Abstract
Recent findings indicate that soy isoflavones and their metabolites may play a role in mitigating postmenopausal bone loss. Equol, a metabolite of the soy isoflavone daidzein produced by intestinal bacteria, has shown some potential, but only 30–50% of the U.S. population is capable of converting dietary daidzein to equol. There are limited data on the pharmacokinetics of dietary racemic equol and its metabolites. This study was conducted to assess the levels of equol and its conjugates in plasma for a 24 h period resulting from oral administration of dietary daidzein and racemic equol in ovariectomized Sprague–Dawley rats. Plasma samples were analyzed for conjugated and free forms of equol using LC-MS/MS. The maximum plasma concentration (Cmax) and time to reach it (tmax) for total equol (conjugated and unconjugated) were 8815 ± 2988 nmol/L and 2.17 ± 2.91 h and 3682 ± 2675 nmol/L and 20.67 ± 4.67 h, for dietary equol and daidzein, respectively. Although the majority of equol metabolites present were glucuronide conjugates (≥99%), there were low levels of equol monosulfate present. The changes in equol metabolism, specifically equol conjugates, due to the form of equol may play a role in the potential health benefits of equol.
Keywords: pharmacokinetics, equol, soy isoflavones, ovariectomized rats, equol conjugates
Introduction
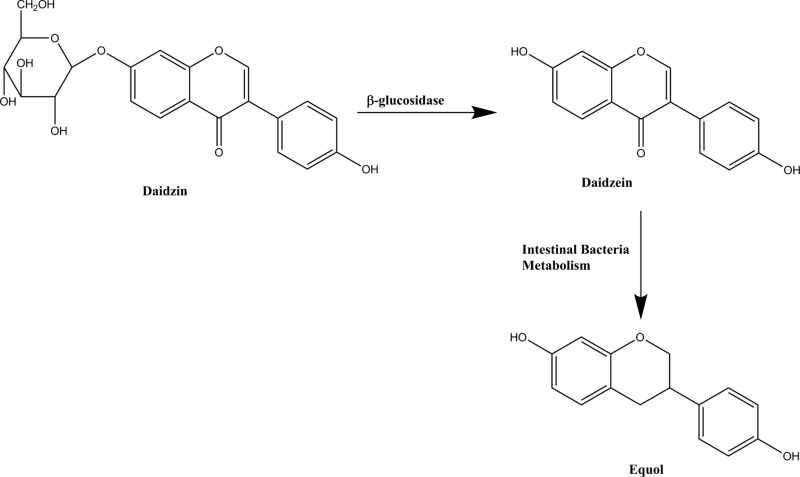
Soy isoflavones and their metabolites have been extensively studied for their various bone and cardiovascular health benefits. In particular, equol, a metabolite of intestinal bacterial conversion from daidzein (Figure 1), has emerged as a bioactive compound with potential for bone health benefits. Equol supplementation is associated with improved bone health in ovariectomized rats including increased trabecular microarchitecture at the proximal femur1 and lumbar spine2 as well as enhanced osteoporotic fracture healing.3 However, results from clinical studies on soy isoflavones have been mixed, which many have attributed to the varied equol-producing ability of the U.S. population.4,5 Equol production is primarily dependent on the intestinal bacterial composition of an individual6 and could play a role in achieving bone health benefits from soy. After classifying participants on equol-producing status, Setchell et al. observed a 2.4% increase in lumbar spine bone mineral density of equol producers (45% of participants) compared to a 0.6% increase in non-equol producers with a 2 year soy isoflavone intervention.
Figure 1.
Schematic of equol production from intestinal bacteria metabolism of daidzein.
In addition to equol-producing ability, the form of circulating equol may also affect potential health benefits. The majority of circulating isoflavones are present primarily as glucuronidated conjugates with low amounts of sulfated7−9conjugates, which could exert various health effects as well. Daidzein sulfate conjugates, but not daidzein, inhibited sterol sulfatase, which is involved in the development of breast cancer in vitro.10 Daidzein and genistein glucuronides have also been shown to augment activation of human natural killer cells in vitro and could aid in improved immunity defense against various cancers.11 To date, there is limited information on the conjugation profile of equol. Schwen and colleagues reported high glucuronidation of S-equol in male Sprague–Dawley rats as well as monkeys.12
Although there has been much research on the metabolism of soy isoflavones and their conjugates in rodents13−19 and humans,20−26 there are limited data on equol metabolism. Two studies explored the transport, absorption, and metabolism of equol in vitro.12,27S-Equol is the only enantiomer produced by human intestinal bacteria from daidzein.28 Pharmacokinetic studies of dietary equol in young healthy adults29 and postmenopausal women30,31 showed rapid absorption of equol. Although there are a few studies of S-equol pharmacokinetics, there is only one in vivo study that evaluated enantiomer forms of equol on metabolism. Setchell et al. demonstrated the bioavailability of equol depends on the enantiomeric form of equol (R-equol, S-equol, racemic equol),29 which subsequently may affect health implications.
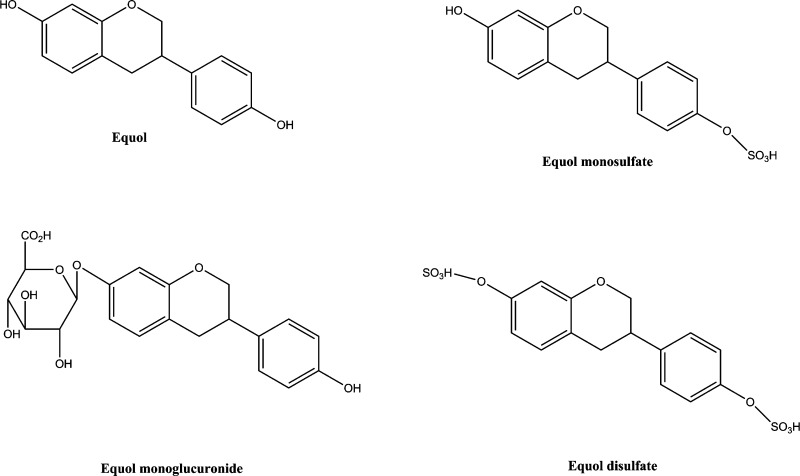
The aim of this pharmacokinetic study was to compare circulating levels of equol and its conjugates (Figure 2) from dietary racemic equol versus intestinal production from dietary daidzein in ovariectomized female Sprague–Dawley rats, a postmenopausal rodent model.
Figure 2.
Chemical structures of equol and its conjugates.
Materials and Methods
Animal Procedures
Three-month-old female Sprague–Dawley rats were purchased from Harlan (Indianapolis, IN, USA) and underwent ovariectomy (OVX) surgery. Rats were maintained on an AIN-93 M diet32 with corn oil replacing soybean oil (to eliminate the potential presence of phytoestrogens in diet) for 1 week before undergoing pharmacokinetics. Animals were housed in individual cages in temperature- and humidity-controlled rooms with a 12 h–12 h on–off light cycle. After a 2 day acclimation period, animals were grouped into pairs and divided into two treatment groups: dietary daidzein (n = 6 pairs) and dietary equol (n = 6 pairs). Animals were matched on the basis of body weight, and pairs were selected to ensure similar body weight average across treatment groups.
Chemicals
Daidzein was obtained from a commercial source (Indofine Chemical Co., Hillsborough, NJ, USA). Racemic equol (50% R-equol, 50% S-equol) as well as equol sulfate conjugates (equol monosulfate and disulfate) were generously provided by the Helferich (Division of Nutritional Sciences, University of Illinois, Urbana, IL, USA) and Botting laboratories (Department of Chemistry, St. Andrew’s University, Fife, UK), respectively. Synthesis details including purity for racemic equol33 and equol conjugates34 are described in earlier work. All other reagents were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Materials
For formulation of oral gavage solutions, both daidzein and equol were suspended in 4.5% starch solution for single oral doses of 10 and 2 mg/mL, respectively. The oral equol dose represents a daily intake of 200 mg racemic equol/kg diet based on a physiologically relevant level for dietary racemic equol seen in earlier research in our laboratory.35 The oral daidzein dose (1000 mg daidzein/kg diet) was selected due to the fact it produced similar circulating equol levels as seen with the dietary dose of 200 mg racemic equol/kg diet.
Pharmacokinetics
Animals were implanted with jugular catheters for blood collection 2 days before pharmacokinetics. Animals received a single oral gavage solution (2 mL) of dietary daidzein (10 mg/mL) or dietary equol (2 mg/mL). Blood (0.2 mL) was drawn via jugular catheter from each rat at the following time points: 0, 0.5, 1, 2, 3, 4, 6, 8, 12, 16, 20, and 24 h. For each pair of animals, blood was pooled at each time point (total of 0.4 mL). Animals weighed between 200 and 250 g. To replenish animals from blood loss, fluids (50:50, saline/5% dextrose, 2 mL) were given after the 3 h time point. After the last blood collection, animals were euthanized by CO2 inhalation. All procedures were approved by the Purdue University Animal Care and Use Committee (Protocol 08-112).
Sample Preparation, Extraction and Analysis
Plasma samples were processed through two separate sample preparations to determine total aglycone and equol conjugate concentrations. To determine total plasma aglycone equol, samples underwent enzymatic hydrolysis as described previously.36 In brief, two conjugates, phenolphthalein β-glucuronide (PHG) and 4-methylumbelliferone sulfate (4-MUS), were added to all samples as markers of effective hydrolysis along with an internal standard, chrysin (CHYS). Isoflavones were recovered by diethyl ether extraction. The ether extracts were evaporated under a stream of nitrogen and reconstituted with 100 μL of an 80% aqueous methanol solution for analysis.
To determine circulating levels of free equol and equol sulfate conjugates, plasma samples (200 μL) underwent protein precipitation. Methanol containing 1% acetic acid solution and a mixed set of internal standards (10 μM 4-MUS–PHG–CHYS) were added to each sample in a ratio of 4:1. Samples were centrifuged for 10 min at 3000g and 4 °C. The supernatants were used for analysis.
Total unconjugated equol, free equol, equol monosulfate, and equol disulfate concentrations were identified and quantified using a highly sensitive and specific electrospray ionization liquid chromatography–multiple reaction ion monitoring (MRM) mass spectrometry method as previously described37 with chromatography conditions detailed in earlier work35 and with the following modifications: equol monosulfate and disulfate were detected using their precursor to product ion transitions of m/z 321/121 and m/z 401/321, respectively. Identification and quantitation of equol and its sulfate conjugates were determined on the basis of comparison of MS/MS fragmentation pattern and retention time to reference material. LC-MS/MS method did not distinguish between R- and S-equol enantiomers. Due to limited availability of known standard for equol glucuronide, it was identified by its m/z 417/241 transition, and concentrations were determined using the following equation, which assumes that only equol sulfate and glucuronide conjugates are present:
| 1 |
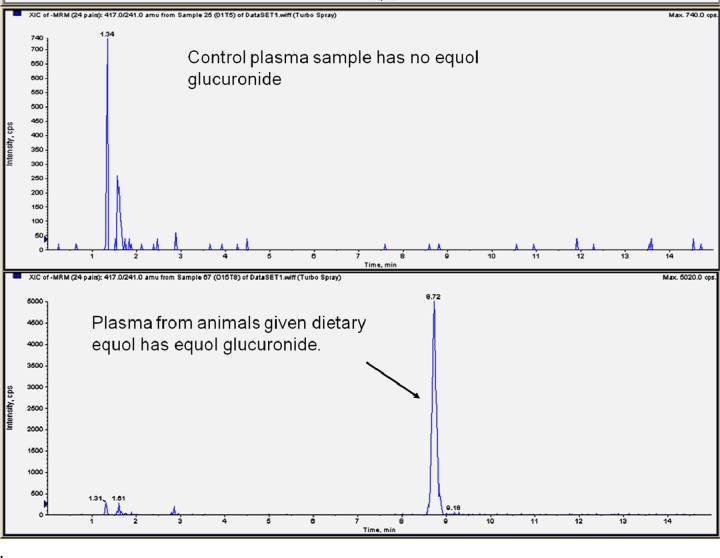
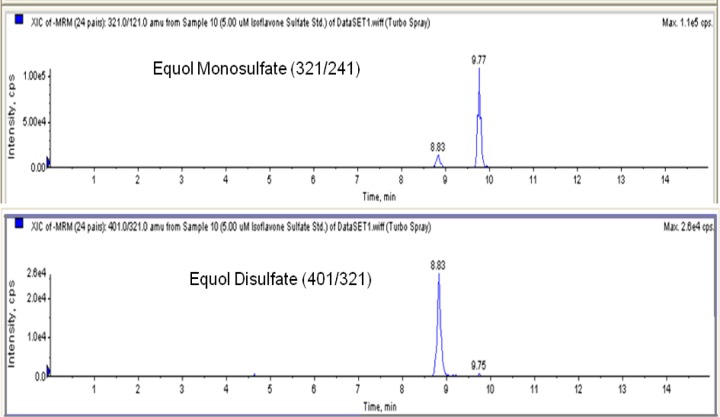
The limit of detection for all analytes of interest was 5 nM. All parameters for metabolite identification including retention times and m/z transitions are listed in Table 1. To validate calculation for equol glucuronide conjugates and ensure no other metabolites were present, a control plasma sample was analyzed along with a sample from the dietary equol group. All pertinent m/z transitions were monitored. Representative chromatograms of equol glucuronide and equol sulfates are shown in Figures 3 and 4.
Table 1. Equol Metabolites Detected in Plasma of Ovarectiomized Rats Fed a Single Oral Dose of Dietary Daidzein (n = 4 Pairs, 10 mg/mL) or Equol (n = 5 Pairs, 2 mg/mL).
| metabolite | retention time (min) | m/z transition |
|---|---|---|
| equol | 10.8 | 241/119 |
| equol disulfate | 8.83 | 401/321 |
| equol glucuronide | 8.72 | 417/241 |
| equol monosulfate | 9.76 | 321/121 |
Figure 3.
MS/MS chromatogram of control sample and product ion mass spectra of plasma samples from rats fed dietary equol (2 mg/mL). The presence of equol glucuronides was determined by monitoring the m/z 417/241 transition using a triple-quadrupole mass spectrometer (4000 QTRAP, ABSCIEX, Framingham, MA, USA) with chromatography details described previously.35
Figure 4.
Representative MS/MS chromatogram of a 5 μM isoflavone sulfate standard containing equol monosulfate and disulfate.
Pharmacokinetic Parameters
The following pharmacokinetic parameters were determined using noncompartmental methods (WinNonlin Pro version 4.01, Pharsight Corp., Mountain View, CA, USA): Cmax (maximum concentration), HL (elimination half-life), tmax (time of maximum concentration), AUC0–24 (area under plasma concentration time curve from 0 to 24h), CL/F (clearance rate), and V/F (volume of distribution).
Results and Discussion
Pharmacokinetic parameters for equol metabolites from dietary daidzein and racemic equol are detailed in Tables 2 and 3. There were higher levels of equol glucuronides than equol monosulfate for both dietary daidzein and racemic equol groups. There were no detectable levels of equol disulfate.
Table 2. Pharmacokinetic Parameters of Equol Metabolites from a Single Oral Gavage of Dietary Daidzein Administered to Ovariectomized Rats (n = 4–6 Pairs)a.
| metabolite | tmax (h) | Cmax (nM) | AUC0–24 (nM·h) |
|---|---|---|---|
| total equol | 20.67 ± 4.67 | 3682 ± 2675 | 24346 ± 8778 |
| unconjugated equol | 20.00 ± 4.90 | 1.21 ± 0.64 | 9.34 ± 8.43 |
| equol monosulfate | 20.00 ± 3.27 | 3.15 ± 3.04 | 19.16 ± 17.76 |
| equol glucuronides | 20.67 ± 4.67 | 3349 ± 2799 | 22438 ± 9907 |
Values are means ± SD. Pharmacokinetic parameters: tmax (time of maximum concentration), Cmax (maximum concentration), AUC0–24 (area under plasma concentration time curve from 0 to 24 h).
Table 3. Pharmacokinetic Parameters of Equol Metabolites from a Single Oral Gavage of Dietary Equol Administered to Ovariectomized Rats (n = 5–6 Pairs)a.
| metabolite | HL (h) | tmax (h) | Cmax (nmol/L) | AUC0–24 (nmol/L·*h) | CL/F (L/h) | V/F (L) |
|---|---|---|---|---|---|---|
| total equol | 21.9 ± 8.02 | 2.17 ± 2.91 | 8815 ± 2988 | 104337 ± 20531 | 0.08 ± 0.03 | 2.32 ± 0.26 |
| unconjugated equol | 9.41 ± 3.93 | 4.42 ± 4.50 | 9.46 ± 3.56 | 96.0 ± 41.9 | 179 ± 138 | 1,937 ± 692 |
| equol monosulfate | 14.4 ± 9.26 | 3.20 ± 2.95 | 23.9 ± 8.54 | 234 ± 97 | 43.7 ± 11.7 | 912 ± 703 |
| equol glucuronides | 23.0 ± 2.91 | 2.17 ± 2.91 | 8775 ± 2976 | 104082 ± 20516 | 0.08 ± 0.03 | 2.38 ± 0.50 |
Values are means ± SD. Pharmacokinetic parameters: HL (elimination half-life), tmax (time of maximum concentration), Cmax (maximum concentration), AUC0–24 (area under plasma concentration time curve from 0 to 24 h), CL/F (clearance rate) = D/AUC0–24, V/F (volume of distribution)
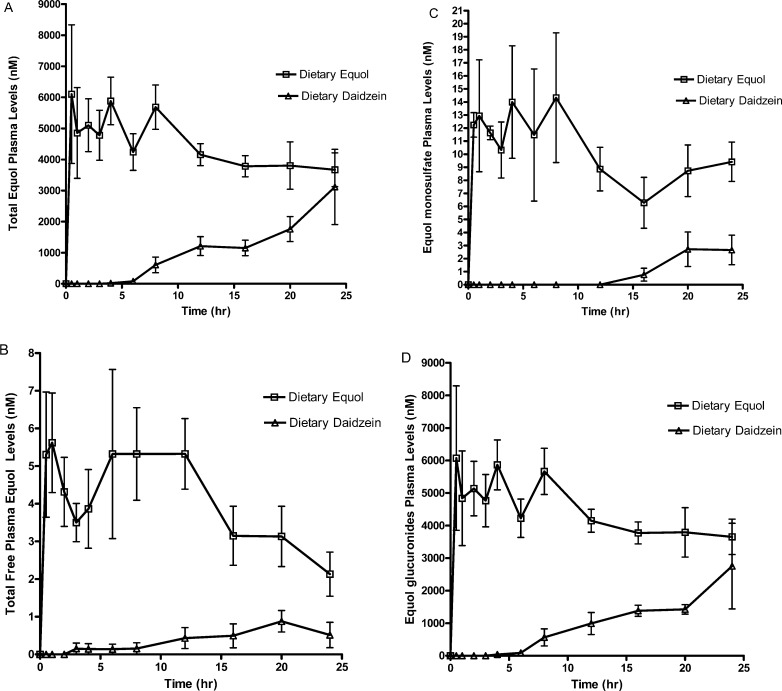
There were significantly higher maximum concentrations of all equol metabolites from dietary racemic equol compared to S-equol produced from dietary daidzein. Plasma concentrations of equol metabolites peaked around 2–3 h for dietary racemic equol and appeared in large levels at 8–13 h for dietary daidzein. The late appearance of equol metabolites with dietary daidzein was expected due to time required for bacterial conversion of daidzein to equol in the large intestine and therefore is a measure of intestinal transit.
Dietary equol led to rises in circulating equol and equol conjugates at 1, 4–5, and 8–9 h (Figure 5). The initial rise at 1 h is attributed to its rapid absorption in the small intestine, which is also seen in both in vitro27 and in vivo30 studies of equol metabolism. The second and third peaks at 4–5 and 8–9 h may be indicators of enterohepatic recycling and additional absorption occurring in the large intestine, respectively. Increases in equol metabolite levels during 16–24 h could also reflect enterohepatic recirculation, as previously reported with other isoflavones.18 Small rises in equol metabolite levels could also be due to high biological variation. Additional studies with greater animals are needed to confirm if plasma increases are significant. Equol conjugates had lower clearance rates (CL/F) than unconjugated equol, which is unexpected due to polarity of conjugates, and warrants further investigation.
Figure 5.
Pharmacokinetic curves over a 24 h period in ovarectiomized rats after a single oral dose of dietary daidzein (n = 6 pairs) (10 mg/mL) or equol (n = 6 pairs) (2 mg/mL) for plasma (A) total aglycone equol, (B) unconjugated or free equol, (C) equol monosulfate, and (D) equol glucuronides. The equol glucuronide was calculated as the difference between the total plasma equol concentration and the sum of the plasma unconjugated and sulfated equol.
The first appearance of equol metabolites (total equol and equol glucuronides) after administration of dietary daidzein occurred around 7–8 h (Figure 5A,D). This corresponds to findings from previous studies.15,17,38 In the dietary daidzein treatment group, total equol, unconjugated equol, and equol glucuronide levels from dietary daidzein rose between 8 and 13 h and all metabolites peaked between 20 and 24 h, consistent with earlier work assessing the bioavailability of daidzein and genistein conjugates in rats.15 None of the equol metabolites reached a plateau after 24 h (Figure 5). There were few data to accurately assess HL, CL/F, and V/F of equol metabolite produced from dietary daidzein. Both findings suggest that a longer time frame is needed to adequately assess the pharmacokinetic profile of equol metabolites from daidzein consumption.
Our results indicate there were higher levels of circulating equol metabolites with dietary racemic equol compared to equol produced from dietary daidzein in ovariectomized rats. We also observed poor clearance of equol metabolites as well as high enterohepatic recirculation as seen previously with other soy isoflavones.18 Most of the circulating equol was conjugated equol, specifically equol glucuronides, which agrees with findings from other in vivo studies. Pharmacokinetics studies of S-equol in various species (rat, monkey, human) show similar metabolism including rapid absorption.12,29,30 Future studies could utilize animal models to determine mechanisms of action for various forms of equol. More elucidation on the impact of the forms of equol (various enantiomers, dietary vs intestinal production) as well as the effect of equol conjugates on health benefits of equol is needed.
Acknowledgments
We thank Pamela Lachcik and Emily Hohman for their assistance with the study as well as William Helferich and the Helferich Laboratory for providing racemic equol and Nigel Botting and the Botting Laboratory for providing equol sulfate standards.
This study was funded by the National Institutes of Health Purdue–UAB Botanical Center for Age-Related Diseases (NIH Grants P50 AT00477, P50 AT00477-07S1). Funds for the mass spectrometer used in the study were provided by a NIH Shared Instrumentation Grant to S.B. (S10 RR19261).
The authors declare the following competing financial interest(s): Stephen Barnes has a U.S. patent on the use of conjugated isoflavones and prevention of osteoporosis. Connie Weaver serves as an advisory board member of Pharmavite.
Funding Statement
National Institutes of Health, United States
References
- Tezval M.; Sehmisch S.; Seidlova-Wuttke D.; Rack T.; Kolios L.; Wuttke W.; Stuermer K. M.; Stuermer E. K. Changes in the histomorphometric and biomechanical properties of the proximal femur of ovariectomized rat after treatment with the phytoestrogens genistein and equol. Planta Med. 2010, 763235–240. [DOI] [PubMed] [Google Scholar]
- Rachon D.; Seidlova-Wuttke D.; Vortherms T.; Wuttke W. Effects of dietary equol administration on ovariectomy induced bone loss in Sprague-Dawley rats. Maturitas 2007, 583308–315. [DOI] [PubMed] [Google Scholar]
- Kolios L.; Sehmisch S.; Daub F.; Rack T.; Tezval M.; Stuermer K. M.; Stuermer E. K. Equol but not genistein improves early metaphyseal fracture healing in osteoporotic rats. Planta Med. 2009, 755459–465. [DOI] [PubMed] [Google Scholar]
- Setchell K. D.; Brown N. M.; Lydeking-Olsen E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J. Nutr. 2002, 132123577–3584. [DOI] [PubMed] [Google Scholar]
- Setchell K. D.; Lydeking-Olsen E. Dietary phytoestrogens and their effect on bone: evidence from in vitro and in vivo, human observational, and dietary intervention studies. Am. J. Clin. Nutr. 2003, 783 Suppl.593S–609S. [DOI] [PubMed] [Google Scholar]
- Xu X.; Harris K. S.; Wang H. J.; Murphy P. A.; Hendrich S. Bioavailability of soybean isoflavones depends upon gut microflora in women. J. Nutr. 1995, 12592307–2315. [DOI] [PubMed] [Google Scholar]
- Adlercreutz H.; van der Wildt J.; Kinzel J.; Attalla H.; Wahala K.; Makela T.; Hase T.; Fotsis T. Lignan and isoflavonoid conjugates in human urine. J. Steroid Biochem. Mol. Biol. 1995, 52197–103. [DOI] [PubMed] [Google Scholar]
- Shelnutt S. R.; Cimino C. O.; Wiggins P. A.; Badger T. M. Urinary pharmacokinetics of the glucuronide and sulfate conjugates of genistein and daidzein. Cancer Epidemiol. Biomarkers Prev. 2000, 94413–419. [PubMed] [Google Scholar]
- Thomas B. F.; Zeisel S. H.; Busby M. G.; Hill J. M.; Mitchell R. A.; Scheffler N. M.; Brown S. S.; Bloeden L. T.; Dix K. J.; Jeffcoat A. R. Quantitative analysis of the principle soy isoflavones genistein, daidzein and glycitein, and their primary conjugated metabolites in human plasma and urine using reversed-phase high-performance liquid chromatography with ultraviolet detection. J. Chromatogr., B: Biomed. Sci. Appl. 2001, 7602191–205. [DOI] [PubMed] [Google Scholar]
- Wong C. K.; Keung W. M. Daidzein sulfoconjugates are potent inhibitors of sterol sulfatase (EC 3.1.6.2). Biochem. Biophys. Res. Commun. 1997, 2333579–583. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Song T. T.; Cunnick J. E.; Murphy P. A.; Hendrich S. Daidzein and genistein glucuronides in vitro are weakly estrogenic and activate human natural killer cells at nutritionally relevant concentrations. J. Nutr. 1999, 1292399–405. [DOI] [PubMed] [Google Scholar]
- Schwen R. J.; Nguyen L.; Jackson R. L. Elucidation of the metabolic pathway of S-equol in rat, monkey and man. Food Chem. Toxicol. 2012, 5062074–2083. [DOI] [PubMed] [Google Scholar]
- Fang N.; Yu S.; Badger T. M. Characterization of isoflavones and their conjugates in female rat urine using LC/MS/MS. J. Agric. Food Chem. 2002, 5092700–2707. [DOI] [PubMed] [Google Scholar]
- Holder C. L.; Churchwell M. I.; Doerge D. R. Quantification of soy isoflavones, genistein and daidzein, and conjugates in rat blood using LC/ES-MS. J. Agric. Food Chem. 1999, 4793764–3770. [DOI] [PubMed] [Google Scholar]
- King R. A. Daidzein conjugates are more bioavailable than genistein conjugates in rats. Am. J. Clin. Nutr. 1998, 686 Suppl.1496S–1499S. [DOI] [PubMed] [Google Scholar]
- King R. A.; Broadbent J. L.; Head R. J. Absorption and excretion of the soy isoflavone genistein in rats. J. Nutr. 1996, 1261176–182. [DOI] [PubMed] [Google Scholar]
- Sepehr E.; Cooke G. M.; Robertson P.; Gilani G. S. Effect of glycosidation of isoflavones on their bioavailability and pharmacokinetics in aged male rats. Mol. Nutr. Food Res. 2009, 53Suppl. 1S16–S26. [DOI] [PubMed] [Google Scholar]
- Sfakianos J.; Coward L.; Kirk M.; Barnes S. Intestinal uptake and biliary excretion of the isoflavone genistein in rats. J. Nutr. 1997, 12771260–1268. [DOI] [PubMed] [Google Scholar]
- Soucy N. V.; Parkinson H. D.; Sochaski M. A.; Borghoff S. J. Kinetics of genistein and its conjugated metabolites in pregnant Sprague-Dawley rats following single and repeated genistein administration. Toxicol. Sci. 2006, 901230–240. [DOI] [PubMed] [Google Scholar]
- Anupongsanugool E.; Teekachunhatean S.; Rojanasthien N.; Pongsatha S.; Sangdee C. Pharmacokinetics of isoflavones, daidzein and genistein, after ingestion of soy beverage compared with soy extract capsules in postmenopausal Thai women. BMC Clin. Pharmacol. 2005, 5, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerge D. R.; Chang H. C.; Churchwell M. I.; Holder C. L. Analysis of soy isoflavone conjugation in vitro and in human blood using liquid chromatography-mass spectrometry. Drug Metab. Dispos. 2000, 283298–307. [PubMed] [Google Scholar]
- Hosoda K.; Furuta T.; Yokokawa A.; Ogura K.; Hiratsuka A.; Ishii K. Plasma profiling of intact isoflavone metabolites by high-performance liquid chromatography and mass spectrometric identification of flavone glycosides daidzin and genistin in human plasma after administration of kinako. Drug Metab. Dispos. 2008, 3681485–1495. [DOI] [PubMed] [Google Scholar]
- Rufer C. E.; Bub A.; Moseneder J.; Winterhalter P.; Sturtz M.; Kulling S. E. Pharmacokinetics of the soybean isoflavone daidzein in its aglycone and glucoside form: a randomized, double-blind, crossover study. Am. J. Clin. Nutr. 2008, 8751314–1323. [DOI] [PubMed] [Google Scholar]
- Setchell K. D.; Brown N. M.; Desai P.; Zimmer-Nechemias L.; Wolfe B. E.; Brashear W. T.; Kirschner A. S.; Cassidy A.; Heubi J. E. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J. Nutr. 2001, 1314 Suppl.1362S–1375S. [DOI] [PubMed] [Google Scholar]
- Shelnutt S. R.; Cimino C. O.; Wiggins P. A.; Ronis M. J.; Badger T. M. Pharmacokinetics of the glucuronide and sulfate conjugates of genistein and daidzein in men and women after consumption of a soy beverage. Am. J. Clin. Nutr. 2002, 763588–594. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Wang G. J.; Song T. T.; Murphy P. A.; Hendrich S. Urinary disposition of the soybean isoflavones daidzein, genistein and glycitein differs among humans with moderate fecal isoflavone degradation activity. J. Nutr. 1999, 1295957–962. [DOI] [PubMed] [Google Scholar]
- Walsh K. R.; Failla M. L. Transport and metabolism of equol by Caco-2 human intestinal cells. J. Agric. Food Chem. 2009, 57188297–8302. [DOI] [PubMed] [Google Scholar]
- Setchell K. D.; Clerici C.; Lephart E. D.; Cole S. J.; Heenan C.; Castellani D.; Wolfe B. E.; Nechemias-Zimmer L.; Brown N. M.; Lund T. D.; Handa R. J.; Heubi J. E. S-equol, a potent ligand for estrogen receptor beta, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora. Am. J. Clin. Nutr. 2005, 8151072–1079. [DOI] [PubMed] [Google Scholar]
- Setchell K. D.; Zhao X.; Jha P.; Heubi J. E.; Brown N. M. The pharmacokinetic behavior of the soy isoflavone metabolite S-(−)equol and its diastereoisomer R-(+)equol in healthy adults determined by using stable-isotope-labeled tracers. Am. J. Clin. Nutr. 2009, 9041029–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setchell K. D.; Zhao X.; Shoaf S. E.; Ragland K. The pharmacokinetics of S-(−)equol administered as SE5-OH tablets to healthy postmenopausal women. J. Nutr. 2009, 139112037–2043. [DOI] [PubMed] [Google Scholar]
- Jackson R. L.; Greiwe J. S.; Desai P. B.; Schwen R. J. Single-dose and steady-state pharmacokinetic studies of S-equol, a potent nonhormonal, estrogen receptor beta-agonist being developed for the treatment of menopausal symptoms. Menopause 2011, 182185–193. [PubMed] [Google Scholar]
- Reeves P. G.; Nielsen F. H.; Fahey G. C. Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123111939–1951. [DOI] [PubMed] [Google Scholar]
- Muthyala R. S.; Ju Y. H.; Sheng S.; Williams L. D.; Doerge D. R.; Katzenellenbogen B. S.; Helferich W. G.; Katzenellenbogen J. A. Equol, a natural estrogenic metabolite from soy isoflavones: convenient preparation and resolution of R- and S-equols and their differing binding and biological activity through estrogen receptors alpha and beta. Bioorg. Med. Chem. 2004, 1261559–1567. [DOI] [PubMed] [Google Scholar]
- Clarke D. B.; Lloyd A. S.; Botting N. P.; Oldfield M. F.; Needs P. W.; Wiseman H. Measurement of intact sulfate and glucuronide phytoestrogen conjugates in human urine using isotope dilution liquid chromatography-tandem mass spectrometry with [13C(3)]isoflavone internal standards. Anal. Biochem. 2002, 3091158–172. [DOI] [PubMed] [Google Scholar]
- Legette L. L.; Martin B. R.; Shahnazari M.; Lee W. H.; Helferich W. G.; Qian J.; Waters D. J.; Arabshahi A.; Barnes S.; Welch J.; Bostwick D. G.; Weaver C. M. Supplemental dietary racemic equol has modest benefits to bone but has mild uterotropic activity in ovariectomized rats. J. Nutr. 2009, 139101908–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund T. E.; Maroni P. D.; Ferucci P. G.; Dayton R.; Barnes S.; Jones K.; Moore R.; Ogden L. G.; Wahala K.; Sackett H. M.; Gray K. J. Long-term dietary habits affect soy isoflavone metabolism and accumulation in prostatic fluid in Caucasian men. J. Nutr. 2005, 13561400–1406. [DOI] [PubMed] [Google Scholar]
- Sites C. K.; Cooper B. C.; Toth M. J.; Gastaldelli A.; Arabshahi A.; Barnes S. Effect of a daily supplement of soy protein on body composition and insulin secretion in postmenopausal women. Fertil. Steril. 2007, 8861609–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allred C. D.; Twaddle N. C.; Allred K. F.; Goeppinger T. S.; Churchwell M. I.; Ju Y. H.; Helferich W. G.; Doerge D. R. Soy processing affects metabolism and disposition of dietary isoflavones in ovariectomized BALB/c mice. J. Agric. Food Chem. 2005, 53228542–8550. [DOI] [PubMed] [Google Scholar]