Abstract
Introduction:
Among smokers, former smokers, and never-smokers, this study aimed to (a) determine the predictive value of smoking expectancy on future smoking status, and (b) test the relative contribution of genes and environment to a person’s ability to accurately predict future smoking status. For smokers, smoking expectancy reflects the intention to continue smoking; for former smokers, it reflects the intention to take up smoking again; and for never-smokers, it reflects the intention to initiate smoking.
Methods:
A longitudinal design was employed in which participants of the Netherlands Twin Register completed 2 consecutive surveys 2 years apart between 1993 and 2011 (3,591 adolescents aged 14–18 years), or between 1993 and 2004 (11,568 adults, aged 18+ years). Smoking expectancy was measured by asking, “Do you think you’ll smoke in a year’s time?”, with answer categories ranging from “certainly not” to “absolutely yes” on a 5-point scale. To determine the predictive value of smoking expectancy, analyses were performed in smokers, former smokers, and never-smokers separately. Data of 2,987 adolescents and 4,911 adult twins were analyzed to estimate heritability. A dichotomous variable reflected the ability to predict future smoking status (correct/incorrect).
Results:
Smoking expectancy significantly predicted future smoking status among former smokers and never-smokers. The ability to accurately predict future smoking status was explained by additive genetic factors for 59% of adolescents and 27% of adults, with the remainder being explained by unique environmental factors.
Conclusions:
A single question on smoking expectancy helps predict future smoking status. Variation in how well subjects predict their future smoking behavior is influenced by genetic factors, especially during adolescence.
INTRODUCTION
Smoking remains a major public health problem worldwide and can cause severe morbidity (CDC, 2004). The World Health Organization has estimated that up to half of all tobacco users will eventually die from a tobacco-related disease (WHO, 2011). Despite these facts, 28% of adult men and 26% of adult women in the Netherlands were smokers in 2010 (Nationaal Kompas Volksgezondheid, 2011), while 16% of adolescents smoked occasionally, and 10% versus 9% of male and female adolescents smoked daily (CBS, 2010a). Smoking cessation leads to a significant decrease in the risk of serious health problems (Taylor, Hasselblad, Henley, Thun, & Sloan, 2002), and a complete understanding of smoking behavior and its predictors might aid in developing successful intervention programs. This study explores the expectancy people have about their own future smoking behavior. Such “smoking expectancy” may predict not only whether smokers continue smoking but also whether former smokers will relapse or never-smokers will initiate smoking.
In general, adolescent never-smokers are more susceptible to the initiation of smoking than (young) adults. In Dutch youngsters, the mean age at first cigarette is 15 years (CBS, 2010b), with 89% of ever-smokers having started smoking before the age of 18 years (Stivoro, 2009). Young males were more likely to initiate smoking than females (Freedman, Nelson, & Feldman, 2012; Nuno, Zhang, Harris, Wilkinson-Lee, & Wilhelm, 2011), and a lower educational level in adolescence was associated with a higher chance of smoking in young adulthood (Mendel, Berg, Windle, & Windle, 2012).
In current smokers, the intention to quit predicted a future quitting attempt and was higher in smokers who smoked for a shorter period of time and/or smoked fewer cigarettes per day (Smit, Fidler, & West, 2011). Whether or not smokers expected to be successful in quitting was also predictive of a future quit attempt (Abdullah et al., 2006). Together, attitude toward quitting smoking, opinions of friends and family about smoking, and the extent to which one believes to be able to quit accounted for approximately 30% of the variance in quitting intentions (Rise, Kovac, Kraft, & Moan, 2008). Past quit attempts and having concerns about the health effects of smoking were predictive of making a quit attempt in the future while succeeding in quitting was influenced by cigarette dependency (Vangeli, Stapleton, Smit, Borland, & West, 2011). Having had health problems in the past increased the intention to quit and the chance to make a quit attempt (Abdullah et al., 2006).
In former smokers, risk factors for relapse include a lower abstinence self-efficacy and a higher frequency of urges to smoke (Herd, Borland, & Hyland, 2009; Yong, Borland, Cooper, & Cummings, 2010). A higher former cigarette dependence increased the chance of relapse following a quit attempt (Yeh, Ellerbeck, & Mahnken, 2012), while a higher educational level and a higher self-reported health were associated with a lower risk or relapse (Augustson et al., 2008; Reid et al., 2010; Wetter et al., 2004).
Smoking expectancy might be able to predict future smoking behavior in never-smokers, current smokers, and former smokers, making it a useful tool for identifying risk groups. Up until now, publications on smoking expectancy (measured by asking if a person thinks he/she will smoke next year) are scarce. In adolescents from New Zealand, a higher age was associated with a higher chance of being a smoker and thus with a higher expectancy to (still) smoke in the future (McCool, Cameron, Petrie, & Robinson, 2003). Smoking adolescents tended to underestimate the chances of continuing, while nonsmoking adolescents underestimated the chances of initiating smoking (Schoenbaum, 2005). In addition, susceptibility to smoking (defined as not being able to rule out the possibility of smoking in a year) was a strong predictor of starting smoking in nonsmoking adolescents (Forrester, Biglan, Severson, & Smolkowski, 2007).
Multiple aspects of smoking behavior are influenced by genetic factors, including smoking initiation, nicotine dependence (Li, Cheng, Ma, & Swan, 2003; Sullivan & Kendler, 1999; Vink, Willemsen, & Boomsma, 2005), and smoking cessation (Broms, Silventoinen, Madden, Heath, & Kaprio, 2006). Genetic factors may also influence people’s ability to accurately predict their own future smoking status and understanding how individual differences can be explained may assist in tailoring of prevention strategies. As for every human complex trait, we expect people to differ in how well they assess their own future smoking behavior. Some might be overly optimistic about their ability to quit, while others may be more capable of predicting their future smoking behavior. Such individual differences are likely to have a heritable component, possibly related to genetically influenced personality traits like optimism. Self-knowledge about ability to quit may also depend on experience and age. The extent to which this knowledge depends on genotype may be age dependent as well, decreasing when people gain more experience about their own behavior. We, therefore, investigate the heritability of predicting future smoking status in both adolescents and adults.
Longitudinal data on smoking expectancy were collected in two large groups of participants from the Netherlands Twin Register (NTR; 3,591 adolescents and 11,568 adults). Within each age group, smoking expectancy for current smokers reflects the intention to continue smoking, while for former smokers, it reflects the intention to take up smoking again, and for never-smokers, the intention to initiate smoking. We aimed to (a) determine the predictive value of smoking expectancy on future smoking status through longitudinal analyses and (b) estimate the relative contribution of genetic and environmental factors to the ability to accurately predict future smoking status through genetic analyses of twin data.
METHODS
Subjects
All participants are enrolled in longitudinal survey studies of the NTR (van Beijsterveldt et al., 2013; Willemsen et al., 2013). The young NTR consists of participants who were recruited as newborn twins from 1987 onwards and their siblings who were included later on. The adult NTR comprises adolescents and adult twins and their family members who were recruited since 1990.
Data were analyzed separately for adolescents (aged 14–18 years) and adults (aged 18+ years). We first selected 3,591 adolescents and 11,568 adult participants who completed at least two successive surveys approximately two years apart. After discarding participants with an unknown smoking status, the adolescent group consisted of 3,114 twins and their siblings (40% male; 4% nontwin; mean age 15.7 years, SD 1.1). Between 1993 and 2011, the adolescents completed two surveys either around 14 and 16 or 16 and 18 years. The adult group contained 10,468 participants (41% male; 53% nontwin; mean age 37.0 years, SD 14.3). Adults completed two or more consecutive surveys in 1993, 1995, 1997, 2000, 2002, and/or 2004. Data on smoking expectancy were collected in all surveys, except in 2004.
For the genetic analyses, data from 2,987 adolescent twins (1,106 complete pairs and 775 twins from incomplete pairs) were available. This group included 422 monozygotic male (MZM), 348 dizygotic male (DZM), 800 monozygotic female (MZF), 499 dizygotic female (DZF), and 918 dizygotic opposite sex (DOS) twins. In the adult group, a total of 4,911 twins (1,911 complete pairs and 1,089 twins from incomplete pairs) were available including 727 MZM, 486 DZM, 1,641 MZF, 903 DZF, and 1,154 DOS twins. Zygosity was based on DNA typing for 27% of the adolescents and 54% of the adult twin pairs. For the remaining pairs, survey questions about similarity between the twins were used. Agreement between zygosity based on survey data and DNA data was 96.1%.
Measures
Smoking expectancy was assessed at baseline (time point 1 [T1]) by asking “Do you think you’ll smoke in a year time?”, with the answers being measured on a 5-point scale ranging from “Certainly not” to “Absolutely yes.” Smoking status (smoker, former smoker, or never-smoker) was established at baseline and at follow up (time point 2 [T2]) by asking “Have you ever smoked?” (answer categories “No,” “A few times just to try,” and “Yes”) and “How often do you smoke now?” (answer categories “I don’t smoke regularly,” “I’ve quit smoking,” “Once a week or less,” “A few times a week,” and “Once a day or more”). In adolescents, only participants who stated that they had smoked more than 50 cigarettes when asked “How many cigarettes have you smoked till now?” could be classified as former smokers. For participants who answered “Yes” when asked “Have you ever smoked?”, but gave no further information on current smoking status or frequency, smoking status was coded as unknown. When participants stated that they (regularly) smoked before but subsequently answered “I have never been a regular smoker” when asked “How often do you smoke now?”, smoking status was also coded as unknown. Participants classified as smokers or former smokers at T1 and as never-smokers at T2 were excluded from analysis (see Supplementary Figure S1). All additional covariates are depicted in Table 1.
Table 1.
Sample Characteristics at Baseline (Time Point 1 [T1]) for Adolescents and Adult Smokers, Former Smokers, and Never-Smokers
| Adolescent group (n = 3,114) | Smokers (n = 196) | Former smokers (n = 50) | Never-smokers (n = 2,868) | Adult group (n = 10,468) | Smokers (n = 2,512) | Former smokers (n = 2,324) | Never-smokers (n = 5,632) | ||
|---|---|---|---|---|---|---|---|---|---|
| Smoking expectancy, n (%) | Certainly not | 7 (3.6) | 9 (18.0) | 2,268 (79.1) | Smoking expectancy, n (%) | Certainly not | 73 (2.9) | 1,814 (78.1) | 5,188 (92.1) |
| Probably not | 32 (16.3) | 15 (30.0) | 419 (14.6) | Probably not | 269 (10.7) | 405 (17.4) | 328 (5.8) | ||
| I don’t know | 63 (32.1) | 15 (30.0) | 163 (5.7) | I don’t know | 681 (27.1) | 96 (4.1) | 94 (1.7) | ||
| Probably | 60 (30.6) | 9 (18.0) | 11 (0.4) | Probably | 1,135 (45.2) | 7 (0.3) | 19 (0.3) | ||
| Absolutely yes | 34 (17.3) | 2 (4.0) | 7 (0.2) | Absolutely yes | 354 (14.1) | 2 (0.1) | 3 (0.1) | ||
| Age, M (SD) | 16.3 (0.9) | 16.0 (1.0) | 15.7 (1.1) | Age, M (SD) | 36.7 (13.7) | 46.2 (12.5) | 33.3 (13.6) | ||
| Sex, n (%) | Male | 68 (34.7) | 23 (46.0) | 1,164 (40.6) | Sex, n (%) | Male | 1,153 (45.9) | 1,042 (44.8) | 2,088 (37.1) |
| Female | 128 (65.3) | 27 (54.0) | 1,704 (59.4) | Female | 1,359 (54.1) | 1,282 (55.2) | 3,541 (62.9) | ||
| Educational achievement, n (%) | Low | 85 (51.5) | 24 (70.6) | 800 (31.2) | Educational achievement, n (%) | Low | 812 (34.0) | 836 (36.9) | 977 (18.5) |
| Intermediate | 47 (28.5) | 5 (14.7) | 728 (28.4) | Intermediate | 854 (35.7) | 676 (29.8) | 1,824 (34.5) | ||
| High | 33 (20.0) | 5 (14.7) | 1,037 (40.4) | High | 724 (30.3) | 753 (33.2) | 2,490 (47.1) | ||
| Health, n (%) | Poor | 1 (0.5) | 0 (0) | 2 (0.1) | Health, n (%) | Poor | 10 (0.4) | 12 (0.5) | 16 (0.3) |
| Fair | 2 (1.0) | 2 (4.2) | 27 (0.9) | Fair | 60 (2.4) | 63 (2.8) | 88 (1.6) | ||
| Reasonable | 34 (17.5) | 4 (8.3) | 187 (6.6) | Reasonable | 342 (13.8) | 318 (14.0) | 457 (8.2) | ||
| Good | 112 (57.7) | 30 (62.5) | 1,505 (52.9) | Good | 1,583 (63.8) | 1,374 (60.3) | 3,331 (59.7) | ||
| Excellent | 45 (23.2) | 12 (25.0) | 1,122 (39.5) | Excellent | 485 (19.6) | 511 (22.4) | 1,691 (30.3) | ||
| Number of cigarettes per day, n (%) | <1 | 25 (13.8) | 5 (12.2) | Number of cigarettes per day, n (%) | <1 | 158 (6.3) | 15 (0.7) | – | |
| 1–5 | 57 (31.5) | 21 (51.2) | 1–5 | 424 (16.9) | 476 (21.1) | – | |||
| 6–10 | 59 (32.6) | 7 (17.1) | 6–10 | 618 (24.7) | 556 (24.6) | – | |||
| 11–20 | 36 (19.9) | 8 (19.5) | 11–20 | 982 (39.2) | 801 (35.5) | – | |||
| 21–30 | 4 (2.2) | 0 (0) | 21–30 | 282 (11.3) | 320 (14.2) | – | |||
| >30 | 0 (0) | 0 (0) | – | >30 | 39 (1.6) | 90 (4.0) | – | ||
| Age at first cigarette, n (%) | <12 years | 13 (7.1) | 6 (12.5) | – | Age at first cigarette, n (%) | <12 years | 87 (5.4) | 65 (5.5) | – |
| 12–13 years | 76 (41.8) | 29 (60.4) | – | 12–13 years | 330 (20.5) | 271 (22.8) | – | ||
| 14–15 years | 79 (43.4) | 12 (25.0) | – | 14–15 years | 569 (35.3) | 384 (32.2) | – | ||
| 16–17 years | 13 (7.1) | 1 (2.1) | – | 16–17 years | 387 (24.0) | 280 (23.5) | – | ||
| >17 years | 1 (0.5) | 0 (0) | – | >17 years | 240 (14.9) | 190 (16.0) | – | ||
| Smoking frequency, n (%) | Once a week or less | 22 (12.1) | – | – | FTND score, M (SD) | 2.7 (2.3) | 2.5 (2.4) | – | |
| Few times a week | 30 (16.5) | – | – | Number of years smoked, M (SD) | 17.8 (12.7) | 15.0 (10.2) | – | ||
| Once a day or more | 130 (71.4) | – | – | Number of times tried quitting, M (SD) | 1.8 (1.9) | 2.1 (2.5) | – |
Note. FTND = Fagerström Test for Nicotine Dependence.
Age was a continuous variable measured in years; sex was measured as 1 (male) and 2 (female); educational achievement was a continuous variable with the following categories: primary school/lower vocational schooling (low), intermediate vocational/upper secondary school (intermediate), and upper vocational/university (high); health/age at first cigarette/number of times tried quitting/number of years smoked/smoking frequency, and number of cigarettes per day were continuous variables; the FTND was a continuous variable measuring severity of nicotine addiction on a scale from 0 to 10 (number of cigarettes per day and FTND in former smokers concerned the period they smoked the heaviest).
A new variable was created reflecting whether someone was able to predict his or her future smoking status. The 5-point scale for smoking expectancy was dichotomized into “No” (answer categories “Certainly not” or “Probably not”) and “Yes” (answer categories “I don’t know,” “Probably,” and “Absolutely yes”), with the latter reflecting the inability to exclude the possibility of smoking, as was previously done by Forrester et al. (2007). This dichotomized variable at baseline was compared with smoking status at follow up, and a dichotomous variable was defined reflecting a correct (0) or an incorrect (1) prediction of future smoking status (see Supplementary Table S1).
Statistical Analysis
Data management and Pearson’s chi-square tests, to test differences in sample characteristics across smoking statuses, were performed using SPSS (version 17.0). To account for family relatedness, chi-square tests were repeated in a subsample of unrelated individuals. Regression analyses were carried out in Stata Statistical Software (version 9.0) and corrected for family clustering by employing the robust cluster option, which uses information on family relatedness to correct for the correlation within the families (i.e., clusters). Logistic regression analysis determined the predictive value of smoking expectancy at T1 (independent variable) on smoking status at T2 (dependent variable). We used a two-step approach to quantify the predictive effect of smoking expectancy over and above commonly used predictors. Smoking expectancy was first regressed on the predictors of smoking behavior at T1 by means of linear regression analysis (see Table 1 for these predictors), after which the resulting residuals were used as a predictor of smoking status at T2 in a second step (residual model). This approach completely eliminates the effects of the predictors of smoking behavior at T1, providing a conservative estimate of the impact of smoking expectancy. Analyses were carried out for smokers, former smokers, and never-smokers, both in adolescents and adults.
The classical twin model was used to estimate the heritability of the ability to predict future smoking status by comparing the correlations of monozygotic (MZ) and dizygotic (DZ) twin pairs. MZ twins share (nearly) 100% of their DNA, while DZ twins share on average 50% of their segregating genes. If the ability to predict future smoking status is influenced by additive genetic factors (A), the correlation between MZ twins is expected to be twice as large as the correlation between DZ twins. When the correlation of DZ twins is larger than half the correlation of MZ twins, the environment that is shared by both twins is also of influence. When the correlation of DZ twins is smaller than half that of MZ twins, genetic nonadditive effects (D) are likely. Structural equation modeling was performed in OpenMx (Boker et al., 2011). Ability to predict future smoking status was analyzed in a threshold model with the underlying liability being a function of genetic and environmental factors (Falconer & Mackay, 1996). A single threshold divides individuals into those who correctly predicted their future smoking status and those who did not. Since smoking initiation at the time of measuring smoking expectancy might affect the chance of making a correct prediction, it was added to the model as a covariate (0: never smoked and 1: ever smoked). The threshold was modeled as follows: T = X + βcovariate, where T is the estimated value of the threshold, X is the value of the threshold when the covariate is 0 (never smoked), and β represents the deviation on the threshold in subjects who initiated smoking.
First, five twin correlations (MZM, DZM, MZF, DZF, and DOS) were estimated in a saturated model. The threshold and the effect (β) of ever smoking were estimated separately for males and females (nine free parameters). Constraints were then imposed on the model in a stepwise manner (models 2, 3, and 4). Next, the influences of additive genetic factors (A), nonadditive or dominance deviations (D), and unique environmental factors (E) were estimated in a univariate ADE model (models 5, 6, and 7). With likelihood ratio tests, the fit of the different nested models was tested by subtracting the negative log-likelihood (−2LL) of a nested model from the −2LL of the more extensive model. The difference in −2LL follows a χ2 distribution with df equal to the difference in df of the two models. In order to achieve the most parsimonious and best-fitting model, constraints were retained whenever they did not significantly deteriorate the fit (p >.05).
RESULTS
Subject Characteristics
Table 1 summarizes the distribution of smoking expectancy across smoking status at baseline in adolescents (p < .001) and adults (p < .001). Most adolescent smokers expected to continue smoking or did not know, while former smokers scored lower on smoking expectancy (58% answered “Certainly not” or “Probably not”). Although the large majority of never-smokers expected that they will “Certainly not” smoke a year from now, more than 6% of never-smoking adolescents say “I don’t know,” “Probably,” or “Absolutely yes.” In adults, smoking expectancy was similarly distributed with a more pronounced difference between former smokers and smokers. The majority of former smoking adults (78.1%) stated that they will certainly not smoke a year from now. Though percentages are lower compared with adolescents, there are some never-smoking adults (2.1%) who expected to start smoking or state they do not know.
In adolescents and adults, never-smokers more often attained a higher education than (former) smokers (p < .001). Subjective health differed significantly across smoking status in adults and adolescents, with never-smokers reporting an “excellent” health more often than former and current smokers (p < .001). In adults, the ratio of males versus females differed across smoking status (p < .001), while in adolescents, there was no significant difference (p = .169).
Transitions in Smoking Behavior Over Time
Adolescents and adults who were smokers at baseline mostly remained smokers at follow up (Figure 1). As might be expected, there was a difference between adult and adolescent never-smokers, with adolescent never-smokers starting smoking at T2 more often than adult never-smokers (8% vs. 3%, respectively). In both adolescents and adults, a small proportion of never-smokers became former smokers at T2, implying they started smoking and stopped in the approximately two years in between baseline and follow up. In former smoking adolescents at T1, a high percentage started smoking again at T2 (70%), while for adults, the percentage of people who relapsed was considerably lower (14%).
Figure 1.
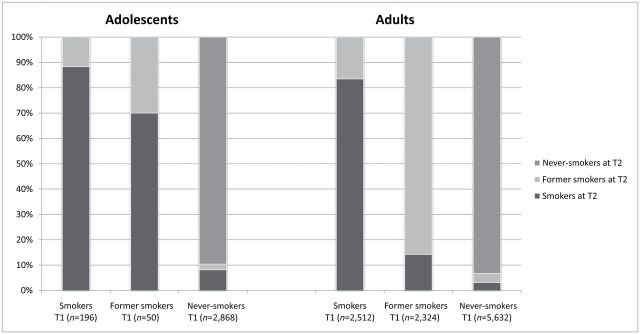
Transitions in smoking status from baseline (time point 1 [T1]) to follow up (time point 2[T2]) depicted for adolescents and adults separately. There is approximately two years in between T1 and T2.
Predictive Value of Smoking Expectancy
Smoking expectancy was a strong predictor of future smoking status in univariate analyses of all groups (see Table 2). Odds ratios (ORs) represent the odds of smoking at T2 (as opposed to not smoking) for an individual who responds one answer category higher than another individual on the scale of smoking expectancy (at T1). Overall, associations were strongest for former smokers (OR 3.02 [confidence interval, CI = 1.37–6.68] in adolescents and 3.01 [CI = 2.51–3.62] in adults) and never-smokers (OR: 3.39 [CI = 2.88–3.98] in adolescents and 4.93 [CI = 4.06–6.01] in adults). When correcting for the impact of age, sex, education, health, and smoking behavior at T1, smoking expectancy remained a significant predictor of future smoking status in never-smokers and former smokers, but not in smokers (OR: 1.46 [CI = 0.74–2.85] in adolescents and 1.04 [CI = 0.82–1.32] in adults). The group of adolescent former smokers was too small to analyze with a residual model. Other than that, results for adolescents and adults were similar.
Table 2.
Results of Logistic Regression Analysis With Smoking Expectancy at Baseline (Time Point 1 [T1]) as the Independent Variable and Smoking Status Approximately Two Years Later (Time Point 2 [T2]) as the Dependent Variable for Adolescents and Adult Smokers, Former Smokers, and Never-Smokers
| Adolescents (n = 3,114) | Smokers (n = 196) | Former smokers (n = 50) | Never-smokers (n = 2,868) | ||||||
| N | OR (95% CIs) | Pseudo R 2 | N | OR (95% CIs) | Pseudo R 2 | N | OR (95% CIs) | Pseudo R 2 | |
| Univariate model | 196 | 2.12 (1.18–3.79) | .09 | 50 | 3.02 (1.37–6.68) | .18 | 2,868 | 3.39 (2.88–3.98) | .12 |
| Residual model | 141 | 1.46 (0.74–2.85) | .02 | – | Not applicable | – | 2,383 | 1.83 (1.56–2.15) | .06 |
| Adults (n = 10,468) | Smokers (n = 2,512) | Former smokers (n = 2,324) | Never-smokers (n = 5,632) | ||||||
| N | OR (95% CIs) | Pseudo R 2 | N | OR (95% CIs) | Pseudo R 2 | N | OR (95% CIs) | Pseudo R 2 | |
| Univariate model | 2,512 | 1.58 (1.41–1.76) | .03 | 2,324 | 3.01 (2.51–3.62) | .08 | 5,632 | 4.93 (4.06–6.01) | .12 |
| Residual model | 591 | 1.04 (0.82–1.32) | .00 | 459 | 2.03 (1.29–3.19) | .03 | 4,844 | 3.44 (2.56–4.61) | .05 |
Note. OR = odds ratio; CI = confidence interval.
OR represents the odds of smoking at T2 (as opposed to not smoking) for an individual who responds one answer category higher than another individual on the scale of smoking expectancy at T1 (categories: “certainly not,” “probably not,” “I don’t know,” “probably,” and “absolutely yes”); pseudo R 2: explained variance, not applicable; too little cases left for analysis; univariate model: smoking expectancy as the independent variable; residual model: smoking expectancy corrected for all predictors of smoking behavior at T1 as the independent variable. Significant ORs are depicted in bold.
Genetic Modeling
Prevalence
Prevalences for the ability to predict future smoking status were significantly different for ever-smokers than never-smokers in both adolescents and adults (see Table 3; model 2). The proportion of adolescents accurately estimating their future smoking status did not differ significantly between boys and girls (model 3). In adolescents, 89% of never-smokers accurately predicted their future smoking status in comparison with 78% of ever-smokers. In adults, 91% of never-smoking men and 94% of never-smoking women predict future smoking status accurately, in comparison with 79% of ever-smoking men and 77% of ever-smoking women.
Table 3.
Structural Equation Models to Explore Genetic and Environmental Influences on the Ability to Accurately Predict Future Smoking Status
| Adolescents (n = 2,987) | Estimated parameters | −2LL | df | Compared with | χ2 | p value |
|---|---|---|---|---|---|---|
| 1. Saturated five-group model | 9 | 2088.83 | 2978 | – | – | – |
| 2. βs covariate set on 0 | 7 | 2110.59 | 2980 | 1 | 21.77 | <.001 |
| 3. Thresholds and βs male = female | 7 | 2090.99 | 2980 | 1 | 2.17 | .34 |
| 4. Correlation MZM = MZF + correlation DZM = DZF = DOS | 4 | 2098.35 | 2983 | 3 | 7.36 | .06 |
| 5. ADE model | 5 | 2098.35 | 2983 | 1 | 29.87 | .23 |
| 6. AE model | 4 | 2098.42 | 2984 | 5 | 0.08 | .78 |
| 7. E model | 3 | 2131.1 | 2985 | 6 | 32.68 | <.001 |
| Adults (n = 4,911) | Estimated parameters | −2LL | df | Compared with | χ2 | p value |
| 1. Saturated five-group model | 9 | 3370.06 | 4902 | – | – | – |
| 2. βs covariate set on 0 | 7 | 3621.51 | 4904 | 1 | 251.45 | <.001 |
| 3. Thresholds and βs male = female | 7 | 3383.23 | 4904 | 1 | 13.18 | <.001 |
| 4. Correlation MZM = MZF + correlation DZM = DZF = DOS | 6 | 3377.43 | 4905 | 1 | 7.38 | .06 |
| 5. ADE model | 7 | 3377.43 | 4905 | 1 | 33.61 | .07 |
| 6. AE model | 6 | 3377.49 | 4906 | 5 | 0.05 | .82 |
| 7. E model | 5 | 3387.6 | 4907 | 6 | 10.11 | <.001 |
Note. DOS = dizygotic opposite sex; DZF = dizygotic female; DZM = dizygotic male; MZF = monozygotic female; MZM = monozygotic male; LL = log-likelihood.
Best-fitting models are depicted in bold; threshold: the value that forms two distinct categories in the underlying liability, which stand for the proportions of individuals who accurately predicted their future smoking status and the individuals who did not; β: the effect of the covariate smoking initiation (0 = “never smoked” and 1 = “ever smoked”) on the threshold. ADE model: additive genetic (A), dominance (D) and unique environmental effects (E) are estimated; AE model: only A and E are estimated; E model: only E is estimated.
Twin Correlations
There were no differences in twin resemblance between men and women (model 4), but twin correlations were higher for MZ (0.58 [CI = 0.40–0.73] for adolescents and 0.27 [CI = 0.09–0.42] for adults) than for DZ pairs (0.17 [CI = −0.03 to 0.35] for adolescents and 0.09 [CI = −0.07 to 0.24] for adults).
Heritability
The pattern of MZ and DZ correlations suggests nonadditive genetic influences, but formal testing indicated that a model including only additive genetic effects was sufficient to explain familial resemblance (model 6). The heritability in adolescents was estimated at 0.59 (CI = 0.41–0.74) with the remaining variance explained by unique environmental influences (0.41; CI = 0.26–0.59). For adults, the heritability was lower with a point estimate of 0.27 (CI = 0.11–0.42) with the largest part of the variance (0.73; CI = 0.58–0.89) explained by unique environmental influences.
DISCUSSION
Smoking expectancy significantly predicted future smoking status over and above commonly used predictors of smoking in former and never-smokers, but not in current smokers. The ability to accurately predict future smoking status was influenced by genetic factors, more so in adolescents than in adults.
In never-smokers, a higher score on smoking expectancy was associated with a higher chance of initiating smoking, both in adolescents and adults. It is the first time that this association has been demonstrated in adults. Similar results have only been reported in never-smoking adolescents, where smoking susceptibility (not being able to rule out the possibility of smoking next year) was a predictor of future smoking status (Forrester et al., 2007). Measuring smoking expectancy is particularly valuable in efforts to prevent smoking initiation in adolescents, as they are most vulnerable to starting smoking (CBS, 2010b; Stivoro, 2009). Not many adult never-smokers started smoking, but we still observed that smoking expectancy was an accurate predictor of future smoking behavior.
In both adolescents and adult smokers, a higher smoking expectancy was associated with a higher chance of remaining a smoker two years later. When taking demographic variables and variables related to smoking into account, associations were still significant in never-smokers and former smokers but not in smokers. This could be due to a relatively small sample size, caused by the fact that some covariate data were only available in a subsample. Previous studies in smokers showed that the intention to quit smoking predicts making a quit attempt (Abdullah et al., 2006; Rise et al., 2008; Smit et al., 2011). A “Motivation To Stop Scale” consisting of one item with seven response categories was able to accurately predict future quitting attempts (Kotz, Brown, & West, 2013). Our measure of smoking expectancy may reflect not only a persons’ willingness to quit smoking but also their estimation of whether or not they will succeed. The importance of self-efficacy in successfully quitting smoking has been shown in several papers (Martinez et al., 2010; Rise et al., 2008; Schnoll et al., 2011).
In former smokers, a higher smoking expectancy resulted in a significantly higher chance of relapse two years later. This result corresponds to findings from the International Tobacco Control survey, which showed that a lower abstinence self-efficacy (measured by asking “How sure are you that you can stay quit?”) was associated with a higher risk of relapse (Herd et al., 2009). Smoking expectancy can be an additional, relatively easy tool to predict which former smokers will start smoking again. Knowledge on who is most vulnerable to relapse is crucial in developing intervention programs because many people attempt to quit smoking, but a lot of them will fail in remaining abstinent (Hughes, Keely, & Naud, 2004; Piasecki, 2006).
When analyzing data from smokers, former smokers, and never-smokers simultaneously and correcting for current smoking status (data not shown), smoking expectancy remained a significant positive predictor for future smoking status in both adolescents (OR: 2.83, p < .001, n = 3,114) and adults (OR: 1.63, p < .001, n = 10,468). This emphasizes the unique predictive effect of smoking expectancy on future smoking status over and above the effect of current smoking status.
Being able to predict future smoking status is explained by genetic factors for 59% and 27% of the variance in adolescents and adults, respectively. Environmental factors explained the remaining portion of the variance. The heritability may be mediated by genetically influenced personality traits such as optimism or sensation seeking. Research in twins has shown that 36% of the variation in optimism can be explained by genetic effects (Mosing, Zietsch, Shekar, Wright, & Martin, 2009) and that heritability estimates for sensation seeking range from 48% to 63% (Koopmans, Boomsma, Heath, & van Doornen, 1995). Optimism may lead people to make a better prediction of their own ability to quit or refrain from smoking, while a high score on sensation seeking may make them more willing to seek out new experiences and change their behavior. In older participants, previous (failed) quitting attempts may have given them more experience, explaining the larger influence of environmental factors. Failure to predict future smoking status in (former) smokers is probably also related to smoking dependence and the inability to quit, with the latter being explained by genetic factors for approximately 50% (Broms et al., 2006; Uhl et al., 2012). Low numbers for the responses “Probably” and “Absolutely yes” prevented us from to re-analyzing the twin data while assigning the response “I don’t know” to the “No” category of the dichotomized version of smoking expectancy.
Heritability is a measure that estimates the contribution of genetic differences to observed differences within a group of individuals (Plomin, DeFries, Knopik, & Neiderhiser, 2013). As well as other behavioral traits, smoking behavior is a complex trait that is influenced by numerous genes and environmental factors. Estimating the heritability of such traits can give new insights into the mechanisms behind the trait (Visscher, Hill, & Wray, 2008). This study shows that genetic factors play a considerable role in the ability to predict future smoking status, especially in younger people. Knowledge that individual differences in smoking trajectories have a heritable component justifies ongoing efforts into the tailoring of prevention strategies.
Heritability estimates were larger in adolescents than in adults (p = .03), while the heritability of substance use typically increases over age (Kendler, Schmitt, Aggen, & Prescott, 2008). Differences in heritability between age cohorts indicate gene by age (gene × age) interaction (Boomsma & Martin, 2002). The influence of environmental factors on the ability to predict future smoking status is larger in adults than in adolescents, lowering the relative contribution of A (heritability). Besides unique environmental factors, E comprises measurement error or “noise.” Changes in social norms can affect the magnitude of genetic influences by maximizing noise (Boardman, Blalock, & Pampel, 2010; Boardman et al., 2011; Shanahan & Hofer, 2005). In adults, social norms on smoking might be more negative, thereby influencing them not to smoke. In adolescents on the other hand, peers stimulate the initiation of smoking (Vitoria, Salgueiro, Silva, & de Vries, 2011). In both age cohorts, there was some suggestion that nonadditive genetic influences might play a role, but although statistical power was sufficient (Posthuma and Boomsma, 2000), these influences were not significant.
A limitation of this study is its reliance on self-reported smoking status. A recent review demonstrated that in 5%–9% of the cases, self-report did not detect someone as a smoker while biochemical validation did (Connor Gorber, Schofield-Hurwitz, Hardt, Levasseur, & Tremblay, 2009). To study the reliability of self-reported smoking status, we used a powerful alternative of a test–retest approach, by studying the similarity within MZ (genetically identical) twin pairs. For several traits, it has been shown that the difference between MZ twins was almost equal to that between two consecutive measurements of the same individual (Den Braber et al., 2013; Vogel & Motulsky, 1996), making the similarity within MZ twin pairs a suitable test of reliability. About 94% of adolescents and 78% of adult twin pairs were concordant for smoking status, implying self-reported smoking status is reliable. The reliability of self-report in adolescents was also shown in a study of 150 Finnish youngsters (M age 15 years), where the sensitivity for detecting smokers was 81%–96% (comparing questionnaire data to biochemical measurements; Kentala, Utriainen, Pahkala, & Mattila, 2004).
Another limitation is that covariate data were not available for the total sample, so the residual model was analyzed in a smaller subsample. Previous studies demonstrated that individuals with missing data (less cooperative subjects) tended to score slightly more unfavorable on lifestyle variables but differences were not significant (Distel et al., 2007; Vink et al., 2004).
This is the first study examining longitudinal data on smoking expectancy in adolescents and adults across smoking status. A recently much debated topic is “precision medicine,” involving tailoring of care/treatment to suit the different genetic backgrounds of patients (Hamburg & Collins, 2010). Smoking expectancy could provide another way of delivering a personalized approach, by effectively tailoring guidance, counseling, and possibly treatment. A big advantage of quizzing people on smoking expectancy is that it is based on a simple question, which can be employed in smokers, former smokers, and never-smokers.
SUPPLEMENTARY MATERIAL
Supplementary Figure S1 and Table S1 can be found online at http://www.ntr.oxfordjournals.org
FUNDING
This study was funded by European Research Council (ERC) 284167, ERC 230374, Zon-Mw 31160008, Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO) 985-10-002, NWO 904-61-193, NWO/Spinoza premie 56-464-14192, NWO 480-04-004, NWO 463-06-001, NWO-VENI 451-04-034, and the National Institute of Diabetes and Digestive and Kidney Diseases (RO1DK092127). GHL was supported by the US National Institutes of Health (DA 018673).
DECLARATION OF> INTERESTS
None declared.
Supplementary Material
REFERENCES
- Abdullah A. S., Ho L. M., Kwan Y. H., Cheung W. L., McGhee S. M., Chan W. H. (2006). Promoting smoking cessation among the elderly: What are the predictors of intention to quit and successful quitting? Journal of Aging and Health, 18, 552–564. 10.1177/0898264305281104 [DOI] [PubMed] [Google Scholar]
- Augustson E. M., Wanke K. L., Rogers S., Bergen A. W., Chatterjee N., Synder K. … Caporaso N. E. (2008). Predictors of sustained smoking cessation: A prospective analysis of chronic smokers from the alpha-tocopherol Beta-carotene cancer prevention study. American Journal of Public Health, 98, 549–555. 10.2105/AJPH.2005.084137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman J. D., Blalock C. L., Pampel F. C. (2010). Trends in the genetic influences on smoking. Journal of Health and Social Behavior, 51, 108–123. 10.1177/0022146509361195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman J. D., Blalock C. L., Pampel F. C., Hatemi P. K., Heath A. C., Eaves L. J. (2011). Population composition, public policy, and the genetics of smoking. Demography, 48, 1517–1533. 10.1007/s13524-011-0057-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boker S., Neale M., Maes H., Wilde M., Spiegel M., Brick T. … Fox J. (2011). OpenMx: An open source extended structural equation modeling framework. Psychometrika, 76, 306–317. 10.1007/S11336-010-9200-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boomsma D. I., Martin N. G. (2002). Gene-environment interactions. In D’haenen H., den Boer J. A., Willner P. (Eds.), Biological psychiatry (pp. 181–187). Chichester, UK: John Wiley & Sons, Ltd. doi:10.1002/0470854871.chxiii [Google Scholar]
- Broms U., Silventoinen K., Madden P. A. F., Heath A. C., Kaprio J. (2006). Genetic architecture of smoking behavior: A study of Finnish adult twins. Twin Research and Human Genetics, 9, 64–72 doi:http://dx.doi.org/10.1375/183242706776403046 [DOI] [PubMed] [Google Scholar]
- CBS (2010a). Gezondheid en welzijn (Health and wellbeing). The Hague, The Netherlands. Centraal Bureau voor Statistiek. Retrieved June 29, 2012, from http://www.cbs.nl/nl-NL/menu/themas/gezondheid-welzijn/publicaties/artikelen/archief/2007/2007-2204-wm.htm [Google Scholar]
- CBS (2010b). CBS Gezondheidsenquête 2010. The Hague, The Netherlands: Centraal Bureau voor Statistiek. Retrieved June 29, 2012, from http://statline.cbs.nl/StatWeb/publication/?VW=T&DM=SLNL&PA=81175NED&LA=NL [Google Scholar]
- CDC (2004). Surgeon General’s Report: The health consequences of smoking. Atlanta, GA: Department of Health and Human Services, Centers for Disease Control and Prevention. [Google Scholar]
- Connor Gorber S., Schofield-Hurwitz S., Hardt J., Levasseur G., Tremblay M. (2009). The accuracy of self-reported smoking: A systematic review of the relationship between self-reported and cotinine-assessed smoking status. Nicotine & Tobacco Research, 11, 12–24. 10.1093/ntr/ntn010 [DOI] [PubMed] [Google Scholar]
- Den Braber A., Bohlken M. M., Brouwer R. M., van ‘t Ent D., Kanai R., Kahn R. S. … Boomsma D. I. (2013). Heritability of subcortical brain measures: A perspective for future genome-wide association studies. NeuroImage, 83, 98–102. 10.1016/j.neuroimage.2013.06.027 [DOI] [PubMed] [Google Scholar]
- Distel M. A., Ligthart L., Willemsen G., Nijholt D. R., Trull T. J., Boomsma D. I. (2007). Personality, health and lifestyle in a questionnaire family study: A comparison between highly cooperative and less cooperative families. Twin Research and Human Genetics, 10, 348–353. http://dx.doi.org/10.1375/twin.10.2.348 [DOI] [PubMed] [Google Scholar]
- Falconer D. S., Mackay T. F. C. (1996). Introduction to quantitative genetics. Harlow, England: Pearson Prentice Hall [Google Scholar]
- Forrester K., Biglan A., Severson H. H., Smolkowski K. (2007). Predictors of smoking onset over two years. Nicotine & Tobacco Research, 9, 1259–1267. 10.1080/14622200701705357 [DOI] [PubMed] [Google Scholar]
- Freedman K. S., Nelson N. M., Feldman L. L. (2012). Smoking initiation among young adults in the United States and Canada, 1998–2010: A systematic review. Preventing Chronic Disease, 9, –E05. http://dx.doi.org/10.5888/pcd9.110037 [PMC free article] [PubMed] [Google Scholar]
- Hamburg M. A., Collins F. S. (2010). The path to personalized medicine. The New England Journal of Medicine, 363, 301–304. 10.1056/NEJMp1006304 [DOI] [PubMed] [Google Scholar]
- Herd N., Borland R., Hyland A. (2009). Predictors of smoking relapse by duration of abstinence: Findings from the International Tobacco Control (ITC) Four Country Survey. Addiction, 104, 2088–2099. 10.1111/j.1360-0443.2009.02732.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J. R., Keely J., Naud S. (2004). Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction, 99, 29–38. 10.1111/j.1360-0443.2004.00540.x [DOI] [PubMed] [Google Scholar]
- Kendler K. S., Schmitt E., Aggen S. H., Prescott C. A. (2008). Genetic and environmental influences on alcohol, caffeine, cannabis, and nicotine use from early adolescence to middle adulthood. Archives of General Psychiatry, 65, 674–682. 10.1001/archpsyc.65.6.674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentala J., Utriainen P., Pahkala K., Mattila K. (2004). Verification of adolescent self-reported smoking. Addictive Behaviors, 29, 405–411. http://dx.doi.org/10.1016/j.addbeh.2003.08.012 [DOI] [PubMed] [Google Scholar]
- Koopmans J. R., Boomsma D. I., Heath A. C., van Doornen L. J. (1995). A multivariate genetic analysis of sensation seeking. Behavior Genetics, 25, 349–356. 10.1007/BF02197284 [DOI] [PubMed] [Google Scholar]
- Kotz D., Brown J., West R. (2013). Predictive validity of the Motivation To Stop Scale (MTSS): A single-item measure of motivation to stop smoking. Drug and Alcohol Dependence, 128, 15–19. http://dx.doi.org/10.1016/j.drugalcdep.2012.07.012 [DOI] [PubMed] [Google Scholar]
- Li M. D., Cheng R., Ma J. Z., Swan G. E. (2003). A meta-analysis of estimated genetic and environmental effects on smoking behavior in male and female adult twins. Addiction, 98, 23–31. 10.1046/j.1360-0443.2003.00295.x [DOI] [PubMed] [Google Scholar]
- Martinez E., Tatum K. L., Glass M., Bernath A., Reynolds P., Schnoll R. A. (2010). Correlates of smoking cessation self-efficacy in a community sample of smokers. Addictive Behaviors, 35, 175–178. http://dx.doi.org/10.1016/j.addbeh.2009.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool J., Cameron L., Petrie K., Robinson E. (2003). Smoking behavior and expectations among Auckland adolescents. The New Zealand Medical Journal, 116, 1176 Retrieved from http://journal.nzma.org.nz/journal/ Last accessed: March 26, 2013 [PubMed] [Google Scholar]
- Mendel J. R., Berg C. J., Windle R. C., Windle M. (2012). Predicting young adulthood smoking among adolescent smokers and nonsmokers. American Journal of Health Behavior, 36, 542–554. http://dx.doi.org/10.5993/AJHB.36.4.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosing M. A., Zietsch B. P., Shekar S. N., Wright M. J., Martin N. G. (2009). Genetic and environmental influences on optimism and its relationship to mental and self-rated health: A study of aging twins. Behavior Genetics, 39, 597–604. 10.1007/s10519-009-9287-7 [DOI] [PubMed] [Google Scholar]
- Nationaal Kompas Volksgezondheid (2011). Gezondheids determinanten – Leefstijl – Roken (Health determinants – Lifestyle – smoking). Bilthoven, The Netherlands: RIVM. Retrieved March 26, 2013, from http://www.nationaalkompas.nl/gezondheidsdeterminanten/leefstijl/roken/hoeveel-mensen-roken/
- Nuno V. L., Zhang Q., Harris R. B., Wilkinson-Lee A. M., Wilhelm M. S. (2011). Smoking susceptibility among students followed from grade six to eight. Addictive Behaviors, 36, 1261–1266. http://dx.doi.org/10.1016/j.addbeh.2011.07.041 [DOI] [PubMed] [Google Scholar]
- Piasecki T. M. (2006). Relapse to smoking. Clinical Psychology Review, 26, 196–215. http://dx.doi.org/10.1016/j.cpr.2005.11.007 [DOI] [PubMed] [Google Scholar]
- Plomin R., DeFries J. C., Knopik V. S., Neiderhiser J. M. (2013). Behavioral genetics. New York, NY: Worth Publishers [Google Scholar]
- Posthuma D., Boomsma D. I. (2000). A note on the statistical power in extended twin designs. Behavior Genetics, 30, 147–158. 10.1023/A:1001959306025 [DOI] [PubMed] [Google Scholar]
- Reid J. L., Hammond D., Boudreau C., Fong G. T., Siahpush M.; ITC Collaboration (2010). Socioeconomic disparities in quit intentions, quit attempts, and smoking abstinence among smokers in four western countries: Findings from the International Tobacco Control Four Country Survey. Nicotine & Tobacco Research, 12, S20–S33. 10.1093/ntr/ntq051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rise J., Kovac V., Kraft P., Moan I. S. (2008). Predicting the intention to quit smoking and quitting behaviour: Extending the theory of planned behaviour. British Journal of Health Psychology, 13, 291–310. 10.1348/135910707X187245 [DOI] [PubMed] [Google Scholar]
- Schnoll R. A., Martinez E., Tatum K. L., Glass M., Bernath A., Ferris D., Reynolds P. (2011). Increased self-efficacy to quit and perceived control over withdrawal symptoms predict smoking cessation following nicotine dependence treatment. Addictive Behaviors, 36, 144–147. http://dx.doi.org/10.1016/j.addbeh.2010.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum M. (2005). The accuracy of teens’ expectations of future smoking. American Journal of Preventive Medicine, 28, 274–280. http://dx.doi.org/10.1016/j.amepre.2004.12.001 [DOI] [PubMed] [Google Scholar]
- Shanahan M. J., Hofer S. M. (2005). Social context in gene-environment interactions: Retrospect and prospect. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 60, 65–76. 10.1093/geronb/60.Special_Issue_1 [DOI] [PubMed] [Google Scholar]
- Smit E. S., Fidler J. A., West R. (2011). The role of desire, duty and intention in predicting attempts to quit smoking. Addiction, 106, 844–851. 10.1111/j.1360-0443.2010.03317.x [DOI] [PubMed] [Google Scholar]
- Stivoro (2009). Rookvrije schoolpleinen (Smoke free school yards). Retrieved June 29, 2012, from http://www.stivoro.nl/Upload/Notitie%20Rookvrije%20Schoolpleinen%20sept%2009%20DEF%20(3).pdf
- Sullivan P. F., Kendler K. S. (1999). The genetic epidemiology of smoking. Nicotine & Tobacco Research, 1, S51–S57. 10.1080/14622299050011811 [DOI] [PubMed] [Google Scholar]
- Taylor D. H., Jr, Hasselblad V., Henley S. J., Thun M. J., Sloan F. A. (2002). Benefits of smoking cessation for longevity. American Journal of Public Health, 92, 990–996. http://dx.doi.org/10.2105/AJPH.92.6.990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl G. R., Walther D., Musci R., Fisher C., Anthony J. C., Storr C. L. … Rose J. E. (2012). Smoking quit success genotype score predicts quit success and distinct patterns of developmental involvement with common addictive substances. Molecular Psychiatry. Advance online publication. 10.1038/mp.2012.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beijsterveldt C. E., Groen-Blokhuis M., Hottenga J. J., Franić S., Hudziak J. J., Lamb D. … Boomsma D. I. (2013). The Young Netherlands Twin Register (YNTR): Longitudinal twin and family studies in over 70,000 children. Twin Research and Human Genetics, 16, 252–267. 10.1017/thg.2012.118 [DOI] [PubMed] [Google Scholar]
- Vangeli E., Stapleton J., Smit E. S., Borland R., West R. (2011). Predictors of attempts to stop smoking and their success in adult general population samples: A systematic review. Addiction, 106, 2110–2121. 10.1111/j.1360-0443.2011.03565.x [DOI] [PubMed] [Google Scholar]
- Vink J. M., Willemsen G., Boomsma D. I. (2005). Heritability of smoking initiation and nicotine dependence. Behavior Genetics, 35, 397–406. 10.1007/s10519-004-1327-8 [DOI] [PubMed] [Google Scholar]
- Vink J. M., Willemsen G., Stubbe J. H., Middeldorp C. M., Ligthart R. S., Baas K. D. … Boomsma D. I. (2004). Estimating non-response bias in family studies: Application to mental health and lifestyle. European Journal of Epidemiology, 19, 623–630. 10.1023/B:EJEP.0000036814.56108.66 [DOI] [PubMed] [Google Scholar]
- Visscher P. M., Hill W. G., Wray N. R. (2008). Heritability in the genomics era–concepts and misconceptions. Nature Reviews Genetics, 9, 255–266. 10.1038/nrg2322 [DOI] [PubMed] [Google Scholar]
- Vitória P. D., Salgueiro M. F., Silva S. A., de Vries H. (2011). Social influence, intention to smoke, and adolescent smoking behaviour longitudinal relations. British Journal of Health Psychology, 16, 779–798. 10.1111/j.2044-8287.2010.02014.x [DOI] [PubMed] [Google Scholar]
- Vogel F., Motulsky A. G. (1996). Human genetics. Problems and approaches. Heidelberg, Germany: Springer-Verlag [Google Scholar]
- Wetter D. W., Cofta-Gunn L., Fouladi R. T., Cinciripini P. M., Sui D., Gritz E. R. (2004). Late relapse/ sustained abstinence among former smokers: A longitudinal study. Preventive Medicine, 39, 1156–1163. http://dx.doi.org/10.1016/j.ypmed.2004.04.028 [DOI] [PubMed] [Google Scholar]
- WHO (2011). WHO REPORT on the global TOBACCO epidemic: Warning about the dangers of tobacco. Geneva, Switzerland: World Health Organization. [Google Scholar]
- Willemsen G., Vink J. M., Abdellaoui A., den Braber A., van Beek J. H., Draisma H. H. … Boomsma D. I. (2013). The Adult Netherlands Twin Register: Twenty-five years of survey and biological data collection. Twin Research and Human Genetics,16, 271–281. 10.1017/thg.2012.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh H. W., Ellerbeck E. F., Mahnken J. D. (2012). Simultaneous evaluation of abstinence and relapse using a Markov chain model in smokers enrolled in a two-year randomized trial. BMC Medical Research Methodology, 12, 95. 10.1037/0012-1649.44.2.446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong H. H., Borland R., Cooper J., Cummings K. M. (2010). Postquitting experiences and expectations of adult smokers and their association with subsequent relapse: Findings from the International Tobacco Control (ITC) Four Country Survey. Nicotine & Tobacco Research, 12, S12–S19. 10.1093/ntr/ ntq127 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


