Abstract
AIM: To clarify the value of combined use of markers for the diagnosis of gallbladder cancer and prediction of its prognosis.
METHODS: Serum cancer antigens (CA)199, CA242, carcinoembryonic antigen (CEA), and CA125 levels were measured in 78 patients with gallbladder cancer (GBC), 78 patients with benign gallbladder diseases, and 78 healthy controls using electrochemiluminescence. CA199, CA242, CEA, and CA125 levels and positive rates were analyzed and evaluated pre- and post-operatively. Receiver operator characteristic curves were used to determine diagnostic sensitivity and specificity of GBC. Survival time analysis, including survival curves, and multivariate survival analysis of a Cox proportional hazards model was performed to evaluate independent prognostic factors.
RESULTS: Serum CA242, CA125, and CA199 levels in the GBC group were significantly higher when compared with those in the benign gallbladder disease and healthy control groups (P < 0.01). With a single tumor marker for GBC diagnosis, the sensitivity of CA199 was the highest (71.7%), with the highest specificity being in CA242 (98.7%). Diagnostic accuracy was highest with a combination of CA199, CA242, and CA125 (69.2%). CA242 could be regarded as a tumor marker of GBC infiltration in the early stage. The sensitivity of CA199 and CA242 increased with progression of GBC and advanced lymph node metastasis (P < 0.05). The 78 GBC patients were followed up for 6-12 mo (mean: 8 mo), during which time serum CA199, CA125, and CA242 levels in the recurrence group were significantly higher than in patients without recurrence (P < 0.01). The post-operative serum CA199, CA125, and CA242 levels in the non-recurrence group were significantly lower than those in the GBC group (P < 0.01). Multivariate survival analysis using a Cox proportional hazards model showed that cancer of the gallbladder neck and CA199 expression level were independent prognostic factors.
CONCLUSION: CA242 is a marker of GBC infiltration in the early stage. CA199 and cancer of the gallbladder neck are therapeutic and prognostic markers.
Keywords: Gallbladder cancer, Tumor marker, Combined detection, Diagnosis, Prognosis
Core tip: Detection of serum tumor markers is simple, and has become a common clinical method for tumor screening. However, when these markers are used individually for the diagnosis of gallbladder cancer, inconsistent results have been obtained. The results of the present study suggest that combined detection of serum cancer antigens cancer antigens (CA)125, CA199, and CA242 can increase the specificity of gallbladder cancer (GBC) diagnosis. CA242 could be regarded as a tumor marker of GBC infiltration in the early stage. Multivariate survival analysis showed that cancer of the gallbladder neck and CA199 expression levels were independent prognostic factors.
INTRODUCTION
Gallbladder cancer (GBC) is one of the most common and aggressive malignant neoplasms of the biliary system. Early-stage GBC lacks typical clinical manifestations, leading to a poor 5-year survival[1-3]. Most patients are at the advanced stage at the time of diagnosis, and thus lose the chance of radical cure. It is therefore important to diagnose GBC earlier. Currently, the diagnosis of GBC mainly depends on non-invasive auxiliary imaging and invasive examination such as laparoscopy and biopsy. However, there is no ideal single tumor marker for the diagnosis and prognosis of GBC[4-6].
Tumor markers such as CEA, cancer antigens (CA) CA125, CA242, and CA199 have been widely used for the diagnosis of different types of cancer (e.g., liver, gastric, colorectal, and pancreatic). However, when these markers are used individually for the diagnosis of GBC, inconsistent results have been obtained[7-10]. A recent study reported that S1P1 overexpression or ERp29 absence is related to carcinogenesis and progression, and are thus potential biomarkers for the early detection of gallbladder adenocarcinoma[11]. We therefore hypothesized as whether the combined use of tumor markers could avoid inconsistent results and increase the diagnostic sensitivity for GBC.
In this study, we detected serum levels of tumor markers CEA, CA125, CA242, and CA199 in 78 patients with GBC, 78 patients with benign gallbladder diseases, and 78 healthy controls. Our results showed that combined detection of these markers could increase the sensitivity for diagnosis and prognosis of GBC.
MATERIALS AND METHODS
Ethics
This study was performed in compliance with the Helsinki Declaration and according to the protocol approved by the Medical Ethics Committee of the authors’ hospitals. All patients and participants were informed of the study and gave voluntary, signed informed consent.
Study subjects
A total of 234 subjects were enrolled in this study: 78 patients with GBC (30 male and 48 female), 78 patients with benign gallbladder diseases (cholecystitis, gallbladder polyps, and gallbladder stones) admitted to Yangpu District Central Hospital and Eastern Hepatobiliary Surgery Hospital (Shanghai, China), and 78 healthy individuals who underwent physical examinations in the same hospitals between January 2010 and September 2012. The 78 GBC patients ranged in age from 45 to 73 years, with an average of 58.7 years. Their body mass index ranged from 19 to 24 kg/m2, with an average of 22. Cancer staging was performed according to the American Joint Committee on Cancer TNM staging system (7th Edition)/ classification system for patients with gallbladder cancer (2010), which showed seven cases of phase II GBC, 10 phase IIIA, 33 phase IIIB, 6 phase IVA, and 22 phase IVB. Of the 78 GBC cases, 73 were adenocarcinomas, including 8 highly differentiated adenocarcinomas, 11 poorly differentiated adenocarcinomas, and 54 moderately differentiated adenocarcinomas. The remaining five cases were carcinoid GBC in two, squamous cell carcinoma in two, and squamous Signet ring cell carcinoma in one. Of the 78 GBC cases, 37 occurred in the gallbladder neck, 23 in the body, 5 in the bottom, and 13 in the duct of gallbladder[12]. All GBC diagnoses were confirmed pathologically. None of the GBC patients had received radiotherapy, chemotherapy, or endocrine therapy before surgery. Metastasis occurred in 53 out of the 78 GBC cases, including 36 cases with adjacent lymph node (LN) metastasis, 17 with distal LN metastasis, and 25 without observable metastasis. The 78 patients in the benign gallbladder disease group included 35 men and 43 women, with a mean age of 55 ± 6.4 years (range: 48-70 years). The 78 healthy controls included 37 men and 41 women, with a mean age of 61.6 ± 6.7 years (range: 40-75 years). Serum CA199, CA242, CEA, and CA125 were detected before and after operation. Surgical modalities for the GBC group are shown in Table 1. Fasting cubital venous blood (5 mL) was drawn in the morning from each of the three groups and centrifuged at 4000 r/min. The supernatant was collected and preserved at -80 °C before use.
Table 1.
Surgical modalities and their relationship with tumor stage in the gallbladder cancer group
| Surgical modality |
TNM stage |
||||
| II | IIIA | IIIB | IVA | IVB | |
| C + N | 6 | 8 | 2 | ||
| C + WR + N | 1 | 6 | 4 | ||
| C + S4aS5 + N | 1 | 7 | 3 | ||
| C + ELH + N | 1 | ||||
| C + ERH + N | 1 | ||||
| C + BD + N | |||||
| C + WR + BD + N | 1 | 6 | 2 | ||
| C + S4As5 + BD + N | 4 | 2 | |||
| C + CH + BD + N | 3 | 1 | |||
| C + S4aS5 + other + N | 1 | 1 | |||
| C + S4aS5 + other + N | 5 | ||||
| C + ERH + BD + other + N | 1 | 1 | |||
| HPD + N | 1 | ||||
| Palliative resection | 1 | 2 | |||
| Simple drainage | 1 | 3 | |||
| Pure exploration | 2 | ||||
BD: Bile duct resection; C: Cholecystectomy; CH: Central hepatectomy; ELH: Extended left hepatectomy; ERH: Extended right hepatectomy; HPD: Hepato-pancreaticoduodenectomy; N: Lymphadenectomy; Other: Other organ tissue resection; S4aS5: Liver resection of segments IVa and V; WR: Wedge resection of the gallbladder fossae.
Detection of serum tumor markers
Serum CA199, CA242, CEA, and CA125 levels were detected by electrochemiluminescence immunoassay (Cobas; Roche Diagnostics, Germany) at the Department of Biliary Surgery of the Eastern Hepatobiliary Surgery Hospital affiliated to the Second Military Medical University, Shanghai, China. The normal reference values were as follows: CEA ≤ 10 μg/L, CA125 ≤ 35 U/mL, CA242 ≤ 15 U/mL, and CA199 ≤ 39 U/mL.
Statistical analysis
The data were expressed as mean ± SD. Measurement data between groups were compared with the t test, while enumerative data were compared with the χ2 test. The prediction value was calculated by receiver operating characteristic (ROC) curve analysis. Survival was analyzed by the Cox proportional hazards model. Statistical analysis was performed using SPSS version 17.0 statistical software (SPSS Inc., Chicago, IL, United States). All tests were two-tailed and P < 0.05 was considered statistically significant.
RESULTS
Serum levels of CEA, CA125, CA199, and CA242
There were significant differences in the mean serum level and positive rate of CA125, CA199, and CA242 between the GBC and the other two groups (P < 0.01). There was no significant difference between the healthy control and benign gallbladder disease groups (P > 0.05). There was no significant difference in serum CEA, CA125, CA199, and CA242 levels with respect to age and sex in the GBC group (P > 0.05). The results are shown in Tables 2, 3 and 4.
Table 2.
Comparison of serum cancer antigen 199, cancer antigen 125, cancer antigen 242, and carcinoembryonic antigen levels
| Group | n | MFI (CEA) | MFI (CA199) | MFI (CA125) | MFI (CA242) |
| Control group | 78 | 3.93 ± 2.04 | 14.97 ± 8.91b | 10.48 ± 6.38b | 9.48 ± 3.43b |
| Benign disease | 78 | 3.83 ± 1.85 | 15.17 ± 7.82c | 12.99 ± 6.99c | 10.19 ± 3.08c |
| GBC | 78 | 9.36 ± 3.58 | 238.17 ± 346.36bc | 55.34 ± 81.78bc | 39.92 ± 45.9bc |
Results are mean ± SD.
P < 0.01 vs control group;
P < 0.05 vs benign disease group. MFI: Mean fluorescence intensity; CA: Cancer antigen; CEA: Carcinoembryonic antigen; GBC: Gallbladder cancer.
Table 3.
Positive rates of carcinoembryonic antigen, cancer antigen 125, cancer antigen 242, and cancer antigen 199 n (%)
| GBC(n = 78) | Benign gallbladder disease (n = 78) | Healthy controls(n = 78) | |
| CEA Pc | 9 (11.5) | 1 (1.2) | 2 (2.5) |
| CA242 Pc | 50 (64.1)b | 2 (2.5) | 1 (1.2) |
| CA125 Pc | 35 (44.8)b | 2 (2.5) | 2 (2.5) |
| CA199 Pc | 56 (71.7)b | 4 (5.0) | 3 (3.8) |
| Combination Pc | 7 (8.9) | 0 (0.0) | 0 (0.0) |
P < 0.01 vs benign gallbladder disease and healthy control group. Combination = Carcinoembryonic antigen (CEA) + cancer antigen CA242 + CA125 + CA199. Pc: Positive cases; GBC: Gallbladder cancer.
Table 4.
Correlations between gallbladder cancer markers, tumor size, location, and staging
| Group | n | MFI (CEA) | MFI (CA199) | MFI (CA125) | MFI (CA242) |
| Age (yr) | |||||
| ≥ 50 | 45 | 9.5 ± 4.3 | 239.1 ± 324.6 | 55.9 ± 86.6 | 39.9 ± 42.9 |
| < 50 | 33 | 9.2 ± 2.7 | 236.9 ± 319.4 | 54.6 ± 78.9 | 39.9 ± 47.3 |
| Sex | |||||
| Male | 30 | 9.3 ± 6.6 | 234.6 ± 356.9 | 54.8 ± 81.7 | 38.5 ± 47.1 |
| Female | 48 | 9.4 ± 3.8 | 240.4 ± 298.7 | 55.7 ± 89.5 | 40.8 ± 49.9 |
| Tumor position | |||||
| Neck | 37 | 8.5 ± 3.9 | 262.2 ± 177.8b | 62.1 ± 47.0b | 42.5 ± 45.9b |
| Body | 23 | 5.2 ± 4.2 | 28.3 ± 20.7 | 56.9 ± 99.7 | 19.0 ± 21.6 |
| Bottom | 5 | 3.3 ± 2.6 | 27.6 ± 2.4b | 14.5 ± 6.5b | 8.5 ± 2.9b |
| Cystic duct | 13 | 21.5 ± 25.1 | 622 ± 196.9 | 49.0 ± 32.9 | 81.7 ± 56.7 |
| Staging | |||||
| II | 7 | 3.2 ± 2.2 | 66.1 ± 6.3a | 17.7 ± 7.7a | 8.4 ± 3.3a |
| IIIA | 10 | 2.0 ± 0.9 | 89.5 ± 8.2 | 47.3 ± 38.2 | 10.2 ± 9.9 |
| IIIB | 33 | 6.9 ± 6.4 | 205.5 ± 33.9a | 59.6 ± 114.1a | 22 ± 22.6a |
| IVA | 6 | 3.5 ± 3.3 | 383.6 ± 55.5a | 58.1 ± 25.5a | 55.2 ± 25.3a |
| IVB | 22 | 19.9 ± 9.1 | 418.7 ± 316.5a | 64.1 ± 50.5a | 86.2 ± 56.9a |
| Tumor size | |||||
| > 5 cm | 20 | 9.6 ± 2.2 | 768.1 ± 272.9a | 82.9 ± 21.9a | 81.8 ± 53.1a |
| ≤ 5 cm | 58 | 9.3 ± 1.2 | 55.4 ± 68.7a | 45.8 ± 10.1a | 25.5 ± 32.9a |
| Pathological type | |||||
| Highly differentiated | 8 | 3.0 ± 2.0 | 68.9 ± 5.9a | 16.7 ± 7.7a | 8.7 ± 3.1a |
| Moderately differentiated | 54 | 6.9 ± 5.9 | 142.3 ± 200.4 | 49.9 ± 9.9 | 33.8 ± 41.3 |
| Poorly differentiated | 11 | 3.5 ± 2.5 | 649.1 ± 209.1a | 84.0 ± 29.4a | 73.4 ± 58.9a |
| Carcinoid | 2 | 20.9 ± 14.6 | 766.3 ± 330.5a | 121.3 ± 45.2a | 62.7 ± 9.4a |
| Squamous cell carcinoma | 2 | 107.5 ± 69.1 | 334.4 ± 14.9a | 116.0 ± 103.1a | 108.5 ± 58.7a |
| Signet ring cell carcinoma | 1 | 31 | 1000a | 89.3a | 69.3a |
Results are mean ± SD.
P < 0.05,
P < 0.01 vs control group. MFI: Mean fluorescence intensity; CA: Cancer antigen; CEA: Carcinoembryonic antigen.
Serum tumor markers in GBC patients with different clinicopathological features
According to the stage, location, and histological differentiation, the GBC group was subclassified by tumor location, stage, size, and pathological type for calculation of the positive rate of the four serum tumor markers using combined qualitative detection by a pathologist. The results showed that CA125, CA199, and CA242 levels in patients with gallbladder neck cancer were significantly higher than in those with cancer in the bottom portion. In addition, there were significant differences in serum CA125, CA199, and CA242 levels between GBC cases at different stages, tumor size, and differentiation (Table 4). Analysis of the sensitivity of the four serum tumor markers at different stages of GBC showed that the sensitivity of CA125, CA199, and CA242 strengthened gradually with the progression of clinical stages. There were significant differences between stages IVA, IVB, and II (P < 0.05). The sensitivity of CA242 in stage II GBC was significantly better than that of CA125 and CA199 (P < 0.05) (Table 5), and therefore, CA242 could be regarded as a tumor marker of GBC infiltration in the early stage.
Table 5.
Analyses of the sensitivity of tumor markers in different stages of gallbladder cancer n (%)
| Clinical stages | Cases (n) | CEA | CA199 | CA242 | CA125 |
| II | 7 | 1 (14.2) | 3 (42.8)a | 4 (57.1)ac | 2 (28.5)a |
| IIIA | 10 | 1 (10) | 5 (50) | 6 (60) | 3 (30) |
| IIIB | 33 | 2 (6) | 18 (54.5) | 20 (60.6) | 14 (42.4) |
| IVA | 6 | 2 (33.3) | 4 (66.6) | 4 (66.6) | 3 (50) |
| IVB | 22 | 3 (13.6) | 16 (72.7) | 16 (72.7) | 13 (59) |
P < 0.05 vs sensitivity of stage IVA, B;
P < 0.05 vs sensitivity of CA125 and CA199. CA: Cancer antigen; CEA: Carcinoembryonic antigen.
GBC diagnostic value of a single vs three tumor markers
CA199 alone had the highest sensitivity of 71.7%, and CA242 alone had the highest specificity of 98.7% for the diagnosis of GBC. CA199 and CA242 had the most exact validity (Table 6). ROC curves are shown in Figure 1. The sensitivity, specificity, and positive predictive values were: 91.0%, 94.9%, and 17.8%, respectively, when any of the three markers exceeded the critical value; 85.9%, 96.2%, and 22.6%, respectively, when any two of the three markers exceeded the critical values; and 69.2%, 100%, and 100%, respectively, when all three markers exceeded the critical values. The sensitivity was 8.9% when all four markers exceeded the critical values. These results suggested that diagnosis of GBC based on combined detection of the tumor markers could increase the specificity, but not sensitivity, of diagnoses (Table 7).
Table 6.
Evaluation of diagnostic value of a single tumor marker in 78 gallbladder cancer cases
| Diagnostic value | n | Sensitivity | Specificity | Positive likelihood ratio | Negative likelihood ratio |
| CEA | 9 | 11.5% | 97.4% | 4.42 | 0.91 |
| CA199 | 56 | 71.7% | 96.1% | 18.4 | 0.29 |
| CA242 | 50 | 64.1% | 98.7% | 49.5 | 0.36 |
| CA125 | 35 | 44.8% | 96.2% | 11.79 | 0.57 |
Sensitivity = true positive/patients × 100%; Specificity = true negative/normal × 100%; Positive likelihood ratio = sensitivity/(1 - specificity); Negative likelihood ratio = (1 - sensitivity)/specificity. CA: Cancer antigen; CEA: Carcinoembryonic antigen.
Figure 1.
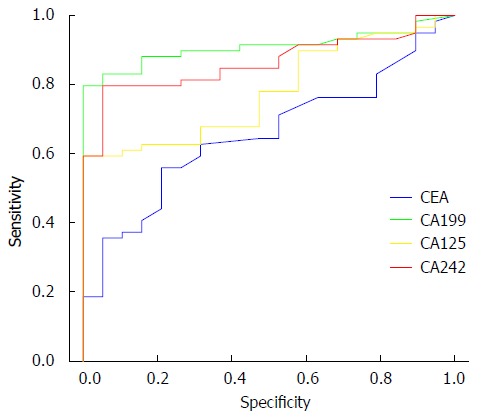
Receiver operating characteristic curve. Receiver operating characteristic curves showing diagnostic performance of cancer antigens (CA) CA199, CA125, CA242, and carcinoembryonic antigen (CEA). The sensitivity of CA199 was the highest (71.7%) and the specificity of CA242 was the highest (98.7%).
Table 7.
Analyses of different combinations of markers in gallbladder cancer diagnosis
| Group | n | 1 item (+) | 2 item (+) | 3 item (+) | 4 item (+) |
| Normal | 78 | 4 (5.1) | 3 (3.8) | 0 (0) | 0 (0) |
| Gallbladder cancer (GBC) | 78 | 71 (91.0) | 67 (85.9) | 54 (69.2) | 7 (8.9) |
| Positive likelihood rate | 17.8% | 22.6% | 100% | 100% |
Correlations of CEA, CA125, CA199, and CA242 expression with LN metastasis in GBC
Serum CEA, CA125, CA199, and CA242 expression in GBC patients with and without LN metastasis was compared. Serum CA125, CA199, and CA242 levels in patients with LN metastasis were significantly higher than those in patients without LN metastasis (P < 0.01). Serum CA125, CA199, and CA242 levels in patients with distal LN metastasis were significantly higher than those in patients with adjacent LN metastasis (P < 0.05) (Table 8).
Table 8.
Correlations between carcinoembryonic antigen, cancer antigen 125, cancer antigen 242, and cancer antigen 199 expression and lymph node metastasis
| Indicators | No LN metastasis(n = 25) | Adjacent LN metastasis (n = 36) | Distal LN metastasis (n = 17) |
| CEA, μg/L | 7.5 ± 3.4 | 9.5 ± 5.9 | 9.8 ± 3.6 |
| CA199, U/mL | 122.2 ± 117.2 | 237.4 ± 189.5b | 491.2 ± 222.5ab |
| CA242, U/mL | 9.5 ± 2.9 | 38.5 ± 15.7b | 58.7 ± 29.6ab |
| CA125 U/mL | 13.5 ± 16.5 | 43.6 ± 37.9b | 61.8 ± 67.8ab |
Results are mean ± SD.
P < 0.05,
P < 0.01 vs patients without lymph node (LN) metastasis. CA: Cancer antigen; CEA: Carcinoembryonic antigen.
Follow-up results
During the follow-up period from 6 mo to 1 year, 17 patients died, and contact was lost with 31 for various reasons. The survival time in each patient was defined as the interval between the date of definitive resection and the date of last follow-up or death. In the 30 patients who completed the follow-up study, six experienced recurrence. Serum CA125, CA199, and CA242 levels in the recurrence group were significantly higher than those in the non-recurrence group 6 mo post-operatively (P < 0.01). Serum CA125, CA199, and CA242 levels in the non-recurrence group were significantly lower than those in the GBC group pre-operatively (P < 0.01) (Table 9). Multivariate survival analysis using the Cox proportional hazards model showed that cancer of the gallbladder neck and CA199 expression level were independent prognostic factors (Table 10). Survival curves are shown in Figure 2.
Table 9.
Carcinoembryonic antigen, cancer antigen 125, cancer antigen 242, and cancer antigen 199 expressions during follow-up
| Groups | n | CEA | CA199 | CA242 | CA125 |
| Non-recurrence group | 22 | 7.34 ± 2.14 | 78.65 ± 86.43d | 12.41 ± 1.25d | 11.96 ± 2.37d |
| Recurrence group | 8 | 9.34 ± 3.04 | 219.74 ± 321.63b | 34.54 ± 8.38b | 53.88 ± 8.2b |
Results are mean ± SD.
P < 0.01 vs non-recurrence group;
P < 0.01 vs gallbladder cancer (GBC) group. CA: Cancer antigen; CEA: Carcinoembryonic antigen.
Table 10.
Results of Cox proportional hazards model for multivariate regression analysis
| Prognostic factor | Parameter estimate | Wald χ2 | P value | HR | 95%CI |
| CEA | -0.043 | 0.498 | 0.48 | 0.957 | 0.849-1.080 |
| CA199 | 0.005 | 12.076 | 0.001 | 1.005 | 1.002-1.009 |
| CA242 | -0.007 | 0.386 | 0.535 | 0.993 | 0.972-1.015 |
| CA125 | 0.003 | 0.714 | 0.398 | 1.003 | 0.996-1.009 |
| Surgical modality | 0.569 | 2.450 | 0.118 | 1.766 | 0.866-3.599 |
| LN metastasis | 1.191 | 3.276 | 0.07 | 3.291 | 0.906-11.957 |
| Position (neck) | -1.578 | 6.358 | 0.012 | 0.206 | 0.061-0.704 |
| Age | -0.010 | 0.073 | 0.787 | 0.990 | 0.922-1.063 |
| Sex | 0.796 | 2.508 | 0.113 | 2.216 | 0.828-5.932 |
Multivariate survival analysis using the Cox proportional hazards model for independent prognostic factors of gallbladder cancer (GBC). P < 0.05 was considered statistically significant. CA: Cancer antigen; CEA: Carcinoembryonic antigen; LN: Lymph node.
Figure 2.
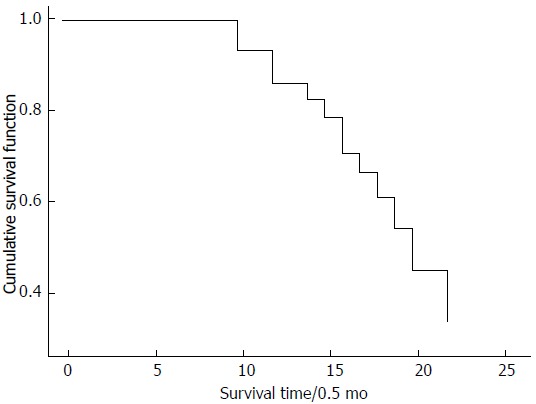
Analysis of overall survival curves. Overall survival curves for 78 patients with gallbladder cancer (GBC). Kaplan-Meier survival curves in GBC patients with a Cox proportional hazards model for multivariate regression analysis.
DISCUSSION
The incidence of GBC has increased in recent years worldwide. How to treat GBC, assess therapeutic effect, evaluate prognosis, and predict post-operative recurrence in the early stage has aroused increasing attention in both clinical studies and practice[13,14]. In this study, we analyzed the diagnostic value of the four common clinical serum tumor markers, CA242, CEA, CA125, and CA199 for GBC, and compared serum levels of these markers in patients with GBC, patients with benign gallbladder disease, and healthy controls. Serum levels of CA242, CA125, and CA199 were significantly higher in the GBC than the benign gallbladder disease and healthy control groups. Serum levels of the four markers were not significantly different between the benign disease and healthy control groups (P = 0.592-0.953). Although we also analyzed the effect of age and sex on serum levels of the four markers in GBC diagnosis, these factors did not change the GBC diagnostic value of the markers. In addition, we investigated correlations of the four markers with the clinicopathological features of GBC. CA242, CA125, and CA199 levels in cancer of the gallbladder neck were significantly higher than in cancer located in the bottom portion. In addition, there were significant differences in the serum level of CA242, CA125, and CA199 between GBC with different differentiation. CA242, CA125, and CA199 increased with the progression of the GBC stage. There was a significant difference in the sensitivity of the four markers between stages IVA, IVB, and II. The results showed that the sensitivity of CA242, CA125, and CA199 (but not CEA) increased gradually with the progression of the clinical stages. The sensitivity of CA199 was the highest, reaching 71.7%, with the highest specificity being that of CA242 at 98.7%. When CA242, CA125, and CA199 exceeded the critical values, the sensitivity was 69.2%, the specificity was 100%, and the positive predictive rate was also 100%. When all four markers exceeded the critical values, the sensitivity was 8.9%. Diagnostic accuracy was highest with a combination of CA242, CA125, and CA199. These findings suggest that the combined use of these markers for the diagnosis of GBC could increase the specificity of diagnosis, but not the sensitivity. The optimal cut-off values of the markers determined by ROC curve analysis could improve the diagnosis of GBC when used together with these markers, resulting in their optimal application and promoting the clinical screening and diagnosis of GBC[15].
We found that serum CA242, CA125, and CA199 levels were significantly higher in patients with LN metastasis than in those without. Serum CA242, CA125, and CA199 levels in patients with distal LN metastasis were significantly higher than in those with adjacent LN metastasis. Most researchers believe that CA199 is a better marker of malignant tumors. Serum CA199 is elevated most obviously in tumors of the digestive system, pancreas, and biliary tract[16]. CA199 is not only a diagnostic indicator, but also a predictor of the therapeutic effect and prognosis of GBC. However, CA199 is not specific for GBC. Therefore, CA199 should be combined with other imaging tests to diagnose GBC[17]. CA125 is a good marker for the diagnosis of cholangiocarcinoma. Qu et al[18], Wu et al[19] and Shukla et al[20] showed that CA125 has a relatively high specificity because it is rarely affected by serological levels of inflammation and liver stones. Our research was not exactly consistent with that of Shukla et al[20]. Combined use of CEA and CA199 or CA125 can improve the diagnosis of cholangiocarcinoma[15,21-26], which is consistent with our study. The positive rate of serum CA242 was high in GBC patients but not in patients with benign gallbladder diseases or the normal control group. Tao et al[27] have suggested that combined detection of α-fetoprotein (AFP) and CA242 could improve the sensitivity and specificity of the diagnosis of cholangiocarcinoma. Rana et al[28] reported that CA242 was better than CEA and CA199 as a tumor marker for the diagnosis of GBC. The expression of CEA is high in most gastrointestinal tumors[29,30]. Stefanović et al[31] found that CEA expression was significantly increased in GBC. However, Vij et al[32] suggested that CEA and AFP had little value for the diagnosis and prognosis of GBC. Our study also showed that CEA had limited value for the diagnosis and prognosis of GBC.
In terms of a single marker for the diagnosis of GBC, CA199 has the highest sensitivity with relatively low specificity, but cannot be used alone as an effective tumor marker to identify GBC. CA242 has the highest specificity, probably because it is rarely affected by the serological level of liver inflammation and stone disease, nor is it affected by the low expression in the pancreatic duct system and pancreatic juice siltation. However, the expression of CA242 is high in almost all malignant tumors, and therefore cannot be used to differentiate between GBC and pancreas cancer[33].
The follow-up study in 30 cases showed that serum CA242, CA125, and CA199 levels in the recurrence group were significantly higher than those in the non-recurrence group. Pre-operation, these levels in the non-recurrence group were lower than those in GBC group as a whole. Multivariate survival analysis using the Cox proportional hazards model showed that cancer of the gallbladder neck and CA199 expression level were independent prognostic factors[34-36].
Post-operative tumor recurrence and metastasis are major causes of death in GBC patients. To achieve a comprehensive and accurate understanding about the probability of post-operative recurrence and metastasis of GBC, efforts have been made to explore more effective clinical predictors. The tumor-related indicators in all individuals cannot be detected systemically and comprehensively due to limited economics and techniques[37], and therefore joint detection of specific tumor markers is of great significance[38].
In summary, the expression of CA242, CA125, and CA199 has an important application in assessing LN metastasis, monitoring recurrence, and clinical staging of tumors. Joint detection of CA242, CA125, and CA199 may prove to be useful for the diagnosis of GBC, assessing therapeutic effects, and predicting a prognosis. CA242 could be a marker of GBC infiltration in the early stage. Cancer of the gallbladder neck and CA199 expression level were independent prognostic factors.
COMMENTS
Background
Early diagnosis and resection of gallbladder cancer (GBC) offers a chance of cure. It is therefore important to diagnose GBC early. However, there is no ideal single tumor marker for the diagnosis and prognosis of GBC. Moreover, when these markers are used individually for the diagnosis of GBC, inconsistent results have been obtained. The aims of this study were to determine whether the combined use of tumor marker cancer antigens (CA)199, CA242, carcinoembryonic antigen (CEA), and CA125 could increase sensitivity for the diagnosis of GBC, as well as to determine the clinicopathological prognostic factors affecting survival and recurrence.
Research frontiers
The authors analyzed the published literature on combined tumor marker detection for early diagnosis, treatment, and prognosis of GBC, and found no other related or similar studies.
Innovations and breakthroughs
Combined detection of serum CA242, CA125, and CA199 had the highest specificity for the diagnosis of GBC. Diagnostic accuracy was highest with a combination of CA242, CA125, and CA199. CA242 can be regarded as a tumor marker of GBC infiltration in the early stage. The sensitivity of CA199 and CA242 increased with the progression of GBC stage, advanced lymph node metastasis, and recurrence. A Cox proportional hazards model for multivariate survival analysis showed that cancer of the gallbladder neck and CA199 expression level were independent prognostic factors.
Applications
The results suggest that combined detection of tumor markers can increase the specificity of GBC diagnosis. CA242 can be regarded as a tumor marker of GBC infiltration in the early stage. CA199 or cancer of the gallbladder neck is a valuable marker for assessing therapeutic effect and predicting prognosis.
Peer review
This was a well-designed study and a well-written paper on the role of tumor markers in the diagnosis of GBC. GBC has a dismal prognosis, and radical surgical resection offers the only chance of cure, but is possible in only a small proportion of patients due to advanced disease, and the rate of recurrence is high. This study contributes to the early diagnosis of the disease, influencing its prognosis.
Footnotes
P- Reviewers: Beltran MA, Kapischke M, Pavlidis TE, Xu Z S- Editor: Gou SX L- Editor: Rutherford A E- Editor: Ma S
References
- 1.Hu L, Wang B, Liu X, Lv Y. Unsuspected gallbladder cancer: a clinical retrospective study. Arch Iran Med. 2013;16:631–635. [PubMed] [Google Scholar]
- 2.Peng HH, Zhang YD, Gong LS, Liu WD, Zhang Y. Increased expression of microRNA-335 predicts a favorable prognosis in primary gallbladder carcinoma. Onco Targets Ther. 2013;6:1625–1630. doi: 10.2147/OTT.S53030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu TY, Tan ZJ, Jiang L, Gu JF, Wu XS, Cao Y, Li ML, Wu KJ, Liu YB. Curcumin induces apoptosis in gallbladder carcinoma cell line GBC-SD cells. Cancer Cell Int. 2013;13:64. doi: 10.1186/1475-2867-13-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okada K, Kijima H, Imaizumi T, Hirabayashi K, Matsuyama M, Yazawa N, Dowaki S, Tobita K, Ohtani Y, Tanaka M, et al. Clinical significance of wall invasion pattern of subserosa-invasive gallbladder carcinoma. Oncol Rep. 2012;28:1531–1536. doi: 10.3892/or.2012.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eil R, Hansen PD, Cassera M, Orloff SL, Sheppard BC, Diggs B, Billingsley KG. Bile duct involvement portends poor prognosis in resected gallbladder carcinoma. Gastrointest Cancer Res. 2013;6:101–105. [PMC free article] [PubMed] [Google Scholar]
- 6.Zhai G, Yan K, Ji X, Xu W, Yang J, Xiong F, Su J, McNutt MA, Yang H. LAPTM4B allele *2 is a marker of poor prognosis for gallbladder carcinoma. PLoS One. 2012;7:e45290. doi: 10.1371/journal.pone.0045290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghosh M, Sakhuja P, Singh S, Agarwal AK. p53 and beta-catenin expression in gallbladder tissues and correlation with tumor progression in gallbladder cancer. Saudi J Gastroenterol. 2013;19:34–39. doi: 10.4103/1319-3767.105922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang D, Yu M, Xu T, Xiong B. Predictive value of serum CEA, CA19-9 and CA125 in diagnosis of colorectal liver metastasis in Chinese population. Hepatogastroenterology. 2013;60:1297–1301. doi: 10.5754/hge121125. [DOI] [PubMed] [Google Scholar]
- 9.He CZ, Zhang KH, Li Q, Liu XH, Hong Y, Lv NH. Combined use of AFP, CEA, CA125 and CAl9-9 improves the sensitivity for the diagnosis of gastric cancer. BMC Gastroenterol. 2013;13:87. doi: 10.1186/1471-230X-13-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zur B, Holdenrieder S, Walgenbach-Brünagel G, Albers E, Stoffel-Wagner B. Method comparison for determination of the tumor markers AFP, CEA, PSA and free PSA between Immulite 2000 XPI and Dimension Vista 1500. Clin Lab. 2012;58:97–105. [PubMed] [Google Scholar]
- 11.Yuan LW, Liu DC, Yang ZL. Correlation of S1P1 and ERp29 expression to progression, metastasis, and poor prognosis of gallbladder adenocarcinoma. Hepatobiliary Pancreat Dis Int. 2013;12:189–195. doi: 10.1016/s1499-3872(13)60030-2. [DOI] [PubMed] [Google Scholar]
- 12.Yang XW, Yang J, Li L, Man XB, Zhang BH, Shen F, Wu MC. Analysis of the relationships between clinicopathologic factors and survival in gallbladder cancer following surgical resection with curative intent. PLoS One. 2012;7:e51513. doi: 10.1371/journal.pone.0051513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang MJ, Song Y, Jang JY, Han IW, Kim SW. Role of radical surgery in patients with stage IV gallbladder cancer. HPB (Oxford) 2012;14:805–811. doi: 10.1111/j.1477-2574.2012.00544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aruga A. Vaccination of biliary tract cancer patients with four peptides derived from cancer-testis antigens. Oncoimmunology. 2013;2:e24882. doi: 10.4161/onci.24882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu H, Yu TN, Cai XJ. Tumor biomarkers: help or mislead in the diagnosis of xanthogranulomatous cholecystitis-analysis of serum CA 19-9, carcinoembryonic antigen, and CA 12-5. Chin Med J (Engl) 2013;126:3044–3047. [PubMed] [Google Scholar]
- 16.Zhou G, Niu L, Chiu D, He L, Xu K. Changes in the expression of serum markers CA242, CA199, CA125, CEA, TNF-α and TSGF after cryosurgery in pancreatic cancer patients. Biotechnol Lett. 2012;34:1235–1241. doi: 10.1007/s10529-012-0908-5. [DOI] [PubMed] [Google Scholar]
- 17.Srivastava K, Srivastava A, Mittal B. Potential biomarkers in gallbladder cancer: present status and future directions. Biomarkers. 2013;18:1–9. doi: 10.3109/1354750X.2012.717105. [DOI] [PubMed] [Google Scholar]
- 18.Qu K, Liu SN, Chang HL, Liu C, Xu XS, Wang RT, Zhou L, Tian F, Wei JC, Tai MH, et al. Gallbladder cancer: a subtype of biliary tract cancer which is a current challenge in China. Asian Pac J Cancer Prev. 2012;13:1317–1320. doi: 10.7314/apjcp.2012.13.4.1317. [DOI] [PubMed] [Google Scholar]
- 19.Wu XS, Shi LB, Li ML, Ding Q, Weng H, Wu WG, Cao Y, Bao RF, Shu YJ, Ding QC, et al. Evaluation of two inflammation-based prognostic scores in patients with resectable gallbladder carcinoma. Ann Surg Oncol. 2014;21:449–457. doi: 10.1245/s10434-013-3292-z. [DOI] [PubMed] [Google Scholar]
- 20.Shukla VK, Gurubachan D, Dixit VK. Diagnostic value of serum CA242, CA 19-9, CA 15-3 and CA 125 in patients with carcinoma of the gallbladder. Trop Gastroenterol. 2006;27:160–165. [PubMed] [Google Scholar]
- 21.Chaube A, Tewari M, Singh U, Shukla HS. CA 125: a potential tumor marker for gallbladder cancer. J Surg Oncol. 2006;93:665–669. doi: 10.1002/jso.20534. [DOI] [PubMed] [Google Scholar]
- 22.Aljiffry M, Walsh MJ, Molinari M. Advances in diagnosis, treatment and palliation of cholangiocarcinoma: 1990-2009. World J Gastroenterol. 2009;15:4240–4262. doi: 10.3748/wjg.15.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen WF, Zhong W, Xu F, Kan T, Geng L, Xie F, Sui CJ, Yang JM. Clinicopathological and prognostic analysis of 429 patients with intrahepatic cholangiocarcinoma. World J Gastroenterol. 2009;15:5976–5982. doi: 10.3748/wjg.15.5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu AX, Knox JJ. Adjuvant therapy for intrahepatic cholangiocarcinoma: the debate continues. Oncologist. 2012;17:1504–1507. doi: 10.1634/theoncologist.2012-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higashi M, Yamada N, Yokoyama S, Kitamoto S, Tabata K, Koriyama C, Batra SK, Yonezawa S. Pathobiological implications of MUC16/CA125 expression in intrahepatic cholangiocarcinoma-mass forming type. Pathobiology. 2012;79:101–106. doi: 10.1159/000335164. [DOI] [PubMed] [Google Scholar]
- 26.Li HY, Zhou SJ, Li M, Xiong D, Singh A, Guo QX, Liu CA, Gong JP. Diagnosis and cure experience of hepatolithiasis-associated intrahepatic cholangiocarcinoma in 66 patients. Asian Pac J Cancer Prev. 2012;13:725–729. doi: 10.7314/apjcp.2012.13.2.725. [DOI] [PubMed] [Google Scholar]
- 27.Tao LY, Cai L, He XD, Liu W, Qu Q. Comparison of serum tumor markers for intrahepatic cholangiocarcinoma and hepatocellular carcinoma. Am Surg. 2010;76:1210–1213. [PubMed] [Google Scholar]
- 28.Rana S, Dutta U, Kochhar R, Rana SV, Gupta R, Pal R, Jain K, Srinivasan R, Nagi B, Nain CK, et al. Evaluation of CA 242 as a tumor marker in gallbladder cancer. J Gastrointest Cancer. 2012;43:267–271. doi: 10.1007/s12029-011-9288-7. [DOI] [PubMed] [Google Scholar]
- 29.Tsouma A, Aggeli C, Lembessis P, Zografos GN, Korkolis DP, Pectasides D, Skondra M, Pissimissis N, Tzonou A, Koutsilieris M. Multiplex RT-PCR-based detections of CEA, CK20 and EGFR in colorectal cancer patients. World J Gastroenterol. 2010;16:5965–5974. doi: 10.3748/wjg.v16.i47.5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ivankovics IG, Fernandes LC, Saad SS, Matos D. Peripheral and mesenteric serum levels of CEA and cytokeratins, staging and histopathological variables in colorectal adenocarcinoma. World J Gastroenterol. 2008;14:6699–6703. doi: 10.3748/wjg.14.6699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stefanović D, Novaković R, Perisić-Savić M, Djordjević Z, Zivanović M, Stajić S. The evaluation of tumor markers levels in determination of surgical procedure in patients with gallbladder carcinoma. Med Pregl. 1993;46 Suppl 1:58–59. [PubMed] [Google Scholar]
- 32.Vij U, Baskaran V. Value of serum CEA and AFP in the diagnosis and prognosis of carcinoma gallbladder. Trop Gastroenterol. 2001;22:227–229. [PubMed] [Google Scholar]
- 33.Zhuang PY, Zhu MJ, Wang JD, Zhou XP, Quan ZW, Shen J. Xanthogranulomatous cholecystitis: a clinicopathological study of its association with gallbladder carcinoma. J Dig Dis. 2013;14:45–50. doi: 10.1111/j.1751-2980.2012.00645.x. [DOI] [PubMed] [Google Scholar]
- 34.Lee HK, Cho MS, Kim TH. Prognostic significance of muc4 expression in gallbladder carcinoma. World J Surg Oncol. 2012;10:224. doi: 10.1186/1477-7819-10-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang RT, Xu XS, Liu J, Liu C. Gallbladder carcinoma: analysis of prognostic factors in 132 cases. Asian Pac J Cancer Prev. 2012;13:2511–2514. doi: 10.7314/apjcp.2012.13.6.2511. [DOI] [PubMed] [Google Scholar]
- 36.Choi BG, Kim CY, Cho SH, Kim HJ, Koh YS, Kim JC, Cho CK, Kim HJ, Hur YH. Impact of lymph node ratio as a valuable prognostic factor in gallbladder carcinoma, focusing on stage IIIB gallbladder carcinoma. J Korean Surg Soc. 2013;84:168–177. doi: 10.4174/jkss.2013.84.3.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andrén-Sandberg A. Molecular biology of gallbladder cancer: potential clinical implications. N Am J Med Sci. 2012;4:435–441. doi: 10.4103/1947-2714.101979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Letelier P, Brebi P, Tapia O, Roa JC. DNA promoter methylation as a diagnostic and therapeutic biomarker in gallbladder cancer. Clin Epigenetics. 2012;4:11. doi: 10.1186/1868-7083-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]


