Abstract
Colorectal cancer (CRC) is one of the most diffuse cancers worldwide and is still a clinical burden. Increasing evidences associate CRC clinical outcome to immune contexture represented by adaptive immune cells. Their type, density and location are summarized in the Immune Score that has been shown to improve prognostic prediction of CRC patients. The non-classical MHC class I human leukocyte antigen-G (HLA-G), is a crucial tumor-driven immune escape molecule involved in immune tolerance. HLA-G and soluble counterparts are able to exert inhibitory functions by direct interactions with inhibitory receptors present on both innate cells such as natural killer cells, and adaptive immune cells as cytotoxic T and B lymphocytes. HLA-G may play a prominent role in CRC strategies to avoid host immunosurveillance. This review highlights the current knowledge on HLA-G contribution in CRC, in related inflammatory diseases and in other type of cancers and disorders. HLA-G genetic setting (specific haplotypes, genotypes and alleles frequencies) and association with circulating/soluble profiles was highlighted. HLA G prognostic and predictive value in CRC was investigated in order to define a novel prognostic immune biomarker in CRC.
Keywords: Colorectal cancer, Human leukocyte antigen-G, Immune score, T lymphocytes, Untranslated regions
Core tip: Colorectal cancer (CRC) prognosis is strictly associated with the immune contexture of tumor microenvironment. IS improves prognostic prediction in CRC. Human leukocyte antigen-G (HLA-G) through its direct inhibitory functions on NK cells and cytotoxic T and B lymphocytes represents a crucial tumor-driven immune escape molecule. This review highlights the current knowledge on HLA-G in CRC and in related inflammatory diseases. HLA-G genetic setting and circulating/soluble profiles need to be defined to comprehend CRC strategies to avoid host immune defences. We suggest that HLA G could represent a novel prognostic immune biomarker to associate with the Immune Score to better characterize host immune response in CRC.
INTRODUCTION
Colorectal cancer (CRC) remains one of the leading causes of cancer death worldwide[1-3]. CRC develops sporadically[4], in the setting of hereditary forms[5], or on the basis of inflammatory bowel disease (IBD)[6]. The adenoma-carcinoma transition is the well-established known model for CRC onset[7] and, its genetic and molecular background have been widely described[5,8]. In the last years, the elaborate exchange among innate-adaptive immune cells of the tumor microenvironment, the local inflammatory state, and the host immune response in solid tumors, generated the concept of cancer immunoediting, characterized by the final escape phase exerted by the cancer from host defence immunity[9]. Increasing evidences demonstrated that solid tumors such as CRC are infiltrated by different adaptive cells of the immune contexture that may influence the progression of the disease[10]. These experimental observations finally provided the design of the Immune Score (IS) that is now considered as a novel and independent prognostic marker, in human cancer as well in CRC[11]. IS, is represented by densities of adaptive immune cells: infiltrating CD8+ cytotoxic T lymphocytes (CTLs), and CD45RO+ memory T cells, detected in center and marginal tumor areas[12]. Higher number of infiltrating CD8+ CTLs and CD45RO+ memory T cells correlates with an improved patient prognosis; lower numbers correlate with tumor relapse[13]. IS demonstrated to have a prognostic value for overall survival (OS) and disease free survival (DFS) in retrospective studies[14], and is currently being submitted for clinical validation in prospective CRC studies, in 16 different Countries world wide[15].
The non-classical HLA-G is considered a tolerogenic molecule due to its inhibitory functions vs T lymphocytes, NK cells and other cell types of immune contexture[16]. HLA-G is only recently been involved in tumor escape mechanisms from the host immune recognition and destruction[17], and is considered a tumor microenvironment molecule[16,18]. HLA-G shows a lower nucleotide variability in coding sequences, while is highly polymorphic in the untranslated regions (UTRs), both in 5’ and 3’ segments[19,20] (Figure 1). Polymorphic sites mainly present in the 3’UTR region, may affect the post transcriptional regulation and biological functions of HLA-G[21]. Indeed, through alternative splicing, HLA-G can be expressed as seven different and specific molecules, four membrane bound (HLA-G1 to -G4) and three soluble (HLA-G5 to -G7)[22]. Most of the published data concern the HLA-G1 molecule and its soluble counterpart HLA-G5[23]. HLA-G1 can be shed generating a soluble isoform (sHLA-G1)[17]. The functional characterizations of the five remaining HLA-G isoforms have been yet not clearly elucidated. HLA-G is over-expressed in CRC[24-26] and is a common signature in other types of cancer, autoimmune disorders, viral infections and transplantations[16-18]. Increase in circulating sHLA-G has been detected in CRC in few studies[27,28] and in other malignancies[23,29-31]. Growing attention is focusing on the genetic setting related to the UTRs, especially the 3’UTR involved in micro RNA (miRNA) binding[32]. Single nucleotide polymorphisms (SNPs) in this region have been associated with the disease risk in cancer[33-35] and other disorders[36,37] (Table 1), but the association with CRC and the prognostic value in this type of malignancy, remain to define. Aim of this review was to investigate the HLA-G as a potential prognostic biomarker in CRC and related inflammatory colon diseases, both at the genetic and circulating profiles (Table 2). Genotypic-phenotypic correlation has been highlighted and its potential role in IS explored (Figure 2).
Figure 1.
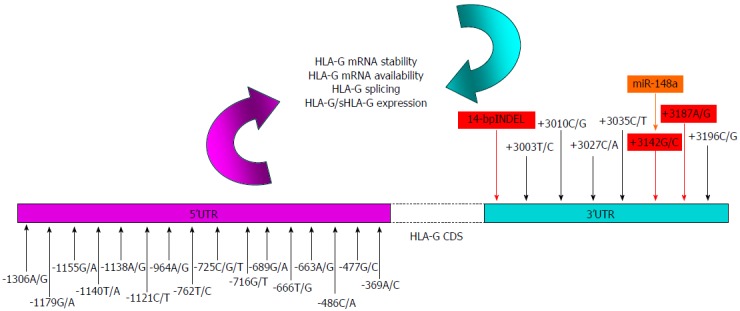
Human leukocyte antigen-G single nucleotide polymorphisms involved in the biological features of the protein: nucleotide variants in the 5’-3’ untranslated regions may influence human leukocyte antigen-G expression levels by modifying the affinity of gene targeted sequences for transcriptional (5’) or post-transcriptional (3’) factors respectively. Polymorphisms in 5’UTR (fucsia) were previously described by Costa et al[113] those in 3’UTR (light blue) by Castelli et al[19]. In red 3’UTR SNPs involved in HLA-G mRNA stability and availability are highlighted. In orange the only one microRNA with a demostrated functional inhibitory role in the HLA-G expression[134] was highlighted . UTR: Untranslated region; SNPs: Single nucleotide polymorphisms; HLA-G: Human leukocyte antigen-G.
Table 1.
List of associations found between single nucleotide polymorphisms/alleles in human leukocyte antigen-G untranslated regions and different pathologies; genotype-phenotype correlations were also reported
| UTRs | SNP | Genotype and/or allele | Disease | Association with1 | UTR SNP and sHLA-G correlation | sHLA-G level | Statistical significance2 | Country | Ref. |
| 3’ | 14 bp INDEL | 14 bp I/I | PE | Increased disease risk | ND | ND | Yes | China | [36] |
| 3’ | 14 bp INDEL | 14 bp I/I | RSA3 | Increased disease risk | ND | ND | No | Denmark | [112] |
| 3’ | +3142 C/G | +3142 GG and G allele | SLE | Increased disease risk | ND | ND | Yes | Brazil | [37] |
| 3’ | 14 bp INDEL | 14 bp I/I | SLE | Increased disease risk | ND | ND | No | Brazil | [37] |
| 3’ | 14 bp INDEL | 14 bp I/I | OvaC | Increased disease risk | ND | ND | Yes | Canada | [33] |
| 3’ | 14 bp INDEL | 14 bp D/D | EsophC | Increased disease risk | ND | ND | Yes | China | [34] |
| 3’ | 14 bp INDEL | 14 bp D/D and D allele | HCC | Increased disease risk | ND | ND | Yes | Brazil | [35] |
| 3’ | 14 bp INDEL | 14 bp I/I and D allele | HCC | Increased disease risk | ND | ND | No | South Korea | [137] |
| 3’ | 14 bp INDEL | 14 bp I/I | Allo-HSCT | Lower OS and DFS | ND | ND | Yes | Italy | [138] |
| 3’ | 14 bp INDEL | 14 bp D/D | RA | MTX therapy (responder group) | Yes | Higher | Yes4 | Italy | [114] |
| 3’ | 14 bp INDEL | 14 bp I/I | RR-MS | sHLA-G | Yes | Lower | Yes4 | Italy | [139] |
| 3’ | +3142 C/G | +3142 GG | RR-MS | sHLA-G | Yes | Lower | Yes4 | Italy | [139] |
| 3’ | 14 bp INDEL | 14 bp I/I | IVF3 | sHLA-G | Yes | Absent | Yes | Denmark | [141] |
| 5’ | -725C/G/T | -725C>G | IVF3 | sHLA-G | Yes | Absent | ND | Denmark | [141] |
| 3’ | 14 bp INDEL | 14 bp I/I | Heart T | sHLA-G | Yes | Lower | Yes | Canada | [142] |
| 3’ | 14 bp INDEL | 14 bp I/I | HD | sHLA-G | Yes | Lower | Yes | China | [143] |
| 3’ | 14 bp INDEL | 14 bp D allele | ERA | Improved disease remission | Yes | Higher | Yes4 | Italy | [144] |
| 5’ | -725C/G/T | -725Callele | RPL3 | sHLA-G | Yes | Lower | Yes | Iraq | [148] |
| 3’ | 14 bp INDEL | 14 bp I/I | PTC | Increased disease risk not found | No | Higher | Yes5 | Italy | [31] |
Data reported in this column are related to the association with SNPs in UTRs; the increased disease risk was compared with a HD control group;
Statistical significance is referred to HLA-G SNP and disease status analysis;
The disease status is related to infertility problems;
A statistical significance was found also between HLA-G SNP and sHLA-G levels;
A statistical significance was found between sHLA-G levels and the disease risk. UTR: Untranslated region; SNP: Single nucleotide polymorphism; sHLA-G: Soluble human leukocyte antigen-G; INDEL: Insertion/deletion polymorphism; I: Insertion; D: Deletion; PE: Pre-eclampsia; RSA: Recurrent spontaneous abortions; SLE: Systemic lupus erythematous; OvaC: Ovarian cancer; EsophC: Esophageal cancer; HCC: Hepatocellular carcinoma; Allo-HSCT: Allogeneic hematopoietic stem cell transplantation; RA: Rheumatoid arthritis; RR-MS: Relapsing-remitting multiple sclerosis; IVF: In vitro fertilization failure; Heart T: Heart transplantation; HD: Healthy blood donors; ERA: Early rheumatoid arthritis; RPL: Recurrent pregnancy loss; PTC: Papillary thyroid carcinoma; OS: Overall survival; DFS: Disease free survival; MTX: Methotrexate; ND: Not determined.
Table 2.
Summary of human leukocyte antigen-G evaluations in colorectal cancer and related colonic diseases of the gastrointestinal tract
| Sample type | HLA-Gs | Methods | Disease, n | Relevances | Ref. |
| Tumor DNA | HLA-G | RT-PCR | CRC, n = 39 | HLA-G mRNA was significantly more expressed in CRC (87.2%) than in the extra neoplastic tissue | [24] |
| Tumor tissue | HLA-G | IHC | CRC, n = 201 | HLA-G is over-expressed in primary CRC sites (64.6%), but not in the normal CRC tissues or benign adenomas | [25] |
| Tumor tissue | HLA-G | IHC | UC, n = 24; CD, n = 19 | HLA-G and IL-10 are highly expressed in UC but not in CD tissue biopsies | [154] |
| Tumor tissue | HLA-G | IHC | CRC, n = 60; DA, n = 67; BC, n = 37; AC, n = 52 | HLA-G is over-expressed in 52 % of CRC lesions and also in 79% of PDAs, 76% in BC and 75% AC | [26] |
| Tumor tissue | HLA-G | IHC | CRC, n = 415 | HLA-G is expressed in > 30% of CRC lesions (data summarize published data collected until 2008) | [16] |
| Tumor tissue | HLA-G | IHC | CRC, n = 154 | HLA-G is expressed in > 30% of CRC lesions (data summarize published data collected until 2005) | [17] |
| Serum | sHLA-G | ELISA | CRC, n = 144 | Higher sHLA-G levels in CRC (median 124.3 U/mL) compared to benign colorectal diseases (cut off value 88.6 U/mL). CEA showed less sensitivity e specificity | [27] |
| Plasma | sHLA-G | ELISA | CRC, n = 37 | sHLA-G as a diagnostic biomarker for the detection of early CRC (median 84 U/mL) with respect to BD (median 34 U/mL) | [28] |
| PBMC | sHLA-G | ELISA | HD, n = 30; CD, n = 10; UC, n = 18 | Spontaneous secretion of sHLA-G from cultured PBMCs of CD but not in UC and BD | [161] |
| Secretion of sHLA-G in CD patient cultures and BD but no in UC, after LPS stimulation | |||||
| Plasma and PBMC | HLA-G | ELISA | UC, n = 27; CD, n = 22 | Immunosuppressive therapy decreases sHLA-G hyperproduction in CD and induces its release in UD, in both plasma and in PBMC culture supernatants | [162] |
HLA-G: Human leukocyte antigen-G; sHLA-G: Soluble HLA-G; CRC: Colorectal cancer; UC: Ulcerative colitis; CD: Crohn’s disease; PDA: Pancreatic ductal adenocarcinoma; BC: Biliary cancer; AC: Ampullary cancer; HD: Healthy blood donors; PBMC: Peripheral blood mononuclear cells; IHC: Immunohistochemistry; LPS: Lipopolysaccharide; CEA: Carcinoembryonic antigen; IL: Interleukin; ELISA: Enzyme-linked immunosorbent assay.
Figure 2.
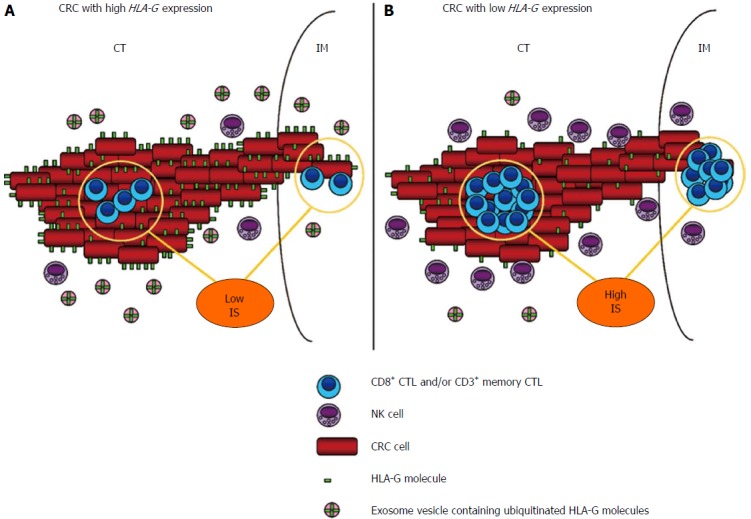
Schematic representation of colorectal cancer tumor microenvironment: potential influence of human leukocyte antigen-G expression on Immune Score and host immune local response. A: High HLA-G expression shows an inhibitory role against host immune defences, represented mostly by natural killer cells and CTLs. Increased HLA-G expression in the local microenvironment is also supported by exosome contribution through a hypothetical trogocytosis release mechanism that mediates HLA-G up-take. Another consequence is a direct association with a low IS value due to HLA-G inhibitory functions on CTLs in CT/IM areas of the tumor, and consequently with a worse patient prognosis (not represented); B: Lower HLA-G expression correlates with a high IS value and a favoureable immune contexture that improves host immune response and patient prognosis (not represented). Representation of IS is referred to recent quality and validation criteria[15] (see text for further clarifications). HLA-G: Human leukocyte antigen-G; CTLs: Cytotoxic T lymphocytes; IS: Immune Score; CT: Center of the tumor; IM: Invasive margin.
CRC AND CHRONIC COLONIC INFLAMMATION
CRC is one of the most diffuse cancers worldwide with about 1.2 million new cases and 600000 deaths recorded annually[1].
Despite improvements and advances in diagnosis, surgery and treatment, CRC is still the 2nd most common cause of cancer death in the United States and other industrialized countries[2,3].
Development of sporadic (88%-94%) and hereditary forms of CRC are mainly related to the accumulation of genetic changes in gatekeeper and caretaker genes like APC and other oncoproteins (K-ras, erb-s, c-src, β-catenin, PI3K) and tumor suppressors (p53, Smad4)[4,5], according to the aberrant crypt foci (ACF)-adenoma-carcinoma transition model[7]. It is well-established that the acquisition of these mutations in a multistep mechanism is part of the genomic instability process that comprehends the chromosomal instability (CIN), microsatellite instability (MSI), and CpG island methylator phenotype (CIMP) pathways[8]. While CIMP exhibits gene silencing due to hypermethylation of CpG islands, in CIN positive tumors (about 70% of sporadic CRCs) an imbalance in chromosome number (aneuploidy), subchromosomal genomic amplifications and a high frequency of loss of heterozygosity (LOH) are observed[38]. About 15% of sporadic CRCs, mostly in the proximal colon anatomic site, are characterized by MSI with a large number of mutations at microsatellite sequences interesting the DNA mismatch repair (MMR) system, so the consequence is the accumulation of thousands of unrepaired mutations[39,40].
Genesis of most of CRCs depends also from environmental factors like intestinal microbiota, dietary habits and lifestyle, associated with the patient genetic background[41]. The chronic colonic inflammation due to active ulcerative colitis (UC) or Crohn’s disease (CD), the two major forms of inflammatory bowel disease (IBD), lead to the colitis-associated cancer (CAC) development that is a CRC subtype[6]. In UC, inflammation is limited to the mucosal layer and usually starts from the rectum spreading then into the colon, while in CD all the layers of gut wall are interested with the terminal ileum and also the colon as the most common sites[42].
IBD subjects are at increased risk to develop the tumor; it is estimated that about 20% of patients affected by chronic UD and CD for a long time, within 30 years, develop CAC; thus CRC risk increases with the duration of the IBD and the severity of inflammation[43].
Pathogenesis of CAC in chronic colitis patients differs from the classical model sustained by sporadic CRC, for a transition from low-high grade dysplasia to carcinoma and also for the sequence of cellular and molecular events[44]. In CRC adenomatous polyposis coli (APC) protein loss of function, is an early event during the formation of precocious adenoma, while it is less frequent and occurs in the late pathogenesis of CAC[45]. p53-loss of heterozygosity (LOH), p53 mutations or loss of function are early molecular events characterizing CAC origin instead of CRC in which they frequently occur in the late adenoma-carcinoma transition[46,47].
IMMUNE CONTEXTURE IN INFLAMMATION AND CRC
The chronic inflammatory status is a common feature of colorectal and colitis-associated tumors. Chronic inflammation is modulated by immune innate/adaptive cells infiltration and immune microenvironment[48,49]. The crucial role of inflammation in tumorigenesis is emerged only recently and now it is estimated that about 15%-20% of cancers are related to an underlying chronic inflammation process[50,51]. CAC is considered a classical inflammation-driven cancer as demonstrated in mice models in presence of dextran sodium sulphate (DSS) and the pro-carcinogen azoxymethane (AOM)[52].
A growing body of evidence is focusing both on relationships between the immune contexture of solid tumors like CRC, and patient prognosis in terms of DFS and OS[53]. Tumor microenvironment is represented by a complex network of stromal, inflammatory and immunocompetent cells. Histopathological analyses show that solid cancers are infiltrated by innate-adaptive immune cells, that have a role in tumor growth[54,55].
The interplay among tumor cells and immune system is highly complex and suggested the concept of cancer immunoediting, a dual process in which the main actors are the host-protective and the tumor-promoting actions of immunity[9,56,57]. Cancer immunoediting occurs in three phases: elimination, equilibrium and escape. In the elimination phase (the modern concept of the older notion “cancer immunosurveillance”)[58], innate and adaptive immunity work together to recognise and destroy nascent tumor cells. If tumor cell variants are not completely eliminated, will enter into an equilibrium phase, in which adaptive immunity controls and stems the growth of clinically undetectable tumor cells and blocks tumor cell immunogenicity. When dormancy of tumoral cells stops, malignant cells with reduced immunogenicity shift into the escape phase and begin to grow and proliferate in an immunologically unrestrained way, establishing an immunosuppressive tumor microenvironment, and becoming clinically detectable. Escape mechanism from immune host control is now considered one of the hallmarks of cancer[59]. Innate immunity is the first line of host defense and it is specialized in counteracting cancer cells and virally infected cells.
Innate immune cells i.e., myeloid derived suppressor cells (MDSCs), macrophages, neutrophils, dendritic cells (DCs), mast cells (MCs), and NK cells, may have a pro- or anti-tumorigenic role in both CRC and CAC[60]. MDSCs under the control of nuclear factor κB (NF-κB), produce interleukin (IL)-6 that activates transcription factor signal transducer and activator of transcription 3 (STAT3) resulting in survival, growth and progression signals in early CAC[61].
Macrophages are responsible mainly for pro-inflammatory cytokines release and are distinguished in two types. Type 1 macrophages (M1) that are activated by interferon (IFN)-γ or tumor necrosis factor (TNF) cytokines, are efficient producers of reactive oxygen and nitrogen species, have an interleukin (IL)-10low IL-12high IL-23high inflammatory phenotype, and tend to negatively control tumor growth[62]. Conversely, type 2 macrophages (M2) share an IL-10high IL-12low IL-23low anti-inflammatory phenotype and stimulate cancer proliferation by secreting immunosuppressive cytokines like IL-10. Production of mediators promoting angiogenesis such as Vascular Endothelial Growth Factor A (VEGFA) and Cyclo-Oxygenase-2 (COX-2)-derived prostaglandin E2[63], hypoxia-dependent upregulation of chemokines (C-X-C motif), induces accumulation of M2 macrophages that is the predominant phenotype in tumor microenvironment[64,65]. A macrophage polarization of tumor-associated macrophages (TAMs) due to the local colonic microenvironment during tumor progression is observed with a switching from M1 (inflammatory) to M2 (anti-inflammatory) type and a gradual NF-κB inhibition[66]. TAMs are one of the major components of leukocyte infiltrates in tumors and represent an independent prognostic factor of poor prognosis in multivariate analysis in different malignancies[67]. In CRC, TAMs correlate with improved OS[68]. Finally, innate immune cells orchestrate a complex inflammatory environment that may be modulated to either stimulate or inhibit CRC proliferation[69,70].
While innate immunity does not involve a specific antigen or peptide or particular tumor-associated antigen recognition, this is a prerequisite for adaptive immunity. Cells of adaptive immune system are mainly represented by B lymphocytes, T helper 1 (Th1) and T helper 2 (Th2) CD4+ cells, CD8+ Cytotoxic T lymphocytes (CTL) cells and CD4+ T regulatory (Treg) cells[71]. Recent findings of memory responses by NK cells suggest that also NK may contribute to adaptive immunity[72]. To destroy cancer cells, CTLs need to recognize an antigen exposed on the tumor cells in association with the human leukocyte antigen (HLA) class I proteins[73]. Only through recognition of this tumor cell antigen/HLA I complex for which their T cell receptor (TCR) is specific, CTLs clonally expand and differentiate in memory T cells[74] (CD45RO+). CD45RO+ comprise CD3+, CD4+ and CD8+ T cells that have been exposed to antigen and respond faster and with an increased intensity after antigen stimulation compared with naïve T cells[54].
Upon activation, CTLs release proteases and lytic components as perforin and mediate disruption of tumor cell membrane and activation of apoptotic pathway. CD4+ T cells respond only to antigens presented by the HLA class II proteins expressed by DCs in secondary or tertiary organs[75]. Many evidences highlight that one of the cancer immunoediting topic is due to the fact that T-cell recognition of tumor antigens drives the immunological destruction of nascent and developing cancer cells. One of the most well-known way used by tumors to escape from specific T-cell recognition mechanisms is down-regulation or total suppression of MHC I molecules, and specific alteration leading to the inefficient presentation of immunodominant antigens[76].
Recently, Matsushita et al[77], demonstrated that the immunoselection by CD8+ T cells of tumor variants lacking strong tumor-specific antigens represents one of the mechanism by which cancer cells escape tumor immunity.
Th1 cells secrete cytokines like IFN-γ and TNF-α, support tissue destruction and CTLs by producing IL-2 required for CD8+ proliferation[78]. Th2 cells produce cytokines such as IL-10, IL-4 and IL-5, and limit CTLs proliferation. Tregs that also highly express CD25 (CD4+CD25+) secrete IL-10 and Tumor Growth Factor (TGF)-β which dampen the immune response[79]. It should be emphasized that while CTLs, Th1 and Th17 inhibit cancer growth, Th2 cells and Tregs stimulate cancer proliferation. Moreover, a shift in Th1 (tumor rejection)/Th2 (tumor promotion) immune response is observed in CRC[80]. Restoring of normal immunological functions and pro-inflammatory cytokines after tumor resection in CRC were demonstrated, highlighting that CRC itself has a direct immunosuppressive effect[81,82].
Overall, immune contexture should be considered as comprising the density of CD8+ CTLs and CD45RO+ memory T cells, their location at the center of the tumor (CT) and invasive margin (IM), combined with the quality of tertiary lymphoid structures (TLS) and additional functionality entities such as Th1-related factors, chemokines, adhesion molecules and cytotoxic factors[83]. Indeed, in CRC, not only Th1 immunity markers (STAT1, IRF1, IFN-γ-SG pathway), cytotoxic markers (Granzyme Perforin, Granulysin, TIA1, Caspase pathway), but also chemoattraction (specific chemokines such as CX3CL1, CXCL10, CXCL9) and adhesion (molecules as ICAM1, VCAM1, MADCAM1) signatures are relevant in influencing the density of infiltrating immune cells[84,85].
CRC AND IMMUNE SCORE
Accumulating evidences since the late 1990s showed an association among tumor-infiltrating lymphocytes (TILs) able to inhibit cancer growth, and improved prognosis in CRC[10,86] and other malignancies such as melanoma[87] and ovarian cancer[88]. A particular phenotype in CRC is represented by MSI type tumors that are associated with a high TILs levels, loss or downregulation of HLA class I, better patient prognosis, a reduced metastatic potential, and a different response to chemotherapy[39,60].
In 2005-2006 the idea that the adaptive immune response plays a role in preventing tumor recurrence in CRC emerged, mostly from the works of Pagès et al[89] and Galon et al[13]. The authors first demonstrated that CRCs with high density of infiltrating and effector memory T cells with a protective immune role, were less prompt to metastasize and can be associated with an increased survival of patients[89]. Subsequently, using the same cohort of patients, relationships among type, density and location of immune cells within the tumor and the clinical outcome, were investigated by using both genomic approaches and immunohistochemistry (IHC)[10]. Through the selection and the evaluation of expression levels of genes involved in inflammation, Th1 adaptive immunity and immunosuppression, Galon et al[13] found a dominant cluster of co-modulating genes for Th1 adaptive immune response (i.e., IFNG, CD8a, GLNY, GZMB, CD3z). Applying specific tissue microarrays and a dedicated image analysis work station, a quantification of total (CD3+) T lymphocytes, CD8+ CTL effectors, associated molecule (GZMB), and CD45RO+ memory T cells, was performed both in CT and IM.
High immune cell densities (CD3+, CD8+, GZMB and CD45RO+) in both CT and IM tumor regions were present in CRC patients without recurrence after adjuvant therapy, while lower densities of the same immune cell types correlated with disease recrudescence. Results highlighted an inverse correlation among expression of these genes and CRC relapse suggesting that Th1 adaptive immunity improve clinical outcome[13]. Camus et al[14] demonstrated the association between loss of coordinated functional immune reaction and the progression of CRC to a metastatic phenotype. These preliminary results demonstrated for the first time that the host immune response plays an important role in determining the outcome of CRC patients. Type, density, and location of immune cells in CRCs increase the prediction accuracy of DFS and OS, and started to represent a superior and independent prognostic parameter with respect to the UICC-TNM well accepted classification[90].
Finally, all these data and evidences, culminated in the concept of “Immune Score” that emerged for the first time in 2011 in the work of Pagès et al[12]. A multivariate Cox proportional hazard regression model was used to assess the hazard ratio of the immune score combination (CD45RO/CD8) in specific tumor regions (CT/IM), together with clinical and histopathological tumor markers. Pagès et al[12], analyzing a large cohort of CRC patients with early (I-II) stage, showed that the combined analysis of cytotoxic (CD8+) and memory (CD45RO+) T cells confirmed its prognostic discriminatory power in the prediction of tumor recurrence and survival[12]. Subsequently, the concept of IS as a clinical prognostic marker improving the standard TNM classification at any stage of CRC, has been established evaluating infiltrating lymphocytes of 599 CRC specimens by Mlecnik et al[91]. Patients with high IS had increased DFS and OS, and patients with a low IS were likely to experience a disease relapse[92]. IS represents a standardized, simple and powerful immune stratification system proposed as a novel prognostic immune marker for routine testing potentially helpful for CRC management to better identify and stratify high-risk patients who would benefit most from adjuvant therapy[12].
Cancer outcomes can vary significantly among patients with the same stage and this could be related to differences in immune cells densities from patient to patient as in CRC[93]. This argues the limit of traditional AJCC/UICC TNM classification in providing limited prognostic information and predicting response to therapy[94]. A worldwide task force representing 22 Institutions from 16 different countries, is working now for IS validation in clinical practise, with the aim to introduce it as a new component of classical cancer classification, that will maybe designate in future as TNM-I (TNM-Immune)[15]. To improve quality and validation in standard laboratories, IS will be quantified by the combination of the two easiest membrane stains CD3 and CD8 to avoid any background noise due to CD45RO and GZMB stains[15]. Special emphasis will be focused in the prognostic significance of validated immunologic parameters in CRC that with the primary goal to validate the prognostic power of the IS in routine settings of stage I/II/III CRC patients and for recurrence prediction for stage II CRC patients[95]. Thus, the bases to revise and renegotiate the clinical outcome based on classical clinical parameters have been seeded[96].
Recently, a great emphasis has been done in an attempt to define the prognostic role of another subtype of tumor infiltrating cells. Treg cells also positive for the nuclear transcription factor protein forkhead box P3 (Foxp3), showed a strong and independent prognostic significance in CRC, superior to CD45RO+ and CD8+ cells[97]. Foxp3+ Tregs cells are generally associated with immunosuppressive properties and poor prognosis in different solid tumors such as hepatocellular[98], prostate[99] and pancreatic carcinoma[100], but conversely were reported to be associated with improved prognosis in CRC[97]. Although Foxp3 is a well accepted marker used to identify tumor infiltrating CD4+ CD25+ Tregs, it is known that a small proportion of Foxp3+ cells may also be CD8+. CD8+ CD25+ Foxp3+ T cells showed suppressive capacities in CRC[101], suggesting that Foxp3+ role should be more explored. Foxp3 expression evaluated in tumor cells was associated with worse outcome of patients in different solid tumors[102-104] but not in CRC, highlighting a new independent prognostic factor. Recently, Foxp3+ expression in tumor cells was compared to Foxp3+ Treg infiltration in CRC, demonstrating for the first time an inverse correlation between the number of Foxp3+ Treg and the level of Foxp3+ in tumor cells, suggesting an anti-proliferative effect of TGF-β on Tregs[105]. Furthermore, patients with high Foxp3+ expression profile in CRC tumor cells were correlated with a poorer prognosis that was not observed for Foxp3+ Treg in the tumor[106].
HLA-G: A CRUCIAL TUMOR-DRIVEN IMMUNE ESCAPE MOLECULE
HLA-G is considered as a tolerogenic molecule exerting its inhibitory functions by direct interaction with different inhibitory receptors of the immunoglobulin family present on NK cells (ILT2/CD85j, KIR2DL4/CD158d), T lymphocytes (ILT2/CD85j, KIR2DL4/CD158d), B cells (ILT2/CD85j), endothelial (CD160), macrophages, monocytes and DCs (ILT2/CD85j, ITL4/CD85d)[16]. HLA-G expression was originally detected in non-pathologic conditions and restricted to extravillous cytotrophoblast, thymic epithelial cells, cornea, pancreas, erythroid and endothelial precursor cells[18]. Recent studies showed that HLA-G proteins can be detected also in pathological conditions, such as in allografts and infiltrating immune cells within transplanted tissues, inflammatory diseases, virus infections and cancer[107-109]. Originally, durings the 1990s, HLA-G role in maternal-fetal tolerance preventing attack of the fetus by the maternal immune system by its interaction with uterine NK cell inhibitory receptors was demonstrated[110]. This binding through KIRDL4 receptor stimulates secretion of cytokines and angiogenic factors from NK cells, which favours implantation and placental vascularisation and development. Trophoblast itself secretes HLA-G modulating balance among these proangiogenic and antiangiogenic factors. The tolerogenic role of HLA-G in pregnancy is strongly supported by the correlations between HLA-G down regulation and preeclampsia/spontaneous abortions events[111-113]. These preliminary evidences observed in maternal-fetal tolerance suggested also that microenvironment factors may modulate HLA-G expression in tissues. Ectopic expression of HLA-G in damaged cells or tissues may be enhanced by stress, nutrient deprivation, hypoxia, hormones such as progesterone, cytokines (GM-CSF, IFNs, IL-10, TNF-α, TGF-β, LIF)[16] and immunosuppressive drugs[114].
HLA-G gene is composed of eight exons and seven introns with a stop codon at exon 6, a quite large 5’UTR extending at least 1.4 kb from ATG, and a 3’UTR[32]. The coding exons transduce only the heavy chain of the molecule and are located on chromosome 6, while β2-microglobulin (β2m) is encoded by a separated gene on chromosome 15. Exon 1 encodes the signal peptide, exons 2, 3 and 4 the extracellular α1, α2 and α3 domains respectively, and exons 5 and 6 the transmembrane and the cytoplasmic domain of the heavy chain. Exon 7 and 8 are not translated. The 3’UTR is included in the exon 8[21]. Classical HLA class I molecules are characterized by nucleotide sequence variations around the peptide-binding cluster encoded by exon 2 (α1 domain) and exon 3 (α2 domain), while HLA-G nucleotide variability spans through exon 2, 3 and 4 (α3 domain)[21,22]. HLA-G coding sequence has a limited genetic variability in contrast to the classical HLA class I molecules that exhibit hundreds of alleles and, to date, 49 different alleles and 15 related proteins have been recognized[115]. On the other hands, 5’UTR and 3’UTR segments are high polymorphic, both influencing HLA-G expression modifying the affinity of gene targeted sequences for transcriptional or post-transcriptional factors, respectively[19] (Figure 1).
Indeed, HLA-G may presents 7 protein isoforms generated by alternative splicing of the primary transcript. 4 isoforms are membrane-bound (HLA-G1, G2, G3 and G4) and 3 are soluble (G5, G6 and G7) species[23]. HLA-G1 and HLA-G5 are the most common isoforms observed, but HLA-G1 was the first isoform to be discovered in healthy tissues and first implicated in materno-fetal tolerance[116]. HLA-G1 is the complete protein similar to the other classical membrane bound HLA-I molecules associated with β2m[23]. Crystal structure of the protein have shown that the full-length HLA-G1 is composed of a heavy chain non-covalently associated with the β2m molecule, and a peptide of about 8-10 amino acids similar to that found in the other classical I HLAs[117]. HLA-G2 isoform has no α2 domain, HLA-G3 has no both α2 and α3 domains, HLA-G4 does not present α3 domain[21]. This high post transduction availability in HLA-G molecules suggests a deeper modulation due to alternative splicing involving mostly 3’UTR region. We speculate that it could be tissue specific considering that miRNA are differentially expressed, and this modulation may be influenced by inflammation status and immune contexture microenvironment. It is known that KIR2DL4 present on NK and T cells binds HLA-G through α1 domain, or ITL-4 (DCs, monocytes and macrophages) and CD8 (T and NK cells) bind via α3 domain, or ITL-2 via α3 domain in association with β2m, however, the exact role of the less common HLA-G isoforms has not been elucidated[118]. HLA-G dimers may also be formed via a Cys42-Cys42 intermolecular disulfide bond on the α1 domains of the heavy chains from two HLA-G monomers. These HLA-G dimers exhibit enhanced binding avidity for ITL2/4-mediated signaling[119]. Soluble and not bound HLA-G5, HLA-G6 and HLA-G7 species have the same extraglobular domains of HLA-G1, HLA-G2 and HLA-G3 respectively[21]. It should be pointed that another form of HLA-G may be generated by proteolytic shedding of the isoform HLA-G1 (sHLA-G1)[120] and potentially anchored HLA-G2-G4, can also be shed from the cell surface if expressed. It should be highlighted that sHLA-G1 is analogue to the sHLA-G5 isoform. Soluble isoforms consequent to secretion or shedding, especially the most common sHLAG5 and sHLA-G1, can be detected in body fluids such as plasma, serum, ascites, cerebro spinal fluids exudates from patients with inflammatory diseases o cancer[17,121,122]. Similarly to membrane bound HLA-G1, sHLA-G exerts immunosuppressive functions vs cells of the immune contexture. Furthermore, sHLA-G produced by immune cells and/or by cancer cells induces apoptosis of activated CD8+ T cells by binding to CD8 and by triggering a Fas/FasL-dependent pathway[119]. Moreover, the release of IL-3, IL-4 and IL-10 is stimulated by sHLA-G[17]. Increased sHLA-G levels compared to healthy controls have been reported in successful pregnancy after in vitro fertilization (IVF)[121], in patients with lymphoproliferative disorders[29], melanoma[30], and different type of cancers[23,31]. sHLA-G has been proposed as a diagnostic biomarker in CRC with increased specificity and sensibility respect to the well known carcinoembryonic antigen (CEA) protein[27]. Due to the possibility that serum sHLA-G is trapped within during the clot formation, to evaluate true biological sHLA-G levels, it is recommended to detect sHLAG in plasma[123]. Anyway, many studies still present data from serum samples.
sHLA-G levels are commonly detected with classical ELISA assay by the use of the monoclonal antibody (MoAb) MEM-G9 which recognizes (the precise epitope is unknown) the native HLA-G molecule in β2m associated forms, HLA-G1 and HLA-G5 soluble and not membrane bound isoforms[124]. It should be noted that recently, Zhao et al[125] demonstrated by flow cytometry that the MEM-G9 antibody it is able to bind also HLA-G3 isoform that is β2m free, thus speculating that an epitope on MEM-G/9 localized on the α1 domain of HLA-G. HLA-G may be expressed at the cell surface and also secreted. Anyway, sHLA-G can also be produced into the cells and subsequently incorporated in microvesicles, the exosomes such as in cancer[126] (Figure 2). In exosomes, HLA-G molecules form high molecular weight complexes through disulfide bridges, share partially an ubiquitinated phenotype and can be released by exosomes as demonstrated recently in vivo[127]. Moreover, very preliminary data coming from the HLA-G Conference held in Paris in 2012, showed that in plasma samples of healthy controls sHLA-G is preferentially exosomal-bound, while in plasma samples of lung cancer patients the level of free “not exosomal-bound” sHLA-G is increased[128]. Intriguing, it was demonstrated a mechanism of protection and immune evasion for HLA-G negative tumor cells that are in proximity of HLA-G positive tumor cells. These HLA-G expressing tumor cells by “trogocytosis” transfer membrane patches containing HLA-G molecules to active and surrounding NK cells[129]. Upon acquisition of HLA-G1-containing membranes from tumor cells, effector NK cells stop proliferating, stop being cytotoxic toward legitimate targets, and behave as regulatory cells capable of inhibiting the cytotoxic functions of other NK cells. This immediate functional inversion from an effector cell to a regulatory cell is directly due to acquired cell-surface HLA-G1[16]. We hypothesize that this mechanism could be related also to exosome sHLA-G release in the extracellular medium (Figure 2) for its re-capture, under the influence of unknown factors, may be chemokines and/or cytokines, but experimental investigations are needed. Moreover, we hypothesize a direct role of HLA-G in affecting the IS value considering its direct inhibitory properties over NK cells and CTLs[130] (Figure 2). This topic could represent a matter of debate in the early future, not only for CRC.
HLA-G MODULATION REFLECTS 5’UTR AND 3’UTR GENETIC VARIABILITY
A growing body of evidence has been focusing in the last years on the 3’UTR polymorphisms and haplotypes, due to the microRNAs (miRNAs) interaction and their influence on expression. In particular, at least eight distinct haplotypes and eight SNPs have been described so far: the14-bp Insertion/Deletion (INDEL), +3003 T/C, +3010 C/G, +3027 C/A, +3035 C/T, +3142 C/G, +3187 A/G, and +3196 C/G[19] (Figure 1). INDEL and +3187 A/G SNPs have been associated with HLA-G mRNA stability and degradation processes even if, the exact mechanisms are not already been well elucidated[21,131]. In presence of the 14-bp Insertion (5’-ATTTGTTCATGCCT-3’) sequence, HLA-G alleles have been associated with lower mRNA production[132]. In the other hand, it has been demonstrated that a smaller fraction of HLA-G mRNA transcripts presenting the 14-bp Insertion (Ins) can be alternative spliced from the mature HLA-G with the removal of 92 bases of exon 8 (include SNPs +3003 and +3010)[19]. mRNA producing smaller mRNA transcripts, reported to be more stable than the complete forms[20]. Interestingly, some AU-rich elements (ARE) are present in the 3’UTR of HLA-G, and it is known that these sequences are recognized by proteins causing rapid changes in mRNA stability[19,32]. The 14-bp sequence begins with AUUUG, and the absence of such motif in the 92 base-deleted transcripts might give an explanation of their resistance to degradation processes[20]. The presence of a guanine in the position +3142 increases the affinity of specific miRNAs (miR-148a, miR-148b and miR-152) to the HLA-G mRNA, decreasing HLA-G expression[32,133] (Figure 1). The influence of +3142G allele was demonstrated by functional studies in which HLA-G high expressing JEG-3 cells were transfected with miR-148a: decreased soluble HLA-G levels were detected[134].
In Table 1 are listed the associations found among HLA-G UTR SNPs and alleles, in particular the 14 bp INDEL polymorphism, and various disorders including cancer. Associations with circulating HLA-G levels were also reported if investigated. Recently, the risk of invasive cancer of uterine cervix was found to be significantly increased in presence of the 14 bp I/I and also the HLA-G*01:01:01:02 genotype in a large Canadian study of 539 women with histologically confirmed HG-CIN and invasive cancer; 833 women with normal cytology served as controls[33]. Moreover, 14 bp I/I genotype correlates with disease progression from high-grade cervical intraepithelial neoplasia (CIN3) to invasive cancer[33]. Previous data provided evidences of HLA-G expression in association with tumor metastasis and poor survival in an ovarian cancer animal model[135], and also with NK cell cytotoxicity inhibition and MMP-15 expression in ovarian cancer cell line[136]. In Kazakh population the risk of developing esophageal carcinoma was significantly higher in individuals carrying the 14 bp Del/Del (D/D) genotype[34], while Teixeira et al[35] showed that the 14 bp (D/D) genotype increases hepatocellular carcinoma (HCC) susceptibility in Brazilian population. Conversely, the 14 bp INDEL was no associated with HCC and liver cirrhosis susceptibility in a Korean study[137]. The 14 bp I/I was also found to be related to lower OS and DFS in patients with haematological malignancies undergoing allogeneic Hematopoietic Stem Cell Transplantation (allo-HSCT) and Methotrexate (MTX) treatment in univariate and multivariate analysis[138]. The 14 bp I/I genotype was also suggested to represent a therapy marker in Rheumatoid Arthritis (RA), able to identify responder patients treated with MTX[114].
To date, an association among the +3142 C>G SNP and a specific disease status, was recently described only in the Systemic Lupus Erythematous (SLE) autoimmune disorder[37] and in Relapsing-Remitting Multiple Sclerosis (RR-MS)[139] (Table 1).
A first attempt to find possible correlations between HLA-G genotype and phenotype (sHLA-G) was performed by Rebmann et al[140] in 2001, analyzing 94 healthy subjects. In particular, individuals carrying the HLA-G*01041 allele had significantly higher sHLA-G levels, while individuals with HLA-G*01031 allele and HLA-G*0105N allele, presented significantly lower of plasmatic and circulating HLA-G[140]. It should be noted that the 5’UTR and 3’UTR HLA-G regions were not investigated. The 14 bp I/I was then associated with significantly lower levels of sHLA-G in blood plasma or serum in different studies[141,142] (Table 1). Chen et al[143] reported a dramatic and significantly lower expression of sHLA-G in plasmatic samples from healthy donors (n = 150) of Chinese etnicity in the presence of the 14 bp I/I genotype with respect to the 14 bp D/D. Another recent investigation performed in MS patients in serum and cerebro spinal fluid (CSF), demonstrated an association among higher sHLA-G levels and +3142 C/C, 14 bp D/D genotypes and lower sHLA-G levels in +3142 G/G, 14 bp I/I combination[139] (Table 1).
Rizzo et al[144] identified a subgroup of Early Rheumatoid Arthritis (ERA) patients characterized by prevalence in 14 bp D/D, 14 bp D/I polymorphisms, and improved disease remission, therefore highlighting a protective role for the 14 bp Del allele. Moreover, the 14 bp Del allele was associated with higher sHLA-G and mHLA-G production and ITL2 expression. In 2010 Castelli et al[19] defined almost eight distinct 3’UTR haplotypes named from UTR-1 to UTR-8, that include the eight common polymorphisms of this nucleotide segment described above. Furthermore, HLA-G alleles were associated with each haplotype, and high Linkage Disequilibrium (LD) among most of the variants was observed, according to Hardy-Weimberg test. In particular, it was evidenced that the 14 bp Ins allele is always associated with the +3142G and +3187A alleles, both previously related with low mRNA availability, thus suggesting the implication of these two polymorphisms in lower mRNA production is associated with 14 bp insertion (Figure 1). The+3187G allele is only associated with the 3’UTR-1 haplotype carrying the 14 bp deletion[19].
It should be emphasized that 4-bp upstream the SNP +3187A/G there is an AUUUA motif, and 9-bp downstream to +3196C/G there is the presence of an UUAUUU motif. These (AU)-rich elements may modulate mRNA degradation and therefore expression level, and are also influenced by close sequence variations. Some authors, according to this nomenclature, started to analyze data from 3’UTR region in terms of haplotypes using different algorithm approaches such as EM algorithm, PHASE method, and FBAT. This haplotype analysis could represent an amazing way to correlate genetic data to the specific phenotypes of the study grouped also in a dominant or in a recessive model, or used to compare a single common haplotype with other phenotypes grouped together. To date, 3’UTR haplotypes analyses were performed in few studies and not regarded to cancer disease and so to CRC[113,131,145].
Recently, the functional impact of 3’UTR and in particular the 14 bp INDEL polymorphism, was deeply investigated by Svendsen et al[146], with particular attention on the processing and stability of the full-length membrane bound transcript of HLA-G1 (mHLA-G1). Authors, transducing different HLA-G1 DNA sequences in K562 human cell line, demonstrated that mRNA from 14 bp I/I had a higher degree of stability than the others, in accordance to the data reported in literature. Moreover, transductants carrying the 14 bp Ins, presented lower sHLA-G1 levels per mHLA-G1 ratio with respect to the constructs lacking the 14 bp Ins, but were the most efficient in inhibiting NK cytotoxicity[146].
In regard to the 5’UTR region, it has been less investigated and only recently, 16 SNPs in the 5’UTR region (Figure 1) and the 14-bp INDEL polymorphism in 3’UTR were analyzed in the same study in Brazilian patients who underwent assisted reproduction treatments (ART), characterized by failure implantation of embryos[113]. Larsen and Hviid[133], presented a complex panel of haplotypes related to the 5’UTR and in part of the 3’UTR regions showing clear LD between several of the polymorphisms centered around two main lineages of HLA-G alleles named G*010101xx and G*010102xx, in accord to WHO classification. The HLA-G promoter region is considered unique among the HLA genes with many regulatory sequences, such as tissue specific regulatory element (TSRE) from position -1350 to -1100, and IFN-stimulated response element (ISRE) from -726 to -725 position[21,147]. The 5’UTR tri-allelic polymorphism -725C/G/T was evaluated in relation to plasma sHLAG concentration in a recent study in Iraqi women with recurrent pregnancy loss[148] A significantly association among lower levels of sHLAG in presence of the CC genotype was found vs the CG and CT condition[148] (Table 1). Of note, the presence in this position of a G nucleotide may alter the methylation profile of CpG di-nucleotides resulting in a modification of gene expression[113], and also influences binding of ISRE or other regulatory elements.
HLA-G IN CRC AND FUTURE APPLICATIONS
Despite the advances in knowledge and increasing interest in the immune contexture involvement, HLA-G has been poorly investigated in CRC and inflammation associated diseases of the gastrointestinal tract. A common mechanism present in CRC and in various types of cancer used by tumor cells to avoid recognition by CTLs, is the HLA class I down-regulation or total loss[149]. Altered HLA I class phenotypes regard reversible down regulation or irreversible mutational genes inactivation and are usually related to LOH of classical HLA-A, -B and -C heavy chains located in the chromosome region 6p21. HLA-G (protein and/or mRNA) is frequently over-expressed in tumors[16,17], including CRC[16,17,24-26] (Table 2). HLA-G expression was associated with malignant transformation and was never detected in the surrounding and closest areas near the tumor[17]. MHC class I loss or down-regulation due probably in defects or alterations in Processing Machinery (APM) components have been found in different malignancies and associated to reduced MHC class I recognition vs tumor-associated antigen (TAA)-specific CTL and disease progression[150]. LOH in the 15q21 region was observed in progressing lesions after immunotheraphy such as in melanoma[151]. Intriguingly, while classical HLA I proteins are frequently down regulate in in about 15% up to 75% of colon carcinoma lesions[150,152,153], HLA-G (related to isoform G1) results over-expressed in CRC malignant and pre-malignant tissues[26] (Table 2), in accord to his role in the host immune escape. Strong positive HLA-G expression was also detected in UD biopsies but no in tissues taken from patients affected by CD, thus it was proposed as a tool to better distinguish these inflammatory diseases[154] (Table 2).
LOH frequency for classical HLA genes in CRC was reported to be 40% evaluating 95 patients[155]. In CRC, higher LOH percentages were found in other chromosomal regions containing tumor suppressor genes i.e., 43%-79% at 18q, 43%-76% at 17p and 17%-43% at 5q[156]. The irreversible total loss of HLA class I is generally referred to mutations affecting the β2m gene that are usually followed to loss of the second copy by LOH within his locus in the 15q21 region[157,158]. Expression of the β2m protein should be taken in consideration due its fundamental role in associate to HLAs molecules for correct antigen presentation. If β2m is lost, stable antigen-HLA class I complexes cannot be produced[156]. Of note, the major function of HLA-G is not antigen presentation and, if β2m is necessary to assemble the most studied HLA-G1, sHLA-G1 and sHLA-G5 isoforms, it should be discussed the function of alternative spliced isoforms lacking β2m. We speculate that HLA-G alternative spliced isoforms without β2m assembly could represent another way to escape from immune control, especially in tumor such as CRC in which β2m downregulation or loss is a frequent event[158]. Moreover, their role should be established and explored (which are their interactors) providing new insides of the HLA-G planet.
Alterations due to LOH have been reported in 25%[159]-35%[156] of CRCs in β2m 15q21 and in 6p21 in 40% of the same patients[156], demonstrating a strong correlation that may impact on disease progression in terms of immune escape exert by the tumor[160]. To date in CRC, HLA-G polymorphisms and haplotypes have not been investigated to find correlations with prognosis or phenotype (circulating sHLA-G proteins) even if, recently, some authors started to report complex and intriguing analysis not related to cancer[147,148].
Data about the soluble HLA-G has been reported only for CRC patients of Chinese ethnicity[27,28] suggesting that sHLA-G should be considered as a good diagnostic tool superior to classical CEA[27], and also a useful indicator to distinguish benign colorectal related disease from CRC. Of note, Cao et al[28] collected and analyzed plasma samples from a limited patient series, while Zhu et al[27] quantified sHLA-G in a quite large cohort of patients in serum that is not the recommended biological sample (Table 2). Both authors did not genotype patients at the germinal level to search for a possible phenotype correlation and/or to assess specific HLA-G alleles and genotypes related to CRC. No correlations were performed with clinical outcome of CRC patients in terms of survival, disease relapse and response to therapy treatment. In our opinion, detection of sHLA-G is not sufficient alone and should be associated to genotyping of the gene, with particular attention on the regulatory untranslated regions that are susceptible to post translational modifications such as methylation, transcription factors and miRNA binding (Figure 1). Moreover, sHLA-G assay should be standardized and defined by precise guidelines to improve its clinical application. Recently, preliminary data that need further investigations, showed that higher sHLA-G expression in mucor secretory vs non-mucosecretory CRC, correlate with worse prognosis of patients[128].
A differential spontaneous sHLA-G secretion from PBMC was reported in CD (high levels of the secrete molecules) and a lack of sHLA-G in UC also after inflammatory stimulus by Lipopolysaccharide (LPS) activation[161] (Table 2). Conversely, opposite results were previously described analyzing HLA-G protein expression by IHC in CD and UC biopsies, with higher expression levels in UC and negative staining in CD samples[154] (Table 2). Subsequently, these controversial data were not confirmed with novel studies. However, authors who reported a lack of sHLA-G in UC[162], showed that immunosuppressive drugs influence the sHLA-G secretion in UC and CD patients, stimulating its release in UC and decrease it in CD[162] (Table 2). Halama et al[163], basing on the evidence that CRC patient prognosis is dependent on the local immune contexture, characterized NK and T cells localization and densities in primary CRC liver metastasis, adenomas and normal tissues. NK cells were rare in tumor tissue independently of HLA class I expression, and also not depending from chemokine levels that were rather elevated and correlated to T cells infiltration[163] Subsequently, for the first time Rocca et al[164], characterized tumor-associated NK cells (TANKs), with respect to autologous peripheral blood NK cells (PB-NKs), from CRC patients, and compared the latter with PB cells from healthy donors. Authors demonstrated an altered phenotype for TANKs in CRC patients, with a low expression of activating receptors and also with an impaired degranulation and release of cytokines (IFN-γ) capacity. It should be pointed that HLA-G evaluation would be of interest in both these analysis, especially considering recent data about sHLA-G role in impair NK cells by (1) modulation of specific chemokine secretion (CXCR3, CX3CR1, and CCR2); (2) functional inhibition on CD94/NKG2A receptor; and (3) modulation of NK chemokines and cytokines secretion[165].
Finally, the host-immune reaction could be the critical element in determining response to therapy, and the effect on the immune response could be the underlying factor behind many of the predictive markers[13]. In ovarian cancer, positive HLA-G cells from peritoneal and pleural effusions decrease in number after chemotherapy and this result correlates with improved survival of the related patients[166]. These data suggest that HLA-G-expressing cells are more susceptible to elimination by the immune response or treatment[162,166].
On the opposite, IFN-α immunotherapy showed to further increase circulating sHLA-G levels in melanoma patients[30], thus in accord to the presence of ISRE element in the 5’UTR region[21]. It is of knowledge that common therapies in cancer (chemotherapy and radiotherapy) have an impact on immune system[54], that could be different depending from the therapy regiment, often associated to different grades of toxicity and neutropenia[167]. A favourable prognosis correlated with TILs in stage III CRC patients treated by surgery alone (n = 851) or 5-fluorouracil (5-FU) adjuvant chemotherapy (n = 305), was observed[168]. Moreover, high densities TILs in metastatic liver lesions at the invasive margin revealed strong association with chemotherapy efficacy and prognosis in advanced CRC patients[169,170]. These studies based on infiltrating lymphocytes in correlation with therapy, have provided the scientific basis to implement the concept of IS and, in the future, we hope that IS validation will provide a well defined tool to assess CRC prognosis and clinical outcome based also on patient treatment. Due to HLA-G functional properties in the direct inhibition of cytotoxic and memory T lymphocytes, we stress the possible association between IS and HLA-G (Figure 2) and therefore patient prognosis.
Moreover, particular HLA-G haplotypes and/or genotypes and allelic variants could be identified as predictive genetic marker of response treatment, to better stratify patients.
In particular, further investigations in large cohort of patients are needed to define: HLA-G haplotypes, genotypes, and alleles frequencies and association with: (1) the risk of CRC (possible distinctions comparing them with those of UD and CD diseases); (2) the prognostic power: relation with OS, DFS, and predictive value of response to therapy; (3) plasma sHLA-G levels as predictive and prognostic markers; and (4) genotype-phenotype correlations among soluble and HLA-G genotypes/alleles (especially 5’UTR and 3’UTR nucleotide variations).
We suggest that HLA-G could represent a novel prognostic immune biomarker with a prominent role in inhibiting host immune response in CRC. We also suggest to assess an association with IS and HLA-G (Figure 2) to better characterize host immune response and complete the immune contexture overview in CRC patients.
Footnotes
Supported by Associazione Italiana per la Ricerca sul Cancro (AIRC), Special Program Molecular Clinical Oncology, 5X1000, No. 12214 (G.T.); European Research Council, Programme ‘‘Ideas’’, Proposal No. 269051 (G.T., F.R.)
P- Reviewers: Arroyo A, Hsiao KCW, Li QQ S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 4.Weitz J, Koch M, Debus J, Höhler T, Galle PR, Büchler MW. Colorectal cancer. Lancet. 2005;365:153–165. doi: 10.1016/S0140-6736(05)17706-X. [DOI] [PubMed] [Google Scholar]
- 5.de Vogel S, Weijenberg MP, Herman JG, Wouters KA, de Goeij AF, van den Brandt PA, de Bruïne AP, van Engeland M. MGMT and MLH1 promoter methylation versus APC, KRAS and BRAF gene mutations in colorectal cancer: indications for distinct pathways and sequence of events. Ann Oncol. 2009;20:1216–1222. doi: 10.1093/annonc/mdn782. [DOI] [PubMed] [Google Scholar]
- 6.Feagins LA, Souza RF, Spechler SJ. Carcinogenesis in IBD: potential targets for the prevention of colorectal cancer. Nat Rev Gastroenterol Hepatol. 2009;6:297–305. doi: 10.1038/nrgastro.2009.44. [DOI] [PubMed] [Google Scholar]
- 7.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 8.Al-Sohaily S, Biankin A, Leong R, Kohonen-Corish M, Warusavitarne J. Molecular pathways in colorectal cancer. J Gastroenterol Hepatol. 2012;27:1423–1431. doi: 10.1111/j.1440-1746.2012.07200.x. [DOI] [PubMed] [Google Scholar]
- 9.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 10.Baier PK, Wimmenauer S, Hirsch T, von Specht BU, von Kleist S, Keller H, Farthmann EH. Analysis of the T cell receptor variability of tumor-infiltrating lymphocytes in colorectal carcinomas. Tumour Biol. 1998;19:205–212. doi: 10.1159/000030008. [DOI] [PubMed] [Google Scholar]
- 11.Galon J, Pagès F, Marincola FM, Thurin M, Trinchieri G, Fox BA, Gajewski TF, Ascierto PA. The immune score as a new possible approach for the classification of cancer. J Transl Med. 2012;10:1. doi: 10.1186/1479-5876-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pagès F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G, Lagorce C, Wind P, Marliot F, Bruneval P, et al. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol. 2009;27:5944–5951. doi: 10.1200/JCO.2008.19.6147. [DOI] [PubMed] [Google Scholar]
- 13.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 14.Camus M, Tosolini M, Mlecnik B, Pagès F, Kirilovsky A, Berger A, Costes A, Bindea G, Charoentong P, Bruneval P, et al. Coordination of intratumoral immune reaction and human colorectal cancer recurrence. Cancer Res. 2009;69:2685–2693. doi: 10.1158/0008-5472.CAN-08-2654. [DOI] [PubMed] [Google Scholar]
- 15.Galon J, Pagès F, Marincola FM, Angell HK, Thurin M, Lugli A, Zlobec I, Berger A, Bifulco C, Botti G, et al. Cancer classification using the Immunoscore: a worldwide task force. J Transl Med. 2012;10:205. doi: 10.1186/1479-5876-10-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carosella ED, Moreau P, Lemaoult J, Rouas-Freiss N. HLA-G: from biology to clinical benefits. Trends Immunol. 2008;29:125–132. doi: 10.1016/j.it.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Rouas-Freiss N, Moreau P, Ferrone S, Carosella ED. HLA-G proteins in cancer: do they provide tumor cells with an escape mechanism. Cancer Res. 2005;65:10139–10144. doi: 10.1158/0008-5472.CAN-05-0097. [DOI] [PubMed] [Google Scholar]
- 18.Carosella ED, Favier B, Rouas-Freiss N, Moreau P, Lemaoult J. Beyond the increasing complexity of the immunomodulatory HLA-G molecule. Blood. 2008;111:4862–4870. doi: 10.1182/blood-2007-12-127662. [DOI] [PubMed] [Google Scholar]
- 19.Castelli EC, Mendes-Junior CT, Deghaide NH, de Albuquerque RS, Muniz YC, Simões RT, Carosella ED, Moreau P, Donadi EA. The genetic structure of 3’untranslated region of the HLA-G gene: polymorphisms and haplotypes. Genes Immun. 2010;11:134–141. doi: 10.1038/gene.2009.74. [DOI] [PubMed] [Google Scholar]
- 20.Rousseau P, Le Discorde M, Mouillot G, Marcou C, Carosella ED, Moreau P. The 14 bp deletion-insertion polymorphism in the 3’ UT region of the HLA-G gene influences HLA-G mRNA stability. Hum Immunol. 2003;64:1005–1010. doi: 10.1016/j.humimm.2003.08.347. [DOI] [PubMed] [Google Scholar]
- 21.Donadi EA, Castelli EC, Arnaiz-Villena A, Roger M, Rey D, Moreau P. Implications of the polymorphism of HLA-G on its function, regulation, evolution and disease association. Cell Mol Life Sci. 2011;68:369–395. doi: 10.1007/s00018-010-0580-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menier C, Rouas-Freiss N, Favier B, LeMaoult J, Moreau P, Carosella ED. Recent advances on the non-classical major histocompatibility complex class I HLA-G molecule. Tissue Antigens. 2010;75:201–206. doi: 10.1111/j.1399-0039.2009.01438.x. [DOI] [PubMed] [Google Scholar]
- 23.Curigliano G, Criscitiello C, Gelao L, Goldhirsch A. Molecular pathways: human leukocyte antigen G (HLA-G) Clin Cancer Res. 2013;19:5564–5571. doi: 10.1158/1078-0432.CCR-12-3697. [DOI] [PubMed] [Google Scholar]
- 24.Fukushima Y, Oshika Y, Nakamura M, Tokunaga T, Hatanaka H, Abe Y, Yamazaki H, Kijima H, Ueyama Y, Tamaoki N. Increased expression of human histocompatibility leukocyte antigen-G in colorectal cancer cells. Int J Mol Med. 1998;2:349–351. doi: 10.3892/ijmm.2.3.349. [DOI] [PubMed] [Google Scholar]
- 25.Ye SR, Yang H, Li K, Dong DD, Lin XM, Yie SM. Human leukocyte antigen G expression: as a significant prognostic indicator for patients with colorectal cancer. Mod Pathol. 2007;20:375–383. doi: 10.1038/modpathol.3800751. [DOI] [PubMed] [Google Scholar]
- 26.Hansel DE, Rahman A, Wilentz RE, Shih IeM, McMaster MT, Yeo CJ, Maitra A. HLA-G upregulation in pre-malignant and malignant lesions of the gastrointestinal tract. Int J Gastrointest Cancer. 2005;35:15–23. doi: 10.1385/ijgc:35:1:015. [DOI] [PubMed] [Google Scholar]
- 27.Zhu CB, Wang CX, Zhang X, Zhang J, Li W. Serum sHLA-G levels: a useful indicator in distinguishing colorectal cancer from benign colorectal diseases. Int J Cancer. 2011;128:617–622. doi: 10.1002/ijc.25372. [DOI] [PubMed] [Google Scholar]
- 28.Cao M, Yie SM, Liu J, Ye SR, Xia D, Gao E. Plasma soluble HLA-G is a potential biomarker for diagnosis of colorectal, gastric, esophageal and lung cancer. Tissue Antigens. 2011;78:120–128. doi: 10.1111/j.1399-0039.2011.01716.x. [DOI] [PubMed] [Google Scholar]
- 29.Sebti Y, Le Maux A, Gros F, De Guibert S, Pangault C, Rouas-Freiss N, Bernard M, Amiot L. Expression of functional soluble human leucocyte antigen-G molecules in lymphoproliferative disorders. Br J Haematol. 2007;138:202–212. doi: 10.1111/j.1365-2141.2007.06647.x. [DOI] [PubMed] [Google Scholar]
- 30.Ugurel S, Rebmann V, Ferrone S, Tilgen W, Grosse-Wilde H, Reinhold U. Soluble human leukocyte antigen--G serum level is elevated in melanoma patients and is further increased by interferon-alpha immunotherapy. Cancer. 2001;92:369–376. doi: 10.1002/1097-0142(20010715)92:2<369::aid-cncr1332>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 31.Dardano A, Rizzo R, Polini A, Stignani M, Tognini S, Pasqualetti G, Ursino S, Colato C, Ferdeghini M, Baricordi OR, et al. Soluble human leukocyte antigen-g and its insertion/deletion polymorphism in papillary thyroid carcinoma: novel potential biomarkers of disease. J Clin Endocrinol Metab. 2012;97:4080–4086. doi: 10.1210/jc.2012-2231. [DOI] [PubMed] [Google Scholar]
- 32.Castelli EC, Moreau P, Oya e Chiromatzo A, Mendes-Junior CT, Veiga-Castelli LC, Yaghi L, Giuliatti S, Carosella ED, Donadi EA. In silico analysis of microRNAS targeting the HLA-G 3’ untranslated region alleles and haplotypes. Hum Immunol. 2009;70:1020–1025. doi: 10.1016/j.humimm.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 33.Ferguson R, Ramanakumar AV, Koushik A, Coutlée F, Franco E, Roger M. Human leukocyte antigen G polymorphism is associated with an increased risk of invasive cancer of the uterine cervix. Int J Cancer. 2012;131:E312–E319. doi: 10.1002/ijc.27356. [DOI] [PubMed] [Google Scholar]
- 34.Chen Y, Gao XJ, Deng YC, Zhang HX. Relationship between HLA-G gene polymorphism and the susceptibility of esophageal cancer in Kazakh and Han nationality in Xinjiang. Biomarkers. 2012;17:9–15. doi: 10.3109/1354750X.2011.633242. [DOI] [PubMed] [Google Scholar]
- 35.Teixeira AC, Mendes-Junior CT, Souza FF, Marano LA, Deghaide NH, Ferreira SC, Mente ED, Sankarankutty AK, Elias-Junior J, Castro-e-Silva O, et al. The 14bp-deletion allele in the HLA-G gene confers susceptibility to the development of hepatocellular carcinoma in the Brazilian population. Tissue Antigens. 2013;81:408–413. doi: 10.1111/tan.12097. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Z, Li Y, Zhang LL, Jia LT, Yang XQ. Association of 14 bp insertion/deletion polymorphism of the HLA-G gene in father with severe preeclampsia in Chinese. Tissue Antigens. 2012;80:158–164. doi: 10.1111/j.1399-0039.2012.01907.x. [DOI] [PubMed] [Google Scholar]
- 37.Consiglio CR, Veit TD, Monticielo OA, Mucenic T, Xavier RM, Brenol JC, Chies JA. Association of the HLA-G gene +3142C& gt; G polymorphism with systemic lupus erythematosus. Tissue Antigens. 2011;77:540–545. doi: 10.1111/j.1399-0039.2011.01635.x. [DOI] [PubMed] [Google Scholar]
- 38.Pino MS, Chung DC. The chromosomal instability pathway in colon cancer. Gastroenterology. 2010;138:2059–2072. doi: 10.1053/j.gastro.2009.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology. 2010;138:2073–2087.e3. doi: 10.1053/j.gastro.2009.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Imai K, Yamamoto H. Carcinogenesis and microsatellite instability: the interrelationship between genetics and epigenetics. Carcinogenesis. 2008;29:673–680. doi: 10.1093/carcin/bgm228. [DOI] [PubMed] [Google Scholar]
- 41.Garagnani P, Pirazzini C, Franceschi C. Colorectal cancer microenvironment: among nutrition, gut microbiota, inflammation and epigenetics. Curr Pharm Des. 2013;19:765–778. [PubMed] [Google Scholar]
- 42.Geboes K. What histologic features best differentiate Crohn’s disease from ulcerative colitis. Inflamm Bowel Dis. 2008;14 Suppl 2:S168–S169. doi: 10.1002/ibd.20598. [DOI] [PubMed] [Google Scholar]
- 43.Harpaz N, Ward SC, Mescoli C, Itzkowitz SH, Polydorides AD. Precancerous lesions in inflammatory bowel disease. Best Pract Res Clin Gastroenterol. 2013;27:257–267. doi: 10.1016/j.bpg.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 44.Rogler G. Chronic ulcerative colitis and colorectal cancer. Cancer Lett. 2014;345:235–241. doi: 10.1016/j.canlet.2013.07.032. [DOI] [PubMed] [Google Scholar]
- 45.Yoshimi K, Tanaka T, Serikawa T, Kuramoto T. Tumor suppressor APC protein is essential in mucosal repair from colonic inflammation through angiogenesis. Am J Pathol. 2013;182:1263–1274. doi: 10.1016/j.ajpath.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 46.Hussain SP, Amstad P, Raja K, Ambs S, Nagashima M, Bennett WP, Shields PG, Ham AJ, Swenberg JA, Marrogi AJ, et al. Increased p53 mutation load in noncancerous colon tissue from ulcerative colitis: a cancer-prone chronic inflammatory disease. Cancer Res. 2000;60:3333–3337. [PubMed] [Google Scholar]
- 47.Cooks T, Pateras IS, Tarcic O, Solomon H, Schetter AJ, Wilder S, Lozano G, Pikarsky E, Forshew T, Rosenfeld N, et al. Mutant p53 prolongs NF-κB activation and promotes chronic inflammation and inflammation-associated colorectal cancer. Cancer Cell. 2013;23:634–646. doi: 10.1016/j.ccr.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Terzić J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–2114.e5. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 49.Ino Y, Yamazaki-Itoh R, Shimada K, Iwasaki M, Kosuge T, Kanai Y, Hiraoka N. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br J Cancer. 2013;108:914–923. doi: 10.1038/bjc.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang K, Grivennikov SI, Karin M. Implications of anti-cytokine therapy in colorectal cancer and autoimmune diseases. Ann Rheum Dis. 2013;72 Suppl 2:ii100–ii103. doi: 10.1136/annrheumdis-2012-202201. [DOI] [PubMed] [Google Scholar]
- 52.Neufert C, Becker C, Neurath MF. An inducible mouse model of colon carcinogenesis for the analysis of sporadic and inflammation-driven tumor progression. Nat Protoc. 2007;2:1998–2004. doi: 10.1038/nprot.2007.279. [DOI] [PubMed] [Google Scholar]
- 53.Koudougou C, Bonneville M, Matysiak-Budnik T, Touchefeu Y. Review article: antitumoural immunity in colorectal cancer - current and potential future implications in clinical practice. Aliment Pharmacol Ther. 2013;38:3–15. doi: 10.1111/apt.12337. [DOI] [PubMed] [Google Scholar]
- 54.Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 55.Fridman WH, Dieu-Nosjean MC, Pagès F, Cremer I, Damotte D, Sautès-Fridman C, Galon J. The immune microenvironment of human tumors: general significance and clinical impact. Cancer Microenviron. 2013;6:117–122. doi: 10.1007/s12307-012-0124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–271. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 57.Vesely MD, Schreiber RD. Cancer immunoediting: antigens, mechanisms, and implications to cancer immunotherapy. Ann N Y Acad Sci. 2013;1284:1–5. doi: 10.1111/nyas.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thomas L. On immunosurveillance in human cancer. Yale J Biol Med. 1982;55:329–333. [PMC free article] [PubMed] [Google Scholar]
- 59.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 60.Grizzi F, Bianchi P, Malesci A, Laghi L. Prognostic value of innate and adaptive immunity in colorectal cancer. World J Gastroenterol. 2013;19:174–184. doi: 10.3748/wjg.v19.i2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22:231–237. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 63.Kojima M, Morisaki T, Uchiyama A, Doi F, Mibu R, Katano M, Tanaka M. Association of enhanced cyclooxygenase-2 expression with possible local immunosuppression in human colorectal carcinomas. Ann Surg Oncol. 2001;8:458–465. doi: 10.1007/s10434-001-0458-x. [DOI] [PubMed] [Google Scholar]
- 64.Riboldi E, Porta C, Morlacchi S, Viola A, Mantovani A, Sica A. Hypoxia-mediated regulation of macrophage functions in pathophysiology. Int Immunol. 2013;25:67–75. doi: 10.1093/intimm/dxs110. [DOI] [PubMed] [Google Scholar]
- 65.Biswas SK, Allavena P, Mantovani A. Tumor-associated macrophages: functional diversity, clinical significance, and open questions. Semin Immunopathol. 2013;35:585–600. doi: 10.1007/s00281-013-0367-7. [DOI] [PubMed] [Google Scholar]
- 66.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest. 2007;117:1155–1166. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cook J, Hagemann T. Tumour-associated macrophages and cancer. Curr Opin Pharmacol. 2013;13:595–601. doi: 10.1016/j.coph.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 68.Zhang QW, Liu L, Gong CY, Shi HS, Zeng YH, Wang XZ, Zhao YW, Wei YQ. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS One. 2012;7:e50946. doi: 10.1371/journal.pone.0050946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Salama P, Platell C. Host response to colorectal cancer. ANZ J Surg. 2008;78:745–753. doi: 10.1111/j.1445-2197.2008.04642.x. [DOI] [PubMed] [Google Scholar]
- 70.Saleh M, Trinchieri G. Innate immune mechanisms of colitis and colitis-associated colorectal cancer. Nat Rev Immunol. 2011;11:9–20. doi: 10.1038/nri2891. [DOI] [PubMed] [Google Scholar]
- 71.Disis ML. Immune regulation of cancer. J Clin Oncol. 2010;28:4531–4538. doi: 10.1200/JCO.2009.27.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Campbell KS, Hasegawa J. Natural killer cell biology: an update and future directions. J Allergy Clin Immunol. 2013;132:536–544. doi: 10.1016/j.jaci.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kloor M, Michel S, von Knebel Doeberitz M. Immune evasion of microsatellite unstable colorectal cancers. Int J Cancer. 2010;127:1001–1010. doi: 10.1002/ijc.25283. [DOI] [PubMed] [Google Scholar]
- 74.Gerlach C, Rohr JC, Perié L, van Rooij N, van Heijst JW, Velds A, Urbanus J, Naik SH, Jacobs H, Beltman JB, et al. Heterogeneous differentiation patterns of individual CD8+ T cells. Science. 2013;340:635–639. doi: 10.1126/science.1235487. [DOI] [PubMed] [Google Scholar]
- 75.Baleeiro RB, Wiesmüller KH, Dähne L, Lademann J, Barbuto JA, Walden P. Direct activation of human dendritic cells by particle-bound but not soluble MHC class II ligand. PLoS One. 2013;8:e63039. doi: 10.1371/journal.pone.0063039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Algarra I, García-Lora A, Cabrera T, Ruiz-Cabello F, Garrido F. The selection of tumor variants with altered expression of classical and nonclassical MHC class I molecules: implications for tumor immune escape. Cancer Immunol Immunother. 2004;53:904–910. doi: 10.1007/s00262-004-0517-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Matsushita H, Vesely MD, Koboldt DC, Rickert CG, Uppaluri R, Magrini VJ, Arthur CD, White JM, Chen YS, Shea LK, et al. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature. 2012;482:400–404. doi: 10.1038/nature10755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liao W, Lin JX, Leonard WJ. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity. 2013;38:13–25. doi: 10.1016/j.immuni.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bianchini R, Bistoni O, Alunno A, Petrillo MG, Ronchetti S, Sportoletti P, Bocci EB, Nocentini G, Gerli R, Riccardi C. CD4(+) CD25(low) GITR(+) cells: a novel human CD4(+) T-cell population with regulatory activity. Eur J Immunol. 2011;41:2269–2278. doi: 10.1002/eji.201040943. [DOI] [PubMed] [Google Scholar]
- 80.Evans C, Dalgleish AG, Kumar D. Review article: immune suppression and colorectal cancer. Aliment Pharmacol Ther. 2006;24:1163–1177. doi: 10.1111/j.1365-2036.2006.03075.x. [DOI] [PubMed] [Google Scholar]
- 81.Galizia G, Lieto E, De Vita F, Romano C, Orditura M, Castellano P, Imperatore V, Infusino S, Catalano G, Pignatelli C. Circulating levels of interleukin-10 and interleukin-6 in gastric and colon cancer patients before and after surgery: relationship with radicality and outcome. J Interferon Cytokine Res. 2002;22:473–482. doi: 10.1089/10799900252952262. [DOI] [PubMed] [Google Scholar]
- 82.Heriot AG, Marriott JB, Cookson S, Kumar D, Dalgleish AG. Reduction in cytokine production in colorectal cancer patients: association with stage and reversal by resection. Br J Cancer. 2000;82:1009–1012. doi: 10.1054/bjoc.1999.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Galon J, Angell HK, Bedognetti D, Marincola FM. The continuum of cancer immunosurveillance: prognostic, predictive, and mechanistic signatures. Immunity. 2013;39:11–26. doi: 10.1016/j.immuni.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 84.Mlecnik B, Tosolini M, Charoentong P, Kirilovsky A, Bindea G, Berger A, Camus M, Gillard M, Bruneval P, Fridman WH, et al. Biomolecular network reconstruction identifies T-cell homing factors associated with survival in colorectal cancer. Gastroenterology. 2010;138:1429–1440. doi: 10.1053/j.gastro.2009.10.057. [DOI] [PubMed] [Google Scholar]
- 85.Atreya I, Neurath MF. Immune cells in colorectal cancer: prognostic relevance and therapeutic strategies. Expert Rev Anticancer Ther. 2008;8:561–572. doi: 10.1586/14737140.8.4.561. [DOI] [PubMed] [Google Scholar]
- 86.Oberg A, Samii S, Stenling R, Lindmark G. Different occurrence of CD8+, CD45R0+, and CD68+ immune cells in regional lymph node metastases from colorectal cancer as potential prognostic predictors. Int J Colorectal Dis. 2002;17:25–29. doi: 10.1007/s003840100337. [DOI] [PubMed] [Google Scholar]
- 87.Clemente CG, Mihm MC, Bufalino R, Zurrida S, Collini P, Cascinelli N. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer. 1996;77:1303–1310. doi: 10.1002/(SICI)1097-0142(19960401)77:7<1303::AID-CNCR12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 88.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pagès F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 90.Sobin LH, Compton CC. TNM seventh edition: what’s new, what’s changed: communication from the International Union Against Cancer and the American Joint Committee on Cancer. Cancer. 2010;116:5336–5339. doi: 10.1002/cncr.25537. [DOI] [PubMed] [Google Scholar]
- 91.Mlecnik B, Tosolini M, Kirilovsky A, Berger A, Bindea G, Meatchi T, Bruneval P, Trajanoski Z, Fridman WH, Pagès F, et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol. 2011;29:610–618. doi: 10.1200/JCO.2010.30.5425. [DOI] [PubMed] [Google Scholar]
- 92.Broussard EK, Disis ML. TNM staging in colorectal cancer: T is for T cell and M is for memory. J Clin Oncol. 2011;29:601–603. doi: 10.1200/JCO.2010.32.9078. [DOI] [PubMed] [Google Scholar]
- 93.Angell H, Galon J. From the immune contexture to the Immunoscore: the role of prognostic and predictive immune markers in cancer. Curr Opin Immunol. 2013;25:261–267. doi: 10.1016/j.coi.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 94.Ascierto PA, Capone M, Urba WJ, Bifulco CB, Botti G, Lugli A, Marincola FM, Ciliberto G, Galon J, Fox BA. The additional facet of immunoscore: immunoprofiling as a possible predictive tool for cancer treatment. J Transl Med. 2013;11:54. doi: 10.1186/1479-5876-11-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hofmeister V, Weiss EH. HLA-G modulates immune responses by diverse receptor interactions. Semin Cancer Biol. 2003;13:317–323. doi: 10.1016/s1044-579x(03)00022-1. [DOI] [PubMed] [Google Scholar]
- 96.Pagès F, Galon J, Dieu-Nosjean MC, Tartour E, Sautès-Fridman C, Fridman WH. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. 2010;29:1093–1102. doi: 10.1038/onc.2009.416. [DOI] [PubMed] [Google Scholar]
- 97.Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, Platell C, Iacopetta B. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27:186–192. doi: 10.1200/JCO.2008.18.7229. [DOI] [PubMed] [Google Scholar]
- 98.Wang F, Jing X, Li G, Wang T, Yang B, Zhu Z, Gao Y, Zhang Q, Yang Y, Wang Y, et al. Foxp3+ regulatory T cells are associated with the natural history of chronic hepatitis B and poor prognosis of hepatocellular carcinoma. Liver Int. 2012;32:644–655. doi: 10.1111/j.1478-3231.2011.02675.x. [DOI] [PubMed] [Google Scholar]
- 99.Flammiger A, Weisbach L, Huland H, Tennstedt P, Simon R, Minner S, Bokemeyer C, Sauter G, Schlomm T, Trepel M. High tissue density of FOXP3+ T cells is associated with clinical outcome in prostate cancer. Eur J Cancer. 2013;49:1273–1279. doi: 10.1016/j.ejca.2012.11.035. [DOI] [PubMed] [Google Scholar]
- 100.Hiraoka N, Onozato K, Kosuge T, Hirohashi S. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res. 2006;12:5423–5434. doi: 10.1158/1078-0432.CCR-06-0369. [DOI] [PubMed] [Google Scholar]
- 101.Chaput N, Louafi S, Bardier A, Charlotte F, Vaillant JC, Ménégaux F, Rosenzwajg M, Lemoine F, Klatzmann D, Taieb J. Identification of CD8+CD25+Foxp3+ suppressive T cells in colorectal cancer tissue. Gut. 2009;58:520–529. doi: 10.1136/gut.2008.158824. [DOI] [PubMed] [Google Scholar]
- 102.Merlo A, Casalini P, Carcangiu ML, Malventano C, Triulzi T, Mènard S, Tagliabue E, Balsari A. FOXP3 expression and overall survival in breast cancer. J Clin Oncol. 2009;27:1746–1752. doi: 10.1200/JCO.2008.17.9036. [DOI] [PubMed] [Google Scholar]
- 103.Tao H, Mimura Y, Aoe K, Kobayashi S, Yamamoto H, Matsuda E, Okabe K, Matsumoto T, Sugi K, Ueoka H. Prognostic potential of FOXP3 expression in non-small cell lung cancer cells combined with tumor-infiltrating regulatory T cells. Lung Cancer. 2012;75:95–101. doi: 10.1016/j.lungcan.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 104.Winerdal ME, Marits P, Winerdal M, Hasan M, Rosenblatt R, Tolf A, Selling K, Sherif A, Winqvist O. FOXP3 and survival in urinary bladder cancer. BJU Int. 2011;108:1672–1678. doi: 10.1111/j.1464-410X.2010.10020.x. [DOI] [PubMed] [Google Scholar]
- 105.Kim M, Grimmig T, Grimm M, Lazariotou M, Meier E, Rosenwald A, Tsaur I, Blaheta R, Heemann U, Germer CT, et al. Expression of Foxp3 in colorectal cancer but not in Treg cells correlates with disease progression in patients with colorectal cancer. PLoS One. 2013;8:e53630. doi: 10.1371/journal.pone.0053630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Finn OJ. Immuno-oncology: understanding the function and dysfunction of the immune system in cancer. Ann Oncol. 2012;23 Suppl 8:viii6–viii9. doi: 10.1093/annonc/mds256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Celsi F, Catamo E, Kleiner G, Tricarico PM, Vuch J, Crovella S. HLA-G/C, miRNAs, and their role in HIV infection and replication. Biomed Res Int. 2013;2013:693643. doi: 10.1155/2013/693643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rouas-Freiss N, LeMaoult J, Moreau P, Dausset J, Carosella ED. HLA-G in transplantation: a relevant molecule for inhibition of graft rejection. Am J Transplant. 2003;3:11–16. doi: 10.1034/j.1600-6143.2003.30103.x. [DOI] [PubMed] [Google Scholar]
- 109.Rouas-Freiss N, Moreau P, Menier C, Carosella ED. HLA-G in cancer: a way to turn off the immune system. Semin Cancer Biol. 2003;13:325–336. doi: 10.1016/s1044-579x(03)00023-3. [DOI] [PubMed] [Google Scholar]
- 110.Roussev RG, Coulam CB. HLA-G and its role in implantation (review) J Assist Reprod Genet. 2007;24:288–295. doi: 10.1007/s10815-007-9148-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nardi Fda S, Slowik R, Wowk PF, da Silva JS, Gelmini GF, Michelon TF, Neumann J, Bicalho Mda G. Analysis of HLA-G polymorphisms in couples with implantation failure. Am J Reprod Immunol. 2012;68:507–514. doi: 10.1111/aji.12001. [DOI] [PubMed] [Google Scholar]
- 112.Hviid TV, Hylenius S, Hoegh AM, Kruse C, Christiansen OB. HLA-G polymorphisms in couples with recurrent spontaneous abortions. Tissue Antigens. 2002;60:122–132. doi: 10.1034/j.1399-0039.2002.600202.x. [DOI] [PubMed] [Google Scholar]
- 113.Costa CH, Gelmini GF, Wowk PF, Mattar SB, Vargas RG, Roxo VM, Schuffner A, Bicalho Mda G. HLA-G regulatory haplotypes and implantation outcome in couples who underwent assisted reproduction treatment. Hum Immunol. 2012;73:891–897. doi: 10.1016/j.humimm.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 114.Rizzo R, Rubini M, Govoni M, Padovan M, Melchiorri L, Stignani M, Carturan S, Ferretti S, Trotta F, Baricordi OR. HLA-G 14-bp polymorphism regulates the methotrexate response in rheumatoid arthritis. Pharmacogenet Genomics. 2006;16:615–623. doi: 10.1097/01.fpc.0000230115.41828.3a. [DOI] [PubMed] [Google Scholar]
- 115.Martinez-Laso J, Herraiz MA, Peñaloza J, Barbolla ML, Jurado ML, Macedo J, Vidart J, Cervera I. Promoter sequences confirm the three different evolutionary lineages described for HLA-G. Hum Immunol. 2013;74:383–388. doi: 10.1016/j.humimm.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 116.Shaikly VR, Morrison IE, Taranissi M, Noble CV, Withey AD, Cherry RJ, Blois SM, Fernández N. Analysis of HLA-G in maternal plasma, follicular fluid, and preimplantation embryos reveal an asymmetric pattern of expression. J Immunol. 2008;180:4330–4337. doi: 10.4049/jimmunol.180.6.4330. [DOI] [PubMed] [Google Scholar]
- 117.Clements CS, Kjer-Nielsen L, Kostenko L, Hoare HL, Dunstone MA, Moses E, Freed K, Brooks AG, Rossjohn J, McCluskey J. Crystal structure of HLA-G: a nonclassical MHC class I molecule expressed at the fetal-maternal interface. Proc Natl Acad Sci USA. 2005;102:3360–3365. doi: 10.1073/pnas.0409676102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shiroishi M, Kuroki K, Ose T, Rasubala L, Shiratori I, Arase H, Tsumoto K, Kumagai I, Kohda D, Maenaka K. Efficient leukocyte Ig-like receptor signaling and crystal structure of disulfide-linked HLA-G dimer. J Biol Chem. 2006;281:10439–10447. doi: 10.1074/jbc.M512305200. [DOI] [PubMed] [Google Scholar]
- 119.Pistoia V, Morandi F, Wang X, Ferrone S. Soluble HLA-G: Are they clinically relevant. Semin Cancer Biol. 2007;17:469–479. doi: 10.1016/j.semcancer.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Howangyin KY, Loustau M, Wu J, Alegre E, Daouya M, Caumartin J, Sousa S, Horuzsko A, Carosella ED, Lemaoult J. Multimeric structures of HLA-G isoforms function through differential binding to LILRB receptors. Cell Mol Life Sci. 2012:Epub ahead of print. doi: 10.1007/s00018-012-1069-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rebmann V, LeMaoult J, Rouas-Freiss N, Carosella ED, Grosse-Wilde H. Quantification and identification of soluble HLA-G isoforms. Tissue Antigens. 2007;69 Suppl 1:143–149. doi: 10.1111/j.1399-0039.2006.763_5.x. [DOI] [PubMed] [Google Scholar]
- 122.Fainardi E, Rizzo R, Melchiorri L, Stignani M, Castellazzi M, Caniatti ML, Baldi E, Tola MR, Granieri E, Baricordi OR. Soluble HLA-G molecules are released as HLA-G5 and not as soluble HLA-G1 isoforms in CSF of patients with relapsing-remitting multiple sclerosis. J Neuroimmunol. 2007;192:219–225. doi: 10.1016/j.jneuroim.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 123.Rudstein-Svetlicky N, Loewenthal R, Horejsi V, Gazit E. HLA-G levels in serum and plasma. Tissue Antigens. 2006;67:111–116. doi: 10.1111/j.1399-0039.2006.00540.x. [DOI] [PubMed] [Google Scholar]
- 124.Rebmann V, Lemaoult J, Rouas-Freiss N, Carosella ED, Grosse-Wilde H. Report of the Wet Workshop for Quantification of Soluble HLA-G in Essen, 2004. Hum Immunol. 2005;66:853–863. doi: 10.1016/j.humimm.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 125.Zhao L, Teklemariam T, Hantash BM. Reassessment of HLA-G isoform specificity of MEM-G/9 and 4H84 monoclonal antibodies. Tissue Antigens. 2012;80:231–238. doi: 10.1111/j.1399-0039.2012.01922.x. [DOI] [PubMed] [Google Scholar]
- 126.Riteau B, Faure F, Menier C, Viel S, Carosella ED, Amigorena S, Rouas-Freiss N. Exosomes bearing HLA-G are released by melanoma cells. Hum Immunol. 2003;64:1064–1072. doi: 10.1016/j.humimm.2003.08.344. [DOI] [PubMed] [Google Scholar]
- 127.Alegre E, Rebmann V, Lemaoult J, Rodriguez C, Horn PA, Díaz-Lagares A, Echeveste JI, González A. In vivo identification of an HLA-G complex as ubiquitinated protein circulating in exosomes. Eur J Immunol. 2013;43:1933–1939. doi: 10.1002/eji.201343318. [DOI] [PubMed] [Google Scholar]
- 128.Loustau M, Wiendl H, Ferrone S, Carosella ED. HLA-G 2012 conference: the 15-year milestone update. Tissue Antigens. 2013;81:127–136. doi: 10.1111/tan.12053. [DOI] [PubMed] [Google Scholar]
- 129.Caumartin J, Favier B, Daouya M, Guillard C, Moreau P, Carosella ED, LeMaoult J. Trogocytosis-based generation of suppressive NK cells. EMBO J. 2007;26:1423–1433. doi: 10.1038/sj.emboj.7601570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dong DD, Yie SM, Li K, Li F, Xu Y, Xu G, Song L, Yang H. Importance of HLA-G expression and tumor infiltrating lymphocytes in molecular subtypes of breast cancer. Hum Immunol. 2012;73:998–1004. doi: 10.1016/j.humimm.2012.07.321. [DOI] [PubMed] [Google Scholar]
- 131.Garcia A, Milet J, Courtin D, Sabbagh A, Massaro JD, Castelli EC, Migot-Nabias F, Favier B, Rouas-Freiss N, Donadi EA, et al. Association of HLA-G 3’UTR polymorphisms with response to malaria infection: a first insight. Infect Genet Evol. 2013;16:263–269. doi: 10.1016/j.meegid.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 132.Hviid TV, Hylenius S, Rørbye C, Nielsen LG. HLA-G allelic variants are associated with differences in the HLA-G mRNA isoform profile and HLA-G mRNA levels. Immunogenetics. 2003;55:63–79. doi: 10.1007/s00251-003-0547-z. [DOI] [PubMed] [Google Scholar]
- 133.Larsen MH, Hviid TV. Human leukocyte antigen-G polymorphism in relation to expression, function, and disease. Hum Immunol. 2009;70:1026–1034. doi: 10.1016/j.humimm.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 134.Tan Z, Randall G, Fan J, Camoretti-Mercado B, Brockman-Schneider R, Pan L, Solway J, Gern JE, Lemanske RF, Nicolae D, et al. Allele-specific targeting of microRNAs to HLA-G and risk of asthma. Am J Hum Genet. 2007;81:829–834. doi: 10.1086/521200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lin A, Zhang X, Xu HH, Xu DP, Ruan YY, Yan WH. HLA-G expression is associated with metastasis and poor survival in the Balb/c nu/nu murine tumor model with ovarian cancer. Int J Cancer. 2012;131:150–157. doi: 10.1002/ijc.26375. [DOI] [PubMed] [Google Scholar]
- 136.Lin A, Xu HH, Xu DP, Zhang X, Wang Q, Yan WH. Multiple steps of HLA-G in ovarian carcinoma metastasis: alter NK cytotoxicity and induce matrix metalloproteinase-15 (MMP-15) expression. Hum Immunol. 2013;74:439–446. doi: 10.1016/j.humimm.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 137.Kim SK, Chung JH, Jeon JW, Park JJ, Cha JM, Joo KR, Lee JI, Shin HP. Association between HLA-G 14-bp insertion/deletion polymorphism and hepatocellular carcinoma in Korean patients with chronic hepatitis B viral infection. Hepatogastroenterology. 2013;60:796–798. doi: 10.5754/hge11180. [DOI] [PubMed] [Google Scholar]
- 138.Chiusolo P, Bellesi S, Piccirillo N, Giammarco S, Marietti S, De Ritis D, Metafuni E, Stignani M, Baricordi OR, Sica S, et al. The role of HLA--G 14-bp polymorphism in allo-HSCT after short-term course MTX for GvHD prophylaxis. Bone Marrow Transplant. 2012;47:120–124. doi: 10.1038/bmt.2011.40. [DOI] [PubMed] [Google Scholar]
- 139.Rizzo R, Bortolotti D, Fredj NB, Rotola A, Cura F, Castellazzi M, Tamborino C, Seraceni S, Baldi E, Melchiorri L, et al. Role of HLA-G 14bp deletion/insertion and +3142C& gt; G polymorphisms in the production of sHLA-G molecules in relapsing-remitting multiple sclerosis. Hum Immunol. 2012;73:1140–1146. doi: 10.1016/j.humimm.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 140.Rebmann V, van der Ven K, Pässler M, Pfeiffer K, Krebs D, Grosse-Wilde H. Association of soluble HLA-G plasma levels with HLA-G alleles. Tissue Antigens. 2001;57:15–21. doi: 10.1034/j.1399-0039.2001.057001015.x. [DOI] [PubMed] [Google Scholar]
- 141.Hviid TV, Rizzo R, Christiansen OB, Melchiorri L, Lindhard A, Baricordi OR. HLA-G and IL-10 in serum in relation to HLA-G genotype and polymorphisms. Immunogenetics. 2004;56:135–141. doi: 10.1007/s00251-004-0673-2. [DOI] [PubMed] [Google Scholar]
- 142.Twito T, Joseph J, Mociornita A, Rao V, Ross H, Delgado DH. The 14-bp deletion in the HLA-G gene indicates a low risk for acute cellular rejection in heart transplant recipients. J Heart Lung Transplant. 2011;30:778–782. doi: 10.1016/j.healun.2011.01.726. [DOI] [PubMed] [Google Scholar]
- 143.Chen XY, Yan WH, Lin A, Xu HH, Zhang JG, Wang XX. The 14 bp deletion polymorphisms in HLA-G gene play an important role in the expression of soluble HLA-G in plasma. Tissue Antigens. 2008;72:335–341. doi: 10.1111/j.1399-0039.2008.01107.x. [DOI] [PubMed] [Google Scholar]
- 144.Rizzo R, Farina I, Bortolotti D, Galuppi E, Rotola A, Melchiorri L, Ciancio G, Di Luca D, Govoni M. HLA-G may predict the disease course in patients with early rheumatoid arthritis. Hum Immunol. 2013;74:425–432. doi: 10.1016/j.humimm.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 145.Sabbagh A, Courtin D, Milet J, Massaro JD, Castelli EC, Migot-Nabias F, Favier B, Rouas-Freiss N, Moreau P, Garcia A, et al. Association of HLA-G 3’ untranslated region polymorphisms with antibody response against Plasmodium falciparum antigens: preliminary results. Tissue Antigens. 2013;82:53–58. doi: 10.1111/tan.12140. [DOI] [PubMed] [Google Scholar]
- 146.Svendsen SG, Hantash BM, Zhao L, Faber C, Bzorek M, Nissen MH, Hviid TV. The expression and functional activity of membrane-bound human leukocyte antigen-G1 are influenced by the 3’-untranslated region. Hum Immunol. 2013;74:818–827. doi: 10.1016/j.humimm.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 147.Hviid TV, Rizzo R, Melchiorri L, Stignani M, Baricordi OR. Polymorphism in the 5’ upstream regulatory and 3’ untranslated regions of the HLA-G gene in relation to soluble HLA-G and IL-10 expression. Hum Immunol. 2006;67:53–62. doi: 10.1016/j.humimm.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 148.Jassem RM, Shani WS, Loisel DA, Sharief M, Billstrand C, Ober C. HLA-G polymorphisms and soluble HLA-G protein levels in women with recurrent pregnancy loss from Basrah province in Iraq. Hum Immunol. 2012;73:811–817. doi: 10.1016/j.humimm.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bukur J, Jasinski S, Seliger B. The role of classical and non-classical HLA class I antigens in human tumors. Semin Cancer Biol. 2012;22:350–358. doi: 10.1016/j.semcancer.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 150.Atkins D, Breuckmann A, Schmahl GE, Binner P, Ferrone S, Krummenauer F, Störkel S, Seliger B. MHC class I antigen processing pathway defects, ras mutations and disease stage in colorectal carcinoma. Int J Cancer. 2004;109:265–273. doi: 10.1002/ijc.11681. [DOI] [PubMed] [Google Scholar]
- 151.Carretero R, Romero JM, Ruiz-Cabello F, Maleno I, Rodriguez F, Camacho FM, Real LM, Garrido F, Cabrera T. Analysis of HLA class I expression in progressing and regressing metastatic melanoma lesions after immunotherapy. Immunogenetics. 2008;60:439–447. doi: 10.1007/s00251-008-0303-5. [DOI] [PubMed] [Google Scholar]
- 152.Momburg F, Degener T, Bacchus E, Moldenhauer G, Hämmerling GJ, Möller P. Loss of HLA-A,B,C and de novo expression of HLA-D in colorectal cancer. Int J Cancer. 1986;37:179–184. doi: 10.1002/ijc.2910370203. [DOI] [PubMed] [Google Scholar]
- 153.Cabrera T, Collado A, Fernandez MA, Ferron A, Sancho J, Ruiz-Cabello F, Garrido F. High frequency of altered HLA class I phenotypes in invasive colorectal carcinomas. Tissue Antigens. 1998;52:114–123. doi: 10.1111/j.1399-0039.1998.tb02274.x. [DOI] [PubMed] [Google Scholar]
- 154.Torres MI, Le Discorde M, Lorite P, Ríos A, Gassull MA, Gil A, Maldonado J, Dausset J, Carosella ED. Expression of HLA-G in inflammatory bowel disease provides a potential way to distinguish between ulcerative colitis and Crohn’s disease. Int Immunol. 2004;16:579–583. doi: 10.1093/intimm/dxh061. [DOI] [PubMed] [Google Scholar]
- 155.Maleno I, Cabrera CM, Cabrera T, Paco L, López-Nevot MA, Collado A, Ferrón A, Garrido F. Distribution of HLA class I altered phenotypes in colorectal carcinomas: high frequency of HLA haplotype loss associated with loss of heterozygosity in chromosome region 6p21. Immunogenetics. 2004;56:244–253. doi: 10.1007/s00251-004-0692-z. [DOI] [PubMed] [Google Scholar]
- 156.Maleno I, Aptsiauri N, Cabrera T, Gallego A, Paschen A, López-Nevot MA, Garrido F. Frequent loss of heterozygosity in the β2-microglobulin region of chromosome 15 in primary human tumors. Immunogenetics. 2011;63:65–71. doi: 10.1007/s00251-010-0494-4. [DOI] [PubMed] [Google Scholar]
- 157.Kloor M, Michel S, Buckowitz B, Rüschoff J, Büttner R, Holinski-Feder E, Dippold W, Wagner R, Tariverdian M, Benner A, et al. Beta2-microglobulin mutations in microsatellite unstable colorectal tumors. Int J Cancer. 2007;121:454–458. doi: 10.1002/ijc.22691. [DOI] [PubMed] [Google Scholar]
- 158.Bicknell DC, Kaklamanis L, Hampson R, Bodmer WF, Karran P. Selection for beta 2-microglobulin mutation in mismatch repair-defective colorectal carcinomas. Curr Biol. 1996;6:1695–1697. doi: 10.1016/s0960-9822(02)70795-1. [DOI] [PubMed] [Google Scholar]
- 159.Park WS, Park JY, Oh RR, Yoo NJ, Lee SH, Shin MS, Lee HK, Han S, Yoon SK, Kim SY, et al. A distinct tumor suppressor gene locus on chromosome 15q21.1 in sporadic form of colorectal cancer. Cancer Res. 2000;60:70–73. [PubMed] [Google Scholar]
- 160.Bernal M, Ruiz-Cabello F, Concha A, Paschen A, Garrido F. Implication of the β2-microglobulin gene in the generation of tumor escape phenotypes. Cancer Immunol Immunother. 2012;61:1359–1371. doi: 10.1007/s00262-012-1321-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rizzo R, Melchiorri L, Simone L, Stignani M, Marzola A, Gullini S, Baricordi OR. Different production of soluble HLA-G antigens by peripheral blood mononuclear cells in ulcerative colitis and Crohn’s disease: a noninvasive diagnostic tool. Inflamm Bowel Dis. 2008;14:100–105. doi: 10.1002/ibd.20281. [DOI] [PubMed] [Google Scholar]
- 162.Zelante A, Borgoni R, Galuppi C, Cifalà V, Melchiorri L, Gullini S, Baricordi O, Rizzo R. Therapy modifies HLA-G secretion differently in Crohn’s disease and ulcerative colitis patients. Inflamm Bowel Dis. 2011;17:E94–E95. doi: 10.1002/ibd.21756. [DOI] [PubMed] [Google Scholar]
- 163.Halama N, Braun M, Kahlert C, Spille A, Quack C, Rahbari N, Koch M, Weitz J, Kloor M, Zoernig I, et al. Natural killer cells are scarce in colorectal carcinoma tissue despite high levels of chemokines and cytokines. Clin Cancer Res. 2011;17:678–689. doi: 10.1158/1078-0432.CCR-10-2173. [DOI] [PubMed] [Google Scholar]
- 164.Rocca YS, Roberti MP, Arriaga JM, Amat M, Bruno L, Pampena MB, Huertas E, Loria FS, Pairola A, Bianchini M, et al. Altered phenotype in peripheral blood and tumor-associated NK cells from colorectal cancer patients. Innate Immun. 2013;19:76–85. doi: 10.1177/1753425912453187. [DOI] [PubMed] [Google Scholar]
- 165.Morandi F, Ferretti E, Castriconi R, Dondero A, Petretto A, Bottino C, Pistoia V. Soluble HLA-G dampens CD94/NKG2A expression and function and differentially modulates chemotaxis and cytokine and chemokine secretion in CD56bright and CD56dim NK cells. Blood. 2011;118:5840–5850. doi: 10.1182/blood-2011-05-352393. [DOI] [PubMed] [Google Scholar]
- 166.Davidson B, Elstrand MB, McMaster MT, Berner A, Kurman RJ, Risberg B, Trope CG, Shih IeM. HLA-G expression in effusions is a possible marker of tumor susceptibility to chemotherapy in ovarian carcinoma. Gynecol Oncol. 2005;96:42–47. doi: 10.1016/j.ygyno.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 167.Cecchin E, D’Andrea M, Lonardi S, Zanusso C, Pella N, Errante D, De Mattia E, Polesel J, Innocenti F, Toffoli G. A prospective validation pharmacogenomic study in the adjuvant setting of colorectal cancer patients treated with the 5-fluorouracil/leucovorin/oxaliplatin (FOLFOX4) regimen. Pharmacogenomics J. 2013;13:403–409. doi: 10.1038/tpj.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Morris M, Platell C, Iacopetta B. Tumor-infiltrating lymphocytes and perforation in colon cancer predict positive response to 5-fluorouracil chemotherapy. Clin Cancer Res. 2008;14:1413–1417. doi: 10.1158/1078-0432.CCR-07-1994. [DOI] [PubMed] [Google Scholar]
- 169.Halama N, Michel S, Kloor M, Zoernig I, Pommerencke T, von Knebel Doeberitz M, Schirmacher P, Weitz J, Grabe N, Jäger D. The localization and density of immune cells in primary tumors of human metastatic colorectal cancer shows an association with response to chemotherapy. Cancer Immun. 2009;9:1. [PMC free article] [PubMed] [Google Scholar]
- 170.Halama N, Michel S, Kloor M, Zoernig I, Benner A, Spille A, Pommerencke T, von Knebel DM, Folprecht G, Luber B, et al. Localization and density of immune cells in the invasive margin of human colorectal cancer liver metastases are prognostic for response to chemotherapy. Cancer Res. 2011;71:5670–5677. doi: 10.1158/0008-5472.CAN-11-0268. [DOI] [PubMed] [Google Scholar]


