Abstract
Antiretroviral pre-exposure prophylaxis (PrEP) has received increasing recognition as a viable prescription-based intervention for people at risk for HIV acquisition. However, little is known about racial biases affecting healthcare providers’ willingness to prescribe PrEP. This investigation sought to explore medical students’ stereotypes about sexual risk compensation among Black versus White men who have sex with men seeking PrEP, and the impact of such stereotypes on willingness to prescribe PrEP. An online survey presented participants (n = 102) with a clinical vignette of a PrEP-seeking, HIV-negative man with an HIV-positive male partner. Patient race was systematically manipulated. Participants reported predictions about patient sexual risk compensation, willingness to prescribe PrEP, and other clinical judgments. Bootstrapping analyses revealed that the Black patient was rated as more likely than the White patient to engage in increased unprotected sex if prescribed PrEP, which, in turn, was associated with reduced willingness to prescribe PrEP to the patient.
Keywords: Race/ethnicity, Men who have sex with men (MSM), Pre-exposure prophylaxis (PrEP), Risk compensation, Healthcare provider
Introduction
Antiretroviral pre-exposure prophylaxis (PrEP) has gained momentum as a promising biomedical prevention strategy for people at high risk for HIV acquisition. In particular, daily oral use of tenofovir with emtricitabine (FTC–TDF, i.e., Truvada®), a well-established and commonly prescribed antiretroviral treatment for people living with HIV, has demonstrated preventive potential in three of five clinical trials to date (iPrEx [1], TDF2 [2], and Partners PrEP [3]), including one trial showing a 44 % relative reduction in HIV incidence among men who have sex with men (MSM) [1]. In July of 2012, the US Food and Drug Administration (FDA) approved daily oral FTC–TDF for prescription as PrEP [4]. However, concern about patient risk compensation (i.e., increased risk-taking in response to a perceived decrease in susceptibility to HIV infection while on PrEP [5]) may decrease healthcare providers’ willingness to prescribe [6], thereby limiting access for people who could benefit from this preventive technology. Additionally, the ambiguity [7–11] and controversy [12, 13] surrounding eligibility criteria for PrEP could enhance the likelihood of discriminatory prescription practices occurring. In light of extant stereotypes of Black MSM as sexually irresponsible and uninhibited [14–16] as well as documented racial discrimination in clinical decisions related to antiretroviral treatment [17–20] and reproductive healthcare [21–24], the current study sought to explore racial bias in providers’ predictions of sexual risk compensation among patients seeking PrEP and implications for their willingness to prescribe.
The Potential for Racial Discrimination in the Prescription of PrEP
Interim PrEP prescription guidance issued by the US Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO) leaves considerable latitude for provider subjectivity in deciding patients’ suitability for PrEP. The CDC indicates that potential PrEP recipients should be judged by providers as being at ongoing, very high risk for acquiring HIV infection, but provides few examples [7–9]. The WHO's published guidance is similarly non-specific [10, 11]. Provider guidelines issued by the drug manufacturer are more detailed in suggesting behaviors and characteristics that may help to identify individuals at high risk (e.g., inconsistent condom use, diagnosis of sexually transmitted infections [STIs], transactional sex) [25]. Providers must nonetheless decide how to weigh these factors in the context of other clinical considerations in determining whether or not to prescribe to a particular individual. Although all medical practice involves the exercise of clinical judgment, PrEP prescription at this early stage of implementation, when specific eligibility criteria and clinical precedents are lacking, may be especially reliant upon provider discretion. Conflicting expert opinions and provider disagreement about PrEP prescription relative to hypothetical patient cases [26, 27] yield preliminary evidence of likely variability in PrEP prescription practices.
Previous research has shown that people are particularly likely to exhibit racial discrimination in contexts in which behavioral norms or situational demands are ambiguous [28, 29], particularly when people can justify their actions based on factors ostensibly unrelated to race [30]. In medical settings specifically, race-based treatment disparities, which are generally prominent throughout healthcare, are more pronounced for procedures that are considered more discretionary [31, 32]. Inequalities in the provision of care are not necessarily driven by psychological biases that operate on a conscious level (i.e., explicit biases)—in fact, such overt racism is declining. Rather, unconscious biases that are subject to unintentional activation (i.e., implicit biases) can affect medical decision-making, compromise the quality of clinical care provided, and ultimately lead to systematic inequalities [32, 33].
Evidence for or against racial discrimination in the prescription of antiretroviral medication as HIV prevention (i.e., PrEP) has yet to emerge given the novelty of PrEP in clinical practice. However, prescription of antiretroviral medication in the context of HIV treatment, particularly in years past, may serve as a high-discretion medical scenario that is analogous to the prescription of antiretroviral medication as PrEP, and could be foretelling of racial discrimination. As with the current PrEP prescription guidance, past prescription guidelines for antiretroviral medication as treatment have been non-definitive and controversial at times [34]; correspondingly, racial disparities in clinical judgment and prescription practices related to HIV treatment have been well documented [17–20, 31].
To date, little is known about racial biases affecting healthcare providers’ PrEP-related clinical decision-making, including their willingness to prescribe PrEP to patients of diverse backgrounds. However, providers’ lack of consensus regarding prescription practices and priority patient groups [12, 13, 35] and the limited specificity of existing guidance [7–11] heighten the risk of discriminatory prescription practices occurring as they have with antiretroviral treatment, posing a potential barrier to access.
Sexual Risk Compensation and Racial Stereotypes
One concern that has been endorsed by general physicians, HIV specialists, and other healthcare providers in relation to prescribing PrEP is the possibility of risk compensation (sometimes referred to as “behavioral disinhibition” or “condom migration”) among PrEP users [6, 13, 27, 36, 37]. The general phenomenon of risk compensation involves a change in behavioral risk-taking that is driven by a perceived change in one's underlying susceptibility to harm [5, 38–40]. In the context of PrEP, sexual risk compensation would occur if a PrEP user increased his or her sexual risk-taking behavior (e.g., reducing condom use, having more partners) based on the assumption that PrEP had lowered his or her likelihood of acquiring HIV [5]. Among healthcare providers, the belief that PrEP could lead to risk compensation has been linked to reduced willingness to prescribe PrEP to high-risk patients [6].
Given the recency of FTC–TDF receiving FDA approval and becoming available for prescription as PrEP [4], present knowledge of PrEP-associated sexual risk compensation is limited. Surveys and qualitative research conducted among potential user groups, including MSM, have documented the potential for increased sexual risk-taking among PrEP users [41–46], and modeling of PrEP's population-level effectiveness has incorporated risk compensation parameters [47, 48]. Behavioral analyses of users of other biomedical HIV prevention technologies, such as male circumcision [40, 49–52], condoms [53], and anti-retroviral treatment (among users who believed that treatment reduced transmission risk) [54], suggest that risk compensation may occur in conjunction with HIV prevention efforts, although findings are mixed.
To date, most of the sexual behavior data available for PrEP users were collected during randomized double-blind clinical trials, during which participants received a combination of other prevention services such as risk reduction counseling, free condoms, referrals for circumcision, and/or screening for other STIs [1–3, 55–57]. Although these trials did not show increased sexual risk behavior among participants, participants were also receiving prevention services that may not always be routinely provided in clinical practice. Furthermore, they were operating under the premise that they were taking a placebo or a pill of uncertain prevention efficacy. Thus, their behavior may not be representative of PrEP users in everyday clinical settings who know that they are taking a pill with established efficacy [58] and who may be treated in the context of a less comprehensive strategy.
Additional empirical data on sexual risk compensation behavior have been collected among individuals in heterosexual serodiscordant partnerships who were originally randomized to receive PrEP as part of a double-blind, placebo-controlled trial [3] and then continued to be supplied with PrEP and monitored for 12 months after condition unblinding and public announcement of study findings [59]. Risk compensation examined in this context as opposed to a double-blind clinical trial may be more predictive of risk compensation among real-world PrEP users since participants were aware that they were indeed taking PrEP (versus a placebo) and could be more confi-dent about its efficacy. Assessment of self-reported sexual behavior during the 12 months of open-label use revealed no significant increase in frequency of unprotected sex with HIV-positive primary partners and only a slight increase in unprotected sex acts with outside partners as compared to the behavioral trends predicted by the 12 months of blinded use. In summary, actual risk compensation patterns among real-world PrEP users have yet to be determined, and behavioral data from clinical trial participants to date do not yield substantial evidence for risk compensation occurring during or subsequent to trial participation.
Thus far, indications of racial disparities in risk compensation are generally lacking in the documented behavior of PrEP trial participants and speculated behavior of potential PrEP users. However, historically rooted stereotypes of Black men as sexually uninhibited and irresponsible [14, 16] may predispose providers to make inequitable judgments about risk compensation among their Black and White patients. Stereotypes are commonly shared beliefs about characteristics of a particular social group; these beliefs serve as bases for judgment and interaction and often operate unconsciously [33, 60]. Furthermore, they may function as justification for existing social disparities [61]. Race-based sexual stereotypes have previously been implicated in healthcare providers’ attitudes and treatment decisions. For example, consistent with Black stereotypes of uncontrolled sexuality, Black patients have been more likely than White patients to receive safe sex or birth control counseling [22, 23] and to be advised to limit their childbearing [24]. Black patients have also experienced racial stereotyping by providers regarding their number of sexual partners and sexual health [62].
Sensationalized portrayals of the “Down Low” lifestyle in the past decade have fueled stereotypes related to promiscuity, unprotected sex, and disease transmission among Black MSM in particular [63, 64], potentially heightening the vulnerability of this demographic to risk compensation stereotyping. One survey of MSM has suggested differences in risk compensation attitudes by race: when asked the minimum level of effectiveness they would require of an HIV prevention pill to be willing to forgo condoms during receptive unprotected anal intercourse with a sero-status-unknown partner, Black and Latino participants were more likely than White participants to report midrange minimums (intended to correspond to actual PrEP efficacy) versus high-range minimums [46]. However, the lack of racial differences in personal predictions of risk compensation reported among other samples of MSM [41] raise doubt about the generalizability of these attitudinal discrepancies.
Moreover, the extent to which attitudinal differences by race would translate to greater sexual risk compensation behavior among Black versus White MSM in actuality is unknown, and questionable given evidence against greater sexual risk-taking among Black MSM in general [65–67]. For example, a 2012 meta-analysis of 174 US studies published over the previous two decades indicated that relative to MSM of other races, Black MSM reported greater condom use during anal intercourse and fewer male sex partners (past year and lifetime) [65]. Thus, stereotypes about sexual promiscuity and irresponsibility associated with Black MSM lack empirical support, but may nonetheless lead healthcare providers to assume that Black MSM will engage in greater sexual risk compensation than White MSM.
The Current Study: Objectives and Hypotheses
The purpose of the current survey-based investigation was to examine medical students’ predictions about sexual risk compensation among Black versus White MSM seeking PrEP, and to examine the indirect effect of patient race on willingness to prescribe via such predictions. To do so, we experimentally manipulated patient race in a case vignette describing a clinical scenario in which an HIV-negative, healthy patient at high risk for HIV acquisition sought PrEP in a primary care setting. We hypothesized that there would be a significant indirect effect of patient race on prescription willingness, such that participants would perceive the Black patient to be more likely than the White patient to engage in sexual risk compensation (i.e., participate in more unprotected anal intercourse after PrEP initiation), which, in turn, would predict reduced willingness to prescribe PrEP to the patient.
Methods
Participants and Procedure
Medical school students in the northeastern US were recruited to participate in an anonymous online survey about attitudes toward prescribing PrEP in January of 2013. An initial email requesting participation and a reminder email sent 11 days later, both of which contained the survey link, were distributed via a listserv to 435 medical students. Students were informed that they would be entered into a drawing for local restaurant gift cards in exchange for their participation.
At the outset of the survey, participants were provided with background information about FTC–TDF (Truvada®) for PrEP, including its purpose, partial efficacy reports in clinical trials, and approval by the FDA. In addition to being given these basic facts, participants were provided with three actual quotes derived from online sources that represented popular arguments in support of PrEP prescription (e.g., “We need new tools to fight this epidemic... PrEP is certainly not for everyone, but it may have a role in bringing HIV-infection rates down.” -Chris Collins, Vice President of American Foundation for AIDS Research [68]) and three quotes in opposition (e.g., “The use of PrEP... carries significant risk that the people who take it haphazardly will mistakenly believe that they are completely protected from HIV and other STDs. These individuals will engage in unprotected sex, which will ultimately lead to an increase in HIV and other infections.” -Michael Weinstein, President of AIDS Healthcare Foundation [69]).
Next, participants were presented with a case vignette, which was adapted from a similar study of bias in providers’ adherence judgments and treatment decisions pertaining to patients living with HIV [17] and further refined based on input from a trained medical doctor with expertise in HIV care. The vignette described a clinical scenario in which an HIV-negative male patient reported engaging in unprotected anal intercourse with an HIV-positive male partner on an ongoing basis and sought a prescription for PrEP (See Box 1). Participants were assigned in a 1:1 ratio to receive one of two versions of the vignette via an automated randomization process. Vignette versions were identical except for the race of the patient specified: Black or White.
Subsequently, participants reported clinical judgments associated with the hypothetical patient (e.g., predicted patient sexual risk compensation, predicted patient adherence, willingness to prescribe PrEP to the patient) and completed measures of racial bias, as well as self-reporting sociodemographic and medical training characteristics. Given that risk compensation in the context of PrEP is an understudied topic, survey items were developed for the purpose of this study. At the conclusion of the survey, participants were debriefed and informed that the quotes represented speculation and that there was no scientific evidence that people taking PrEP engage in more risky sexual behavior. Participants were also provided with a link to the CDC website for more information about PrEP. All study procedures were reviewed and approved by the Yale University Institutional Review Board prior to inception.
Measures
Sociodemographic and Medical Training Characteristics
Survey items inquired about participants’ gender [(1) male/ (2) female/ (3) transgender/ (4) other], race [(1) American Indian or Alaska Native/ (2) Asian/ (3) Black or African American/ (4) Hispanic or Latino/ (5) Native Hawaiian or Pacific Islander/ (6) White/ (7) other], sexual orientation [(1) homosexual (lesbian or gay)/ (2) bisexual/ (3) heterosexual (straight)/ (4) other], age (years), social class [(1) lower/ (2) lower middle/ (3) middle/ (4) upper middle/ (5) upper], and current year of medical school [(1) 1st/ (2) 2nd/ (3) 3rd/ (4) 4th/ (5) other]. In addition, past clinical experience with HIV-positive patients was assessed by asking, “Have you interacted with HIV-positive patients in a clinical setting in the past?”, with response options ranging from (1) never to (4) often.
For all inferential analyses, participants’ race was recoded into two separate dichotomous variables: [(1) Black versus (0) not] and [(1) White versus (0) not] given the patient race manipulation. Gender was dichotomized as (1) female versus (0) male (no participants reported being transgender or of another gender) and sexual orientation was dichotomized as (1) heterosexual (straight) versus (0) not. For correlation, hierarchical linear regression, and bootstrapping analyses, current year of medical school was treated as a continuous variable with response (5) other recoded as missing. Missing values were excluded pairwise for correlation and regression analyses and listwise for the boostrapping analysis.
Clinical Judgments
Predicted Patient Sexual Risk Compensation
Participants’ belief about the likelihood of the patient engaging in more unprotected sex if prescribed PrEP was measured with a single item asking, “How likely would this patient be to have MORE unprotected sex if he started taking Truvada as PrEP?” Response options ranged from (1) not at all likely to (5) extremely likely, with higher scores representing greater predicted likelihood of patient sexual risk compensation.
Predicted Patient Adherence
Participants’ perception of the patient's probable level of adherence to PrEP was assessed with a single item asking, “If you were to prescribe PrEP to the patient, how adherent do you think he would be?” Response options ranged from (1) not at all adherent to (5) extremely adherent, with higher scores representing greater predicted adherence.
Perceived Patient Risk of HIV Infection Without PrEP
Participants’ judgment of the patient's risk of acquiring HIV without PrEP was measured with a single item asking, “How high do you think this patient's risk of getting HIV is WITHOUT PrEP?” Response options ranged from (1) extremely low to (5) extremely high, with higher scores suggesting greater perceived patient risk.
Risk Reduction Associated with PrEP
Participants’ perception of the extent to which PrEP would reduce the patient's risk was calculated by subtracting their response to the item “How high do you think this patient's risk of getting HIV would be if he started taking Truvada as PrEP and did not change his condom practices?” [(1) extremely low to (5) extremely high] from the aforementioned perceived patient risk of HIV infection without PrEP item. The difference between the two items represented risk reduction associated with PrEP, with higher scores indicating greater perceived reduction in patient risk associated with PrEP.
Willingness to Prescribe Prep
Participants’ willingness to prescribe PrEP to the patient described was assessed with a single item asking, “Would you prescribe Truvada as PrEP to this patient?” Response options ranged from (1) definitely not to (5) definitely yes, with higher scores indicating greater willingness to prescribe.
Racial Bias
The following two measures were included to assess racial bias in terms of perceived importance of the patient's request (between-group comparison) and general feelings toward Black versus White patients (individual difference). These items sought to detect race-based devaluation of the patient's request and explicit prejudice, respectively.
Perceived Importance of Patient Request
Participants’ judgment of the importance of the patient's request for PrEP in the clinical scenario was assessed with a single item asking, “How IMPORTANT would this patient's request be to you?” Response options ranged from (1) not at all important to (5) extremely important, with higher scores representing greater perceived importance of the request.
General Feelings Toward Black Versus White Patients
Participants were instructed that “It is natural for healthcare providers to feel more positively toward some types of patients versus others,” and subsequently asked to rate their general feelings toward 16 different categories of patients (e.g., Black patients, medically adherent patients, White patients, physically fit patients, pediatric patients, drug-abusing patients) on a scale ranging from (1) extremely negative to (9) extremely positive. Discrepancies in feelings toward “Black patients” and “White patients” were calculated by subtracting ratings of the former from the latter, with scores greater than 0 indicating more positive feelings toward White patients and scores less than 0 indicating more positive feelings toward Black patients.
Patient Race/Manipulation Check
To ensure that participants had sufficiently attended to the race of the patient in the clinical vignette with which they had been presented, they were asked to choose the race of the patient in the clinical scenario [(1) American Indian or Alaska Native/ (2) Asian/ (3) Black or African American/ (4) Hispanic or Latino/ (5) Native Hawaiian or Pacific Islander/ (6) White/ (7) other/ (8) I don't know]. This item was placed towards the end of the survey, deliberately separated from the vignette-related items by multiple unrelated items, including those pertaining to participant sociodemographic and medical training characteristics.
Analysis
All descriptive and inferential statistical analyses were performed using IBM SPSS Statistics Version 19 software. Independent samples t tests (for continuous variables) and Pearson χ2 tests (for dichotomous variables such as gender) were performed to test for differences in sociodemographic and medical training characteristics between Black-patient and White-patient conditions. Univariate analyses of covariance controlling for participant race (Black versus not and White versus not) were conducted to assess between-group differences in clinical judgment and racial bias scores. To examine bivariate relationships separately for Black-patient and White-patient conditions, intercor-relations between variables were calculated using Phi coefficients (for two dichotomous variables), Spearman rho (for one dichotomous variable and one continuous variable), and Pearson correlation coefficients (for two continuous variables).
Hierarchical linear regression analysis was performed to predict willingness to prescribe PrEP. In the first model, sociodemographic and medical training characteristics as well as study condition (Black patient or White patient) were included. In the second model, clinical judgments pertaining to predicted patient adherence, perceived patient risk of HIV infection without PrEP, and risk reduction associated with PrEP, as well as racial bias (perceived importance of patient request and general feelings toward Black versus White patients), were added. In the final model, predicted patient sexual risk compensation was added.
Bootstrapping was conducted to test predicted patient sexual risk compensation as a mediator [M] between patient race [X] and willingness to prescribe PrEP [Y]. This resampling methodology has received increasing support as the optimal approach for testing indirect effects given its minimization of Type 1 error, lack of assumptions about the normality of the sampling distribution of the indirect effect, and appropriateness for use with small samples [70, 71]. Hayes’ PROCESS macro [72] was used to generate 5,000 bootstrapped samples, from which bias-corrected and accelerated confidence intervals were established to estimate indirect effects. We applied current recommendations for testing indirect effects, which do not require a signifi-cant bivariate relationship between X and Y [70–73]. In particular, we directly quantified the indirect effect of patient race on willingness to prescribe PrEP through predicted patient sexual risk compensation, a procedure considered superior to inferring the effect through a series of tests of the separate paths that compose the mediation model (as in Baron and Kenny's causal steps approach) [71].
Results
Sample Characteristics
Approximately 168 medical students accessed the survey site and completed the consent form, of which 162 (96.4 %) proceeded to the survey and 130 (77.4 %) completed the survey. Of these, 28 (21.5 %) failed the manipulation check: 2 participants in the Black-patient condition and 26 participants in the White-patient condition endorsed “I don't know” on the patient race item. No participants reported an incorrect patient race. Participants who failed the manipulation check were excluded from the primary analyses. Follow-up Pearson χ2 tests and independent samples t tests comparing survey completers who passed versus failed the manipulation check revealed no differences in sociodemographic or medical training characteristics, clinical judgments, or racial bias, with the exception of social class: participants who passed reported a higher social class than those who failed, but both reported means in the middle- to upper middle-class range.
The final sample was comprised of 102 students ranging in age from 20 to 35 years (M = 25.65, SD = 2.81). Additional sociodemographic and medical training characteristics are provided in Table 1. Mean survey completion time was 12 min.
Table 1.
Sociodemographic and descriptive characteristics of the sample (n = 102)
| Characteristic | n (%) |
|---|---|
| Gender | |
| Female | 51 (50.0) |
| Male | 51 (50.0) |
| Race | |
| Asian | 41 (40.2) |
| Black or Afrian American | 3 (2.9) |
| Hispanic or Latino | 4 (3.9) |
| White | 49 (48.0) |
| Other | 5 (4.9) |
| Social class | |
| Lower | 1 (1.0) |
| Lower Middle | 8 (7.8) |
| Middle | 29 (28.4) |
| Upper Middle | 60 (58.8) |
| Upper | 4 (3.9) |
| Sexual orientation | |
| Homosexual (lesbian or gay) | 5 (4.9) |
| Bisexual | 1 (1.0) |
| Heterosexual (straight) | 96 (94.1) |
| Current year of medical school | |
| 1st | 20 (19.6) |
| 2nd | 30 (29.4) |
| 3rd | 18 (17.6) |
| 4th | 21 (20.6) |
| Other | 13 (12.7) |
| Past clinical experience with HIV+ patients | |
| Never | 12 (11.8) |
| Rarely | 38 (37.3) |
| Sometimes | 42 (41.2) |
| Often | 10 (9.8) |
Between-Group Comparisons
Pearson χ2 tests and independent samples t tests revealed no differences between Black-patient and White-patient conditions in terms of age, gender, race (White versus not, Black versus not), social class, sexual orientation (heterosexual versus not), years of medical school, and past clinical experience with HIV-positive patients (p > 0.05).
Table 2 displays total sample means and between-group means comparisons for the primary variables of interest. The only significant between-group difference to emerge was related to predicted patient sexual risk compensation: participants in the Black-patient condition rated the patient as more likely to increase his unprotected sexual behavior if prescribed PrEP compared to participants in the White-patient condition. Specifically, mean scores fell in the “somewhat likely” to “very likely” range for the Black-patient condition, but only the “a little bit likely” to “somewhat likely” range for the White-patient condition.
Table 2.
Means comparisons of Black-patient and White-patient study conditions: clinical judgments and racial bias
| Full sample (n
= 102) |
Condition 1: Black patient
(n = 64) |
Conditon 2: White patient
(n = 38) |
Condition 1 vs. Condition
2a |
|||||
|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | F | p | |
| 1 Predicted patient sexual risk compensation | 3.14 | 1.07 | 3.30 | 1.05 | 2.87 | 1.07 | 4.44 | 0.038* |
| 2 Predicted patient adherence | 3.35 | 0.68 | 3.38 | 0.68 | 3.32 | 0.70 | 0.07 | 0.791 |
| 3 Perceived patient HIV risk without PrEP | 4.17 | 0.77 | 4.23 | 0.71 | 4.05 | 0.87 | 1.55 | 0.216 |
| 4 Risk reduction associated with PrEP | 1.37 | 0.72 | 1.41 | 0.75 | 1.32 | 0.66 | 0.22 | 0.640 |
| 5 Willingness to prescribe PrEP | 3.75 | 0.72 | 3.80 | 0.78 | 3.68 | 0.62 | 0.35 | 0.557 |
| 6 Perceived importance of patient request | 3.95 | 0.55 | 4.02 | 0.60 | 3.84 | 0.44 | 1.69 | 0.197 |
| 7 General feelings toward patients (White patients – Black patients) | 0.07 | 0.57 | 0.08 | 0.45 | 0.05 | 0.73 | 0.11 | 0.740 |
p < .05
** p < .01
Participant race controlled in all analyses
On average, for the combined sample, participants judged the patient to be “somewhat” to “very” adherent and to have a “fairly” to “extremely” high risk of contracting HIV without PrEP. Mean willingness to prescribe to the patient fell in the “maybe” to “probably yes” range, with 0 % of participants reporting they would “definitely not” prescribe and only 2 % reporting they would “probably not” prescribe. On average, participants perceived the patient's request to be “somewhat” to “very” important. Finally, the majority (87.3 %) of participants endorsed equally positive feelings for White and Black patients, with only 2.9 % rating Black patients more positively and 9.8 % rating White patients more positively.
Correlation Analyses
Correlation coefficient values representing bivariate relationships among variables separately by condition are displayed in Table 3. Participants in the Black-patient condition who (a) were not White, (b) reported lower social class, and (c) were less advanced in medical school predicted greater patient risk compensation, as did younger participants in the White-patient condition. Participants in the Black-patient condition who were (a) female, (b) White, and (c) more advanced in medical school were more willing to prescribe PrEP, as were older participants in the White-patient condition.
Table 3.
Summary of intercorrelations between sociodemographic and medical training characteristics, clinical judgments, and racial bias among participants
| Variablea | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 Gender (female) | - | -0.01 | -0.06 | -0.05 | 0.04 | 0.00 | 0.01 | 0.05 | -0.01 | -0.21 | 0.10 | 0.15 | 0.29* | -0.08 | 0.05 |
| 2 Race (Black) | -0.16 | - | -0.19 | 0.05 | 0.00 | -0.11 | 0.10 | 0.23 | 0.11 | 0.04 | 0.21 | 0.18 | -0.07 | -0.01 | -0.05 |
| 3 Race (White) | 0.15 | -0.14 | - | 0.07 | 0.61** | 0.22 | 0.39** | 0.30* | -0.28* | 0.20 | -0.04 | 0.14 | 0.31* | 0.34** | -0.16 |
| 4 Sexual orientation (heterosexual) | -0.17 | 0.03 | 0.14 | - | -0.18 | 0.00 | -0.22 | -0.08 | -0.03 | -0.15 | 0.17 | 0.04 | -0.23 | -0.19 | 0.24 |
| 5 Age | 0.42** | -0.10 | 0.28 | -0.18 | - | 0.00 | 0.38** | 0.36** | -0.04 | 0.16 | -0.10 | -0.01 | 0.22 | 0.30* | -0.22 |
| 6 Social class | 0.27 | -0.30 | 0.51** | 0.16 | 0.24 | - | 0.21 | 0.13 | -0.39** | 0.17 | -0.17 | -0.20 | 0.15 | 0.01 | 0.32* |
| 7 Current year of medical schoolb | 0.09 | -0.08 | 0.25 | -0.24 | 0.45** | 0.11 | - | 0.31* | -0.28* | 0.08 | 0.30* | 0.16 | 0.42** | 0.44** | -0.18 |
| 8 Past clinical experience with HIV+ patients | 0.20 | -0.27 | 0.35* | -0.08 | 0.45** | 0.37* | 0.52** | - | -0.15 | 0.06 | -0.04 | 0.11 | 0.14 | 0.28* | -0.14 |
| 9 Predicted patient sexual risk compensation | -0.31 | 0.20 | -0.04 | -0.20 | -0.32* | -0.09 | -0.10 | -0.20 | - | -0.03 | 0.03 | 0.09 | -0.39** | -0.11 | -0.12 |
| 10 Predicted patient adherence | 0.11 | -0.09 | 0.13 | 0.09 | 0.19 | -0.02 | 0.03 | -0.01 | -0.02 | - | -0.09 | 0.04 | 0.21 | 0.10 | 0.16 |
| 11 Perceived patient risk without PrEP | -0.26 | -0.22 | -0.25 | 0.03 | -0.19 | 0.00 | 0.12 | -0.22 | -0.08 | -0.07 | - | 0.51** | 0.17 | 0.03 | -0.11 |
| 12 Risk reduction associated with PrEP | -0.05 | -0.09 | 0.10 | -0.19 | -0.14 | 0.11 | 0.24 | -0.15 | -0.02 | -0.05 | 0.44** | - | 0.31* | 0.06 | -0.29* |
| 13 Willingness to prescribe PrEP | 0.24 | -0.19 | 0.11 | -0.10 | 0.43** | 0.09 | 0.06 | 0.11 | -0.47** | 0.05 | 0.13 | -0.01 | - | 0.28* | -0.27* |
| 14 Perceived importance of patient request | 0.10 | 0.06 | 0.18 | -0.06 | 0.16 | 0.02 | 0.11 | 0.18 | 0.07 | 0.17 | -0.19 | -0.01 | 0.21 | - | -0.30* |
| 15 General feelings toward patients (White patients – Black patients) | 0.41* | -0.41** | 0.01 | -0.38* | 0.20 | 0.40* | 0.20 | 0.03 | -0.09 | 0.02 | 0.42** | 0.19 | 0.16 | 0.11 | - |
Intercorrelations for participants in the Black-patient condition (n = 64) are presented above the diagonal, and intercorrelations for participants in the White-patient condition (n = 38) are presented below. Phi coefficient values are provided for intercorrelations involving two dichotomous variables, Spearman's rho values are provided for intercorrelations involving one dichotomous and one continuous variable, and Pearson correlation coefficients are provided for intercorrelations involving two continuous variables
* p < .05, ** p < .01
Referent groups are provided parenthetically if applicable
For this variable n = 54 for the Black patient group and n = 35 for the White-patient group. (Participants who reported their current year of medical school to be “other” were excluded so that the variable could be treated as continuous.)
Hierarchical Linear Regression
Results from the hierarchical linear regression analysis are displayed in Table 4. In the first model, female gender was the only independent variable significantly associated with willingness to prescribe PrEP, indicating that women were more willing to prescribe than men when patient race and participant sociodemographic and medical training characteristics were controlled. The second model retained all previous independent variables and added clinical judgments with regard to predicted patient adherence, perceived patient risk of HIV infection without PrEP, and risk reduction associated with PrEP, as well as racial bias in terms of perceived importance of the patient's request and general feelings toward Black versus White patients. In this model, female gender remained the only significant predictor of willingness to prescribe. In the third and final model, which retained all previous independent variables and added predicted patient sexual risk compensation, variables significantly associated with greater willingness to prescribe included female gender, positive feelings toward Black versus White patients, and lower predicted patient sexual risk compensation. The addition of sexual risk compensation to the model resulted in a significant improvement in its predictive ability, accounting for an additional 11 % of variance in willingness to prescribe PrEP. The final model accounted for 32 % of the variance in this variable.
Table 4.
Hierarchical linear regression predicting participant willingness to prescribe PrEP
| Variable | Model 1 |
Model 2 |
Model 3 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unstandardized
coefficients |
Standardized coeficients |
Unstandardized
coeffiients |
Standrdized coeffcients |
Unstanardized
coeffcients |
Standadized coeffiients |
||||||||||
| B | SE | β | t | p | B | SE | β | t | p | B | SE | β | t | p | |
| Gender (female) | 0.35 | 0.15 | 0.25 | 2.36 | 0.021* | 0.42 | 0.15 | 0.29 | 2.82 | 0.006** | 0.37 | 0.14 | 0.26 | 2.64 | 0.010* |
| Race (Black) | -0.24 | 0.45 | -0.06 | -0.53 | 0.595 | -0.33 | 0.43 | -0.08 | -0.77 | 0.441 | -0.19 | 0.40 | -0.04 | -0.46 | 0.643 |
| Race (White) | 0.15 | 0.18 | 0.10 | 0.82 | 0.417 | 0.03 | 0.19 | 0.02 | 0.16 | 0.875 | 0.02 | 0.17 | 0.01 | 0.09 | 0.926 |
| Sexual orientation (heterosexual) | -0.40 | 0.33 | -0.13 | -1.22 | 0.225 | -0.34 | 0.33 | -0.11 | -1.02 | 0.310 | -0.44 | 0.30 | -0.14 | -1.44 | 0.154 |
| Age | 0.02 | 0.03 | 0.08 | 0.66 | 0.513 | 0.03 | 0.03 | 0.11 | 0.88 | 0.380 | 0.03 | 0.03 | 0.11 | 0.97 | 0.335 |
| Social class | 0.05 | 0.11 | 0.05 | 0.50 | 0.622 | 0.16 | 0.11 | 0.17 | 1.46 | 0.147 | 0.09 | 0.10 | 0.09 | 0.87 | 0.386 |
| Current year of medical school | 0.14 | 0.08 | 0.21 | 1.76 | 0.082 | 0.06 | 0.08 | 0.09 | 0.67 | 0.503 | 0.02 | 0.08 | 0.03 | 0.22 | 0.826 |
| Past clinical experience with HIV+ patients | -0.05 | 0.10 | -0.06 | -0.52 | 0.602 | -0.05 | 0.10 | -0.05 | -0.48 | 0.636 | -0.07 | 0.09 | -0.08 | -0.73 | 0.465 |
| Study condition (White patient) | -0.04 | 0.15 | -0.03 | -0.28 | 0.778 | 0.04 | 0.15 | 0.03 | 0.29 | 0.774 | -0.06 | 0.14 | -0.04 | -0.41 | 0.680 |
| Predicted patient adherence | 0.15 | 0.11 | 0.14 | 1.37 | 0.174 | 0.14 | 0.10 | 0.14 | 1.45 | 0.151 | |||||
| Perceived patient HIV risk without PrEP | 0.20 | 0.12 | 0.22 | 1.72 | 0.090 | 0.18 | 0.11 | 0.20 | 1.70 | 0.094 | |||||
| Risk reduction associated with PrEP | 0.07 | 0.12 | 0.07 | 0.62 | 0.538 | 0.11 | 0.11 | 0.10 | 0.96 | 0.339 | |||||
| Perceived importance of patient request | 0.21 | 0.14 | 0.16 | 1.52 | 0.134 | 0.22 | 0.13 | 0.16 | 1.67 | 0.099 | |||||
| General feelings toward patients (White patients – Black patients) | -0.27 | 0.14 | -0.21 | -1.92 | 0.059 | -0.26 | 0.13 | -0.21 | -2.02 | 0.047* | |||||
| Predicted patient sexual risk compensation | -0.25 | 0.07 | -0.37 | -3.81 | <0.001*** | ||||||||||
| Model summary |
Model summary |
Model summary |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | R2 | Adjusted R2 | SE | F | p | R2 | Adjusted R2 | SE | F | p | R2 | Adjusted R2 | SE | |
| 2.38 | 0.020* | 0.21 | 0.12 | 0.68 | 2.52 | 0.005** | 0.32 | 0.20 | 0.65 | 3.75 | <0.001*** | 0.44 | 0.32 | 0.60 | |
| Change statistics |
Change statistics |
Change statistics |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 change | F change | df1, df2 | p | R2 change | F change | df1, df2 | p | R2 change | F change | df1, df2 | p | |
| 0.21 | 2.38 | 9, 79 | 0.020* | 0.11 | 2.41 | 5, 74 | 0.044* | 0.11 | 14.50 | 1, 73 | <0.001*** | |
* p < .05, ** p < .01, *** p < .001
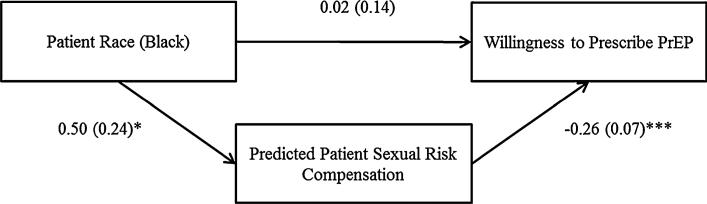
Bootstrapping Analysis of Indirect Effect
Bootstrapping analyses revealed a significant indirect effect of patient race on participant willingness to prescribe PrEP via predicted patient sexual risk compensation, as indicated by bias-corrected and accelerated confidence intervals that did not straddle zero [95 % CIs (–0.344, –0.024)]. Thus, participants judged the Black patient to be more likely than the White patient to increase his rate of unprotected sex if prescribed PrEP, which, in turn, was associated with reduced willingness to prescribe PrEP to the patient. (See Fig. 1.)
Fig. 1.
Model of the indirect effect of patient race on participant willingness to prescribe PrEP. Unstandardized coefficients and standard errors [B (SE)] are included for all paths. Participant sociodemographic and medical training characteristics (gender, race, sexual orientation, age, social class, current year of medical school, and past clinical experience with HIV-positive patients); other clinical judgments (predicted patient adherence, perceived patient risk of HIV infection without PrEP, and risk reduction associated with PrEP); and racial bias (perceived importance of patient request and general feelings toward Black versus White patients) were statistically controlled in the analysis
Discussion
Results of this vignette-based study indicate the potential for inequitable access to PrEP across racial groups due to provider bias, even when other potential barriers to access (e.g., insurance coverage/cost or access to care) are absent. Medical students judged a Black patient to be more likely to engage in PrEP-associated sexual risk compensation compared to a White patient, and this judgment was associated with reduced willingness to prescribe PrEP in a hypothetical primary care scenario. Further, sexual risk compensation emerged as a key consideration relative to prescription willingness, accounting for substantial variance above and beyond other clinical judgments such as anticipated patient adherence, beliefs about a patient's risk without PrEP, and the perceived risk reduction capacity of PrEP. This is concerning given the apparent vulnerability of this clinical perception to racial bias.
Predictions of greater unprotected sex with PrEP for the Black patient relative to the White patient are consistent with stereotypes of uncontrolled sexuality and sexual risk that have been documented in association with Black men and Black MSM in particular [14–16]. The identified disparity supports some Black MSM's perceptions of health-care providers having race-based and sexual orientation-based preconceptions about their behavior [74]. Stereotypes are a form of automatic cognition that operate outside of awareness [60, 75]; they are learned through repeated exposure to external stimulus pairings and retained via implicit memory [76]. Thus, the stereotype that may be operating among medical students in this study likely reflects an association promoted by the broader culture within which they are immersed, and does not necessarily represent a consciously held attitude.
Data assessing the behavior of PrEP users who have not been involved in clinical trials are not yet available; evidence is lacking, therefore, to confirm or refute participants’ predictions regarding sexual risk compensation among PrEP users of either race. However, it is important to note that there is extensive behavioral evidence to contradict stereotypes of greater sexual risk-taking among Black MSM [65–67]. Thus, any provider assumptions about Black MSM being more prone to engaging in sexual risk behavior, and thus more inclined to exhibit risk compensation with PrEP, would not be substantiated by empirical knowledge to date.
Implications for Intervention
Given the potential utility of PrEP for people at high risk for HIV acquisition, identifying and addressing potential barriers to access is important. As long as PrEP requires a prescription, healthcare providers with prescription licenses will function as gatekeepers. Importantly, these gate-keepers include HIV specialists and other types of care providers alike [77]. To ensure the broadest possible access, implementation of PrEP ought to occur in a variety of clinical settings, such as private practices, HIV clinics, emergency rooms, voluntary counseling and testing centers, STI clinics, and community health clinics [78]. Providers across the spectrum will thus confront decisions about PrEP prescription, and their decisions may be vulnerable to stereotypes.
Interventions designed to identify and combat stereotypes that could adversely affect the equitable distribution of PrEP ought to be implemented broadly to capture medical providers of all ranks and clinical settings who encounter patients at risk for HIV acquisition. Ideally, widespread change in the sexual representations of Black MSM would occur on a societal level to counteract existing stereotypes. However, to the extent that these stereotypes persist, interventions are needed to prevent them from negatively affecting clinical judgment and practice.
Previous research has demonstrated that stereotypes are less likely to be activated in tasks that emphasize individuation (i.e., focusing on individual characteristics of a patient) over social categorization (i.e., basing perceptions of a patient on his/her group membership; [79]). Accordingly, individuation has been encouraged as a cognitive strategy to reduce bias in provider judgment and practice [80]. Provider-patient partnership building that generates a sense of common group identity has also been recommended as a means of inhibiting the activation of racial stereotypes [33, 80] and has yielded enhanced patient trust and improved clinical outcomes among Black patients when implemented via behavioral intervention [81]. Another recommendation for combating race-based discrimination in clinical practice involves raising providers’ awareness about the prevalence of such discrimination and the vulnerability of their own clinical judgment to unconscious racial biases [33, 80]. For example, given the current findings, increasing providers’ cognizance of the sexual risk compensation stereotype may cue them to recognize the stereotypical basis of their own assumptions about sexual behavior among the Black PrEP-seeking patients whom they encounter. Of note, in light of evidence suggesting provider bias can actually be exacerbated by awareness of such bias, consciousness-raising interventions should incorporate opportunities for self-affirmation as a buffer to such potentially threatening content [82].
Medical students are an important group to target for intervention given their clinical involvement during medical school, accessibility for didactic training, and status as future care providers who, theoretically, have many years of practice ahead of them. During their upcoming years of medical service, many are likely to encounter patients who could benefit from PrEP. Accordingly, raising awareness about provider vulnerability to stereotypes and implications for discriminatory practice is crucial at this stage of training [31, 83], and the tactics identified above could be utilized successfully with medical students. Geiger [31] advocates for active integration of relevant educational material into medical coursework, stating, “The problems and nature of stereotyping and bias need to be taught and discussed repeatedly at every level of the undergraduate and graduate medical curriculum, not merely as a part of a cultural competency curriculum devoted to the beliefs and behaviors of different groups of patients, but also as efforts at self-awareness and the culture of medicine itself” (p. 443). Other educators have echoed his call for medical training that promotes introspection and critical reflection in the context of cultural competence education as well as emphasizing individual differences within a given group to avoid perpetuating stereotypes [84, 85].
Limitations and Future Research
Several limitations of the current study warrant mention and set the stage for future research. First, the sample was comprised of medical students, restricting the extent to which we can predict attitudes or behavior among independently practicing physicians during the initial phase of PrEP roll-out based on these findings. Replication of this study with a different sampling frame could explore the relevance of patient race to predictions of risk compensation and PrEP prescription practices among current providers.
Additionally, the sample size did not allow for separate analyses within participant racial groups; thus, our findings are drawn from a multiethnic sample with race controlled statistically. Future research with a larger sample could examine whether these processes differ among providers of different racial/ethnic groups, as patient-provider racial concordance has previously been associated with antiretroviral treatment [18].
The focal population in the current study was MSM. Despite the observed difference in predicted sexual risk compensation by race, the vast majority of study participants in both conditions acknowledged there being some likelihood that the patient might engage in sexual risk compensation (97 % of patients in the Black-patient condition and 87 % in the White-patient condition reported the patient was “a little bit,” “somewhat,” “very,” or “extremely” likely to engage in more unprotected sex if prescribed PrEP). The extent to which the pervasive assumption of risk compensation reflects stereotypes of promiscuity applied to the gay male community at large, beliefs about sexual tendencies of men more generally, or assumptions about human nature irrespective of gender and sexual orientation is indeterminable based on the two vignettes used in the current study. Future vignette-based research manipulating not only patient race, but also gender, sexual orientation, and other sociodemographic characteristics, could shed light on any systematic biases in judgments of sexual risk compensation associated with other social groups.
Between-subjects analyses in this study suggested the existence of a stereotype, or cognitive attribution of a particular behavioral characteristic, to Black patients, as opposed to general racial prejudice (i.e., a negative affective/evaluative response towards Black patients) [75, 86]. In fact, the vast majority of participants (87 %) expressed no explicit bias in their self-reported feelings toward Black versus White patients. Given that implicit measures may be more sensitive to racial bias and more predictive of poorer medical care than explicit measures [33, 87], inclusion of implicit, within-subject measures of stereotypes and prejudice in future research examining racial disparities in clinical decision-making related to PrEP may yield greater insight into underlying cognitive mechanisms.
Our results provide evidence for racial bias in the context of a hypothetical clinical scenario, but the extent to which these findings would translate to bias in real-world clinical settings is unknown. That said, clinical vignettes have received support as a cost-effective method of assessing clinical decision-making while controlling for case mix and structural factors [88, 89], and they have been recommended specifically for research on variation in clinical practice based on patient sociodemographic characteristics [88]. Further, the validity of clinical vignettes as indicators of actual clinical practice has generally been supported by previous research comparing physicians’ case conceptualizations pertaining to clinical vignettes (including diagnoses, tests to order, and treatment plans) with their clinical care for standardized patients presenting with identical symptoms [89]. Nonetheless, it will be important to systematically monitor clinical perceptions and prescription practices relative to characteristics of real patients as PrEP becomes more commonly utilized. It is crucial to understand how providers may predict and respond to the potential for risk compensation behavior among their patients.
With increasing community uptake of PrEP, it will also be important to assess actual patterns of risk compensation among PrEP users. If future analyses document PrEP-associated risk compensation among real-life users, prescribing providers will play a paramount role in delivering counseling and other behavioral interventions to complement PrEP use. The FDA-approved Risk Evaluation and Mitigation Strategy for Truvada® PrEP already relies heavily on prescribing providers to communicate with patients regarding condom use and safer-sex practices [25]. Research identifying risk factors for participation in sexual risk compensation could inform providers’ approach to PrEP delivery, including the provision of additional supports as needed, thereby enhancing the potential for PrEP to be a safe and effective method of prevention for many different people at high risk for HIV acquisition.
Concluding Remarks
“An AIDS-free generation requires antiretroviral deployment for treatment and prevention ... denying antiretroviral provision for prevention efforts is ... a transgression of human rights” (Singh, 2013, emphasis added [90]). To date, concerns about equitable access to PrEP have been voiced with regard to structural barriers such as cost, medical monitoring requirements, and resource allocation [90–94], yet little attention has been dedicated to social barriers, including the potential for discrimination in the provision of PrEP by healthcare providers. Our findings on racial bias and clinical judgment are particularly timely, as PrEP is first being introduced to providers and awareness could be raised in the process. Current definitions of the target population remain elusive and controversial [12, 95], and there is a recognized need for assessment protocols to direct initial eligibility screening, ensure ongoing monitoring of PrEP suitability, and guide discontinuation of PrEP [78, 96]. As the CDC works towards addressing these concerns through the development of comprehensive guidelines for providers, integration of content that increases awareness about provider bias generally and racial stereotypes regarding risk compensation specifically could reduce the potential for discriminatory prescription practices to occur, maximizing access to PrEP for the diversity of people who stand to benefit from it.
Box 1.
Case vignette to which participants responded in online survey
Mr. J is a 31-year-old, Black (White) man who is HIV-negative. He presents to you, his primary care physician, requesting a prescription for Truvada, stating that he wants to take the medication to help prevent himself from getting HIV. He has insurance that would cover the prescription.
Mr. J currently has one male sex partner, who has been diagnosed as HIV-positive and with whom he is monogamous. During previous appointments, you have discussed HIV risk with him and encouraged him to use condoms. However, he does not always use them, resulting in repeated episodes of unprotected sex with his partner.
HIV antibody and RNA lab tests confirm that Mr. J is HIV-negative. He has never had any STDs. He has no physical complaints. He has never had surgery or been hospitalized. His medical history is otherwise unremarkable.
Mr. J does not use alcohol, tobacco, or other drugs. He has no known drug allergies. He does not currently take any medications.
Acknowledgments
Sarah K. Calabrese and Valerie A. Earnshaw were supported by Award Number T32MH020031 from the National Institute of Mental Health. Research reported in this publication was supported by the National Institute of Mental Health under Award Number P30MH062294. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health. The authors wish to thank the restaurant owners who generously donated gift cards in support of this study. In addition, they are grateful to Dr. Jaimie Meyer for contributing her medical expertise in developing the clinical vignette, Dr. Nancy Angoff for facilitating recruitment, and the medical students for their participation.
Contributor Information
Sarah K. Calabrese, Center for Interdisciplinary Research on AIDS, Yale University, 135 College Street, Suite 200, New Haven, CT 06510, USA
Valerie A. Earnshaw, Center for Interdisciplinary Research on AIDS, Yale University, 135 College Street, Suite 200, New Haven, CT 06510, USA
Kristen Underhill, Center for Interdisciplinary Research on AIDS, Yale University, 135 College Street, Suite 200, New Haven, CT 06510, USA; Yale Law School, Yale University, New Haven, CT, USA.
Nathan B. Hansen, Center for Interdisciplinary Research on AIDS, Yale University, 135 College Street, Suite 200, New Haven, CT 06510, USA College of Public Health, University of Georgia, Athens, GA, USA.
John F. Dovidio, Department of Psychology, Yale University, New Haven, CT, USA
References
- 1.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587–99. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367(5):423–34. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 3.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.US Food and Drug Administration [1 Oct 2013];FDA News Release: FDA approves first drug for reducing the risk of sexually acquired HIV infection. 2013 http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm312210.htm.
- 5.Hogben M, Liddon N. Disinhibition and risk compensation: scope, definitions, and perspective. Sex Transm Dis. 2008;35(12):1009–10. doi: 10.1097/OLQ.0b013e31818eb752. [DOI] [PubMed] [Google Scholar]
- 6.Tripathi A, Ogbuanu C, Monger M, Gibson JJ, Duffus WA. Pre-exposure prophylaxis for HIV infection: healthcare providers’ knowledge, perception, and willingness to adopt future implementation in the southern US. South Med J. 2012;105(4):199–206. doi: 10.1097/SMJ.0b013e31824f1a1b. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention Interim guidance: preexposure prophylaxis for the prevention of HIV infection in men who have sex with men. MMWR Morb Mortal Wkly Rep. 2011;60(3):65–8. [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention Interim guidance for clinicians considering the use of preexposure prophylaxis for the prevention of HIV infection in heterosexually active adults. MMWR Morb Mortal Wkly Rep. 2012;61(31):586–9. [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention Update to Interim Guidance for Preexposure Prophylaxis (PrEP) for the Prevention of HIV Infection: PrEP for injecting drug users. MMWR Morb Mortal Wkly Rep. 2013;62(23):463–5. [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization [1 Oct 2013];Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. http://www.who.int/hiv/pub/guidelines/arv2013/download/en/index.html. [PubMed]
- 11.World Health Organization . Guidance on pre-exposure oral prophylaxis (PrEP) for serodiscordant couples, men and transgender women who have sex with men at high risk of HIV: recommendations for use in the context of demonstration projects. World Health Organization; Geneva: 2012. [PubMed] [Google Scholar]
- 12.Arnold EA, Hazelton P, Lane T, et al. A qualitative study of provider thoughts on implementing pre-exposure prophylaxis (PrEP) in clinical settings to prevent HIV infection. PLoS One. 2012;7(7):e40603. doi: 10.1371/journal.pone.0040603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Puro V, Palummieri A, De Carli G, Piselli P, Ippolito G. Attitude towards antiretroviral pre-exposure prophylaxis (PrEP) prescription among HIV specialists. BMC Infect Dis. 2013;13(1):217–24. doi: 10.1186/1471-2334-13-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bowleg L, Teti M, Massie JS, Patel A, Malebranche DJ, Tschann JM. ‘What does it take to be a man? What is a real man?’: ideologies of masculinity and HIV sexual risk among Black heterosexual men. Cult Health Sex. 2011;13(5):545–59. doi: 10.1080/13691058.2011.556201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bowleg L. “Once you've blended the cake, you can't take the parts back to the main ingredients”: Black gay and bisexual men's descriptions and experiences of intersectionality. Sex Roles. 2013;68(11–12):754–67. [Google Scholar]
- 16.Valentine JA. Impact of attitudes and beliefs regarding African American sexual behavior on STD prevention and control in African American communities: unintended consequences. Sex Transm Dis. 2008;35(12):S23–9. doi: 10.1097/OLQ.0b013e31818d3cc7. [DOI] [PubMed] [Google Scholar]
- 17.Bogart LM, Catz SL, Kelly JA, Benotsch EG. Factors influencing physicians’ judgments of adherence and treatment decisions for patients with HIV disease. Med Decis Making. 2001;21(1):28–36. doi: 10.1177/0272989X0102100104. [DOI] [PubMed] [Google Scholar]
- 18.King WD, Wong MD, Shapiro MF, Landon BE, Cunningham WE. Does racial concordance between HIV-positive patients and their physicians affect the time to receipt of protease inhibitors? J Gen Intern Med. 2004;19(11):1146–53. doi: 10.1111/j.1525-1497.2004.30443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shapiro MF, Morton SC, McCaffrey DF, et al. Variations in the care of HIV-infected adults in the United States—results from the HIV cost and services utilization study. JAMA. 1999;281(24):2305–15. doi: 10.1001/jama.281.24.2305. [DOI] [PubMed] [Google Scholar]
- 20.Stone VE. Physician contributions to disparities in HIV/AIDS care: the role of provider perceptions regarding adherence. Curr HIV/AIDS Rep. 2005;2:189–93. doi: 10.1007/s11904-005-0015-5. [DOI] [PubMed] [Google Scholar]
- 21.Harrison DD, Cooke CW. An elucidation of factors influencing physicians’ willingness to perform elective female sterilization. Obstet Gynecol. 1988;72(4):565–70. [PubMed] [Google Scholar]
- 22.Borrero S, Schwarz EB, Creinin M, Ibrahim S. The impact of race and ethnicity on receipt of family planning services in the United States. J Womens Health. 2009;18(1):91–6. doi: 10.1089/jwh.2008.0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D'Amore MM, Cheng DM, Allensworth-Davies D, Samet JH, Saitz R. Disparities in safe sex counseling & behavior among individuals with substance dependence: a cross-sectional study. Reprod Health. 2012;9:35. doi: 10.1186/1742-4755-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Downing RA, LaVeist TA, Bullock HE. Intersections of ethnicity and social class in provider advice regarding reproductive health. Am J Public Health. 2007;97(10):1803–7. doi: 10.2105/AJPH.2006.092585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilead Sciences Inc [1 Oct 2013];Truvada: Risk Evaluation and Mitigation Strategy. http://www.fda.gov/downloads/Drugs/DrugSafety/Post marketDrugSafetyInformationforPatiePostmarketDrugS/UCM312304.pdf.
- 26.Abdool Karim SS, Gray GE, Martinson N. Clinical decisions. Preexposure prophylaxis for HIV prevention. N Engl J Med. 2012;367(5):462–5. doi: 10.1056/NEJMclde1207706. [DOI] [PubMed] [Google Scholar]
- 27.Maznavi K, Hardy D, Bredeek F. Pre-exposure prophylaxis (PrEP) for HIV: an online survey of HIV healthcare providers evaluating their knowledge, perception, and prescription of PrEP. Infectious Diseases Society of America Annual Meeting; Boston: 2011. [Google Scholar]
- 28.Gaertner SL, Dovidio JF. Understanding and addressing contemporary racism: from aversive racism to the common ingroup identity model. J Soc Issues. 2005;61(3):615–39. [Google Scholar]
- 29.Aberson CL, Ettlin TE. The aversive racism paradigm and responses favoring African Americans: meta-analytic evidence of two types of favoritism. Soc Justice Res. 2004;17(1):25–46. [Google Scholar]
- 30.Dovidio JF, Gaertner SL. Aversive racism. Adv Exp Soc Psychol. 2004;36:1–52. [Google Scholar]
- 31.Geiger HJ. Racial and ethnic disparities in diagnosis and treatment: a review of the evidence and a consideration of causes. In: Smedley A, Stith A, Nelson A, editors. Unequal treatment: donfronting racial and ethnic disparities in health care. National Academic Press; Washington, DC: 2003. pp. 417–54. [PubMed] [Google Scholar]
- 32.Dovidio JF, Penner LA, Albrecht TL, Norton WE, Gaertner SL, Shelton JN. Disparities and distrust: the implications of psychological processes for understanding racial disparities in health and health care. Soc Sci Med. 2008;67(3):478–86. doi: 10.1016/j.socscimed.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 33.Dovidio JF, Fiske ST. Under the radar: how unexamined biases in decision-making processes in clinical interactions can contribute to health care disparities. Am J Public Health. 2012;102(5):945–52. doi: 10.2105/AJPH.2011.300601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thorner AR, Rosenberg ES. Early versus delayed antiretroviral therapy in patients with HIV infection—a review of the current guidelines from an immunological perspective. Drugs. 2003;63(13):1325–37. doi: 10.2165/00003495-200363130-00001. [DOI] [PubMed] [Google Scholar]
- 35.Mimiaga MJ, White JM, Krakower DS, Biello KB, Mayer KH. Suboptimal awareness and comprehension of published preexposure prophylaxis efficacy results among physicians in Massachusetts. AIDS Care. 2013 doi: 10.1080/09540121.2013.845289. Epub 2013/10/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.White JM, Mimiaga MJ, Krakower DS, Mayer KH. Evolution of Massachusetts physician attitudes, knowledge, and experience regarding the use of antiretrovirals for HIV prevention. AIDS Patient Care STDs. 2012;26(7):395–405. doi: 10.1089/apc.2012.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Senn H, Wilton J, Sharma M, Fowler S, Tan DH. Knowledge of and opinions on HIV preexposure prophylaxis among front-line service providers at Canadian AIDS service organizations. AIDS Res Hum Retroviruses. 2013;29(9):1183–9. doi: 10.1089/aid.2013.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hedlund J. Risky business: safety regulations, risk compensation, and individual behavior. Inj Prev. 2000;6:82–90. doi: 10.1136/ip.6.2.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cassell MM, Halperin DT, Shelton JD, Stanton D. Risk compensation: the Achilles’ heel of innovations in HIV prevention? BMJ. 2006;332(7541):605–7. doi: 10.1136/bmj.332.7541.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eaton LA, Kalichman S. Risk compensation in HIV prevention: implications for vaccines, microbicides, and other biomedical HIV prevention technologies. Curr HIV/AIDS Rep. 2007;4(4):165–72. doi: 10.1007/s11904-007-0024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Golub SA, Kowalczyk W, Weinberger CL, Parsons JT. Preexposure prophylaxis and predicted condom use among high-risk men who have sex with men. J Acquir Immune Defic Syndr. 2010;54(5):548–55. doi: 10.1097/QAI.0b013e3181e19a54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith DK, Toledo L, Smith DJ, Adams MA, Rothenberg R. Attitudes and program preferences of African-American urban young adults about pre-exposure prophylaxis (PrEP). AIDS Educ Prev. 2012;24(5):408–21. doi: 10.1521/aeap.2012.24.5.408. [DOI] [PubMed] [Google Scholar]
- 43.Underhill K, Morrow KM, Operario D, Ducharme R, Kuo C, Mayer KH. Project PrEP talk: an in-depth qualitative analysis of PrEP acceptability, expectations and risk compensation beliefs among United States MSM.. XIX International AIDS Conference; Washington, DC. 2012. [Google Scholar]
- 44.Krakower D, Mimiaga M, Rosenberger J, et al. Anticipated risk compensation with pre-exposure prophylaxis use among North American men who have sex with men using an internet social network.. XIX International AIDS Conference; Washington, DC. 2012. [Google Scholar]
- 45.Brooks RA, Landovitz RJ, Kaplan RL, Lieber E, Lee SJ, Barkley TW. Sexual risk behaviors and acceptability of HIV pre-exposure prophylaxis among HIV-negative gay and bisexual men in sero-discordant relationships: a mixed methods study. AIDS Patient Care STDS. 2012;26(2):87–94. doi: 10.1089/apc.2011.0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koblin BA, Mansergh G, Frye V, et al. Condom-use decision making in the context of hypothetical pre-exposure prophylaxis efficacy among substance-using men who have sex with men: Project MIX. J Acquir Immune Defic Syndr. 2011;58(3):319–27. doi: 10.1097/QAI.0b013e31822b76d2. [DOI] [PubMed] [Google Scholar]
- 47.Paltiel AD, Freedberg KA, Scott CA, et al. HIV preexposure prophylaxis in the United States: impact on lifetime infection risk, clinical outcomes, and cost-effectiveness. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:806–15. doi: 10.1086/597095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Desai K, Sansom SL, Ackers ML, Stewart SR, Hall HI, Hu DJ, et al. Modeling the impact of HIV chemoprophylaxis strategies among men who have sex with men in the United States: HIV infections prevented and cost-effectiveness. AIDS. 2008;22:1829–39. doi: 10.1097/QAD.0b013e32830e00f5. [DOI] [PubMed] [Google Scholar]
- 49.Gray R, Kigozi G, Kong X, et al. The effectiveness of male circumcision for HIV prevention and effects on risk behaviors in a posttrial follow-up study. AIDS. 2012;26(5):609–15. doi: 10.1097/QAD.0b013e3283504a3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kong X, Kigozi G, Nalugoda F, et al. Assessment of changes in risk behaviors during 3 years of posttrial follow-up of male circumcision trial participants uncircumcised at trial closure in Rakai, Uganda. Am J Epidemiol. 2012;176(10):875–85. doi: 10.1093/aje/kws179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kalichman S, Eaton L, Pinkerton S. Circumcision for HIV prevention: failure to fully account for behavioral risk compensation. PLoS Med. 2007;4(3):e138. doi: 10.1371/journal.pmed.0040138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gust DA, Kretsinger K, Pals SL, Gaul ZJ, Hefflefinger JD, Begley EB, et al. Male circumcision as an HIV prevention intervention in the US: influence of health care providers and potential for risk compensation. Prev Med. 2011;52(3–4):270–3. doi: 10.1016/j.ypmed.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 53.Pinkerton SD. Sexual risk compensation and HIV/STD transmission: empirical evidence and theoretical considerations. Risk Anal. 2001;21(4):727–36. doi: 10.1111/0272-4332.214146. [DOI] [PubMed] [Google Scholar]
- 54.Crepaz N, Hart TA, Marks G. Highly active antiretroviral therapy and sexual risk behavior: a meta-analytic review. JAMA. 2004;292(2):224–36. doi: 10.1001/jama.292.2.224. [DOI] [PubMed] [Google Scholar]
- 55.Guest G, Shattuck D, Johnson L, et al. Changes in sexual risk behavior among participants in a PrEP HIV prevention trial. Sex Transm Dis. 2008;35(12):1002–8. [PubMed] [Google Scholar]
- 56.Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367(5):411–22. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381(9883):2083–90. doi: 10.1016/S0140-6736(13)61127-7. [DOI] [PubMed] [Google Scholar]
- 58.Underhill K. Study designs for identifying risk compensation behavior among users of biomedical HIV prevention technologies: balancing methodological rigor and research ethics. Soc Sci Med. 2013;94:115–23. doi: 10.1016/j.socscimed.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mugwanya KK, Donnell D, Celum C, Thomas KK, Ndase P, Mugo N, et al. Sexual behaviour of heterosexual men and women receiving antiretroviral pre-exposure prophylaxis for HIV prevention: a longitudinal analysis. Lancet Infect Dis. 2013 doi: 10.1016/S1473-3099(13)70226-3. Epub 2013/10/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Greenwald AG, Banaji MR. Implicit social cognition: attitudes, self-esteem, and stereotypes. Psychol Rev. 1995;102(1):4–27. doi: 10.1037/0033-295x.102.1.4. [DOI] [PubMed] [Google Scholar]
- 61.Jost JT, Banaji MR. The role of stereotyping in system-justification and the production of false consciousness. Br J Soc Psychol. 1994;33:1–27. [Google Scholar]
- 62.Thorburn S, Bogart LM. African American women and family planning services: perceptions of discrimination. Women Health. 2005;42(1):23–39. doi: 10.1300/J013v42n01_02. [DOI] [PubMed] [Google Scholar]
- 63.Saleh LD, Operario D. Moving beyond “the Down Low”: a critical analysis of terminology guiding HIV prevention efforts for African American men who have secretive sex with men. Soc Sci Med. 2009;68(2):390–5. doi: 10.1016/j.socscimed.2008.09.052. [DOI] [PubMed] [Google Scholar]
- 64.Ford CL, Whetten KD, Hall SA, Kaufman JS, Thrasher AD. Black sexuality, social construction, and research targeting ‘the Down Low’ (‘the DL’). Ann Epidemiol. 2007;17(3):209–16. doi: 10.1016/j.annepidem.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 65.Millett GA, Peterson JL, Flores SA, et al. Comparisons of disparities and risks of HIV infection in black and other men who have sex with men in Canada, UK, and USA: a meta-analysis. Lancet. 2012;380(9839):341–8. doi: 10.1016/S0140-6736(12)60899-X. [DOI] [PubMed] [Google Scholar]
- 66.Millett GA, Peterson JL, Wolitski RJ, Stall R. Greater risk for HIV infection of black men who have sex with men: a critical literature review. Am J Public Health. 2006;96(6):1007–19. doi: 10.2105/AJPH.2005.066720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Feldman MB. A critical literature review to identify possible causes of higher rates of HIV infection among young Black and Latino men who have sex with men. J Natl Med Assoc. 2010;102(12):1206–21. doi: 10.1016/s0027-9684(15)30776-8. [DOI] [PubMed] [Google Scholar]
- 68.Young S. [1 Oct 2013];Panel recommends approving Truvada to prevent HIV infection CNN Health. 2012 Jul 16; http://www.cnn.com/2012/05/10/health/hiv-drug.
- 69.AIDS Healthcare Foundation [1 Oct 2013];AHF asks, “What's rotten at FDA” in new Politico Playbook ads. 2012 http://www.aidshealth.org/archives/news/ahf-asks-whats-rotten-at-fda-in-politico-playbook.
- 70.Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychol Methods. 2002;7(4):422–45. [PubMed] [Google Scholar]
- 71.Hayes AF. Beyond Baron and Kenny: statistical mediation analysis in the new millennium. Commun Monogr. 2009;76(4):408–20. [Google Scholar]
- 72.Hayes AF. An introduction to mediation, moderation, and conditional process analysis: a regression-based approach. Guilford Press; New York: 2013. [Google Scholar]
- 73.MacKinnon DP, Fairchild AJ. Current Directions in Mediation Analysis. Curr Dir Psychol Sci. 2009;18(1):16–20. doi: 10.1111/j.1467-8721.2009.01598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Malebranche DJ, Peterson JL, Fullilove RE, Stackhouse RW. Race and sexual identity: perceptions about medical culture and healthcare among Black men who have sex with men. J Natl Med Assoc. 2004;96(1):97–107. [PMC free article] [PubMed] [Google Scholar]
- 75.Amodio DM, Devine PG. Stereotyping and evaluation in implicit race bias: evidence for independent constructs and unique effects on behavior. J Pers Soc Psychol. 2006;91(4):652–61. doi: 10.1037/0022-3514.91.4.652. [DOI] [PubMed] [Google Scholar]
- 76.Amodio DM, Ratner KG. A memory systems model of implicit social cognition. Curr Dir Psychol Sci. 2011;20(3):143–8. [Google Scholar]
- 77.Mera RM, Rawlings MK, Pechonkina A, Rooney JF, Peschel T, Cheng A. Status of Truvada (TVD) for HIV pre-exposure prophylaxis (PrEP) in the United States: an early drug utilization analysis.. Interscience Conference on Antimicrobial Agents and Chemotherapy; Denver. 2013. [Google Scholar]
- 78.Underhill K, Operario D, Skeer M, Mimiaga M, Mayer K. Packaging PrEP to prevent HIV: an integrated framework to plan for pre-exposure prophylaxis implementation in clinical practice. J Acquir Immune Defic Syndr. 2010;55(1):8–13. doi: 10.1097/qai.0b013e3181e8efe4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wheeler ME, Fiske ST. Controlling racial prejudice: social-cognitive goals affect amygdala and stereotype activation. Psychol Sci. 2005;16(1):56–63. doi: 10.1111/j.0956-7976.2005.00780.x. [DOI] [PubMed] [Google Scholar]
- 80.Burgess D, van Ryn M, Dovidio J, Saha S. Reducing racial bias among health care providers: lessons from social-cognitive psychology. J Gen Intern Med. 2007;22(6):882–7. doi: 10.1007/s11606-007-0160-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Penner LA, Gaertner S, Dovidio JF, et al. A social psychological approach to improving the outcomes of racially discordant medical interactions. J Gen Intern Med. 2013;28(9):1143–9. doi: 10.1007/s11606-013-2339-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Burgess DJ. Addressing racial healthcare disparities: how can we shift the focus from patients to providers? J Gen Intern Med. 2011;26(8):828–30. doi: 10.1007/s11606-011-1748-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Teal CR, Shada RE, Gill AC, et al. When best intentions aren't enough: helping medical students develop strategies for managing bias about patients. J Gen Intern Med. 2010;25(Suppl 2):S115–8. doi: 10.1007/s11606-009-1243-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Boutin-Foster C, Foster JC, Konopasek L. Viewpoint: physician, know thyself: the professional culture of medicine as a framework for teaching cultural competence. Acad Med. 2008;83(1):106–11. doi: 10.1097/ACM.0b013e31815c6753. [DOI] [PubMed] [Google Scholar]
- 85.Novack DH, Epstein RM, Paulsen RH. Toward creating physician-healers: fostering medical students’ self-awareness, personal growth, and well-being. Acad Med. 1999;74(5):516–20. doi: 10.1097/00001888-199905000-00017. [DOI] [PubMed] [Google Scholar]
- 86.Newheiser AK, Dovidio JF. Individual differences and intergroup bias: divergent dynamics associated with prejudice and stereotyping. Pers Individ Dif. 2012;53(1):70–4. [Google Scholar]
- 87.Greenwald AG, Poehlman TA, Uhlmann EL, Banaji MR. Understanding and using the implicit association test: III. Meta-analysis of predictive validity. J Pers Soc Psychol. 2009;97(1):17–41. doi: 10.1037/a0015575. [DOI] [PubMed] [Google Scholar]
- 88.Veloski J, Tai S, Evans AS, Nash DB. Clinical vignette-based surveys: a tool for assessing physician practice variation. Am J Med Qual. 2005;20(3):151–7. doi: 10.1177/1062860605274520. [DOI] [PubMed] [Google Scholar]
- 89.Peabody JW, Luck J, Glassman P, Dresselhaus TR, Lee M. Comparison of vignettes, standardized patients, and chart abstraction: a prospective validation study of 3 methods for measuring quality. JAMA. 2000;283(13):1715–22. doi: 10.1001/jama.283.13.1715. [DOI] [PubMed] [Google Scholar]
- 90.Singh JA. Antiretroviral resource allocation for HIV prevention. AIDS. 2013;27(6):863–5. doi: 10.1097/QAD.0b013e32835f2b30. [DOI] [PubMed] [Google Scholar]
- 91.Cohen SE, Liu AY, Bernstein KT, Philip S. Preparing for HIV pre-exposure prophylaxis: lessons learned from post-exposure prophylaxis. Am J Prev Med. 2013;44(Suppl 2):S80–5. doi: 10.1016/j.amepre.2012.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Galindo GR, Walker JJ, Hazelton P, et al. Community member perspectives from transgender women and men who have sex with men on pre-exposure prophylaxis as an HIV prevention strategy: implications for implementation. Implement Sci. 2012;7:116. doi: 10.1186/1748-5908-7-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jay JS, Gostin LO. Ethical challenges of preexposure prophylaxis for HIV. JAMA. 2012;308(9):867–8. doi: 10.1001/2012.jama.10158. [DOI] [PubMed] [Google Scholar]
- 94.Rennie S. Ethical use of antiretroviral resources for HIV prevention in resource poor settings. Dev World Bioeth. 2013;13(2):79–86. doi: 10.1111/dewb.12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Underhill K, Operario D, Mimiaga MJ, Skeer MR, Mayer KH. Implementation science of pre-exposure prophylaxis: preparing for public use. Curr HIV/AIDS Rep. 2010;7(4):210–9. doi: 10.1007/s11904-010-0062-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Krakower D, Mayer KH. Engaging healthcare providers to implement HIV pre-exposure prophylaxis. Curr Opin HIV AIDS. 2012;7(6):593–9. doi: 10.1097/COH.0b013e3283590446. [DOI] [PMC free article] [PubMed] [Google Scholar]