Abstract
Background
A prolonged QT interval can lead to malignant ventricular arrhythmias and sudden cardiac death, and has frequently been found in end-stage liver disease (ESLD). However, myocardial repolarization lability has not yet been fully investigated. We evaluated the QT variability index (QTVI), a marker of temporal inhomogeneity in ventricular repolarization and an abnormality associated with re-entrant malignant ventricular arrhythmias. We determined whether QTVI is affected by the head-up tilt test in ESLD.
Methods
We assessed 36 ESLD patients and 12 control subjects without overt heart disease before and after the 70-degree head-up tilt test. The electrocardiography signal (lead II) was recorded on a computer with an analog-to-digital converter. The RR interval (RRI) and QT interval were measured after recording 5 min of the digitized electrocardiography. Then, the QT intervals were corrected with Bazett's formula (QTc). QTVI was calculated through the following formula: QTVI = log10 [(QTv/QTm2)/(RRIv/RRIm2)], QTv/RRIv: variance of QTI/RRI, QTm/RRIm: mean of QT interval/RRI.
Results
Cirrhotic patients exhibited an elevated QTVI. In particular, Child class C patients had a significantly increased QTVI compared to Child class A patients and the control subjects in the supine position. However, the head-up tilt test did not cause a significant difference in QTVI in relation to the severity of ESLD.
Conclusions
Myocardial repolarization lability was significantly altered in end-stage liver disease. Our data suggest that the severity of ESLD is associated with the degree of the alteration in the QT variability index.
Keywords: End-stage liver disease, Head-up tilt, QT interval, QT variability index
Introduction
Liver cirrhosis is associated with a broad spectrum of cardiovascular changes, ranging from subclinical alterations to hyperdynamic circulation and cirrhotic cardiomyopathy [1,2]. The major electrocardiographic change in cirrhosis is the prolongation of the QT interval, which is related to the severity of cirrhosis, and is independent of its etiology [3,4,5]. The prolongation of QT interval is associated with malignant ventricular arrhythmia and sudden cardiac death in end-stage liver disease (ESLD) and myocardial infarction [5,6,7]. Many factors are responsible for the prolongation of the QT interval in acquired conditions, such as cardiac, neurological, and electrolyte disturbances, as well as autonomic imbalance from sympathetic nervous system hyperactivity [8,9]. These factors can be associated with an alteration in the QT interval in patients with ESLD.
The QT variability index (QTVI) is a non-invasive measure of beat-to-beat QT interval fluctuations that easily assesses temporal myocardial repolarization lability [10,11]. QTVI is a well-established global assessment tool for risk of sudden cardiac death, not only in high-risk groups but also in patients with mild-to-moderate arrhythmic risk [10,11,12]. QTVI is also influenced by alternation in the RR intervals in the autonomic nervous system or its direct effect on the QT interval itself [13]. Autonomic cardiovascular dysfunction and impaired baroreceptor reflex are well known to occur in ESLD [1,2,14]. Abnormal sympathetic activity plays a role in electrophysiological change and cardiac dysfunction in ESLD, including the prolongation of the QT interval [14,15]. QTVI is a measure of cardiac sympathetic activity to some extent, and it increases in healthy subjects with postural change and infusion of isoproterenol [16]. We speculated on the alteration of QTVI after a head-up tilt test in patients with ESLD with autonomic dysfunction. The head-up tilt test affects the autonomic nervous system, suggesting that there is parasympathetic withdrawal or sympathetic tone activation [17].
This study aimed to determine whether QTVI is affected by the head-up tilt test in cirrhotic patients, depending on the severity of their disease.
Materials and Methods
Twelve control subjects and 36 cirrhotic patients, who were scheduled for living-related liver transplantation, were enrolled (Table 1). The Declaration of Helsinki and the Ethics Committee of our institute accepted the study protocol, and informed consent was obtained from all subjects. The etiology of the ESLD was hepatitis B. Patients were assessed according to Child's classification and the Child score assessment was done within 7 days of the study. Patients who had a history of cardiorespiratory disease, valvular heart disease, or septal defects, or those with abnormalities in conduction or with arrhythmias, and those treated with drugs known to prolong the QT interval, were excluded from the study. Each Child score group had 12 patients. Because there were no relevant previous studies, after the conclusion of the study, a post hoc power calculation with Gpower 3.1 (Dusseldorf, Germany) used the outcomes observed between the groups to show that the sample size was sufficient to detect a difference.
Table 1.
Demographic Data
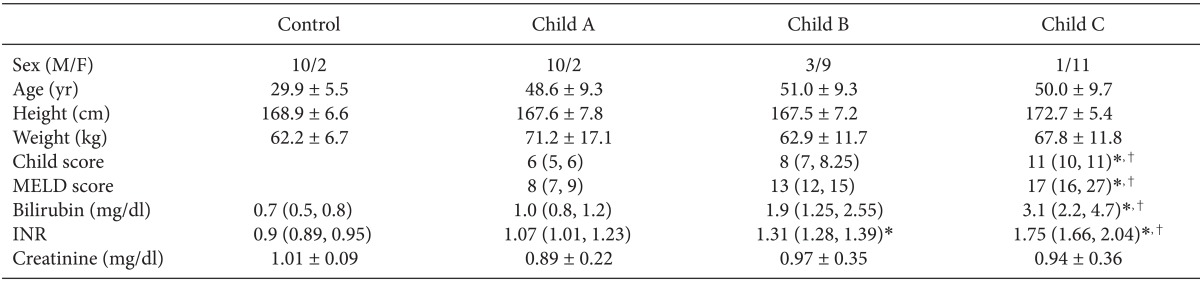
Values are the mean ± SD, median (25%, 75%), or numbers. MELD: model for end-stage liver disease, INR: international normalization ratio. *P < 0.05 vs. control, †P < 0.05 vs. Child class A patient.
Study subjects were placed in the supine position in a quiet room with an ambient temperature ranging from 24-26℃ at least 2 h after they had last eaten. Lead II was used to monitor heart rate on an electrocardiography (ECG, Hewlett-Packard 78352A, CA, USA). Beat-to-beat ECG signals were digitized and collected at 500 Hz using an on-line personal computer that was interfaced with an analog-to-digital converter (DI-720U, Dataq instruments, MA, USA).
After 20 minutes of lying down on the tilt table, baseline value for the ECG was recorded continuously for 10 minutes during spontaneous breathing. At the end of this period, all study subjects were passively erected to a 70° head-up tilt position within 15 seconds. A 10 minute, head-up tilting ECG was recorded, following a stabilization period of 10 minutes.
All ECG data were reviewed visually, and any segments containing signal loss, noise, or extrasystole were discarded. The QT interval was measured in lead II with the automatic method using Labchart® 6 Pro (version 6.1.3, ADInstruments, Dunedin, NewZealand) and its accuracy was verified through a manual measurement. The values of the QT interval for every four beats were averaged for 5 minutes. The heart rate adjusted QT interval (corrected QT interval, QTc) was calculated with Bazett's formula (QTc = QT interval/RR interval1/2 (RRI)). QTVI was calculated by the following formula [10]:
QTVI = log10 [(QTv/QTm2)/(RRIv/RRIm2)],
where QTv is the variance of QT interval; QTm is the mean of QT interval;
RRIv is the variance of RRI, and RRIm is the mean of RRI.
All data were presented as the mean ± SD or median. Logarithmic transformations of the QT variables were performed because the absolute values were not normally distributed. Repeated measurements of a two-way analysis of variance with multiple comparisons among groups (Holm-Sidak test) were performed, based on liver cirrhosis severity and the tilt test. A P value < 0.05 was considered statistically significant.
Results
The demographic characteristics of all study subjects are presented in Table 1. The study was completed without any complaints including light-headedness.
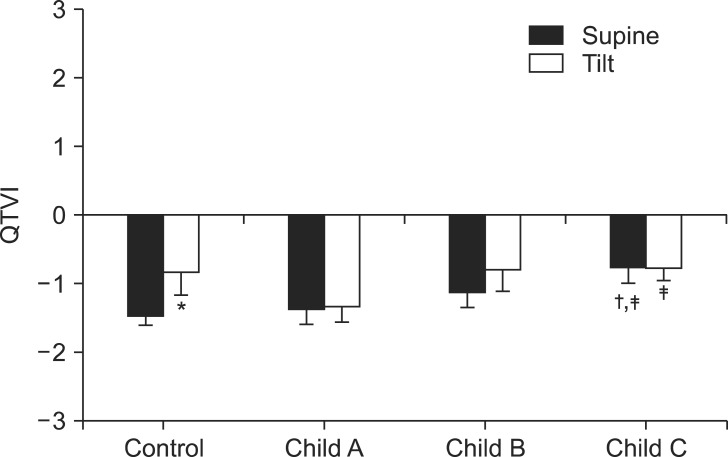
QTVI in Child class C patients increased significantly compared to both the control group and, Child class A patients in the supine position (Fig. 1). QTVI increased significantly in the control group after the head-up tilt test. Moreover, QTVI in Child class C patients was also significantly higher than that in Child class A patients after the head-up tilt test. In addition, the QT interval was significantly prolonged in Child class B and C patients compared with the control group in the supine position (Table 2). After the head-up tilt test, the QT interval decreased significantly in Child class A and B patients. QTc prolongation has been frequently found in liver cirrhosis (0 vs 22% in the supine and head-up tilt positions, respectively). The prevalence of an abnormal QTc (QTc ≥ 450 ms) was 2 (16%), 1 (8%), and 5 (42%), respectively, in each Child class in the supine position.
Fig. 1.
Change in QTVI (QT variability index) in the supine and head-up tilt (HUT) positions in control and end-stage liver disease patients. QTVI significantly increases according to severity of the end-stage liver disease. QTVI significantly increases after HUT in the control group. *P < 0.05 vs. supine position, †P < 0.05 vs. control subjects, ‡P < 0.05 vs. Child class A patients.
Table 2.
QT Parameters before and after the Head Up Tilt Test
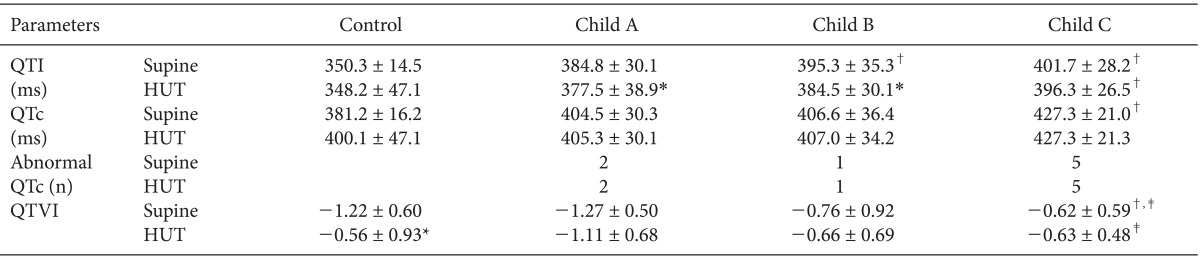
Values are the mean ± SD or numbers. QTI: QT interval, HUT: head-up tilt, QTc: corrected QT interval using Bazett's formula, abnormal QTc: QTc ≥ 450 ms, QTVI: QT variability index. *P < 0.05 vs. supine position, †P < 0.05 vs. control subjects, ‡P < 0.05 vs. Child class A patients.
Discussion
In this study, we found that QTVI significantly increased according to the severity of liver disease. The head-up tilt test significantly increased QTVI in the control group, but QTVI in cirrhotic patients did not change after the head-up tilt test. However, it significantly shortened the QT interval in Child class A and B patients.
The QT interval refers to the period from the earliest activation of ventricular myocardium to the end of the latest repolarization. Prolongation of the QT interval is related to sudden cardiac death, malignant ventricular arrhythmias, and poor survival in patients afflicted with many diseases, such as myocardial infarction and cardiac dysfunction [6,7]. It is well known that QTc is prolonged in ESLD [1,2,3,4,5]. The prolongation of QTc in cirrhotic patients is independent of etiology, and associated with an increased risk of sudden cardiac death in alcoholic liver cirrhosis [5,18]. Bernardi et al. [3] reported that QTc prolongation was correlated with the Child score; additionally, patients with an abnormal QTc (> 440 ms) had a significantly lower survival rate in ESLD. In this study, the QT interval and QTc were significantly prolonged in cirrhotic patients in both positions. Many factors may be responsible for the prolonged QTc in cirrhotic patients, such as electrolyte disturbances (hypokalemia, hypomagnesemia, and hypocalcemia), alcohol ingestion, and autonomic imbalance with sympathetic dominance [5,8,9]. These factors are associated with cirrhotic cardiomyopathy [1,2]. In particular, sympathetic hyperactivity and delayed myocardial repolarization due to K+ channel abnormality may contribute to QTc prolongation in ESLD [1,3]. A QTc prolongation is not a contraindication of liver transplantation, but reversible causes, such as an electrolyte abnormality, should be promptly corrected [2].
The QTVI provides a noninvasive measure of beat-by-beat QT interval fluctuations the ECG [11]. It represents the relationship between the QT interval and heart rate variability (HRV) and temporal myocardial repolarization lability [10]. Increased repolarization lability is related to ventricular malignant arrhythmias, sudden cardiac death, and mortality due to heart failure [10,11,19,20]. An increased QTVI has also been documented in patients with acute myocardial infarction, left ventricular dysfunction, or in patients facing sudden cardiac death [10,11,21]. All of these conditions are present in malignant arrhythmic events; therefore, an increased QTVI might reflect increased vulnerability to arrhythmia [10,11]. Less information is available on QTVI in cirrhotic patients. We found that QTVI increased according to the ESLD severity. After the head-up tilt test, QTVI decreased negativity in the control group, but it did not change in cirrhotic patients. Increased QTVI was related to increases in the QT variability (numerator in QTVI) and decreases in HRV, with out-of-proportion unchanged or slightly increased QT variance. An increase in QTVI could be caused by a structural change in the myocardium and an alteration in the autonomic nervous system associated with cirrhotic cardiomyopathy. The head-up tilt test results in an increase in the low frequency/high frequency ratio (LF/HF ratio) or a decrease in HF because of enhanced sympathetic activity or withdrawal of the parasympathetic activity [17]. QTVI also reflects the sympathetic activity to some extent, showing as increase in healthy subjects with a postural change from supine to standing [16]. Our finding in the control group is in agreement with the results of Yeragani et al. [16]. Therefore, we investigated the alteration of QTVI after the head-up tilt test in ESLD patients with autonomic dysfunction. Autonomic dysfunction in cirrhosis exhibits impairment of the cardiovascular autonomic reflexes and is associated with an increase in the severity and duration of liver disease [22,23]. In addition, autonomic dysfunction is associated with reduced HRV, and is related to abnormal circulatory regulation [14,24]. The head-up tilt test did not increase QTVI in the cirrhotic patients. Although we could not clearly explain this finding, the head-up tilt test may not trigger enough stimuli in cirrhotic patients because they already have sympathetic hyperactivity. The sympathetic nervous dysfunction in ESLD is noted through impaired end-organ responsiveness due to decrease production of neurotransmitters, or receptor down-regulation [25].
This study has several limitations. First, as we studied only a limited number of patients with liver cirrhosis. Hence, its results must be confirmed with a larger number of cirrhotic patients. In addition, we did not assess the relationship between increased QTVI and the risk of arrhythmia. Future studies are needed to ascertain morbidity related to QTVI.
In conclusion, myocardial repolarization lability was significantly altered in end-stage liver disease. Our data suggest that the severity of liver cirrhosis is associated with increased risk of arrhythmogenesis.
References
- 1.Zardi EM, Abbate A, Zardi DM, Dobrina A, Margiotta D, Van Tassell BW, et al. Cirrhotic cardiomyopathy. J Am Coll Cardiol. 2010;56:539–549. doi: 10.1016/j.jacc.2009.12.075. [DOI] [PubMed] [Google Scholar]
- 2.Moller S, Hove JD, Dixen U, Bendtsen F. New insights into cirrhotic cardiomyopathy. Int J Cardiol. 2013;167:1101–1108. doi: 10.1016/j.ijcard.2012.09.089. [DOI] [PubMed] [Google Scholar]
- 3.Bernardi M, Calandra S, Colantoni A, Trevisani F, Raimondo ML, Sica G, et al. Q-T interval prolongation in cirrhosis: prevalence, relationship with severity, and etiology of the disease and possible pathogenetic factors. Hepatology. 1998;27:28–34. doi: 10.1002/hep.510270106. [DOI] [PubMed] [Google Scholar]
- 4.Mohamed R, Forsey PR, Davies MK, Neuberger JM. Effect of liver transplantation on QT interval prolongation and autonomic dysfunction in end-stage liver disease. Hepatology. 1996;23:1128–1134. doi: 10.1002/hep.510230529. [DOI] [PubMed] [Google Scholar]
- 5.Day CP, James OF, Butler TJ, Campbell RW. QT prolongation and sudden cardiac death in patients with alcoholic liver disease. Lancet. 1993;341:1423–1428. doi: 10.1016/0140-6736(93)90879-l. [DOI] [PubMed] [Google Scholar]
- 6.Schouten EG, Dekker JM, Meppelink P, Kok FJ, Vandenbroucke JP, Pool J. QT interval prolongation predicts cardiovascular mortality in an apparently healthy population. Circulation. 1991;84:1516–1523. doi: 10.1161/01.cir.84.4.1516. [DOI] [PubMed] [Google Scholar]
- 7.Algra A, Tijssen JG, Roelandt JR, Pool J, Lubsen J. QTc prolongation measured by standard 12-lead electrocardiography is an independent risk factor for sudden death due to cardiac arrest. Circulation. 1991;83:1888–1894. doi: 10.1161/01.cir.83.6.1888. [DOI] [PubMed] [Google Scholar]
- 8.Surawicz B. Electrolytes and the Electrocardiogram. Am J Cardiol. 1963;12:656–662. doi: 10.1016/0002-9149(63)90255-8. [DOI] [PubMed] [Google Scholar]
- 9.Oka H, Mochio S, Sato K, Isogai Y. Correlation of altered Q-T interval and sympathetic nervous system dysfunction in diabetic autonomic neuropathy. Eur Neurol. 1994;34:23–29. doi: 10.1159/000117003. [DOI] [PubMed] [Google Scholar]
- 10.Berger RD, Kasper EK, Baughman KL, Marban E, Calkins H, Tomaselli GF. Beat-to-beat QT interval variability: novel evidence for repolarization lability in ischemic and nonischemic dilated cardiomyopathy. Circulation. 1997;96:1557–1565. doi: 10.1161/01.cir.96.5.1557. [DOI] [PubMed] [Google Scholar]
- 11.Atiga WL, Calkins H, Lawrence JH, Tomaselli GF, Smith JM, Berger RD. Beat-to-beat repolarization lability identifies patients at risk for sudden cardiac death. J Cardiovasc Electrophysiol. 1998;9:899–908. doi: 10.1111/j.1540-8167.1998.tb00130.x. [DOI] [PubMed] [Google Scholar]
- 12.Piccirillo G, Cacciafesta M, Lionetti M, Nocco M, Di Giuseppe V, Moise A, et al. Influence of age, the autonomic nervous system and anxiety on QT-interval variability. Clin Sci (Lond) 2001;101:429–438. [PubMed] [Google Scholar]
- 13.Cappato R, Alboni P, Pedroni P, Gilli G, Antonioli GE. Sympathetic and vagal influences on rate-dependent changes of QT interval in healthy subjects. Am J Cardiol. 1991;68:1188–1193. doi: 10.1016/0002-9149(91)90192-n. [DOI] [PubMed] [Google Scholar]
- 14.Genovesi S, Prata Pizzala DM, Pozzi M, Ratti L, Milanese M, Pieruzzi F, et al. QT interval prolongation and decreased heart rate variability in cirrhotic patients: relevance of hepatic venous pressure gradient and serum calcium. Clin Sci (Lond) 2009;116:851–859. doi: 10.1042/CS20080325. [DOI] [PubMed] [Google Scholar]
- 15.Frith J, Newton JL. Autonomic dysfunction in chronic liver disease. Liver Int. 2009;29:483–489. doi: 10.1111/j.1478-3231.2009.01985.x. [DOI] [PubMed] [Google Scholar]
- 16.Yeragani VK, Pohl R, Jampala VC, Balon R, Kay J, Igel G. Effect of posture and isoproterenol on beat-to-beat heart rate and QT variability. Neuropsychobiology. 2000;41:113–123. doi: 10.1159/000026642. [DOI] [PubMed] [Google Scholar]
- 17.Vybiral T, Bryg RJ, Maddens ME, Boden WE. Effect of passive tilt on sympathetic and parasympathetic components of heart rate variability in normal subjects. Am J Cardiol. 1989;63:1117–1120. doi: 10.1016/0002-9149(89)90089-1. [DOI] [PubMed] [Google Scholar]
- 18.Lazzeri C, La Villa G, Laffi G, Vecchiarino S, Gambilonghi F, Gentilini P, et al. Autonomic regulation of heart rate and QT interval in nonalcoholic cirrhosis with ascites. Digestion. 1997;58:580–586. doi: 10.1159/000201505. [DOI] [PubMed] [Google Scholar]
- 19.Piccirillo G, Magri D, Matera S, Magnanti M, Torrini A, Pasquazzi E, et al. QT variability strongly predicts sudden cardiac death in asymptomatic subjects with mild or moderate left ventricular systolic dysfunction: a prospective study. Eur Heart J. 2007;28:1344–1350. doi: 10.1093/eurheartj/ehl367. [DOI] [PubMed] [Google Scholar]
- 20.Haigney MC, Zareba W, Gentlesk PJ, Goldstein RE, Illovsky M, McNitt S, et al. QT interval variability and spontaneous ventricular tachycardia or fibrillation in the Multicenter Automatic Defibrillator Implantation Trial (MADIT) II patients. J Am Coll Cardiol. 2004;44:1481–1487. doi: 10.1016/j.jacc.2004.06.063. [DOI] [PubMed] [Google Scholar]
- 21.Vrtovec B, Starc V, Starc R. Beat-to-beat QT interval variability in coronary patients. J Electrocardiol. 2000;33:119–125. doi: 10.1016/s0022-0736(00)80068-0. [DOI] [PubMed] [Google Scholar]
- 22.Oliver MI, Miralles R, Rubies-Prat J, Navarro X, Espadaler JM, Sola R, et al. Autonomic dysfunction in patients with non-alcoholic chronic liver disease. J Hepatol. 1997;26:1242–1248. doi: 10.1016/s0168-8278(97)80458-8. [DOI] [PubMed] [Google Scholar]
- 23.Fleckenstein JF, Frank S, Thuluvath PJ. Presence of autonomic neuropathy is a poor prognostic indicator in patients with advanced liver disease. Hepatology. 1996;23:471–475. doi: 10.1002/hep.510230311. [DOI] [PubMed] [Google Scholar]
- 24.Iga A, Nomura M, Sawada Y, Ito S, Nakaya Y. Autonomic nervous dysfunction in patients with liver cirrhosis using 123I-metaiodobenzylguanidine myocardial scintigraphy and spectrum analysis of heart-rate variability. J Gastroenterol Hepatol. 2003;18:651–659. doi: 10.1046/j.1440-1746.2003.03044.x. [DOI] [PubMed] [Google Scholar]
- 25.Laffi G, Lagi A, Cipriani M, Barletta G, Bernardi L, Fattorini L, et al. Impaired cardiovascular autonomic response to passive tilting in cirrhosis with ascites. Hepatology. 1996;24:1063–1067. doi: 10.1053/jhep.1996.v24.pm0008903376. [DOI] [PubMed] [Google Scholar]