Abstract
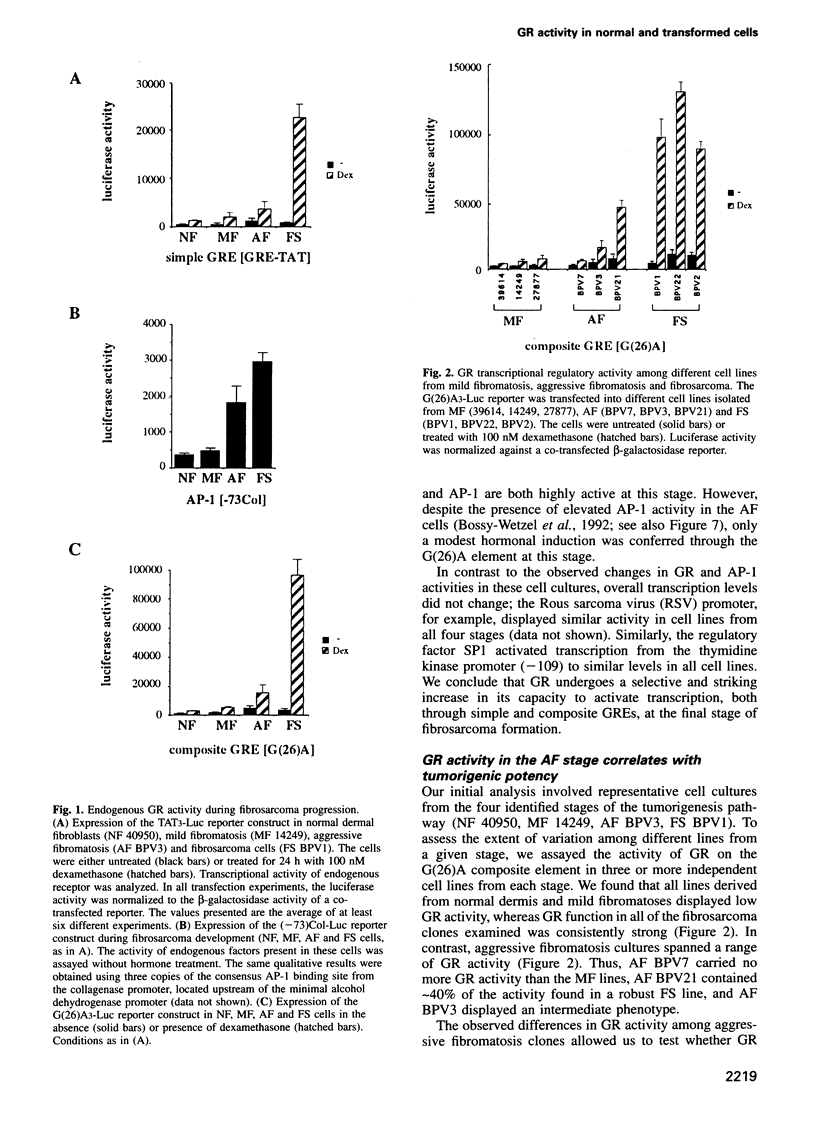
In transgenic mice harboring the bovine papillomavirus genome, fibrosarcomas arise along an experimentally accessible pathway in which normal dermal fibroblasts progress through two pre-neoplastic stages, mild and aggressive fibromatosis, followed by a final transition to the tumor stage. We found that the glucocorticoid receptor (GR) displays only modest transcriptional regulatory activity in cells derived from the three non-tumor stages, whereas it is highly active in fibrosarcoma cells. Upon inoculation into mice, the aggressive fibromatosis cells progress to tumor cells that have high GR activity; thus, the increased transcriptional regulatory activity of GR correlates with the cellular transition to the tumor stage. The intracellular levels of GR, as well as its hormone-dependent nuclear translocation and specific DNA binding activities, are unaltered throughout the progression. Strikingly, the low GR activity observed in the pre-neoplastic stages cannot be overcome by exogenous GR introduced by co-transfection. Moreover, comparisons of primary embryo fibroblasts and their transformed derivatives revealed a similar pattern--modest GR activity, unresponsive to overexpressed GR protein, in the normal cells was strongly increased in the transformed cells. Likewise, the retinoic acid receptor (RAR) displayed similar differential activity in the fibrosarcoma pathway. Thus, the oncogenic transformation of fibroblasts, and likely other cell types, is accompanied by a striking increase in the activities of transcriptional regulators such as GR and RAR. We suggest that normal primary cells have a heretofore unrecognized capability to limit the magnitude of induction of gene expression.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews N. C., Faller D. V. A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res. 1991 May 11;19(9):2499–2499. doi: 10.1093/nar/19.9.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel P., Baumann I., Stein B., Delius H., Rahmsdorf H. J., Herrlich P. 12-O-tetradecanoyl-phorbol-13-acetate induction of the human collagenase gene is mediated by an inducible enhancer element located in the 5'-flanking region. Mol Cell Biol. 1987 Jun;7(6):2256–2266. doi: 10.1128/mcb.7.6.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Molecular themes in oncogenesis. Cell. 1991 Jan 25;64(2):235–248. doi: 10.1016/0092-8674(91)90636-d. [DOI] [PubMed] [Google Scholar]
- Bohen S. P., Yamamoto K. R. Isolation of Hsp90 mutants by screening for decreased steroid receptor function. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11424–11428. doi: 10.1073/pnas.90.23.11424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrow J., Goddard A. D., Sheer D., Solomon E. Molecular analysis of acute promyelocytic leukemia breakpoint cluster region on chromosome 17. Science. 1990 Sep 28;249(4976):1577–1580. doi: 10.1126/science.2218500. [DOI] [PubMed] [Google Scholar]
- Bossy-Wetzel E., Bravo R., Hanahan D. Transcription factors junB and c-jun are selectively up-regulated and functionally implicated in fibrosarcoma development. Genes Dev. 1992 Dec;6(12A):2340–2351. doi: 10.1101/gad.6.12a.2340. [DOI] [PubMed] [Google Scholar]
- Burnstein K. L., Cidlowski J. A. Regulation of gene expression by glucocorticoids. Annu Rev Physiol. 1989;51:683–699. doi: 10.1146/annurev.ph.51.030189.003343. [DOI] [PubMed] [Google Scholar]
- Dejean A., Bougueleret L., Grzeschik K. H., Tiollais P. Hepatitis B virus DNA integration in a sequence homologous to v-erb-A and steroid receptor genes in a hepatocellular carcinoma. Nature. 1986 Jul 3;322(6074):70–72. doi: 10.1038/322070a0. [DOI] [PubMed] [Google Scholar]
- Denis M. G., Chadéneau C., Blanchardie P., Lustenberger P. Biological effects of glucocorticoid hormones on two rat colon adenocarcinoma cell lines. J Steroid Biochem Mol Biol. 1992 Mar;41(3-8):739–745. doi: 10.1016/0960-0760(92)90415-f. [DOI] [PubMed] [Google Scholar]
- Diamond M. I., Miner J. N., Yoshinaga S. K., Yamamoto K. R. Transcription factor interactions: selectors of positive or negative regulation from a single DNA element. Science. 1990 Sep 14;249(4974):1266–1272. doi: 10.1126/science.2119054. [DOI] [PubMed] [Google Scholar]
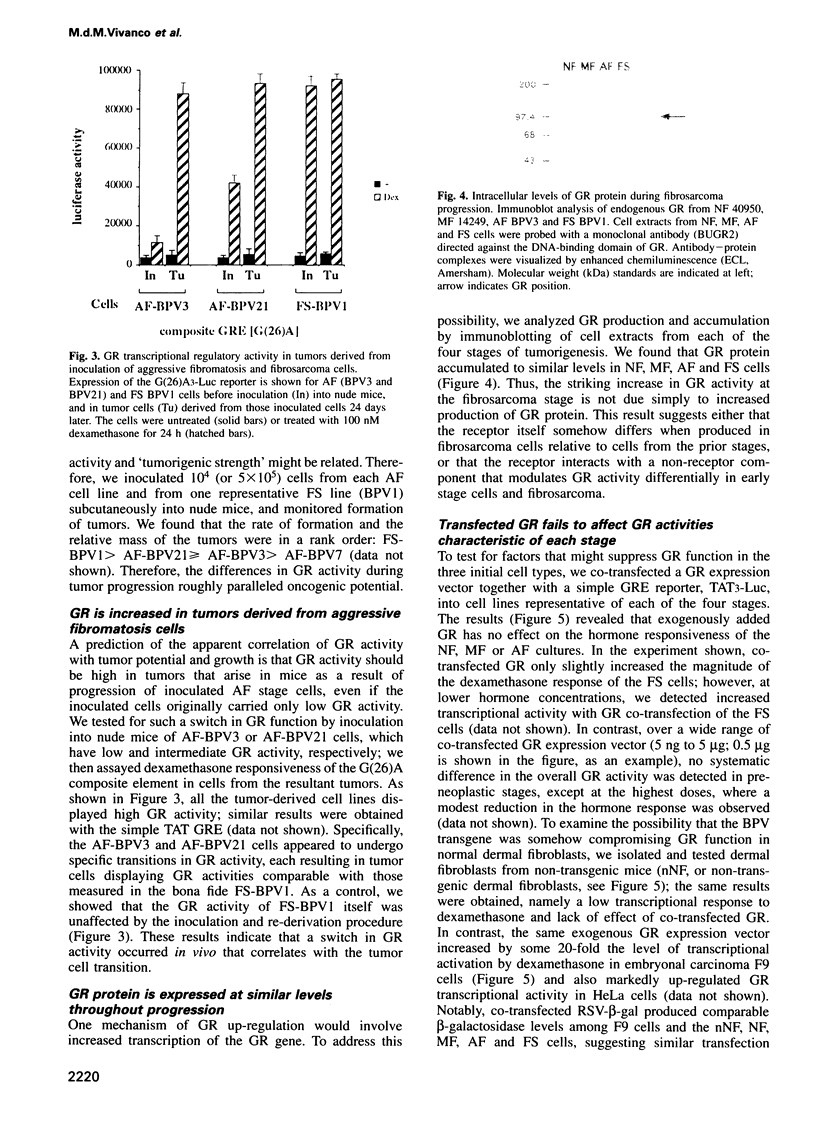
- Doucas V., Spyrou G., Yaniv M. Unregulated expression of c-Jun or c-Fos proteins but not Jun D inhibits oestrogen receptor activity in human breast cancer derived cells. EMBO J. 1991 Aug;10(8):2237–2245. doi: 10.1002/j.1460-2075.1991.tb07760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droms K. A., Hanson L. A., Malkinson A. M., Beer D. G. Altered dexamethasone responsiveness and loss of growth control in tumorigenic mouse lung cell lines. Int J Cancer. 1993 Apr 1;53(6):1017–1022. doi: 10.1002/ijc.2910530627. [DOI] [PubMed] [Google Scholar]
- Dürst M., Gallahan D., Jay G., Rhim J. S. Glucocorticoid-enhanced neoplastic transformation of human keratinocytes by human papillomavirus type 16 and an activated ras oncogene. Virology. 1989 Dec;173(2):767–771. doi: 10.1016/0042-6822(89)90595-3. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funder J. W., Pearce P. T., Smith R., Smith A. I. Mineralocorticoid action: target tissue specificity is enzyme, not receptor, mediated. Science. 1988 Oct 28;242(4878):583–585. doi: 10.1126/science.2845584. [DOI] [PubMed] [Google Scholar]
- Gametchu B., Harrison R. W. Characterization of a monoclonal antibody to the rat liver glucocorticoid receptor. Endocrinology. 1984 Jan;114(1):274–279. doi: 10.1210/endo-114-1-274. [DOI] [PubMed] [Google Scholar]
- Gaub M. P., Bellard M., Scheuer I., Chambon P., Sassone-Corsi P. Activation of the ovalbumin gene by the estrogen receptor involves the fos-jun complex. Cell. 1990 Dec 21;63(6):1267–1276. doi: 10.1016/0092-8674(90)90422-b. [DOI] [PubMed] [Google Scholar]
- Godowski P. J., Picard D., Yamamoto K. R. Signal transduction and transcriptional regulation by glucocorticoid receptor-LexA fusion proteins. Science. 1988 Aug 12;241(4867):812–816. doi: 10.1126/science.3043662. [DOI] [PubMed] [Google Scholar]
- Godowski P. J., Rusconi S., Miesfeld R., Yamamoto K. R. Glucocorticoid receptor mutants that are constitutive activators of transcriptional enhancement. Nature. 1987 Jan 22;325(6102):365–368. doi: 10.1038/325365a0. [DOI] [PubMed] [Google Scholar]
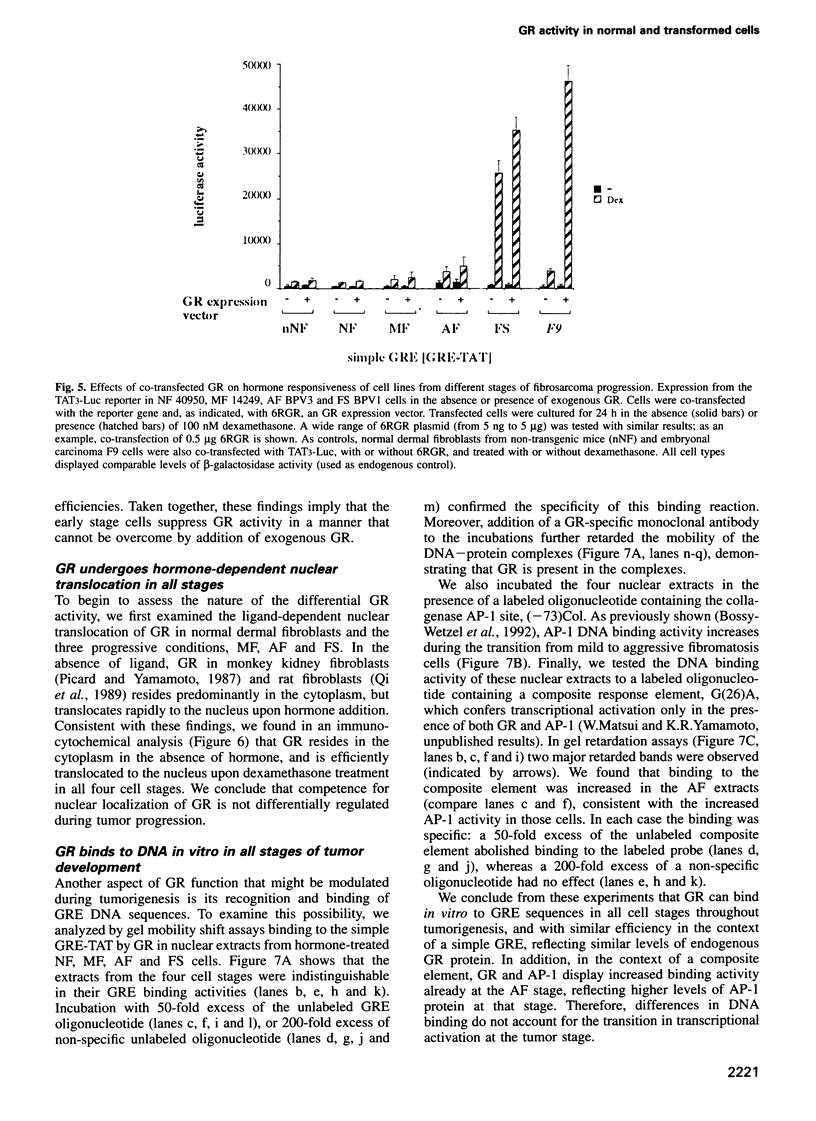
- Ham J., Parker M. G. Regulation of gene expression by nuclear hormone receptors. Curr Opin Cell Biol. 1989 Jun;1(3):503–511. doi: 10.1016/0955-0674(89)90012-4. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Dissecting multistep tumorigenesis in transgenic mice. Annu Rev Genet. 1988;22:479–519. doi: 10.1146/annurev.ge.22.120188.002403. [DOI] [PubMed] [Google Scholar]
- Hashimoto S., Schmid W., Schütz G. Transcriptional activation of the rat liver tyrosine aminotransferase gene by cAMP. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6637–6641. doi: 10.1073/pnas.81.21.6637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeck W., Groner B. Hormone-dependent phosphorylation of the glucocorticoid receptor occurs mainly in the amino-terminal transactivation domain. J Biol Chem. 1990 Apr 5;265(10):5403–5408. [PubMed] [Google Scholar]
- Hunter T. Cooperation between oncogenes. Cell. 1991 Jan 25;64(2):249–270. doi: 10.1016/0092-8674(91)90637-e. [DOI] [PubMed] [Google Scholar]
- Hunter T., Karin M. The regulation of transcription by phosphorylation. Cell. 1992 Aug 7;70(3):375–387. doi: 10.1016/0092-8674(92)90162-6. [DOI] [PubMed] [Google Scholar]
- Jantzen H. M., Strähle U., Gloss B., Stewart F., Schmid W., Boshart M., Miksicek R., Schütz G. Cooperativity of glucocorticoid response elements located far upstream of the tyrosine aminotransferase gene. Cell. 1987 Apr 10;49(1):29–38. doi: 10.1016/0092-8674(87)90752-5. [DOI] [PubMed] [Google Scholar]
- Jonat C., Rahmsdorf H. J., Park K. K., Cato A. C., Gebel S., Ponta H., Herrlich P. Antitumor promotion and antiinflammation: down-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell. 1990 Sep 21;62(6):1189–1204. doi: 10.1016/0092-8674(90)90395-u. [DOI] [PubMed] [Google Scholar]
- Jordan V. C., Murphy C. S. Endocrine pharmacology of antiestrogens as antitumor agents. Endocr Rev. 1990 Nov;11(4):578–610. doi: 10.1210/edrv-11-4-578. [DOI] [PubMed] [Google Scholar]
- Jordan V. G., Morrow M. An appraisal of strategies to reduce the incidence of breast cancer. Stem Cells. 1993 Jul;11(4):252–262. doi: 10.1002/stem.5530110401. [DOI] [PubMed] [Google Scholar]
- Kandel J., Bossy-Wetzel E., Radvanyi F., Klagsbrun M., Folkman J., Hanahan D. Neovascularization is associated with a switch to the export of bFGF in the multistep development of fibrosarcoma. Cell. 1991 Sep 20;66(6):1095–1104. doi: 10.1016/0092-8674(91)90033-u. [DOI] [PubMed] [Google Scholar]
- Kaspers G. J., Pieters R., Klumper E., De Waal F. C., Veerman A. J. Glucocorticoid resistance in childhood leukemia. Leuk Lymphoma. 1994 Apr;13(3-4):187–201. doi: 10.3109/10428199409056282. [DOI] [PubMed] [Google Scholar]
- Kerppola T. K., Curran T. DNA bending by Fos and Jun: the flexible hinge model. Science. 1991 Nov 22;254(5035):1210–1214. doi: 10.1126/science.1957173. [DOI] [PubMed] [Google Scholar]
- Lindgren V., Sippola-Thiele M., Skowronski J., Wetzel E., Howley P. M., Hanahan D. Specific chromosomal abnormalities characterize fibrosarcomas of bovine papillomavirus type 1 transgenic mice. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5025–5029. doi: 10.1073/pnas.86.13.5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall C. J. Tumor suppressor genes. Cell. 1991 Jan 25;64(2):313–326. doi: 10.1016/0092-8674(91)90641-b. [DOI] [PubMed] [Google Scholar]
- Mattei M. G., Simon-Chazottes D., Hirai S., Ryseck R. P., Galcheva-Gargova Z., Guénet J. L., Mattei J. F., Bravo R., Yaniv M. Chromosomal localization of the three members of the jun proto-oncogene family in mouse and man. Oncogene. 1990 Jan;5(1):151–156. [PubMed] [Google Scholar]
- Miesfeld R., Godowski P. J., Maler B. A., Yamamoto K. R. Glucocorticoid receptor mutants that define a small region sufficient for enhancer activation. Science. 1987 Apr 24;236(4800):423–427. doi: 10.1126/science.3563519. [DOI] [PubMed] [Google Scholar]
- Miner J. N., Yamamoto K. R. The basic region of AP-1 specifies glucocorticoid receptor activity at a composite response element. Genes Dev. 1992 Dec;6(12B):2491–2501. doi: 10.1101/gad.6.12b.2491. [DOI] [PubMed] [Google Scholar]
- Oikarinen A., Kylmäniemi M., Autio-Harmainen H., Autio P., Salo T. Demonstration of 72-kDa and 92-kDa forms of type IV collagenase in human skin: variable expression in various blistering diseases, induction during re-epithelialization, and decrease by topical glucocorticoids. J Invest Dermatol. 1993 Aug;101(2):205–210. doi: 10.1111/1523-1747.ep12363823. [DOI] [PubMed] [Google Scholar]
- Ortí E., Mendel D. B., Smith L. I., Munck A. Agonist-dependent phosphorylation and nuclear dephosphorylation of glucocorticoid receptors in intact cells. J Biol Chem. 1989 Jun 15;264(17):9728–9731. [PubMed] [Google Scholar]
- Pearce D., Yamamoto K. R. Mineralocorticoid and glucocorticoid receptor activities distinguished by nonreceptor factors at a composite response element. Science. 1993 Feb 19;259(5098):1161–1165. doi: 10.1126/science.8382376. [DOI] [PubMed] [Google Scholar]
- Picard D., Khursheed B., Garabedian M. J., Fortin M. G., Lindquist S., Yamamoto K. R. Reduced levels of hsp90 compromise steroid receptor action in vivo. Nature. 1990 Nov 8;348(6297):166–168. doi: 10.1038/348166a0. [DOI] [PubMed] [Google Scholar]
- Picard D., Yamamoto K. R. Two signals mediate hormone-dependent nuclear localization of the glucocorticoid receptor. EMBO J. 1987 Nov;6(11):3333–3340. doi: 10.1002/j.1460-2075.1987.tb02654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poduslo S. E. Induction of ketone body enzymes in glial cells. Arch Biochem Biophys. 1989 Aug 1;272(2):318–322. doi: 10.1016/0003-9861(89)90225-7. [DOI] [PubMed] [Google Scholar]
- Qi M., Hamilton B. J., DeFranco D. v-mos oncoproteins affect the nuclear retention and reutilization of glucocorticoid receptors. Mol Endocrinol. 1989 Aug;3(8):1279–1288. doi: 10.1210/mend-3-8-1279. [DOI] [PubMed] [Google Scholar]
- Sakai D. D., Helms S., Carlstedt-Duke J., Gustafsson J. A., Rottman F. M., Yamamoto K. R. Hormone-mediated repression: a negative glucocorticoid response element from the bovine prolactin gene. Genes Dev. 1988 Sep;2(9):1144–1154. doi: 10.1101/gad.2.9.1144. [DOI] [PubMed] [Google Scholar]
- Schena M., Lloyd A. M., Davis R. W. A steroid-inducible gene expression system for plant cells. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10421–10425. doi: 10.1073/pnas.88.23.10421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schena M., Yamamoto K. R. Mammalian glucocorticoid receptor derivatives enhance transcription in yeast. Science. 1988 Aug 19;241(4868):965–967. doi: 10.1126/science.3043665. [DOI] [PubMed] [Google Scholar]
- Schüle R., Rangarajan P., Kliewer S., Ransone L. J., Bolado J., Yang N., Verma I. M., Evans R. M. Functional antagonism between oncoprotein c-Jun and the glucocorticoid receptor. Cell. 1990 Sep 21;62(6):1217–1226. doi: 10.1016/0092-8674(90)90397-w. [DOI] [PubMed] [Google Scholar]
- Schüle R., Umesono K., Mangelsdorf D. J., Bolado J., Pike J. W., Evans R. M. Jun-Fos and receptors for vitamins A and D recognize a common response element in the human osteocalcin gene. Cell. 1990 May 4;61(3):497–504. doi: 10.1016/0092-8674(90)90531-i. [DOI] [PubMed] [Google Scholar]
- Sippola-Thiele M., Hanahan D., Howley P. M. Cell-heritable stages of tumor progression in transgenic mice harboring the bovine papillomavirus type 1 genome. Mol Cell Biol. 1989 Mar;9(3):925–934. doi: 10.1128/mcb.9.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. A., Parkinson D. R., Cheson B. D., Friedman M. A. Retinoids in cancer therapy. J Clin Oncol. 1992 May;10(5):839–864. doi: 10.1200/JCO.1992.10.5.839. [DOI] [PubMed] [Google Scholar]
- Solomon E., Borrow J., Goddard A. D. Chromosome aberrations and cancer. Science. 1991 Nov 22;254(5035):1153–1160. doi: 10.1126/science.1957167. [DOI] [PubMed] [Google Scholar]
- Strawhecker J. M., Pelling J. C. Inhibition of mouse skin tumorigenesis by dexamethasone occurs through a Ha-ras-independent mechanism. Carcinogenesis. 1992 Nov;13(11):2075–2080. doi: 10.1093/carcin/13.11.2075. [DOI] [PubMed] [Google Scholar]
- Truss M., Beato M. Steroid hormone receptors: interaction with deoxyribonucleic acid and transcription factors. Endocr Rev. 1993 Aug;14(4):459–479. doi: 10.1210/edrv-14-4-459. [DOI] [PubMed] [Google Scholar]
- Vivanco Ruiz M. M., Bugge T. H., Hirschmann P., Stunnenberg H. G. Functional characterization of a natural retinoic acid responsive element. EMBO J. 1991 Dec;10(12):3829–3838. doi: 10.1002/j.1460-2075.1991.tb04952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B., Kinzler K. W. The multistep nature of cancer. Trends Genet. 1993 Apr;9(4):138–141. doi: 10.1016/0168-9525(93)90209-z. [DOI] [PubMed] [Google Scholar]
- Vogt P. K., Bos T. J. jun: oncogene and transcription factor. Adv Cancer Res. 1990;55:1–35. doi: 10.1016/s0065-230x(08)60466-2. [DOI] [PubMed] [Google Scholar]
- Walsh D., Avashia J. Glucocorticoids in clinical oncology. Cleve Clin J Med. 1992 Sep-Oct;59(5):505–515. doi: 10.3949/ccjm.59.5.505. [DOI] [PubMed] [Google Scholar]
- Wilding G. The importance of steroid hormones in prostate cancer. Cancer Surv. 1992;14:113–130. [PubMed] [Google Scholar]
- Yang-Yen H. F., Chambard J. C., Sun Y. L., Smeal T., Schmidt T. J., Drouin J., Karin M. Transcriptional interference between c-Jun and the glucocorticoid receptor: mutual inhibition of DNA binding due to direct protein-protein interaction. Cell. 1990 Sep 21;62(6):1205–1215. doi: 10.1016/0092-8674(90)90396-v. [DOI] [PubMed] [Google Scholar]
- Yoshinaga S. K., Yamamoto K. R. Signaling and regulation by a mammalian glucocorticoid receptor in Drosophila cells. Mol Endocrinol. 1991 Jun;5(6):844–853. doi: 10.1210/mend-5-6-844. [DOI] [PubMed] [Google Scholar]
- Zhang H. Y., Young A. P. A single upstream glucocorticoid response element juxtaposed to an AP1/ATF/CRE-like site renders the chicken glutamine synthetase gene hormonally inducible in transfected retina. J Biol Chem. 1991 Dec 25;266(36):24332–24338. [PubMed] [Google Scholar]
- de Thé H., Chomienne C., Lanotte M., Degos L., Dejean A. The t(15;17) translocation of acute promyelocytic leukaemia fuses the retinoic acid receptor alpha gene to a novel transcribed locus. Nature. 1990 Oct 11;347(6293):558–561. doi: 10.1038/347558a0. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]