Abstract

The biopharmaceutics classification system (BCS) and biopharmaceutics drug distribution classification system (BDDCS) are complementary classification systems that can improve, simplify, and accelerate drug discovery, development, and regulatory processes. Drug permeability has been widely accepted as a screening tool for determining intestinal absorption via the BCS during the drug development and regulatory approval processes. Currently, predicting clinically significant drug interactions during drug development is a known challenge for industry and regulatory agencies. The BDDCS, a modification of BCS that utilizes drug metabolism instead of intestinal permeability, predicts drug disposition and potential drug–drug interactions in the intestine, the liver, and most recently the brain. Although correlations between BCS and BDDCS have been observed with drug permeability rates, discrepancies have been noted in drug classifications between the two systems utilizing different permeability models, which are accepted as surrogate models for demonstrating human intestinal permeability by the FDA. Here, we recommend the most applicable permeability models for improving the prediction of BCS and BDDCS classifications. We demonstrate that the passive transcellular permeability rate, characterized by means of permeability models that are deficient in transporter expression and paracellular junctions (e.g., PAMPA and Caco-2), will most accurately predict BDDCS metabolism. These systems will inaccurately predict BCS classifications for drugs that particularly are substrates of highly expressed intestinal transporters. Moreover, in this latter case, a system more representative of complete human intestinal permeability is needed to accurately predict BCS absorption.
Keywords: biopharmaceutics classification system (BCS), intestinal absorption, biopharmaceutics drug disposition classification system (BDDCS), drug metabolism, permeability rate, PAMPA, Caco-2
Introduction
Many promising drug candidates fail during drug discovery and development due to unacceptable toxicity and inefficacies caused by unfavorable absorption, distribution, metabolism, and excretion (ADME) properties.1 It is exceedingly desirable for these compounds to be disqualified early in the drug discovery phase when they are new molecular entities (NMEs), rather than later, during the much more costly drug development phases. Hence, it is of vital importance to implement strategies that identify toxicity and ADME properties of NMEs during early screening for candidate prioritization and elimination, thereby benefiting the drug discovery and development process immensely.
Ever since its inception in 1995, the biopharmaceutics classification system (BCS) has been an invaluable tool for predicting intestinal drug absorption following oral administration. The BCS framework classifies compounds into four groups according to their aqueous solubility and their intestinal permeability: Class I (high solubility, high permeability), Class II (low solubility, high permeability), Class III (high solubility, low permeability), and Class IV (low solubility, low permeability). Amidon and co-workers2 found a strong correlation between human jejunal permeability rate measures (Peff) determined from intestinal perfusion studies in humans and the fraction of dose absorbed obtained from pharmacokinetic or mass balance studies in humans. They observed that a drug substance had a high intestinal permeability rate when the extent of absorption (Fa) was ≥90% of the oral dose.
Based on these findings, the U.S. Food and Drug Administration (FDA) implemented a BCS Guidance3 that supports waivers of bioequivalence clinical studies of highly permeable, highly soluble BCS Class 1 drugs. The FDA BCS Guidance3 describes several permeability methodologies for determining BCS classification and demonstrating bioequivalence. The BCS class can be determined by measuring human effective permeability rates (Peff) across the jejunal membrane, or alternatively, apparent permeability rates (Papp) across in vitro epithelial cell monolayers, such as the human intestinal cell line Caco-2, relative to a high permeability reference standard (e.g., metoprolol). Only limited jejunal permeability studies in humans have been conducted with published information available for about 30 drugs, likely due to the complexity and high costs of each procedure.4 Subsequently, the most popular human intestinal permeability screening method has used in vitro Caco-2 cell monolayers.5 Even though the BCS is beneficial for obtaining waivers for bioequivalence studies in humans, it can also be very useful in predicting the absorption of NMEs for candidate lead selection in early drug discovery programs.6
The biopharmaceutics drug disposition classification system (BDDCS) is advantageous to early drug discovery programs for predicting an NME’s drug disposition characteristics and potentially clinically significant drug interactions that may arise in the intestine, liver, and brain.7−9 The BDDCS framework is a modification of the BCS that utilizes drug metabolism (by Phase 1 oxidative and Phase 2 conjugative processes) rather than intestinal permeability.7 As such, it classifies compounds into four groups according to their aqueous solubility and their extent of metabolism: Class I (high solubility, extensive metabolism), Class II (low solubility, extensive metabolism), Class III (high solubility, poor metabolism), and Class IV (low solubility, poor metabolism). Benet and colleagues10 recognized that orally administered drugs that were ≥90% metabolized by Phase 1 and Phase 2 processes had to be ≥90% absorbed. Thus, they recommended that the extent of drug metabolism can be an alternative method for supporting a biowaiver for BCS Class 1 drugs.
As a result of the good correlation between high intestinal permeability rates, high intestinal absorption, and extensive metabolism, the permeability methods for determining BCS classification have also been used for BDDCS classification.11−14 However, discrepancies between BCS and BDDCS classification among the various permeability methods have been observed whereby assignment by one method was in accordance with BDDCS but not with BCS and vice versa.11−17 Of 14 drugs with reported high intestinal permeability extent and poor metabolism,13 we could find published Caco-2 permeability rate measurements for 11 drugs. Of these 11 drugs, 8 (73%) were poorly permeable based on their in vitro data, thereby agreeing with their BDDCS classification and disagreeing with their BCS classification.15,16 Even though a number of publications have acknowledged the presence of carrier-mediated and paracellular processes in the Caco-2 cellular system, we have previously discussed how the Caco-2 cellular system is deficient in carrier-mediated and paracellular mechanisms.18 Therefore, we hypothesize that the extent of drug metabolism (BDDCS permeability) is particularly correlated with passive transcellular permeability rate, whereas the extent of drug absorption (BCS permeability) is correlated with complete human intestinal permeability. To test this hypothesis, we evaluate correlations of BCS and BDDCS with permeability rate data from studies across the human jejunum in vivo, Caco-2 cell monolayers, and the parallel artificial membrane permeation assay (PAMPA) membrane, which is devoid of carrier-mediated and paracellular processes.19 Consequently, we present recommendations on the most appropriate permeability models for accurately predicting BCS and BDDCS classifications in early drug discovery programs.
Methods
Compilation of Permeability Rate Data Sets
A literature search was performed to compile all the available human intestinal (jejunal) permeability rate measures of drugs from published human studies (Supporting Information Table 1). Metoprolol was chosen as the high permeability reference standard cut-off as it has been previously used by the BCS in the same role.2 Thus, drugs exhibiting permeability rates greater than or equal to the corresponding value of metoprolol are considered high permeability rate drugs. Conversely, drugs with permeability rates less than the corresponding value of metoprolol are classified as low permeability rate drugs.
Another literature search was performed to compile multiple data sets of in vitro permeability rate measures. We have previously shown how using Caco-2 permeability rate values measured from different laboratories can skew correlations.18 Therefore, in order to avoid interlaboratory variation and selection bias, each data set contained Caco-2 permeability rate values of at least 23 drugs, including metoprolol, that were measured by the same laboratory (Supporting Information Table 2). Upon extensive evaluation of the literature, permeability rate data sets could be found for multiple variations of the PAMPA model. For this study, permeability rate data sets of measurements for at least 35 drugs, including metoprolol, were selected from four different PAMPA models: (i) traditional,20 (ii) a lipid/oil/lipid trilayer,20 (iii) a biomimetic layer,21 and (iv) a hydrophilic filter membrane PAMPA assay22 (Supporting Information Table 3).
Correlations of BCS and BDDCS with Drug Permeability Rate Measures
To facilitate a comparison between BCS and BDDCS classifications using drug permeability rate data, information on the extents of absorption and metabolism measures were compiled from standard references23−26 and verified with their original references (Supporting Information Table 4). Drugs for which absorption data could not be found, such as those administered nonorally, were disqualified and excluded from the study. Each qualifying drug was evaluated for its permeability rate in relation to its extent of absorption and its extent of metabolism. Here, a drug is classified as having a high extent of absorption or metabolism in humans when the extent is ≥90%, and a low extent of absorption or metabolism when the extent is <90%. Comparisons were then made between the class based on permeability rate versus the classes based on the extent of absorption and the extent of metabolism. Nonlinear regression analyses were performed using GraphPad Prism software version 4.03 (GraphPad Software, Inc., San Diego, CA) for the data sets of human absorption or metabolism with their respective permeability rate measures. Drugs that exhibit permeability rates greater than that of metoprolol, but are <90% absorbed or <90% metabolized in humans, are termed false positives. False negatives, on the other hand, are drugs that are ≥90% absorbed or ≥90% metabolized but have experimental human intestinal permeability rates that are lower than that of metoprolol.
Results
Comparison of BCS and BDDCS Classifications Using Human Intestinal Permeability Rate Measures
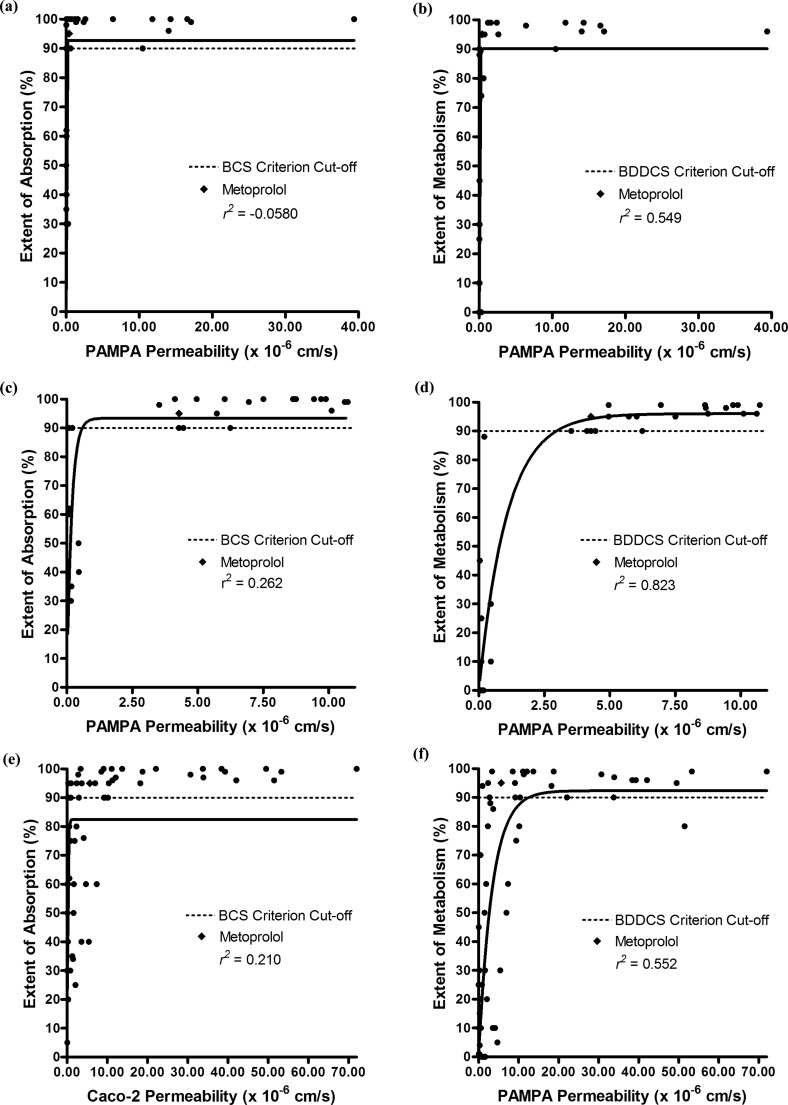
The available human intestinal (jejunal) permeability rates for 30 drugs in the literature4 are summarized in Supporting Information Table 1, with estimates of their extent of absorption and extent of metabolism values in Supporting Information Table 4. Enalapril and valacyclovir are prodrugs whose extents of absorption and permeability rate measures were made for the active species rather than the dosed prodrug. As a result, these drugs were excluded from our analyses. The list contains 14 high permeability rate drugs (that have permeability rates greater than or equal to the corresponding value for metoprolol) and 14 low permeability rate drugs (that have permeability rates less than the corresponding value for metoprolol). Figure 1 allows for a side-by-side comparison of BCS and BDDCS for the 28 drugs with published human intestinal permeability rate measures. Figure 1a depicts a graph of the human intestinal permeability rates versus the extent of absorption, whereas Figure 1b shows these permeability numbers versus the extent of metabolism.
Figure 1.
Relationships between the extent of absorption, extent of metabolism, and human intestinal permeability rates for 28 drugs. (a) Correlation plot of the extent of absorption with the human jejunal permeability rate. (b) Correlation plot of the extent of metabolism with the human intestinal permeability rate. (c) Correlation plot of the extent of metabolism with the extent of absorption.
When using the BCS criterion of an extent of absorption ≥90%, the human intestinal permeability rates accurately predicted the extent of absorption for 26 out of 28 (93%) drugs. The ≥90% extent of absorption criterion accurately correlated with all of the high permeability rate drugs, and the <90% extent of absorption criterion accurately correlated with 12 out of 14 (86%) low permeability rate drugs. Isotretinoin and losartan are two false negatives. Both drugs are considered low permeability rate drugs because their human intestinal permeability rates are lower than that of metoprolol. However, the complete absorption of losartan and 90% absorption of isotretinoin would meet the ≥90% BCS absorption criterion.
When using the BDDCS criterion of an extent of metabolism ≥90%, the human intestinal permeability rates accurately predicted the extent of metabolism for 24 out of 28 (86%) drugs. The ≥90% extent of metabolism criterion accurately correlated with 12 out of 14 (86%) high permeability rate drugs, and the <90% extent of metabolism criterion accurately correlated with 12 out of 14 (86%) low permeability rate drugs. Isotretinoin and losartan are the same false negatives found in both the BCS absorption and the BDDCS metabolism predictions. Both drugs are considered low permeability rate drugs because their human intestinal permeability rates are lower than that of metoprolol. However, their low permeability rates would incorrectly predict their extent of metabolism measures to be low. The BDDCS metabolism predictions resulted in two false positives. Amiloride and cephalexin are both poorly metabolized, even though they exhibit high human intestinal permeability rates relative to metoprolol. Their high permeability rates accurately predicted their high extents of absorption. It should be noted that when a drug is both ≥90% absorbed and ≥90% metabolized, it always has a high intestinal permeability rate.
The false negatives and false positives from the BCS and BDDCS predictions can be seen in Figures 1a and 1b. The two plots are very similar in appearance. From Figure 1b, one can see few drugs within the 30% to 70% extent of metabolism range, a general conclusion noted by Wu and Benet.7 However, as seen in Figure 1a, a number of drugs fall in the 30% to 70% absorption range. Figure 1c depicts the relationship between the extent of metabolism and the extent of absorption for the 30 drugs listed in Supporting Information Table 1. The correlation coefficient (r2) for the correlation in Figure 1c is 0.630. If the 10 compounds with 100% absorption are omitted, the r2 for the remaining 18 compounds decreases to 0.390. Hence, there is generally a poor correlation between the extent of absorption and the extent of metabolism. However, drugs that are ≥90% metabolized accurately predict an extent of absorption ≥90%.
Comparison of BCS and BDDCS Classifications Using Caco-2 Permeability Rate Measures
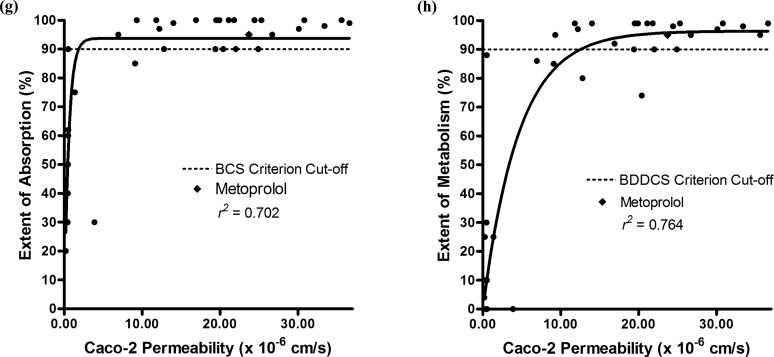
Supporting Information Table 2 shows a summary of four data sets each comprising at least 23 drugs, including metoprolol, and their Caco-2 permeability rate measures taken from four different laboratories.27−30 Figure 2 depicts the correlation plots of the Caco-2 permeability rate measures of each data set in relation to their extents of absorption and extents of metabolism. For each data set, the r2 of the correlation of Caco-2 permeability rate is higher with the extent of metabolism than with the extent of absorption: 0.490 in Figure 2b vs −0.693 in 2a, 0.738 in Figure 2d vs 0.0757 in 2c, 0.918 in Figure 2f vs 0.912 in 2e, and 0.764 in Figure 2h vs 0.702 in 2g.
Figure 2.
Comparison of BCS and BDDCS classifications with Caco-2 permeability rate measures. Correlation plots of the extents of absorption (a) and metabolism (b) with Caco-2 permeability rate data from Alsenz and Haenel.27 Correlation plots of the extents of absorption (c) and metabolism (d) with Caco-2 permeability rate data from Irvine et al.28 Correlation plots of the extents of absorption (e) and metabolism (f) with Caco-2 permeability rate data from Li et al.29 Correlation plots of the extents of absorption (g) and metabolism (h) with Caco-2 permeability rate data from Yazdanian et al.30 The solid line represents the best fit of the nonlinear regression.
When using the BCS criterion of an extent of absorption ≥90%, the Caco-2 permeability rates accurately predicted the extent of absorption for a mean of 68 ± 14% of the drugs. The ≥90% extent of absorption criterion accurately correlated with all of the high permeability rate drugs, and the <90% extent of absorption criterion accurately correlated with a mean of 56 ± 15% of the low permeability rate drugs. When using the BDDCS criterion of an extent of metabolism ≥90%, the Caco-2 permeability rates accurately predicted the extent of metabolism for a mean of 75 ± 9% of the drugs. The ≥90% extent of metabolism criterion accurately correlated with all of the high permeability rate drugs, and the <90% extent of absorption criterion accurately correlated with a mean of 65 ± 9% of the low permeability rate drugs. In order to evaluate if the high number of false positives in the BCS and BDDCS predictions was due to metoprolol being too strict of a high permeability reference standard, we also conducted BCS and BDDCS predictions using a high permeability cut-off that was 30% lower than metoprolol’s permeability rate. Using this adjusted cut-off, the predictions improved with the <90% extent of absorption criterion accurately correlated with a mean of 73 ± 6% of the low permeability rate drugs and the <90% extent of metabolism criterion accurately correlated with a mean of 78 ± 8% of the low permeability rate drugs. There were no false negatives found in the BCS absorption and BDDCS metabolism predictions. Hence, all high permeability rate drugs (that have permeability rates greater than or equal to the corresponding value for metoprolol) in Caco-2 accurately predict a ≥90% extent of absorption and metabolism in humans.
Comparison of BCS and BDDCS Classifications Using PAMPA Permeability Rate Measures
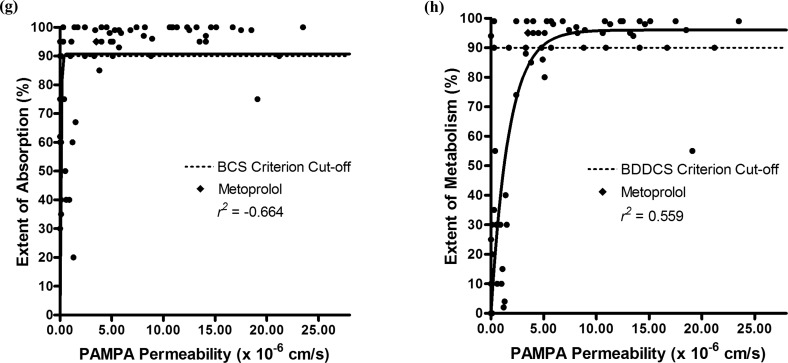
The permeability rate measures for at least 35 drugs, including metoprolol, measured by four different PAMPA models, (i) traditional,20 (ii) a lipid/oil/lipid trilayer,20 (iii) a biomimetic layer,21 and (iv) a hydrophilic filter membrane PAMPA assay,22 are summarized in Supporting Information Table 3. Figure 3 displays the correlation plots of the permeability rate measures for each PAMPA model in relation to their extents of absorption and extents of metabolism. In all four models, the PAMPA permeability rate measures had a stronger correlation with the extent of metabolism than with the extent of absorption: 0.549 in Figure 3b vs −0.0580 in 3a, 0.823 in Figure 3d vs 0.262 in 3c, 0.552 in Figure 3f vs 0.210 in 3e, and 0.559 in Figure 3h vs −0.664 in 3g.
Figure 3.
Comparison of BCS and BDDCS classifications with PAMPA permeability rate measures. Correlation plots of the extent of absorption (a) and metabolism (b) with PAMPA permeability rate data from a traditional model.20 Correlation plots of the extents of absorption (c) and metabolism (d) with PAMPA permeability rate data from a lipid/oil/lipid trilayer model.20 Correlation plots of the extents of absorption (e) and metabolism (f) with PAMPA permeability rate data from a biomimetic layer model.21 Correlation plots of the extents of absorption (g) and metabolism (h) with PAMPA permeability rate data from a hydrophilic filter membrane assay.22 The solid line represents the best fit of the nonlinear regression.
Out of the four models, the BCS absorption and BDDCS metabolism criteria were best predicted via the lipid/oil/lipid trilayer model.20 When using the BCS criterion of an extent of absorption ≥90%, the PAMPA permeability rates in this model accurately predicted the extent of absorption for 84% of the drugs. The ≥90% extent of absorption criterion accurately correlated with all of the high permeability rate drugs, and the <90% extent of absorption criterion accurately correlated with 64% of the low permeability rate drugs. When using the BDDCS criterion of an extent of metabolism ≥90%, the PAMPA permeability rates in this model accurately predicted the extent of metabolism for 88% of the drugs. The ≥90% extent of metabolism criterion accurately correlated with all of the high permeability rate drugs, and the <90% extent of metabolism criterion accurately correlated with 71% of the low permeability rate drugs. The number of false positives in BCS absorption predictions with the PAMPA permeability rate data was higher relative to the BDDCS metabolism predictions (five compared to four). Because some of the false positives are due to metoprolol being too strict of a high permeability rate reference standard, a high permeability rate cut-off value that was 30% lower than that of metoprolol’s was estimated. Using this adjusted high permeability rate measure, there was one false positive in the BCS prediction and no false positives in the BDDCS prediction. There were no false negatives found in the BCS absorption and BDDCS metabolism predictions for this model. Hence, using this PAMPA model, all high permeability rate drugs (that have permeability rates greater than or equal to that of metoprolol) accurately predict a ≥90% extent of absorption and metabolism in humans.
Discussion
Use of BDDCS in the BCS FDA Guidance for Industry
For this study, we revisit the original human intestinal (jejunal) permeability rate measures that were used to illustrate a correlation with human intestinal absorption, and we demonstrate for the first time a correlation with human drug metabolism. The intestinal permeability rate accurately predicted the BCS absorption (high versus low) for 93% of the 28 drugs, all of the high permeability rate drugs, and 86% of the low permeability rate drugs. The intestinal permeability rate accurately predicted the BDDCS metabolism (high versus low) for 86% of the 28 drugs, 86% of the high permeability rate drugs, and 86% of the low permeability rate drugs. It is evident that when both criteria are used, a high intestinal permeability rate is always accurately predicted.
For such simple categorizations in BCS and BDDCS, we note that the extent of absorption and the extent of metabolism both do a remarkable job in predicting the human intestinal permeability rates of drugs. Both criteria result in similar outcomes when predicting high intestinal permeability rates. They both share two false negatives, losartan and isotretinoin. Here, it may be that metoprolol is too strict a permeability standard with a 95% extent of absorption, and a compound with a 90% extent of absorption may be more suitable as the cut-off. Labetalol has been proposed as an alternative,31 but it may not be ideal because its permeability has been recently shown to be concentration dependent.32 Thus, work should be underway to find a more suitable alternative permeability reference marker to metoprolol that is about 90% absorbed primarily by passive diffusion.
Amiloride and cephalexin are false positives of the BDDCS metabolism prediction, where the BDDCS metabolism could not accurately predict amiloride’s and cephalexin’s high intestinal permeability rates because neither are >10% metabolized.33,34 Both of their permeabilities across the intestinal membrane have been characterized to be primarily due more to carrier-mediated than passive processes.35,36 These are two examples where we see how different permeability rates are needed to accurately predict BCS absorption and BDDCS metabolism. Amiloride and cephalexin’s high intestinal permeability rates (inclusive of both carrier-mediated and passive processes) accurately predict their high extents of absorption, whereas their low passive transcellular permeability rates accurately predict their low extents of metabolism.
We show that generally drug metabolism does not predict drug absorption. However, there are cases when drug metabolism can be helpful in predicting the BCS criterion of a high extent of absorption. An extent of metabolism ≥90% accurately predicted the extent of absorption ≥90% in all cases. It has been proposed by Benet and co-workers10 that the following criteria be used to define ≥90% metabolized for marketed drugs:
Following a single oral dose to humans, administered at the highest dose strength, mass balance of the Phase 1 oxidative and Phase 2 conjugative drug metabolites in the urine and feces, measured either as unlabeled, radioactive labeled, or non-radioactive labeled substances, account for ≥90% of the drug dosed. This is the strictest definition for a waiver based on metabolism. For an orally administered drug to be ≥90% metabolized by Phase 1 oxidative and Phase 2 conjugative processes, it is obvious that the drug must be absorbed.
Because there is a good correlation in extensive metabolism predicting extensive absorption (although not necessarily vice versa), the recommendation for regulatory agencies to add the extent of drug metabolism (i.e., ≥90% metabolized) as an alternative method for the extent of drug absorption (i.e., ≥90% absorbed) seems appropriate in defining Class 1 drugs suitable for a waiver of in vivo studies of bioequivalence.
Drug Discovery Considerations When Making BCS and BDDCS Predictions
Academic, industrial, and regulatory scientists have attempted to predict either BCS and BDDCS classifications11−13 or a provisional biopharmaceutics classification system14 that combines the two using the same permeability method. These predictions can successfully achieve accuracies when their classes agree (i.e., both are high or low) and can be unsuccessful when their classes disagree (i.e., one is high and the other is low). Because cases of high metabolism (i.e., ≥90% metabolized) will only exist with high absorption (i.e., ≥90% absorbed), discontinuity will only occur when absorption is high (i.e., ≥90% absorbed) and metabolism is low (i.e., <90% metabolized). In the latter case, we point out that only using the most suitable permeability method will likely lead to the correct class prediction.
In our 2005 publication,7 we initially suggested that ≥70% may be the appropriate cut-off for being labeled extensively metabolized. Here, we show that ≥90% is a more rigorous cut-off for always correctly showing the correlation with permeability rates greater than metoprolol and ≥90% metabolism. We do note that the majority of the compounds in Supporting Information Tables 2 and 3 exhibiting permeability rates less than that of metoprolol but showing greater than 70% metabolism are primarily or initially metabolized by non-CYP processes (i.e., acebutolol, acetylsalicylic acid, zidovudine), but not all are (i.e., indomethacin, timolol).
The PAMPA and Caco-2 systems are attractive in vitro systems for high-throughput permeability screening, known to be deficient in transporter expression and paracellular mechanisms (if not completely devoid of them as in the case of PAMPA). Hence, they are excellent for characterizing the passive transcellular permeability rates of compounds in early drug discovery programs. We show that PAMPA and Caco-2 permeability rate measures have a stronger correlation and higher prediction accuracy with BDDCS metabolism than with BCS absorption. This agrees with our hypothesis that passive transcellular permeability rates accurately predict BDDCS metabolism, whereas complete human intestinal permeability rates accurately predict BCS absorption. We do not find this surprising because previous correlations of lipophilicity have been observed with passive transcellular permeability37 and with drug metabolism.38
When the passive transcellular permeability rate is high (i.e., PAMPA or Caco-2 permeability rate is high), the human intestinal permeability rate (or extent of absorption) should also be high, regardless of whether carrier-mediated transport is occurring. We observed this to be consistently true for our Caco-2 data sets independent of their laboratories of origin. However, we only found a single PAMPA model to be consistent in this finding. Although we cannot confirm the differences between each PAMPA model due to the information being proprietary, we feel that the lipid/oil/lipid PAMPA membrane20 may be more representative of transcellular membrane transport than other models that include hydrophilic membrane transport.21,22 Thus, we believe that the Caco-2 cell line, and only some PAMPA models, may be appropriate for screening and supporting drugs that are potentially eligible for a biowaiver or for predicting their biopharmaceutics (whether BCS or BDDCS) classifications.
A number of outliers were observed when using low PAMPA and Caco-2 permeability rate measures to predict a low extent (<90%) of absorption and slightly fewer when predicting a low (<90%) extent of metabolism. It has been previously noted that metoprolol may be too strict of a high permeability rate measures. We also observed that there were many drugs that were predicted as false positives that were slightly lower than metoprolol’s permeability rate but had high extents of absorption and metabolism. When we conducted an analysis using a permeability rate value that was 30% lower than that of metoprolol’s, we found many of these previous false positives to be correctly predicted using this adjusted high permeability rate cut-off. In the original BCS paper,2 it was suggested that metoprolol was suitable as a high permeability reference standard because its extent of absorption is close to 90%. However, there are numerous sources indicating that its extent of absorption (as well as extent of metabolism) is closer to 95%,26 supporting that a more suitable high permeability reference standard with an extent measure closer to 90% should be investigated.
Even with a less strict permeability rate cut-off, there are still false positives that can arise in the BCS and BDDCS predictions when using PAMPA and Caco-2 systems. We observed that the remaining false positives in the BCS predictions were also very hydrophilic in nature (Supporting Information Table 4). We have previously identified that many of these hydrophilic false positives (e.g., amiloride, cephalexin, levodopa, and zidovudine) are substrates of highly expressed intestinal transporters (e.g., amino acid, peptide, and nucleoside transporters) that are deficient in these in vitro systems.18 It should be cautioned, though, that these systems may be deficient in accurately predicting the high intestinal permeability rates of all NMEs that are particularly substrates of these highly expressed intestinal transporters. We have previously recommended that drug discovery programs utilize other permeability methods that are more representative of carrier-mediated and passive processes for screening these NMEs, such as the human or rat intestine.18
In evaluating the remaining false positives in the BDDCS predictions using PAMPA and Caco-2 systems, we found these compounds to be also very hydrophilic in nature (Supporting Information Table 4). We observed that many of these false positives were extensively metabolized either primarily or initially by non-CYP metabolism, for example, acebutolol,39 acetylsalicylic acid,40 bromocriptine,41 ketoprofen,42 labetolol,43 levodopa,44 salicylic acid,45 and zidovudine.46 This indicates that the metabolism of some very hydrophilic compounds may not be accurately predicted by these systems and alternative methods should be used to verify their BDDCS classification.
Acknowledgments
This work was supported in part by NIH grant GM-61390. During the course of this work, C.A.L. was supported in part by an AFPE Pre-Doctoral Fellowship and an NIH Kirschstein-NRSA Training Grant T32-GM007175.
Supporting Information Available
Permeability rate, class, and extent measures. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Li A. P. Screening for Human ADME/Tox Drug Properties in Drug Discovery. Drug Discov. Today 2001, 6, 357–366. [DOI] [PubMed] [Google Scholar]
- Amidon G. L.; Lennernas H.; Shah V. P.; Crison J. R. A Theoretical Basis for a Biopharmaceutic Drug Classification: The Correlation of In Vitro Drug Product Dissolution and In Vivo Bioavailability. Pharm. Res. 1995, 12, 413–420. [DOI] [PubMed] [Google Scholar]
- Waiver of In Vivo Bioavailability and Bioequivalence Studies for Immediate-Release Solid Oral Dosage Forms Based on a Biopharmaceutics Classification System. FDA Guidance for Industry; Food and Drug Administration: Rockville, MD, 2000. [Google Scholar]
- Lennernas H. Intestinal Permeability and its Relevance for Absorption and Elimination. Xenobiotica 2007, 37, 1015–1051. [DOI] [PubMed] [Google Scholar]
- Ungell A. L. Caco-2 Replace or Refine?. Drug Discov. Today Tech. 2004, 1, 423–430. [DOI] [PubMed] [Google Scholar]
- Varma M. V.; Khandavilli S.; Ashokraj Y.; Jain A.; Dhanikula A.; Sood A.; Thomas N. S.; Pillai O.; Sharma P.; Gandhi R.; Agrawal S.; Nair V.; Panchagnula R. Biopharmaceutic Classification System: a Scientific Framework for Pharmacokinetic Optimization in Drug Research. Curr. Drug Metab. 2004, 5, 375–388. [DOI] [PubMed] [Google Scholar]
- Wu C. Y.; Benet L. Z. Predicting Drug Disposition via Application of BCS: Transport/Absorption/Elimination Interplay and Development of a Biopharmaceutics Drug Disposition Classification System. Pharm. Res. 2005, 22, 11–23. [DOI] [PubMed] [Google Scholar]
- Shugarts S.; Benet L. Z. The Role of Transporters in the Pharmacokinetics of Orally Administered Drugs. Pharm. Res. 2009, 26, 2039–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broccatelli F.; Larregieu C. A.; Cruciani G.; Oprea T. I.; Benet L. Z. Improving the Prediction of the Brain Disposition for Orally Administered Drugs Using BDDCS. Adv. Drug Delivery Rev. 2012, 64, 95–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benet L. Z.; Amidon G. L.; Barends D. M.; Lennernas H.; Polli J. E.; Shah V. P.; Stavchansky S. A.; Yu L. X. The Use of BDDCS in Classifying the Permeability of Marketed Drugs. Pharm. Res. 2008, 25, 483–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi T.; Ramachandran C.; Bermejo M.; Yamashita S.; Yu L. X.; Amidon G. L. A Provisional Biopharmaceutical Classification of the Top 200 Oral Drug Products in the United States, Great Britain, Spain, and Japan. Mol. Pharmaceutics 2006, 3, 631–643. [DOI] [PubMed] [Google Scholar]
- Dahan A.; Miller J. M.; Amidon G. L. Prediction of Solubility and Permeability Class Membership: Provisional BCS Classification of the World’s Top Oral Drugs. AAPS J 2009, 11, 740–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M. L.; Yu L. The Use of Drug Metabolism for Prediction of Intestinal Permeability. Mol. Pharmaceutics 2009, 6, 74–81. [DOI] [PubMed] [Google Scholar]
- Varma M. V.; Gardner I.; Steyn S. J.; Nkansah P.; Rotter C. J.; Whitney-Pickett C.; Zhang H.; Di L.; Cram M.; Fenner K. S.; El-Kattan A. F. pH-Dependent Solubility and Permeability Criteria for Provisional Biopharmaceutics Classification (BCS and BDDCS) in Early Drug Discovery. Mol. Pharmaceutics 2012, 9, 1199–1212. [DOI] [PubMed] [Google Scholar]
- Larregieu C. A.; Benet L. Z.. The Confounding FDA Redefinition of Intestinal Permeability in Terms of Extent of Absorption. AAPS J. 2009, 11, Abstract W1840. [Google Scholar]
- Benet L. Z.; Larregieu C. A. The FDA Should Eliminate the Ambiguities in the Current BCS Biowaiver Guidance and Make Public the Drugs for Which BCS Biowaivers Have Been Granted. Clin. Pharmacol. Ther. 2010, 88, 405–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M. L.; Amidon G. L.; Benet L. Z.; Lennernas H.; Yu L. X. The BCS, BDDCS, and Regulatory Guidances. Pharm. Res. 2012, 28, 1774–1778. [DOI] [PubMed] [Google Scholar]
- Larregieu C. A.; Benet L. Z. Drug Discovery and Regulatory Considerations for Improving In Silico and In Vitro Predictions that Use Caco-2 as a Surrogate for Human Intestinal Permeability Measurements. AAPS J. 2013, 15, 483–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kansy M.; Senner F.; Gubernator K. Physicochemical High Throughput Screening: Parallel Artificial Membrane Permeation Assay in the Description of Passive Absorption Processes. J. Med. Chem. 1998, 41, 1007–1010. [DOI] [PubMed] [Google Scholar]
- Chen X.; Murawski A.; Patel K.; Crespi C. L.; Balimane P. V. A Novel Design of Artificial Membrane for Improving the PAMPA Model. Pharm. Res. 2008, 25, 1511–1520. [DOI] [PubMed] [Google Scholar]
- Sugano K.; Takata N.; Machida M.; Saitoh K.; Terada K. Prediction of Passive Intestinal Absorption Using Bio-Mimetic Artificial Membrane Permeation Assay and the Paracellular Pathway Model. Int. J. Pharm. 2002, 241, 241–251. [DOI] [PubMed] [Google Scholar]
- Zhu C.; Jiang L.; Chen T. M.; Hwang K. K. A Comparative Study of Artificial Membrane Permeability Assay for High Throughput Profiling of Drug Absorption Potential. Eur. J. Med. Chem. 2002, 37, 399–407. [DOI] [PubMed] [Google Scholar]
- Benet L. Z.; Øie S.; Schwarz J. B.. Design and Optimization of Dosage Regimens: Pharmacokinetic Data. In Goodman and Gilman’s The Pharmacological Basis of Therapeutics, 9th ed.; Hardman J. G.; Limbird L. E.; Molinoff P. B.; Ruddon R. W.; Gilman A. G.; McGraw Hill: New York, 1996; pp 1707–1792. [Google Scholar]
- Thummel K. E.; Shen D. D.. Design and Optimization of Dosage Regimens: Pharmacokinetic Data. In Goodman and Gilman’s The Pharmacological Basis of Therapeutics, 10th ed.; Hardman J. G., Limbird L. E., Eds.; McGraw Hill: New York, 2001; pp 1924–2023. [Google Scholar]
- Lacy C. F.; Armstrong L. L.; Goldman M. P.; Lance L. L.. Drug Information Handbook: A Comprehensive Resource for All Clinicians and Healthcare Professionals, 19th ed.; Lexi-Comp, Inc.: Hudson, OH, 2010; p 16–1655. [Google Scholar]
- DRUGDEX® System [Internet database]. Greenwood Village, Colo: Thomson Reuters (Healthcare) Inc.; http://www.rubali.com/new/index.php?option=com_content&view=article&id=6&Itemid=121 (accessed Feb 2014); updated periodically. [Google Scholar]
- Alsenz J.; Haenel E. Development of a 7-day, 96-Well Caco-2 Permeability Assay with High-Throughput Direct UV Compound Analysis. Pharm. Res. 2003, 20, 1961–1969. [DOI] [PubMed] [Google Scholar]
- Irvine J. D.; Takahashi L.; Lockhart K.; Cheong J.; Tolan J. W.; Selick H. E.; Grove J. R. MDCK (Madin-Darby Canine Kidney) Cells: A Tool for Membrane Permeability Screening. J. Pharm. Sci. 1999, 88, 28–33. [DOI] [PubMed] [Google Scholar]
- Li C.; Liu T.; Cui X.; Uss A. S.; Cheng K. C. Development of In Vitro Pharmacokinetic Screens Using Caco-2, Human Hepatocyte, and Caco-2/Human Hepatocyte Hybrid Systems for the Prediction of Oral Bioavailability in Humans. J. Biomol. Screening 2007, 12, 1084–1091. [DOI] [PubMed] [Google Scholar]
- Yazdanian M.; Glynn S. L.; Wright J. L.; Hawi A. Correlating Partitioning and Caco-2 Cell Permeability of Structurally Diverse Small Molecular Weight Compounds. Pharm. Res. 1998, 15, 1490–1494. [DOI] [PubMed] [Google Scholar]
- Volpe D. A.; Faustino P. J.; Ciavarella A. B.; Asafu-Adjaye E. B.; Ellison C. D.; Yu L. X.; Hussain A. S. Classification of Drug Permeability with a Caco-2 Cell Monolayer Assay. Clin. Res. Regul. Affairs 2007, 24, 39–47. [Google Scholar]
- Incecayir T.; Tsume Y.; Amidon G. L. Comparison of the Permeability of Metoprolol and Labetalol in Rat, Mouse, and Caco-2 Cells: Use as a Reference Standard for BCS Classification. Mol. Pharmaceutics 2013, 10, 958–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss P.; Hersey R. M.; Dujovne C. A.; Bianchine J. R. The Metabolism of Amiloride Hydrochloride in Man. Clin. Pharmacol. Ther. 1969, 10, 401–406. [DOI] [PubMed] [Google Scholar]
- Bergan T.; Midtvedt T.; Erikssen J. Human Pharmacokinetics of Cephalexin. Pharmacology 1970, 4, 264–472. [DOI] [PubMed] [Google Scholar]
- Wright S. H.; Wunz T. M. Amiloride Transport in Rabbit Renal Brush-Border Membrane Vesicles. Am. J. Physiol. 1989, 256, F462–F468. [DOI] [PubMed] [Google Scholar]
- Dantzig A. H.; Bergin L. Uptake of the Cephalosporin, Cephalexin, by a Dipeptide Transport Carrier in the Human Intestinal Cell Line, Caco-2. Biochim. Biophys. Acta 1990, 1027, 211–217. [DOI] [PubMed] [Google Scholar]
- Camenisch G.; Alsenz J.; van de Waterbeemd H.; Folkers G. Estimation of Permeability by Passive Diffusion through Caco-2 Cell Monolayers Using the Drugs’ Lipophilicity and Molecular Weight. Eur. J. Pharm. Sci. 1998, 6, 317–324. [PubMed] [Google Scholar]
- Lewis D. F. Human Cytochromes P450 Associated with the Phase 1 Metabolism of Drugs and Other Xenobiotics: A Compilation of Substrates and Inhibitors of the CYP1, CYP2 and CYP3 Families. Curr. Med. Chem. 2003, 10, 1955–1972. [DOI] [PubMed] [Google Scholar]
- Lilja J. J.; Raaska K.; Neuvonen P. J. Effects of Grapefruit Juice on the Pharmacokinetics of Acebutolol. Br. J. Clin. Pharmacol. 2005, 60, 659–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings A. J.; King M. L. Urinary Excretion of Acetylsalicylic Acid in Man. Nature 1966, 209, 620–621. [DOI] [PubMed] [Google Scholar]
- Matsubayashi K.; Matsumoto H.; Fukui Y. Contribution of Cytochrome P450 3A Pathway to Bromocriptine Metabolism and Effects of Ferrous Iron and Hypoxia-Re-Oxygenation on its Elimination in the Perfused Rat Liver. J. Pharm. Pharmacol. 1997, 49, 551–557. [DOI] [PubMed] [Google Scholar]
- Jamali F.; Brocks D. R. Clinical Pharmacokinetics of Ketoprofen and its Enantiomers. Clin. Pharmacokinet. 1990, 19, 197–217. [DOI] [PubMed] [Google Scholar]
- McNeil J. J.; Louis W. J. Clinical Pharmacokinetics of Labetalol. Clin. Pharmacokinet. 1984, 9, 157–167. [DOI] [PubMed] [Google Scholar]
- Kumakura Y.; Cumming P. PET Studies of Cerebral Levodopa Metabolism: a Review of Clinical Findings and Modeling Approaches. Neuroscientist 2009, 15, 635–650. [DOI] [PubMed] [Google Scholar]
- Alpen E. L.; Mandel H. G.; Rodwell V. W.; Smith P. K. The Metabolism of C14 Carboxyl Salicylic Acid in the Dog and in Man. J. Pharmacol. Exp. Ther. 1951, 102, 150–155. [PubMed] [Google Scholar]
- Veal G. J.; Back D. J. Metabolism of Zidovudine. Gen. Pharmacol. 1995, 26, 1469–1475. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.