Abstract
Chiral polychlorinated biphenyls (PCBs) display variable atropisomeric enrichment in wildlife and animal models, especially at higher trophic levels. These differences in PCBs’ chiral signatures are, at least in part, due to species-dependent oxidation of PCBs to hydroxylated PCB metabolites (OH-PCBs). Here, we investigate the hypothesis that the cytochrome P450 (P450) enzyme-mediated oxidation of chiral PCBs results in species-dependent differences in the chiral signatures of OH-PCBs (i.e., the direction and extent of OH-PCBs’ atropisomeric enrichment). To investigate this hypothesis, we incubated PCB 136, a representative chiral PCB, with pooled human liver microsomes (HLMs) or liver microsomes from male guinea pig, hamster, monkey, mouse, and rabbit or female dog and determined average profiles and chiral signatures of the OH-PCBs. 2,2′,3,3′,6,6′-Hexachlorobiphenyl-4-ol (4–136) was the major metabolite in incubations with HLMs and monkey and rabbit microsomes. 2,2′,3,3′,6,6′-Hexachlorobiphenyl-5-ol (5–136) was the major metabolite formed by microsomes from all other species. Both 4–136 and 5–136 were formed atropselectively in all microsomal incubations; however, the direction and extent of the atropisomeric enrichment of both OH-PCB metabolites showed considerable differences across microsomal preparations obtained from different species. These differences in OH-PCBs’ atropisomeric enrichment may not only be toxicologically relevant but may also be useful to study sources and transport of OH-PCBs in the environment.
Introduction
Polychlorinated biphenyls (PCBs) are a class of industrial chemicals and unintentional byproducts of industrial processes banned under the Stockholm Convention on Persistent Organic Pollutants. PCBs remain an environmental and human health concern because of their ongoing, inadvertent production, their environmental persistence, and the presence of PCBs in the environment, diet, and in human serum and tissues.1−4 Nineteen PCB congeners and various hydroxylated (and other) metabolites with three or four ortho chlorine substituents and an unsymmetrical substitution pattern in both phenyl rings are chiral.5 These PCB derivatives exist as nonsuperimposable rotational isomers, called atropisomers, which are mirror images of each other. Chiral PCB congeners, in particular congeners with a 2,3,6 substitution pattern in one phenyl ring, have been linked to neurodevelopmental toxicity in humans and laboratory animals and shown to cause effects on neurotransmitter functions in the central nervous system and alter cellular processes related to calcium signaling.6,7
Chiral PCBs are present in commercial PCB mixtures as racemates (i.e., a 1:1 mixture of atropisomers) and, due to PCBs chemical and thermal stability, are released into the environment as racemates. Studies of the atropisomeric enrichment of chiral PCBs reveal near racemic signatures in diet, house dust, and air, but highly variable atropisomeric enrichment in wildlife, especially at higher tropic levels, and humans (reviewed in ref (5)). Because physical and chemical transport (e.g., passive diffusion) and transformation processes (e.g., photodegradation) are not atropselective, the variable atropisomeric enrichment of PCBs in environmental samples is due to atropselective biotransformation and/or biological transport processes. PCBs can undergo atropselective bacterial biodegradation8,9 and are atropselectively metabolized in plant10 and animal models (reviewed in ref (5)). Laboratory studies have shown that cytochrome P450 enzymes, such as different CYP2B isoforms,11,12 can atropselectively metabolize PCBs to OH-PCBs and, thus, contribute to nonracemic signatures of PCBs at higher trophic levels.
OH-PCBs are found in many species,13−19 including humans,2,3 and represent an environmental and human health concern. In mammals, OH-PCBs can adversely affect neurodevelopment by altering processes related to calcium signaling20,21 or thyroid function.22 Structure–activity relationship studies show that the OH-PCB metabolites of chiral PCBs are ryanodine receptor- (RyR-)active and display different modes of action depending on the position of the hydroxyls group on the biphenyl moiety in vitro.21 OH-PCB profiles are highly species dependent23,24 and can display interindividual variability.25 These differences in OH-PCB profiles are due to differences in the isoform composition, expression, and activities of PCB and OH-PCB metabolizing enzymes (e.g., P450 enzymes, sulfotransferases, glucuronosyl transferases, and others). Furthermore, differences in the composition and OH-PCB binding affinity of various transport proteins (e.g., transthyretin) may contribute to species and interindividual differences in OH-PCB profiles in vivo. Since PCBs are atropselectively metabolized by P450 enzymes to OH-PCBs, it is likely that, similar to the parent PCBs,5 P450 enzyme-mediated metabolism contributes to differences in the atropisomeric enrichment of chiral OH-PCBs in wildlife and humans. Consistent with this hypothesis, we have recently reported differences in the atropselective formation of OH-PCBs in rats and mice in in vitro metabolism studies;26 however, systematic laboratory and environmental studies of the atropselective formation of OH-PCBs by P450 enzymes from different species have not been reported to date.
The present study investigates the atropselective formation of OH-PCB from 2,2′,3,3′,6,6′-hexachlorobiphenyl (PCB 136) by liver microsomes from humans and several other mammalian species. Pooled liver microsomes from naïve animals were used to assess representative OH-PCB profiles and chiral signatures in the species investigated. PCB 136 was selected for this study as a prototypical chiral PCB congener of environmental relevance.
Experimental Section
Liver Microsomes
Untreated beagle dog (female), Cynomolgus monkey (male), New Zealand rabbit (male), golden Syrian hamster (male), Hartley albino guinea pig (male), and human liver microsomes pooled from 50 donors with mixed age, sex, and race (HLMs) were purchased from Xenotech (Lenexa, KS, USA). Microsomes obtained from female instead of male beagle dogs were used because of the higher enzymatic activity of the respective microsomal preparations. Mouse liver microsomes were prepared by pooling livers from saline and corn oil treated male C57BI/6 mice and characterized as described previously.27 The microsomal cytochrome P450 content is described in the Supporting Information (Tables S1 and S2).
Chemicals
Dimethyl sulfoxide (DMSO), sodium phosphate dibasic (Na2HPO4), sodium phosphate monobasic (NaH2PO4), magnesium chloride (MgCl2), tetrabutylammonium sulfite, sodium sulfite, and pesticide grade solvents were obtained from Fisher Scientific (Pittsburgh, PA, USA). Nicotinamide adenine dinucleotide phosphate reduced (NADPH) was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Racemic PCB 136 was synthesized by the Ullmann coupling of 2,3,6-trichloro-1-iodobenzene,28 and the atropisomers of PCB 136 were separated using two serially connected Nucleodex β-PM columns (Macherey-Nagel, Düren, Germany). The enantiomeric fractions (EF = Area(+)-PCB 136 /(Area(+)-PCB 136 + Area(−)-PCB 136)) of (−)-PCB 136 and (+)-PCB 136 were 0.01 and 1.00, respectively.27 2,2′,3,3′,6,6′-Hexachlorobiphenyl-4-ol (4–136), 2,2′,3,3′,6,6′-hexachlorobiphenyl-5-ol (5–136), 4,5-dimethoxy-2,2′,3,3′,6,6′-hexachlorobiphenyl, and 2,2′,3′,4,6,6′-hexachloro-3-methoxybiphenyl were prepared as described elsewhere.29 Recovery standards (2,3,4,4′,5,6-hexachlorobiphenyl, PCB 166; 2,3,4′,5,6-pentachlorobiphenyl, PCB 117; 2,3,3′,4,5,5′-hexachlorobiphenyl-4-ol, 4′-159) and the internal standard (2,2′,3,4,4′,5,6,6′-octachlorobiphenyl, PCB 204) were purchased from Accustandard (New Haven, CT).
Metabolism Experiments
Incubation conditions were initially optimized for microsomal protein content and NAPDH concentration using human and dog liver microsomes as described previously.30 Subsequently, time-course experiments were performed in triplicate with the optimized experimental condition. Briefly, an incubation mixture (12 mL) consisting of phosphate buffer (0.1 M, pH 7.4), NADPH (0.5 mM in HLMs or 1.5 mM in all animal microsomes), magnesium chloride (3 mM), and hepatic microsomal protein (1.0 mg/mL for all animal microsomes or 0.5 mg/mL for human microsomes) was preincubated for 5 min at 37 ± 1 °C in a shaking water bath. PCB 136 in DMSO (0.5%) was added with a final concentration of 50 μM. These incubation conditions, including the high PCB 136 concentrations, were selected to ensure the formation of sufficient OH-PCB quantities for atropselective analyses. Experiments with HLMs used (±)-, (−)-, or (+)-PCB 136. (±)-PCB 136 was used in incubations with microsomes from all animal species. An aliquot (2 mL) of the incubation mixture was removed after 5, 10, 15, 20, 25, and 30 min. A total of 2 mL of ice cold sodium hydroxide (0.5 M) was added to each aliquot to stop the reaction. For the 0 min time point, a separate sample (1990 μL) was preincubated for 5 min as described above, followed by sequential addition of the sodium hydroxide (2 mL) and PCB 136 solution (10 μL). Control incubations without microsomes or NADPH or containing heat-inactivated microsomes were performed in parallel.
Extraction of PCB 136 and Its Hydroxylated Metabolites
Extraction of PCB 136 and its hydroxylated metabolites was performed using a published method.30 In short, surrogate standards (500 ng of PCB 117 in animal microsomes or PCB 166 in human microsomes; 274 ng of 4′-159) were added to each sample, followed by hydrochloric acid (6 M, 1 mL) and 2-propanol (3 mL). The samples were extracted with hexane-MTBE (1:1 v/v, 5 mL) and hexane (3 mL). The combined organic extracts were washed with an aqueous KCl solution (1%, 3 mL). After removal of the organic phase, the KCl phase was re-extracted with hexane (3 mL), and the combined extracts were reduced under a gentle stream of nitrogen to ∼1 mL. The hydroxylated metabolites were derivatized with diazomethane and subjected to a sulfur cleanup as described previously.31 PCB 204 (200 ng) was added as an internal standard prior to analysis.
Gas Chromatographic Determinations
Levels of OH-PCB 136 metabolites were determined using an Agilent 6890N gas chromatograph with a 63Ni-μECD detector and a DB1-MS capillary column (60 m × 0.25 mm ID × 0.25 μm film thickness; Agilent, Santa Clara, CA, USA).30 OH-PCB levels were adjusted for milligram microsomal protein. Relative rates of OH-PCB formation were determined in the linear range of metabolite formation (i.e., 5 min) by adjusting the amount of OH-PCB by the total P450 content.32 The limits of detection and background PCBs levels are listed in Table S3.
To further verify the formation of specific metabolites, samples from 30 min incubations were analyzed on an Agilent 7890A gas chromatograph with a 5975 C mass selective detector in both total and selective ion monitoring modes with an HP-5 MS column (30 m × 0.32 mm I.D., 0.25 μm film thickness; Agilent) following a published method.26,33
Atropselective analysis of the derivatized hydroxylated PCB 136 atropisomers was performed using an Agilent 7890A gas chromatograph with a 63Ni μECD detector. The atropisomers of 5–136 and 4–136 were separated on Chirasil-Dex (CD column, 25 m × 0.25 mm ID × 0.25 μm film thickness; Varian, Palo Alto, CA, US) and Cyclosil-B columns (CB column, 30 m × 0.25 mm ID × 0.25 μm film thickness; Agilent, Santa Clara, CA, US), respectively, following a published method.30,34 Enantiomeric fractions (EFs) were determined as EF = A2/(A1 + A2), where A1 and A2 are the peak areas of the first and second eluting atropisomers, respectively. The resolution of 5–136 and 4–136 atropisomers were 0.69 and 0.74, respectively.
Statistical Analysis
The species dependent formation of metabolites was studied using one way ANOVA and PROC in the statistical analysis package SAS (version 9.3, SAS Institute, Cary, NC, USA). Metabolites formation and EF values were compared by Tukey’s Studentized Range (HSD) Test. A paired t test was used to compare the EF values of 5–136 and 4–136 to racemic standards and the formation rates of 4–136 and 5–136 between each species. A p value <0.05 was used to indicate a significant difference between species.
Results
PCB 136 Metabolism by HLMs
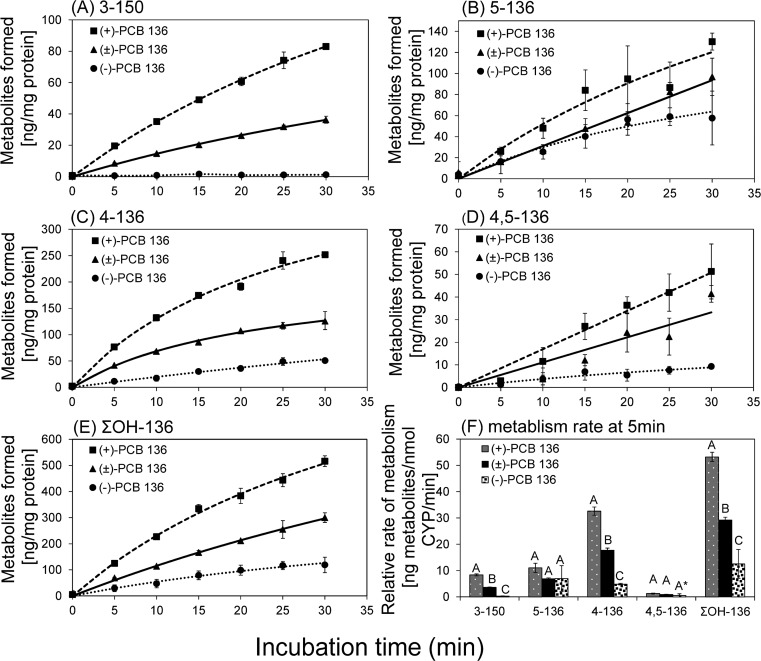
Racemic PCB 136 or its atropisomers were incubated with HLMs to investigate if potentially neurotoxic OH-PCBs are formed atropselectively in humans. Only a small percentage (<1%) of the total PCB 136 was converted to OH-PCBs under the incubation conditions (Table S4). 4–136 and 5–136 were the major metabolites for racemic PCB 136 and pure PCB 136 atropisomers, with more 4–136 being formed (5–136/4–136 ratio = 0.39 after 5 min, Table S5). 4,5–136 and the 1,2-shift product of PCB 136 (3–150) were minor metabolites. One unknown metabolite peak (m/z = 420.0) was observed at a later retention time, indicating the formation of a second dihydroxylated metabolite (Figure S1). The amounts of 3–150, 4–136, 5–136, and 4,5–136 increased with time in all HLM incubations and depended on the PCB atropisomer composition (Figure 1). For all metabolites, the rate of formation followed the order (+)-PCB 136 > racemic PCB 136 > (−)-PCB 136 (Figure 1 and Table S4).
Figure 1.
The time-dependent formation of (A) 3–150, (B) 5–136, (C) 4–136, (D) 4, 5–136, and (E) ΣOH-136 followed the rank order (+)-PCB 136 > (±)-PCB 136 > (−)-PCB 136 in incubations with human liver microsomes. (F) The relative rates of OH-PCB formation showed a significantly slower metabolite formation in the incubations with (−)-PCB 136 compared to incubations with (+)-PCB 136 and (±)-PCB 136. Different letters indicate statistically significant differences in the OH-PCB formation rates (p < 0.05) as determined by a Tukey student range test using SAS. *p = 0.05 for comparison of the 4,5–136 formation rates between incubations with (+)- and (−)-PCB 136. The values are mean ± standard deviation (n = 3).
PCB 136 Metabolism by Animal Microsomes
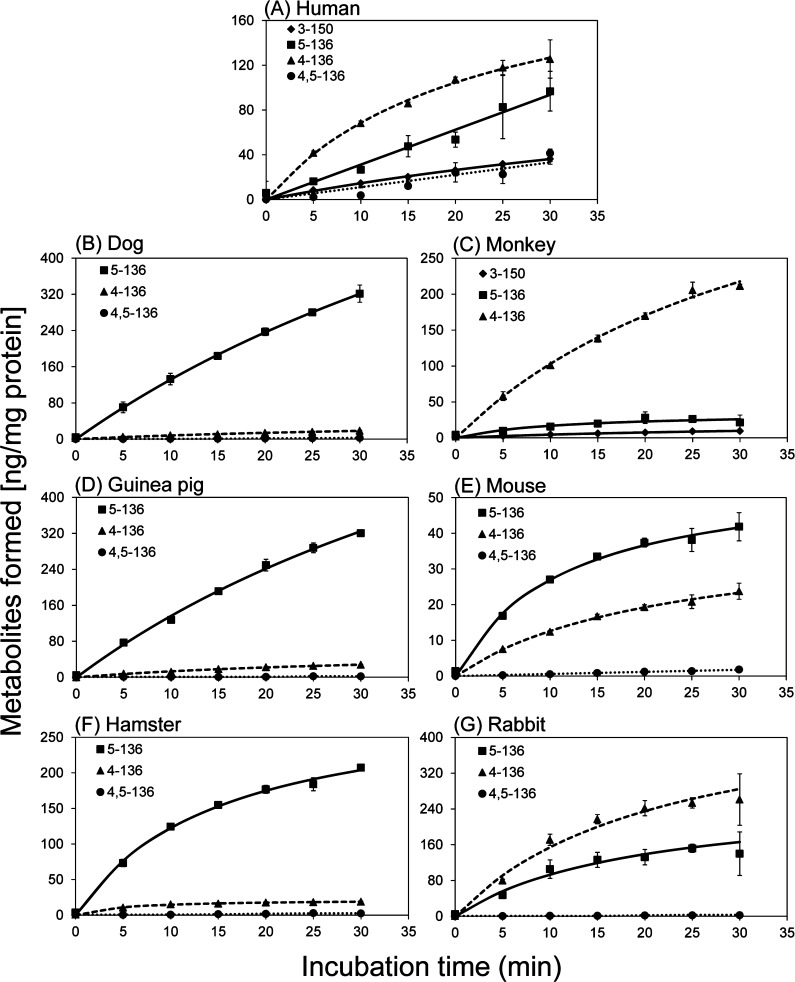
Racemic PCB 136 was incubated with liver microsomes obtained from different species (i.e., male monkey, guinea pig, mouse, hamster, and rabbit; female dog) to explore differences in typical metabolite profiles and chiral signatures between humans and toxicologically relevant mammalian species. Only a small percentage of PCB 136 (<3%) was converted to OH-PCBs (Table S4). Similar to experiments with HLMs, 4–136 was the major metabolite formed in incubations with microsomes obtained from male monkeys and rabbits (Figure 2), with 5–136/4–136 ratios of 0.17 and 0.64 at 5 min, respectively (Table S5). In contrast, 5–136 was the major metabolite in incubations using microsomes from dogs, guinea pigs, mice, and hamsters. After a 5 min incubation time, the 5–136/4–136 ratios were 14, 10, 2.2, and 6.9 for incubations with dog, guinea pig, mouse, and hamster microsomes, respectively (Table S5). 4,5–136 was a minor metabolite observed in the incubations with microsomes prepared from dogs, guinea pigs, mice, hamsters, and rabbits. The formation of 3–150, but not 4,5–136, was observed in microsomes obtained from monkeys. The formation of these OH-PCB metabolites was confirmed by GC-MS (Figure S1).
Figure 2.
Time- and species-dependent formation of OH-PCBs in incubations of PCB 136 with liver microsomes from (A) humans (pooled), (B) dog, (C) monkey, (D) guinea pig, (E) mouse, (F) hamster, and (G) rabbit. 4–136 was the major metabolite in incubations using human, monkey and rabbit liver microsomes. 5–136 was the major metabolite in experiments with dog, guinea pig, mouse, and hamster liver microsomes. The 1,2-shift metabolite, 3–150, was only observed in incubations using human and monkey liver microsomes. The values are mean ± standard deviation (n = 3).
Relative Rates of OH-PCB Formation
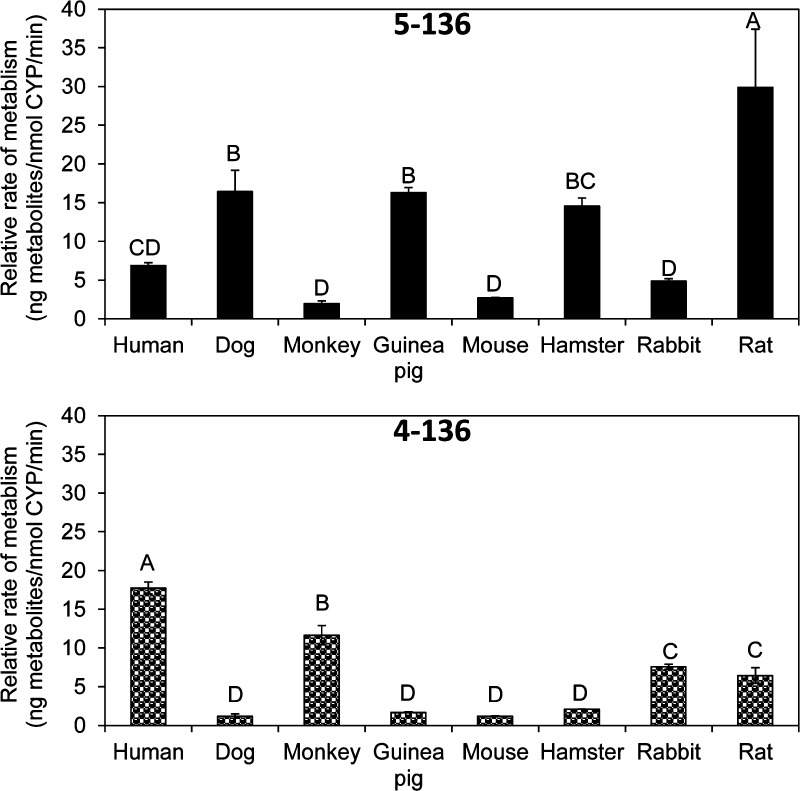
The relative rates of formation of 5–136 and 4–136 were determined for the 5 min incubation time by expressing OH-PCB levels per nanomole of total P450 content. This adjustment accounts for the differences in total P450 content between different microsomal preparation and allows a comparison across species (Figure 3). The rates of formation of 5–136 followed the order rat (estimated based on published data, see ref (30)) > dog ∼ guinea pig ∼ hamster > human > monkey ∼ mouse ∼ rabbit. However, the rate of 5–136 formation by HLMs was only significantly different compared to incubations using microsomes obtained from dogs and guinea pigs. The rate of 5–136 formation by HLMs was similar compared to the rate observed in incubations with microsomes from hamster, monkey, mouse, and rabbit. The rate of 4–136 formation by HLMs was significantly faster compared to experiments using microsomes from other species and followed the order human > monkey > rabbit ∼ rat > dog ∼ guinea pig ∼ mouse ∼ hamster (Figure 3). The formation rate of 5–136 was significantly different from that of 4–136 in all species after paired t test within species.
Figure 3.
Comparison of the formation rates of (A) 5–136 and (B) 4–136 in incubations with liver microsomes obtained from different species. The rat data were taken from ref (30). Metabolites formation rates are adjusted by total P450 content. Different letters indicate statistically significant differences in the OH-PCB formation rates (p < 0.05) as determined by a Tukey student range test using SAS. The values are mean ± standard deviation (n = 3). The formation rate of 4–136 was significantly different from that of 5–136 within each species (p < 0.05; paired t test).
Atropisomeric Enrichment of OH-PCB 136
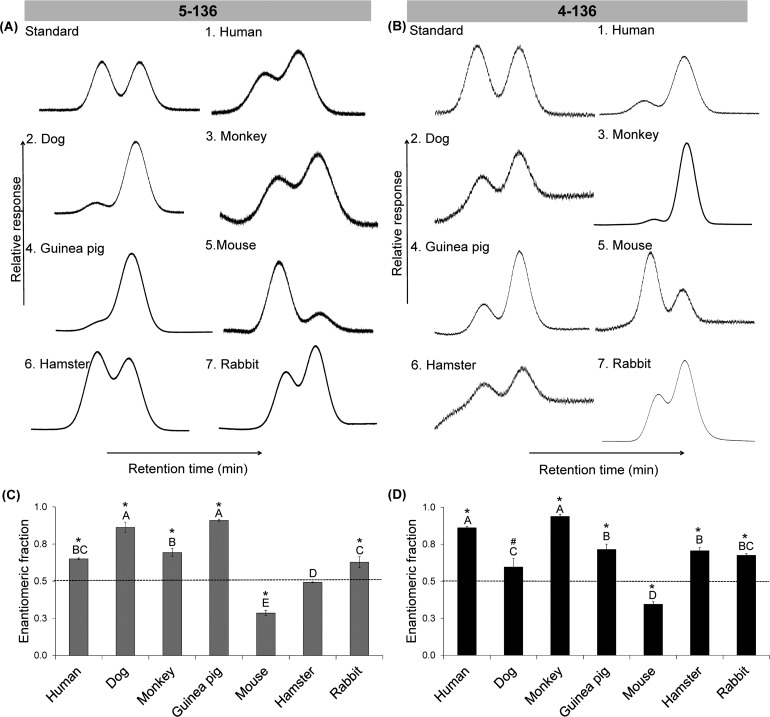
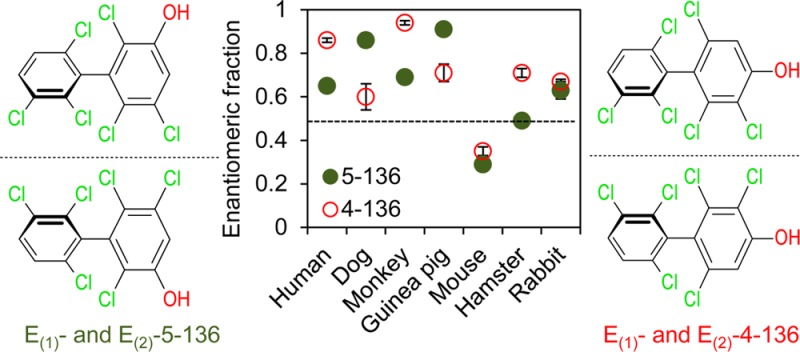
The atropisomeric enrichment of OH-PCB 136 metabolites was determined in microsomal incubations with racemic PCB 136 using atropselective gas chromatography. The objective was to determine if OH-PCBs are formed atropselectively in incubations with HLM and how this enrichment differs compared to toxicologically relevant species. The second eluting atropisomers of 5–136 (E2-5–136), which is formed from (+)-PCB 136, was enriched in incubations using human, dog, monkey, guinea pig, and rabbit microsomes (Figure 4A). The atropselective formation of the 5–136 resulted in near constant EF values with time (data not shown). Therefore, EF values at 5 min were statistically analyzed and presented in Figure 4C.
Figure 4.
The atropisomeric enrichment of 5–136 and 4–136 formed from liver microsomes is species-dependent. (A) Representive chromatograms showing an enrichment of the second eluting 5–136 atropisomer in incubations with human (pooled), dog, monkey, guinea pig, and rabbit liver microsomes and an enrichment of the first eluting 5–136 atropisomer in experiments with mouse and hamster liver microsomes. (B) Representive chromatograms showing an enrichment of the second eluting 4–136 atropisomer in incubations with human (pooled), dog, monkey, guinea pig, hasmster, and rabbit liver microsomes and an enrichment of the first eluting 4–136 atropisomer in experiments with mouse liver microsomes. Enantiomeric fractions of (C) 5–136 and (D) 4–136. Different letters indicate statistically significant differences in the EF values (p < 0.05) as determined by a Tukey student range test using SAS. *EF values significantly different from control (p < 0.05, paired t test). #EF values of 4–136 in incubations with dog microsomes showed a trend to significance from control (p = 0.054). The values are mean ± standard deviation (n = 3).
The extent of the atropisomeric enrichment of 5–136 in microsomal incubations followed the order dog ∼ guinea pig > monkey ∼ human ∼ rabbit (Figure 4C). Interestingly, the first eluting atropisomer of 5–136 (E1-5–136), the 5–136 metabolite formed from (-)-PCB 136, displayed atropisomeric enrichment in experiments with mouse and, to a lesser extent, hamster microsomes. The EF values of 5–136 were significantly different from the racemic standard, with the exception of incubations using hamster microsomes. Similar to 5–136, the second eluting atropisomers of 4–136 (E2-4–136), a metabolite formed from (+)-PCB 136, was enriched in incubations using human, dog, monkey, guinea pig, hamster, and rabbit microsomes (Figure 4B). The extent of the atropisomeric enrichment of 4–136 formed in microsomal incubations followed the order human ∼ monkey > guinea pig ∼ hamster ∼ rabbit > dog. In contrast, the first eluting atropisomer of 4–136 (E1-4–136), which is formed from (−)-PCB 136, was enriched in experiments with mouse microsomes. The EF values of 4–136 were significantly different from racemic standards, with incubation using dog microsomes displaying only a trend of E2-4–136 enrichment (p = 0.054; Figure 4D).
Discussion
The present study uses hepatic microsomes to gain insights into typical OH-PCB 136 metabolite profiles and chiral signatures formed by P450 enzymes in different mammalian species, including humans. 4–136 and 5–136 were the two major monohydroxylated PCB 136 metabolites formed atropselectively by HLMs, which is consistent with an earlier study by Schnellmann and co-workers.35 In addition, a few other mono- and dihydroxylated PCB metabolites were observed as minor metabolites. This includes 3–150, a 1,2-shift product of PCB 136 formed via an arene oxide intermediate. The formation of such a 1,2-shift metabolite by HLMs has not been reported previously. The 5–136/4–136 ratios in our study ranged from 0.4 to 0.8:1 (for incubation times from 5 to 30 min). On the basis of our re-evaluation of the published mass spectra, a metabolite ratio of 1.3:1 was observed by Schnellmann and co-workers.35 This difference in the metabolite profile is most likely due to differences in the P450 enzyme composition of the respective HLMs. The relative rate of formation of all OH-PCBs was different for incubations using (+)-PCB 136, (−)-PCB 136, and racemic PCB 136, with (+)-PCB 136 being more rapidly oxidized compared to (−)-PCB 136. These atropisomer-specific differences in the OH-PCB formation rates explain the atropisomeric enrichment of PCBs observed in in vitro studies12 and are consistent with a role of P450 enzymes in their atropisomeric enrichment observed human samples.5
Analogous to HLMs, 5–136, 4–136, and 4,5–136 were formed by most animal microsomal preparations studied. There is considerable evidence that CYP2B enzymes are involved in the oxidation of PCB 136 and structurally related PCB congeners in the meta position. Studies with recombinant enzymes demonstrate that rat CYP2B111,12,29 and dog CYP2B1129 selectively oxidize PCB 136 to 5–136. CYP2B1 also metabolizes 4-OH-PCBs and 5-OH-PCBs to the corresponding 4,5-dihydroxylated metabolites, such as 4,5–136.11,29 Warner and co-workers demonstrated that PCB 136 is oxidized by human CYP2B6 to a single, unidentified OH-PCB.12 This OH-PCB metabolite is most likely 5–136 because CYP2B6 oxidizes other PCBs in the meta position.25,36 In contrast, rabbit CYP2B4 and CYP2B5 do not metabolize PCB 136.29 The P450 isoforms responsible for the formation of 4–136 remain elusive, as CYP3A enzymes are probably not involved in its formation in rats or mice.26,30,34 We also observed no change in 4–136 levels in liver microsomes after induction of CYP1A enzymes in rats pretreated with PCB 126, which suggests that 4–136 is not formed by CYP1A enzymes (Wu and Lehmler, unpublished data).
While essentially the same metabolites were formed by liver microsomes from different species, the ratios, relative formation rates, and chiral signatures of the OH-PCBs differed considerably depending on the species. Experiments with HLMs displayed the fastest formation rate for 4–136 and one of the lowest formation rates for 5–136 of all microsomal preparations investigated. As a result, 4–136 was the major metabolite formed in HLM incubations. It is important to emphasize that our result represents an average OH-PCB profile formed by a pool of liver microsomes from 50 individual donors; however, there can be considerable interindividual variability in humans due to genetic polymorphisms, diseases, and exposure to other xenobiotics. For example, a recent PCB 146 metabolism experiment with HLMs from individual donors revealed considerable interindividual metabolism of PCB in humans associated with CYP2B6 activity.25
4–136 was also the major metabolite in incubations using microsomes from monkeys and rabbits. In contrast, 5–136 was the major metabolite in experiments with microsomes from dogs, guinea pigs, mice, hamsters, and, as reported previously, rats.30 The faster formation of 5–136 in rat compared to dog microsomes in the current study is consistent with the differences in the oxidation of PCB 136 reported by Waller et al. for recombinant rat CYP2B1 and dog CYP2B11.29 5–136 and 4–136 are also the major PCB 136 metabolites formed in rats after intraperitoneal administration of PCB 136,37 with a rank order of 5–136 > 4–136. These species differences in the OH-PCB ratios and formation rates are not surprising because of the considerable interspecies differences in the constitutive expression and catalytic activity of P450 enzymes.38 It is important to emphasize that our results represent OH-PCB ratios and formation rates obtained with pooled microsomal preparations from naïve animals. As with humans, genetic and environmental factors can result in interindividual variability in the OH-PCB profiles and formation rates in these species. However, these differences are likely small in toxicologically relevant animal models because the animals under investigation are inbred and maintained under rigorously controlled environmental and dietary conditions. More significant interindividual variability in the P450 enzyme-mediated oxidation of chiral PCBs is likely to occur in wildlife.
The present study also revealed considerable differences in the atropisomeric enrichment of 5–136 and 4–136 formed by microsomal preparations obtained from different species. Interestingly, the E2-5–136 and E2-4–136 were enriched in incubations with liver microsomes from most species, albeit to a different extent. Microsomes from mice were a notable exception, because E1-5–136 and E1-4–136 were enriched. The same direction of the atropisomeric enrichment of 5–136 and 4–136 has been observed in mouse tissue slices.26 It is currently unclear to which extent our in vitro metabolism studies predict chiral OH-PCB signatures in vivo. In particular, subsequent metabolism (e.g., sulfation and glucuronidation) and transport of OH-PCBs may modulate the atropisomeric enrichment of OH-PCBs in vivo; however, the atropselectivity of these biological processes has not been investigated to date.
Both E1-5–136 and E1-4–136 are formed from (−)-PCB 136.30 Consequently, most mammalian species, including humans, metabolize and eliminate the (−)-PCB 136 atropisomer less rapidly than the (+)-PCB 136 atropisomer. In contrast, mice metabolize (−)-PCB 136 more rapidly, at least compared to the other mammalian species investigated in our study. Consistent with this interpretation, (+)-PCB 136 undergoes atropisomeric enrichment in mice,5 whereas only a slight enrichment of (−)-PCB 136 is observed in rats.37 This observation is important because (−)-PCB 136, but not (+)-PCB 136, is a potent sensitizer of RyRs and alters neuronal connectivity via a RyR-dependent mechanism.20,39 It is therefore possible that differences in the metabolism of PCB 136 atropisomers across species, sex, or individuals play a role in the developmental neurotoxicity of PCB 136 and structurally related congeners.
The toxicological relevance of the atropisomeric enrichment of OH-PCB metabolites of PCB 136 and other chiral PCBs is currently unknown and warrants further investigation. Like the parent PCBs,20,40 pure OH-PCB atropisomers may display atropselectivity toward cellular targets and, thus, cause atropselective toxicity in wildlife and humans. Developmental neurotoxicity is a particular concern in humans because OH-PCBs cross the placenta22 and accumulate in fetal target tissues.41 OH-PCBs have several modes of action and, for example, disrupt cellular calcium homeostasis by mechanisms involving RyRs21 or cause thyroid dysfunction.7,22 OH-PCB 136 metabolites and structurally related, chiral OH-PCBs have not been detected in humans, partly because suitable analytical standards are not readily available; however, their parent compounds can be present at high levels in indoor air,42,43 including in school buildings in the United States.44 It is therefore likely that potentially neurotoxic, chiral OH-PCBs are present in humans, especially in school children and other susceptible human populations.1,4
Similar to the parent PCBs,5 our observation that the atropisomeric enrichment of OH-PCBs is highly species-dependent will be useful for source apportionment studies of OH-PCB. OH-PCBs have not only been detected in laboratory animals37 and humans,2,3 but also in species at different tropic levels, such as fish,13 sea birds,14 marine mammals,15−17 and plants.18,19 Although studies with liver microsomes demonstrate that P450 enzymes are involved in the formation of OH-PCBs in many species,45,46 several studies demonstrate the presence of OH-PCBs in abiotic samples. For example, OH-PCBs are present in technical Aroclors.47 OH-PCBs are also formed by the reaction of OH radicals with PCBs and have been detected in surface water and precipitation.48 As with PCBs, chiral OH-PCB formed by abiotic processes will be racemic, whereas OH-PCBs in biological samples will be nonracemic due to atropselective biological transport and biotransformation processes. Consequently, chiral signatures can be used to distinguish abiotic from biotic OH-PCB sources. Furthermore, species-dependent differences on chiral signatures may be useful to study how OH-PCBs move through aquatic and terrestrial food webs. Similarly, chiral signatures are a powerful tool to study the movement of chiral PCBs through aquatic and terrestrial food webs.5
Acknowledgments
The authors would like to thank Jarline Encarnacion Medina for help with studies involving human liver microsomes. The project described was supported by NIH grants ES05605, ES013661, and ES017425 from the National Institute of Environmental Health Sciences. The PCB 136 metabolite standards were a generous gift from E.A. Mash and S.C. Waller of the Synthetic Chemistry Facility Core of the Southwest Environmental Health Sciences Center, funded by NIH grant ES06694.
Supporting Information Available
The Supporting Information includes characteristics of the human and animal liver mixed function oxidase system, limits of detection, atropisomer resolution and background levels of PCB 136 and metabolites, percent of PCB 136 converted to OH-PCB 136, summary of 5–136 to 4–136 metabolite ratios, and GC-MS chromatograms confirming metabolite formation. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Megson D.; O’Sullivan G.; Comber S.; Worsfold P. J.; Lohan M. C.; Edwards M. R.; Shields W. J.; Sandau C. D.; Patterson D. G. J. Elucidating the structural properties that influence the persistence of PCBs in humans using the National Health and Nutrition Examination Survey (NHANES) dataset. Sci. Total Environ. 2013, 461–462C, 99–107. [DOI] [PubMed] [Google Scholar]
- Marek R. F.; Thorne P. S.; Wang K.; Dewall J.; Hornbuckle K. C. PCBs and OH-PCBs in serum from children and mothers in urban and rural U.S. communities. Environ. Sci. Technol. 2013, 4773353–3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirtu A. C.; Jaspers V. L. B.; Cernat R.; Neels H.; Covaci A. Distribution of PCBs, their hydroxylated metabolites, and other phenolic contaminants in human serum from two European countries. Environ. Sci. Technol. 2010, 4482876–2883. [DOI] [PubMed] [Google Scholar]
- Mitchell M. M.; Woods R.; Chi L. H.; Schmidt R. J.; Pessah I. N.; Kostyniak P. J.; LaSalle J. M. Levels of select PCB and PBDE congeners in human postmortem brain reveal possible environmental involvement in 15q11-q13 duplication autism spectrum disorder. Environ. Mol. Mutagen. 2012, 538589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmler H.-J.; Harrad S. J.; Hühnerfuss H.; Kania-Korwel I.; Lee C. M.; Lu Z.; Wong C. S. Chiral polychlorinated biphenyl transport, metabolism, and distribution: A review. Environ. Sci. Technol. 2010, 4482757–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessah I. N.; Cherednichenko G.; Lein P. J. Minding the calcium store: Ryanodine receptor activation as a convergent mechanism of PCB toxicity. Pharmacol. Ther. 2010, 1252260–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodavanti P. R. S.; Curras-Collazo M. C. Neuroendocrine actions of organohalogens: Thyroid hormones, arginine vasopressin, and neuroplasticity. Front. Neuroendocrinol. 2010, 314479–496. [DOI] [PubMed] [Google Scholar]
- Pakdeesusuk U.; Jones W. J.; Lee C. M.; Garrison A. W.; O’Niell W. L.; Freedman D. L.; Coates J. T.; Wong C. S. Changes in enantiomeric fractions (EF) during microbial reductive dechlorination of PCB 132, PCB 149, and Aroclor 1254 in Lake Hartwell sediment microcosms. Environ. Sci. Technol. 2003, 3761100–1107. [DOI] [PubMed] [Google Scholar]
- Singer A. C.; Wong C. S.; Crowley D. E. Differential enantioselective transformation of atropisomeric polychlorinated biphenyls by multiple bacterial strains with different inducing compounds. Appl. Environ. Microbiol. 2002, 68115756–5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai G.; Hu D.; Lehmler H. J.; Schnoor J. L. Enantioselective biotransformation of chiral PCBs in whole poplar plants. Environ. Sci. Technol. 2011, 4562308–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z.; Kania-Korwel I.; Lehmler H.-J.; Wong C. S. Stereoselective formation of mono- and di-hydroxylated polychlorinated biphenyls by rat cytochrome P450 2B1. Environ. Sci. Technol. 2013, 472112184–12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner N. A.; Martin J. W.; Wong C. S. Chiral polychlorinated biphenyls are biotransformed enantioselectively by mammalian cytochrome P-450 isozymes to form hydroxylated metabolites. Environ. Sci. Technol. 2009, 433114–121. [DOI] [PubMed] [Google Scholar]
- Buckman A. H.; Wong C. S.; Chow E. A.; Brown S. B.; Solomon K. R.; Fisk A. T. Biotransformation of polychlorinated biphenyls (PCBs) and bioformation of hydroxylated PCBs in fish. Aquat. Toxicol. 2006, 782176–185. [DOI] [PubMed] [Google Scholar]
- Jorundsdottir H.; Lofstrand K.; Svavarsson J.; Bignert A.; Bergman A. Organochlorine compounds and their metabolites in seven Icelandic seabird species - a comparative study. Environ. Sci. Technol. 2010, 4493252–3259. [DOI] [PubMed] [Google Scholar]
- Weijs L.; Das K.; Siebert U.; van Elk N.; Jauniaux T.; Neels H.; Blust R.; Covaci A. Concentrations of chlorinated and brominated contaminants and their metabolites in serum of harbour seals and harbour porpoises. Environ. Int. 2009, 356842–850. [DOI] [PubMed] [Google Scholar]
- McKinney M. A.; De Guise S.; Martineau D.; Beland P.; Lebeuf M.; Letcher R. J. Organohalogen contaminants and metabolites in beluga whale (Delphinapterus leucas) liver from two Canadian populations. Environ. Toxicol. Chem. 2006, 2551246–1257. [DOI] [PubMed] [Google Scholar]
- Routti H.; Letcher R. J.; Arukwe A.; Van Bavel B.; Yoccoz N. G.; Chu S.; Gabrielsen G. W. Biotransformation of PCBs in relation to phase I and II xenobiotic-metabolizing enzyme activities in ringed seals (Phoca hispida) from Svalbard and the Baltic Sea. Environ. Sci. Technol. 2008, 42238952–8958. [DOI] [PubMed] [Google Scholar]
- Liu J.; Hu D.; Jiang G.; Schnoor J. L. In vivo biotransformation of 3,3′,4,4′-tetrachlorobiphenyl by whole plants–poplars and switchgrass. Environ. Sci. Technol. 2009, 43197503–7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai G.; Lehmler H. J.; Schnoor J. L. Hydroxylated metabolites of 4-monochlorobiphenyl and its metabolic pathway in whole poplar plants. Environ. Sci. Technol. 2010, 44103901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessah I. N.; Hansen L. G.; Albertson T. E.; Garner C. E.; Ta T. A.; Do Z.; Kim K. H.; Wong P. W. Structure-activity relationship for noncoplanar polychlorinated biphenyl congeners toward the ryanodine receptor-Ca2+ channel complex type 1 (RyR1). Chem. Res. Toxicol. 2006, 19192–101. [DOI] [PubMed] [Google Scholar]
- Niknam Y.; Feng W.; Cherednichenko G.; Dong Y.; Joshi S. N.; Vyas S. M.; Lehmler H.-J.; Pessah I. N. Structure-activity relationship of select meta- and para-hydroxylated non-dioxin-like polychlorinated biphenyls: From single RyR1 channels to muscle dysfunction. Toxicol. Sci. 2013, 1362500–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerts I. A.; Assink Y.; Cenijn P. H.; Van Den Berg J. H.; Weijers B. M.; Bergman A.; Koeman J. H.; Brouwer A. Placental transfer of a hydroxylated polychlorinated biphenyl and effects on fetal and maternal thyroid hormone homeostasis in the rat. Toxicol. Sci. 2002, 682361–371. [DOI] [PubMed] [Google Scholar]
- Kunisue T.; Tanabe S. Hydroxylated polychlorinated biphenyls (OH-PCBs) in the blood of mammals and birds from Japan: Lower chlorinated OH-PCBs and profiles. Chemosphere 2009, 747950–961. [DOI] [PubMed] [Google Scholar]
- Bergman A.; Klasson-Wehler E.; Kuroki H. Selective retention of hydroxylated PCB metabolites in blood. Environ. Health Perspect. 1994, 1025464–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta C.; Haraguchi K.; Kato Y.; Endo T.; Koga N. Species difference in the metabolism of 2,2′,3,4′,5,5′-hexachlorobiphenyl (CB146) by animal and human liver microsomes. Fukuoka Igaku Zasshi 2013, 1044161–169. [PubMed] [Google Scholar]
- Wu X.; Duffel M. W.; Lehmler H.-J. Oxidation of polychlorinated biphenyls by liver tissue slices from phenobarbital-pretreated mice is congener-specific and atropselective. Chem. Res. Toxicol. 2013, 26111642–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania-Korwel I.; Hrycay E. G.; Bandiera S. M.; Lehmler H. J. 2,2′,3,3′,6,6′-Hexachlorobiphenyl (PCB 136) atropisomers interact enantioselectively with hepatic microsomal cytochrome P450 enzymes. Chem. Res. Toxicol. 2008, 2161295–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh N.; Parkin S.; Lehmler H. J. The Ullmann coupling reaction: A new approach to tetraarylstannanes. Organometallics 2006, 25174207–4214. [Google Scholar]
- Waller S. C.; He Y. A.; Harlow G. R.; He Y. Q.; Mash E. A.; Halpert J. R. 2,2′,3,3′,6,6′-Hexachlorobiphenyl hydroxylation by active site mutants of cytochrome P450 2B1 and 2B11. Chem. Res. Toxicol. 1999, 128690–699. [DOI] [PubMed] [Google Scholar]
- Wu X.; Pramanik A.; Duffel M. W.; Hrycay E. G.; Bandiera S. M.; Lehmler H. J.; Kania-Korwel I. 2,2′,3,3′,6,6′-Hexachlorobiphenyl (PCB 136) is enantioselectively metabolized to hydroxylated metabolites by rat liver microsomes. Chem. Res. Toxicol. 2011, 24122249–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania-Korwel I.; Hornbuckle K. C.; Peck A.; Ludewig G.; Robertson L. W.; Sulkowski W. W.; Espandiari P.; Gairola C. G.; Lehmler H. J. Congener specific tissue distribution of Aroclor 1254 and a highly chlorinated environmental PCB mixture in rats. Environ. Sci. Technol. 2005, 39103513–3520. [DOI] [PubMed] [Google Scholar]
- Lupton S. J.; McGarrigle B. P.; Olson J. R.; Wood T. D.; Aga D. S. Human liver microsome-mediated metabolism of brominated diphenyl ethers 47, 99, and 153 and identification of their major metabolites. Chem. Res. Toxicol. 2009, 22111802–1809. [DOI] [PubMed] [Google Scholar]
- Kania-Korwel I.; Duffel M. W.; Lehmler H. J. Gas chromatographic analysis with chiral cyclodextrin phases reveals the enantioselective formation of hydroxylated polychlorinated biphenyls by rat liver microsomes. Environ. Sci. Technol. 2011, 45229590–9596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X.; Kania-Korwel I.; Chen H.; Stamou M.; Dammanahalli K.; Duffel M.; Lein P. J.; Lehmler H.-J. Metabolism of 2,2′,3,3′,6,6′-hexachlorobiphenyl (PCB 136) atropisomers in tissue slices from phenobarbital or dexamethasone-induced rats is sex-dependent. Xenobiotica 2013, 4311933–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnellmann R.; Putnam C.; Sipes I. Metabolism of 2,2′,3,3′,6,6′-hexachlorobiphenyl and 2,2′,4,4′,5,5′-hexachlorobiphenyl by human hepatic microsomes. Biochem. Pharmacol. 1983, 32213233–3239. [DOI] [PubMed] [Google Scholar]
- Ariyoshi N.; Oguri K.; Koga N.; Yoshimura H.; Funae Y. Metabolism of highly persistent PCB congener, 2,4,5,2′,4′,5′-hexachlorobiphenyl, by human CYP2B6. Biochem. Biophys. Res. Commun. 1995, 2122455–460. [DOI] [PubMed] [Google Scholar]
- Kania-Korwel I.; Vyas S.; Song Y.; Lehmler H. J. Gas chromatographic separation of methoxylated polychlorinated biphenyl atropisomers. J. Chromatogr. A 2008, 12071–2146–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D. F.; Loannides C.; Parke D. V. Cytochromes P450 and species differences in xenobiotic metabolism and activation of carcinogen. Environ. Health Perspect. 1998, 10610633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D.; Kania-Korwel I.; Ghogha A.; Chen H.; Stamou M.; Bose D. D.; Pessah I. N.; Lehmler H.-J.; Lein P. J.. PCB 136 Atropselectively Alters Morphometric and Functional Parameters of Neuronal Connectivity in Cultured Rat Hippocampal Neurons via Ryanodine Receptor-Dependent Mechanisms Toxicol. Sci. 2014, doi: 10.1093/toxsci/kft334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmler H. J.; Robertson L. W.; Garrison A. W.; Kodavanti P. R. S. Effects of PCB 84 enantiomers on [3H] phorbol ester binding in rat cerebellar granule cells and 45Ca2+-uptake in rat cerebellum. Toxicol. Lett. 2005, 1563391–400. [DOI] [PubMed] [Google Scholar]
- Lucier G. W.; McDaniel O. S.; Schiller C. M.; Matthews H. B. Structural requirements for the accumulation of chlorinated biphenyl metabolites in the fetal rat intestine. Drug Metab. Dispos. 1978, 65584–590. [PubMed] [Google Scholar]
- Harrad S.; Ren J.; Hazrati S.; Robson M. Chiral signatures of PCB#s 95 and 149 in indoor air, grass, duplicate diets and human faeces. Chemosphere 2006, 6381368–1376. [DOI] [PubMed] [Google Scholar]
- Jamshidi A.; Hunter S.; Hazrati S.; Harrad S. Concentrations and chiral signatures of polychlorinated biphenyls in indoor and outdoor air and soil in a major UK conurbation. Environ. Sci. Technol. 2007, 4172153–2158. [DOI] [PubMed] [Google Scholar]
- Thomas K.; Xue J.; Williams R.; Jones P.; Whitaker D.. Polychlorinated biphenyls (PCBs) in school buildings: Sources, environmental levels, and exposures; United States Environmental Protection Agency, Office of Research and Development, National Exposure Research Laboratory: Washington, DC, 2012. http://www.epa.gov/wastes/hazard/tsd/pcbs/pubs/caulk/pdf/pcb_EPA600R12051_final.pdf.
- Richardson K. L.; Schlenk D. Biotransformation of 2,2′,5,5′-tetrachlorobiphenyl (PCB 52) and 3,3′,4,4′-tetrachlorobiphenyl (PCB 77) by liver microsomes from four species of sea turtles. Chem. Res. Toxicol. 2011, 245718–725. [DOI] [PubMed] [Google Scholar]
- McKinney M. A.; De Guise S.; Martineau D.; Béland P.; Arukwe A.; Letcher R. J. Biotransformation of polybrominated diphenyl ethers and polychlorinated biphenyls in beluga whale (Delphinapterus leucas) and rat mammalian model using an in vitro hepatic microsomal assay. Aquat. Toxicol. 2006, 77187–97. [DOI] [PubMed] [Google Scholar]
- Marek R. F.; Martinez A.; Hornbuckle K. C. Discovery of hydroxylated polychlorinated biphenyls (OH-PCBs) in sediment from a lake Michigan waterway and original commercial Aroclors. Environ. Sci. Technol. 2013, 47158204–8210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno D.; Darling C.; Alaee M.; Campbell L.; Pacepavicius G.; Teixeira C.; Muir D. Detection of hydroxylated polychlorinated biphenyls (OH-PCBs) in the abiotic environment: Surface water and precipitation from Ontario, Canada. Environ. Sci. Technol. 2007, 4161841–1848. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.