been used effectively in past studies of antibiotic use and antibiotic resistance.26,27 Data obtained included age, sex, race, the hospital of admission (ie, HUP or PPMC), origin at the time of hospital admission (eg, home or transfer from another facility), hospital location at the time of infection (eg, intensive care unit [ICU] or medical floor), and number of hospital and ICU-days prior to infection. The presence of the following comorbid conditions was documented: diabetes mellitus, renal insufficiency (creatinine level >2.0 mg/dL or the requirement of hemodialysis), malignancy, prior organ transplantation, human immunodeficiency virus, neutropenia (absolute neutrophil count <500/mm3), and use of an immunosuppressive agent or steroids in the 30 days prior to infection. The Charlson comorbidity index was calculated for each subject.28
All inpatient antimicrobial therapy administered during the same hospitalization up to 30 days prior to infection was obtained. Antimicrobial agents were categorized by specific agents (if only 1 agent of a given class was used) or by class, including aminoglycosides (eg, amikacin, gentamicin, and tobramycin), other penicillins (eg, penicillin, ampicillin, and ampicillin/sulbactam), extended-spectrum cephalosporins (eg, ceftriaxone, cefotaxime, and cefepime), other cephalosporins (eg, cefazolin), and macrolides (eg, azithromycin and erythromycin).29 In analyses, antimicrobial use was categorized as “use” versus “no use” during the prior 30 days.
Statistical Methods
Cases and controls were first characterized by all potential risk factors, including demographics, comorbid conditions, and prior antibiotic use. Bivariable analyses were then conducted to determine the association between potential risk factors and RVS, with a primary focus on the association between prior vancomycin use and RVS. For the primary analysis, RVS was defined using E-test MICs. Categorical values were compared using the Fisher exact test. An odds ratio (OR) and 95% confidence interval (CI) were calculated to evaluate the strength of any association. Continuous variables were compared using the Wilcoxon rank-sum test.30
Stratified analyses were then performed to identify where data were sparse and to elucidate where confounding and interactions were likely to exist in multivariable analyses. In particular, we specifically performed stratified analyses based on methicillin susceptibility (eg, MSSA vs MRSA), as we hypothesized that this risk factor might be an important confounder or effect modifier. Interaction was assumed to be present when the test for heterogeneity between the ORs for different strata yielded a significant result (P < .05). The Mantel-Haenszel test for summary statistics was used to evaluate the effects of each variable of interest as a possible confounder.31
Multivariable analyses were performed using multiple logistic regression.32 The building of the multivariable model began with the inclusion of the key risk factor variable of interest (ie, prior vancomycin use). All variables with a P value less than 0.20 on bivariable analyses were considered for inclusion in the multivariable explanatory model,33 as were variables noted to be confounders on stratified analyses. A variable remained in the final model if its inclusion resulted in more than a 15% change in the effect size for the primary association of interest (ie, vancomycin use and RVS).34
As a secondary analysis, the above analyses were repeated using RVS defined using broth dilution MICs (rather than E-test). A comparison of E-test MIC values and broth dilution MIC values also was performed using the Spearman rank correlation. For all calculations, a 2-tailed P value less than 0.05 was considered significant. All statistical calculations were performed using standard programs in Stata, version 10.0 (StataCorp).
RESULTS
Over the 18-month study period, there were 392 unique patients with a blood culture positive for S. aureus, 202 (51.5%) with MSSA, and 190 (48.5%) with MRSA. Two hundred ninety-two (74.4%) of these patients had bacteremia present on or within 48 hours of admission. The median age of patients was 57 (range, 18–95), 241 (61.5%) were males, and 193 (49.2%) were white. Among all subjects, 274 (69.9%) were hospitalized at HUP, and 118 (30.1%) were hospitalized at PPMC.
The distribution of vancomycin MICs as determined by E-test among the 392 subjects was 17 (4.3%) with MIC at most 0.5 μg/mL, 83 (21.2%) with MIC of 0.75 μg/mL, 158 (40.3%) with MIC of 1.0 μg/mL, 123 (31.4%) with MIC of 1.5 μg/mL, and 11 (2.8%) with MIC of 2.0 μg/mL. Accordingly, 134 (34.2%) had RVS as defined by E-test MIC greater than 1.0 μg/mL. Fifty-eight of 202 patients (28.7%) with MSSA had RVS, and 76 of 190 patients (40.0%) with MRSA had RVS (P = .02).
In bivariable analyses, several variables were noted to be significantly associated with RVS (Table 1). Inpatient vancomycin use within the prior 30 days was associated with RVS (OR, 2.08; 95% CI, 1.00–4.32; P = .046). The other antibiotics associated with RVS were agents with broad-spectrum anti-gram-negative and anaerobic activity. Onset of infection more than 48 hours after hospital admission and location in the ICU more than 48 hours prior to infection were each independently associated with RVS, while age (P = .67), Charlson score (P = .88), and exposure in the prior 30 days to steroids (P = .74) or other immunosuppressive agents (P = .84) were not.
TABLE 1.
Results of Bivariable Analysis of Risk Factors for Reduced Vancomycin Susceptibility in Staphylococcus aureus Bacteremia
| Variable | Case patients (n = 134) | Control patients (n = 258) | OR (95% CI) | P |
|---|---|---|---|---|
| Hospital length of stay prior to infection, median (IQR), days | 0 (0–4) | 0 (0–1) | 1.00 (0.99–1.01) | .02 |
| Stay at Penn Presbyterian Medical Centera | 48 (35.8) | 70 (27.1) | 1.50 (0.93–2.40) | .08 |
| Nonwhite race | 56 (43.4) | 129 (51.2) | 0.71 (0.45–1.12) | .13 |
| Hospital onset of infectionb | 40 (29.8) | 60 (23.3) | 1.40 (0.85–2.30) | .18 |
| Location in ICU >48 hours prior to infection | 23 (17.2) | 27 (10.5) | 1.77 (0.92–3.37) | .08 |
| Inpatient antimicrobial use in prior 30 days | ||||
| Vancomycin | 19 (14.2) | 19 (7.4) | 2.08 (1.00–4.32) | .046 |
| Piperacillin/tazobactam | 3 (2.2) | 1 (0.4) | 5.89 (0.47–310.10) | .12 |
| Extended-spectrum cephalosporinc | 12 (9.0) | 12 (4.6) | 2.02 (0.80–5.06) | .12 |
| Aztreonam | 3 (2.2) | 1 (0.4) | 5.89 (0.46–310.10) | .12 |
| Levofloxacin | 21 (15.7) | 15 (5.8) | 3.01 (1.42–6.51) | .003 |
| Aminoglycosided | 10 (7.5) | 7 (2.7) | 2.89 (0.96–9.15) | .04 |
| Metronidazole | 14 (10.4) | 16 (6.2) | 1.76 (0.77–4.00) | .16 |
NOTE. Data are no. (%) of patients, unless otherwise indicated. Only variables with P less than .20 are shown. The Fisher exact test was used for categorical variables and the Wilcoxon rank-sum test for continuous variables. CI, confidence interval; ICU, intensive care unit; IQR, interquartile range; OR, odds ratio.
As opposed to the Hospital of the University of Pennsylvania.
Onset of infection more than 48 hours after hospital admission.
Ceftriaxone, cefotaxime, or cefepime.
Amikacin, gentamicin, tobramycin.
In unadjusted stratified analyses, there was significant effect modification by methicillin susceptibility (P = .04). Specifically, a significant association between prior vancomycin use and RVS was noted for subjects with MSSA (OR, 5.60; 95% CI, 1.41–26.27) but not for subjects with MRSA (OR, 1.12; 95% CI, 0.43–2.79).
In multivariable analyses, prior vancomycin use remained an independent risk factor for RVS, but this association differed significantly by methicillin susceptibility (Table 2). After controlling for other significant confounders (ie, the hospital to which the patient was admitted and inpatient use of levofloxacin in the prior 30 days), the association between prior vancomycin use and RVS was significant for patients with MSSA infection (adjusted OR, 4.02; 95% CI, 1.11–14.50) but not for patients with MRSA infection (adjusted OR, 0.87; 95% CI, 0.36–2.13).
TABLE 2.
Multivariable Model of Risk Factors for Reduced Vancomycin Susceptibility in Staphylococcus aureus Bacteremia
| Variable | Adjusted OR (95% CI) | P |
|---|---|---|
| Inpatient vancomycin use in prior 30 daysa | 4.02 (1.11–14.50)b | .03 |
| Stay at Penn Presbyterian Medical Centerc | 1.46 (0.92–2.32) | .10 |
| Inpatient levofloxacin use in prior 30 daysd | 2.42 (1.14–5.14) | .02 |
| MRSAe | 1.79 (1.14–2.83) | .01 |
| Interaction between vancomycin use and methicillin susceptibility | 0.22 (0.05–1.00) | .05 |
NOTE. CI, confidence interval; MRSA, methicillin-resistant S. aureus; OR, odds ratio; SE, standard error.
Coefficient and OR reflect those subjects with methicillin-susceptible S. aureus (MSSA).
Adjusted OR (95% CI) for the association between inpatient vancomycin use in prior 30 days and reduced vancomycin susceptibility was 4.02 (1.11–14.50) in patients with MSSA infection and 0.87 (0.36–2.13) in patients with MRSA infection.
As opposed to the Hospital of the University of Pennsylvania.
As opposed to no inpatient levofloxacin use in prior 30 days.
As opposed to MSSA.
In a secondary analysis, these analyses were repeated with RVS defined using broth dilution MIC cutoffs. The distribution of vancomycin MICs as determined by broth dilution among the 392 subjects was 1 (0.3%) with MIC of 0.25 μg/mL, 78 (19.9%) with MIC of 0.5 μg/mL, 240 (61.2%) with MIC of 0.75 μg/mL, 62 (15.8%) with MIC of 1.0 μg/mL, and 11 (2.8%) with MIC of 1.5 μg/mL. Accordingly, 313 (79.8%) had RVS as defined by broth dilution MIC greater than 0.5 μg/mL. There was a significant, albeit weak, correlation between vancomycin E-test MICs and vancomycin broth dilution MICs (Spearman correlation, 0.50; P < .0001). Inpatient vancomycin use within the prior 30 days was not significantly associated with RVS as defined by broth dilution on either bivariable (OR, 1.39; 95% CI, 0.56–3.44) or multivariable (OR, 1.02; 95% CI, 0.38–2.72) analyses.
The distribution of daptomycin MICs as determined by E-test among the 392 subjects was 34 (8.7%) with MIC of 0.125 μg/mL, 85 (21.7%) with MIC of 0.25 μg/mL, 215 (54.8%) with MIC of 0.5 μg/mL, 48 (12.2%) with MIC of 0.75 μg/mL, 5 (1.3%) with MIC of 1.0 μg/mL, 3 (0.7%) with MIC of 1.5 μg/mL, 1 (0.3%) with MIC of 2.0 μg/mL, and 1 (0.3%) with MIC of 4.0 μg/mL. The distribution of daptomycin MICs as determined by broth dilution among the 392 subjects was 176 (44.9%) with MIC of 0.25 μg/mL, 200 (51.0%) with MIC of 0.5 μg/mL, 14 (3.6%) with MIC of 1.0 μg/mL, and 2 (0.5%) with MIC of 2.0 μg/mL. Of the 392 subjects, 27 screened positive for glycopeptide heteroresistance, and 20 were con-firmed. Comparisons of daptomycin MICs as determined by E-test and broth dilution, as well as rates of glycopeptide heteroresistance between cases and controls, are shown in Figure 1.
FIGURE 1.
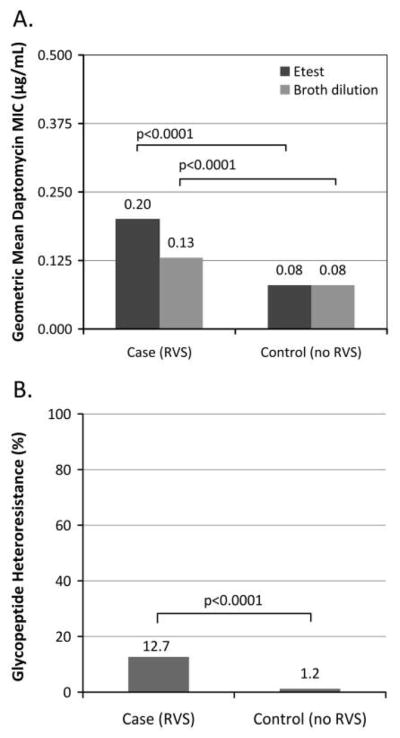
A, Difference in daptomycin E-test and broth dilution minimum inhibitory concentrations in Staphylococcus aureus bloodstream isolates with and without reduced vancomycin susceptibility (RVS). B, Difference in glycopeptide heteroresistance in S. aureus bloodstream isolates with and without RVS.
DISCUSSION
In this 18-month, health system–wide study involving 392 patients with S. aureus bacteremia, we found that 134 (34.2%) S. aureus isolates demonstrated RVS. The proportion of patients with RVS differed significantly between those with MSSA (28.7%) as compared with MRSA (40.0%) infection. In the final multivariable model controlling for hospital to which the patient was admitted and use of levofloxacin in the prior 30 days, the association between prior vancomycin use and RVS was statistically significant only for patients with MSSA, not those with MRSA.
Only a limited number of studies have examined the relationship between prior vancomycin use and RVS. In contrast to our findings, one study evaluating 105 patients with only MRSA bloodstream isolates found that prior vancomycin use was an independent predictor of RVS in MRSA isolates as defined by a vancomycin E-test MIC greater than or equal to 1.5.15 The OR on multivariable analysis for recent vancomycin exposure, however, had a very wide confidence interval (1.1–80.7), likely reflecting limited sample size.15 Also, the high prevalence of RVS (73.3%) observed in that study suggests an underlying cohort quite distinct from our study population.15 A second smaller study of 81 MRSA bacteremias also found an independent association between prior vancomycin use and RVS; however, vancomycin MIC cutoffs were determined by microbroth dilution and not E-test, and that study cohort was enriched for prior vancomycin intolerance or failure.16
A novel finding identified in our study, and one not investigated previously, was that prior vancomycin use was a significant risk factor for RVS only for patients with MSSA infection, not those with MRSA infection. One explanation for this finding could be an increased microbial fitness of MSSA strains with RVS (MSSA-RVS) when compared with MRSA strains with RVS (MRSA-RVS).35 If it is assumed that patients can be simultaneously colonized with both MRSA and MSSA strains,36,37 prior vancomycin use might provide selective pressure for the development of both MSSA-RVS and MRSA-RVS in patients with such exposure. Due to increased microbial fitness, MSSA-RVS strains would predominate over MRSA-RVS to become the main S. aureus strain in such patients. Therefore, prior vancomycin use would appear to be a risk factor for MSSA-RVS but not MRSA-RVS.
An alternative explanation could be that RVS may induce the loss of mecA in some MRSA strains. In vitro it has been observed that induction of high-level vancomycin resistance in MRSA strains by serial passage resulted in mecA deletion and restoration of fitness when compared with mecA-containing bacteria.38 Whether this occurs in vivo and with low-level vancomycin resistance is unknown but possible. In patients with either MRSA colonization or MSSA colonization, prior vancomycin use could provide selective pressure for the development of MRSA-RVS or MSSA-RVS, respectively. If the development of RVS in MRSA strains promoted the loss of mecA, those MRSA-RVS would revert to MSSA-RVS with resultant enhanced bacterial fitness. Therefore, prior vancomycin use could be a risk factor for MSSA-RVS but not MRSA-RVS. Elucidating the exact microbiological explanation of our findings was beyond the scope of our study, but either of these hypotheses could be tested further in prospective studies. Regardless, this association between prior vancomycin use and MSSA-RVS further supports the idea that beta-lactams should be the preferred agents for treating MSSA bloodstream infections when possible.
An additional finding in our study was the observed association between prior fluoroquinolone use and RVS. While we cannot fully explain this association, prior fluoroquinolone use has been associated with MRSA in the past.39
Another novel finding in our study, and one not previously investigated, was that the relationship between prior vancomycin use and RVS differed dramatically depending on the MIC method used to define RVS. There was a significant association between prior vancomycin use and RVS when vancomycin MICs were determined by E-test but not when determined by broth dilution. This reflects the poor correlation observed in our study between the E-test and broth dilution MIC testing methods, described elsewhere.40,41 Previous studies evaluating RVS that have relied on E-test alone have used varying MIC cutoffs to define RVS, with most using the cutoff used in our study (ie, >1.0 μg/mL),6,7,15 while others have used at least 1.0 μg/mL9 or even 3 categories of susceptibility levels.10,16 Taken together, these observations highlight the need for a more uniform approach when defining RVS so that results may be compared across studies. More research is needed to investigate which MIC method and cutoff value are superior for defining RVS. The optimal approach will likely depend on which is most closely linked with clinical outcomes.
Our study had several potential limitations. Although selection bias is of potential concern, we identified study subjects through the clinical microbiology laboratory that processed and cultured all specimens obtained at HUP and PPMC during the study period, so no potential subjects should have been overlooked. Additionally, every eligible patient was included as either a case or a control. Misclassification of case-control status was also reduced, as antibiotic susceptibility profiles were completed prior to initiation of the study, so that the distinction between case patients and control patients could not have influenced the identification of antibiotic resistance patterns.
Misclassification of exposure status is of potential concern, given our focus on antibiotic use during the same hospitalization up to 30 days prior to infection. Patients developing bacteremia on or shortly after hospital admission may have had antibiotic use during a previous hospitalization or as an outpatient that we were unable to quantify. Fortunately, only 22% of all study patients had a previous hospitalization in the 30 days prior to admission, and this proportion was similar among patients who developed their bacteremia less than 48 hours after admission, as compared with more than 48 hours. In addition, the potential for uncontrolled confounding exists, because certain variables (eg, the presence of indwelling devices) could not be assessed. Finally, our study was conducted in a large tertiary care medical center and a smaller urban community hospital; the results may not be generalizable to dissimilar institutions or geographical locations.
In conclusion, we found that 34.2% of patients with S. aureus bacteremia had RVS. The association between prior vancomycin use and RVS was statistically significant only for patients with MSSA, not those with MRSA. This suggests that a complex relationship between the clinical and molecular epidemiology of RVS in S. aureus may exist and warrants further exploration in a prospective study.
Acknowledgments
Martha Edelstein provided excellent technical assistance.
Financial support This work was supported by a research grant from Cubist Pharmaceuticals to E.L.
Footnotes
Potential conflicts of interest. E.L. has received past grant support from Merck, AstraZeneca, and 3M. All other authors report no conflicts of interest relevant to this article. All authors submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest, and the conflicts that the editors consider relevant to this article are disclosed here.
References
- 1.Cosgrove SE, Qi Y, Kaye KS, Harbarth S, Karchmer AW, Carmeli Y. The impact of methicillin resistance in Staphylococcus aureus bacteremia on patient outcomes: mortality, length of stay, and hospital charges. Infect Control Hosp Epidemiol. 2005;26(2):166–174. doi: 10.1086/502522. [DOI] [PubMed] [Google Scholar]
- 2.McDonald LC. Trends in antimicrobial resistance in health care-associated pathogens and effect on treatment. Clin Infect Dis. 2006;42(suppl 2):S65–S71. doi: 10.1086/499404. [DOI] [PubMed] [Google Scholar]
- 3.Kollef MH. Limitations of vancomycin in the management of resistant staphylococcal infections. Clin Infect Dis. 2007;45(suppl 3):S191–S195. doi: 10.1086/519470. [DOI] [PubMed] [Google Scholar]
- 4.Steinkraus G, White R, Friedrich L. Vancomycin MIC creep in non-vancomycin-intermediate Staphylococcus aureus (VISA), vancomycin-susceptible clinical methicillin-resistant S. aureus (MRSA) blood isolates from 2001–05. J Antimicrob Chemother. 2007;60(4):788–794. doi: 10.1093/jac/dkm258. [DOI] [PubMed] [Google Scholar]
- 5.Wang G, Hindler JF, Ward KW, Bruckner DA. Increased vancomycin MICs for Staphylococcus aureus clinical isolates from a university hospital during a 5-year period. J Clin Microbiol. 2006;44(11):3883–3886. doi: 10.1128/JCM.01388-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hidayat LK, Hsu DI, Quist R, Shriner KA, Wong-Beringer A. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Arch Intern Med. 2006;166(19):2138–2144. doi: 10.1001/archinte.166.19.2138. [DOI] [PubMed] [Google Scholar]
- 7.Lodise TP, Graves J, Evans A, et al. Relationship between vancomycin MIC and failure among patients with methicillin-resistant Staphylococcus aureus bacteremia treated with vancomycin. Antimicrob Agents Chemother. 2008;52(9):3315–3320. doi: 10.1128/AAC.00113-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moise-Broder PA, Sakoulas G, Eliopoulos GM, Schentag JJ, Forrest A, Moellering RC., Jr Accessory gene regulator group II polymorphism in methicillin-resistant Staphylococcus aureus is predictive of failure of vancomycin therapy. Clin Infect Dis. 2004;38(12):1700–1705. doi: 10.1086/421092. [DOI] [PubMed] [Google Scholar]
- 9.Sakoulas G, Moise-Broder PA, Schentag J, Forrest A, Moellering RC, Jr, Eliopoulos GM. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J Clin Microbiol. 2004;42(6):2398–2402. doi: 10.1128/JCM.42.6.2398-2402.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soriano A, Marco F, Martinez JA, et al. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis. 2008;46(2):193–200. doi: 10.1086/524667. [DOI] [PubMed] [Google Scholar]
- 11.Wang J-L, Wang J-T, Sheng W-H, Chen Y-C, Chang S-C. Nos-ocomial methicillin-resistant Staphylococcus aureus (MRSA) bacteremia in Taiwan: mortality analyses and the impact of vancomycin, MIC = 2 mg/L, by the broth microdilution method. BMC Infect Dis. 2010;10(1):159. doi: 10.1186/1471-2334-10-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moise PA, Sakoulas G, Forrest A, Schentag JJ. Vancomycin in vitro bactericidal activity and its relationship to efficacy in clearance of methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2007;51(7):2582–2586. doi: 10.1128/AAC.00939-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moise PA, North D, Steenbergen JN, Sakoulas G. Susceptibility relationship between vancomycin and daptomycin in Staphylococcus aureus: facts and assumptions. Lancet Infect Dis. 2009;9(10):617–624. doi: 10.1016/S1473-3099(09)70200-2. [DOI] [PubMed] [Google Scholar]
- 14.Patel JB, Jevitt LA, Hageman J, McDonald LC, Tenover FC. An association between reduced susceptibility to daptomycin and reduced susceptibility to vancomycin in Staphylococcus aureus. Clin Infect Dis. 2006;42(11):1652–1653. doi: 10.1086/504084. [DOI] [PubMed] [Google Scholar]
- 15.Lodise TP, Miller CD, Graves J, et al. Predictors of high vancomycin MIC values among patients with methicillin-resistant Staphylococcus aureus bacteraemia. J Antimicrob Chemother. 2008;62(5):1138–1141. doi: 10.1093/jac/dkn329. [DOI] [PubMed] [Google Scholar]
- 16.Moise PA, Smyth DS, El-Fawal N, et al. Microbiological effects of prior vancomycin use in patients with methicillin-resistant Staphylococcus aureus bacteraemia. J Antimicrob Chemother. 2007;61(1):85–90. doi: 10.1093/jac/dkm445. [DOI] [PubMed] [Google Scholar]
- 17.Clinical and Laboratory Standards Institute (CLSI) Performance Standards for Antimicrobial Susceptibility Testing—Eighteenth Informational Supplement. Wayne, PA: CLSI; 2008. CLSI document M100–S18. [Google Scholar]
- 18.CLSI. Performance Standards for Antimicrobial Disk Susceptibility Tests: Approved Standard. 9. Wayne, PA: CLSI; 2006. CLSI document M2-A9. [Google Scholar]
- 19.CLSI. Methods for Dilutional Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: Approved Standard. 7. Wayne, PA: CLSI; 2006. CLSI document M7-A7. [Google Scholar]
- 20.Bannerman TL, Peacock SJ. Staphylococcus, micrococcus, and other catalase-positive cocci. In: Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA, editors. Manual of Clinical Microbiology. Washington, DC: ASM; 2007. pp. 390–411. [Google Scholar]
- 21.Biodisk A. [Accessed February 18, 2011];E-test Technical Manual. http://www.abbiodisk.com/bd_litt_etm.html.
- 22.Satola SW, Farley MM, Anderson KF, Patel JB. Comparison of detection methods for heteroresistant vancomycin-intermediate Staphylococcus aureus, with the population analysis profile method as the reference method. J Clin Microbiol. 2011;49(1):177–183. doi: 10.1128/JCM.01128-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fitzgibbon MM, Rossney AS, O’Connell B. Investigation of reduced susceptibility to glycopeptides among methicillin-resistant Staphylococcus aureus isolates from patients in Ireland and evaluation of agar screening methods for detection of heterogeneously glycopeptide-intermediate S. aureus. J Clin Microbiol. 2007;45(10):3263–3269. doi: 10.1128/JCM.00836-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wootton M, MacGowan AP, Walsh TR, Howe RA. A multicenter study evaluating the current strategies for isolating Staphylococcus aureus strains with reduced susceptibility to glycopeptides. J Clin Microbiol. 2006;45(2):329–332. doi: 10.1128/JCM.01508-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18–e55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 26.Gasink LB, Fishman NO, Weiner MG, Nachamkin I, Bilker WB, Lautenbach E. Fluoroquinolone-resistant Pseudomonas aeruginosa: assessment of risk factors and clinical impact. Am J Med. 2006;119(6):e519–e525. doi: 10.1016/j.amjmed.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 27.Quain RD, Barton TD, Fishman NO, Weiner MG, Lautenbach E. Coadministration of oral levofloxacin with agents that impair its absorption: potential impact on emergence of resistance. Int J Antimicrob Agents. 2005;26(4):327–330. doi: 10.1016/j.ijantimicag.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 28.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 29.MacAdam H, Zaoutis TE, Gasink LB, Bilker WB, Lautenbach E. Investigating the association between antibiotic use and antibiotic resistance: impact of different methods of categorising prior antibiotic use. Int J Antimicrob Agents. 2006;28(4):325–332. doi: 10.1016/j.ijantimicag.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 30.Kleinbaum DG, Kupper LL, Morgenstern H. Epidemiologic Research: Principles and Quantitative Methods. New York: Van Nostrand Reinhold; 1982. [Google Scholar]
- 31.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–748. [PubMed] [Google Scholar]
- 32.Hosmer DO, Lemeshow SL. Applied Logistic Regression. New York: Wiley; 1989. [Google Scholar]
- 33.Sun GW, Shook TL, Kay GL. Inappropriate use of bivariable analysis to screen risk factors for use in multivariable analysis. J Clin Epidemiol. 1996;49(8):907–916. doi: 10.1016/0895-4356(96)00025-x. [DOI] [PubMed] [Google Scholar]
- 34.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129(1):125–137. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 35.Ender M, McCallum N, Adhikari R, Berger-Bachi B. Fitness cost of SCCmec and methicillin resistance levels in Staphylococcus aureus. Antimicrob Agents Chemother. 2004;48(6):2295–2297. doi: 10.1128/AAC.48.6.2295-2297.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bloemendaal AL, Fluit AC, Jansen WT, et al. Colonization with multiple Staphylococcus aureus strains among patients in European intensive care units. Infect Control Hosp Epidemiol. 2009;30(9):918–920. doi: 10.1086/605640. [DOI] [PubMed] [Google Scholar]
- 37.Mongkolrattanothai K, Gray BM, Mankin P, et al. Simultaneous carriage of multiple genotypes of Staphylococcus aureus in children. J Med Microbiol. 2010;60(3):317–322. doi: 10.1099/jmm.0.025841-0. [DOI] [PubMed] [Google Scholar]
- 38.Noto MJ, Fox PM, Archer GL. Spontaneous deletion of the methicillin resistance determinant, mecA, partially compensates for the fitness cost associated with high-level vancomycin resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 2008;52(4):1221–1229. doi: 10.1128/AAC.01164-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weber SG, Gold HS, Hooper DC, Karchmer AW, Carmeli Y. Fluoroquinolones and the risk for methicillin-resistant Staphylococcus aureus in hospitalized patients. Emerg Infect Dis. 2003;9(11):1415–1422. doi: 10.3201/eid0911.030284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sader HS, Fritsche TR, Jones RN. Daptomycin bactericidal activity and correlation between disk and broth microdilution method results in testing of Staphylococcus aureus strains with decreased susceptibility to vancomycin. Antimicrob Agents Chemother. 2006;50(7):2330–2336. doi: 10.1128/AAC.01491-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keel RA, Sutherland CA, Aslanzadeh J, Nicolau DP, Kuti JL. Correlation between vancomycin and daptomycin MIC values for methicillin-susceptible and methicillin-resistant Staphylococcus aureus by 3 testing methodologies. Diagn Microbiol Infect Dis. 2010;68(3):326–329. doi: 10.1016/j.diagmicrobio.2010.08.006. [DOI] [PubMed] [Google Scholar]


