Abstract
Background
Development and progression of multiple myeloma is dependent on the bone marrow (BM) microenvironment, and within the BM, a number of factors are secreted, including the Wnt ligands. Bone marrow stromal cells (BMSC) secrete Wnt ligands that activate Wnt signaling in multiple myeloma. The canonical Wnt pathway which is, mediated through the transcriptional effector β-catenin (β-cat) is commonly deregulated in many cancers. Cells with active β-cat-regulated transcription (CRT) are protected against apoptosis; conversely inhibition of CRT may prevent cell proliferation.
Materials and Methods
In this study, we tested the efficacy of recently described inhibitors of CRT (iCRTs; oxazole and thiazole) for their selective antagonistic effect on Wnt-β-cat response in MM cells MM1, U266, BMSC and primary BMMC obtained from patient samples (n=16).
Results
We demonstrate that iCRTs we used block Wnt/β-cat reporter activity, down regulate β-cat expression and inhibit cell proliferation in a dose dependent manner with an optimal dose closer to 15 µM. Our data further indicate that iCRTs do not influence the expression of the upstream components of the Wnt pathway DKK1 at the optimal dose, suggesting that iCRTs may specifically target β-cat in MM cells. Additionally, iCRT-treatment of MM cells co-cultured with BMSC showed an inhibitory effect on VEGF and cell migration.
Conclusion
This study provides the first in vitro data evaluation of newly described iCRTs as potential Wnt-β-cat/VEGF pathway antagonists in multiple myeloma.
Keywords: Multiple myeloma, Wnt, β-catenin, transcription, VEGF and DKK1
Multiple Myeloma (MM) is the second most frequent hematological cancer in the US and is characterized by the clonal proliferation of neoplastic plasma cells in association with elevated serum monoclonal protein levels. Clinical manifestations of MM include lytic bone lesions, anemia, immunodeficiency, and renal impairment. It is evident from earlier studies that maintenance of MM within the bone marrow (BM) microenvironment largely depends on a number of factors including cytokines IL-6 and the family of Wnt ligands that are secreted by the BM stromal cells (BMSCs) (1–5). Wnt ligand specifically activates the canonical Wnt pathway which is mediated through the key transcriptional effector β-catenin (β-cat). In unstimulated cells, β-cat is phosphorylated by the Axin/APC/GSK3β-mediated destruction complex (DC), and is a target for ubiquitination and subsequent proteasome-mediated degradation. Wnt induction blocks the activity of the DC, thereby resulting in the cytosolic accumulation of non-phosphorylated, active β-cat, which translocates to the nucleus and together with T-cell factor/Lymphoid Enhancer Factor (TCF/LEF) transcriptional factors, activates transcription of downstream target genes. The Wnt pathway has been shown to play an important role in the regulation of cancer cell proliferation and differentiation (2, 6–8). Previous studies have shown that malignant MM plasma cells over express β-cat, including its N-terminally non-phosphorylated form, suggesting that β-cat/TCF-mediated transcription may be active in MM cells (9). Studies using both in vitro and in vivo models have shown that Wnt-β-cat signaling mediates critical events in the development of MM and thus indicates related phenotypic changes in plasma cells(10). Although a recent study reports the therapeutic efficacy of bortezomib via Wnt-independent stabilization of β-cat (11), a role for Wnt signaling in MM remains unclear. Dickkopf-1 (DKK1), a soluble inhibitor of Wnt/β catenin signaling, functions by binding to the Wnt co-receptor LRP5 to regulate its function on the cell surface in MM cells (12). However, deregulation of CRT in cancer development makes the β-cat-TCF complex as an ideal target for therapeutic approaches (13–15). Given the dual role of Wnt in normal bone formation and in myeloma disease, our interest was to test the chemosensitivity of recently identified small molecule inhibitors of β-cat regulated transcription (iCRTs) that are designed to specifically target β-cat/TCF-regulated transcription (16). Using human MM cell lines and patient derived BMMC that express nuclear β-cat, we report that iCRTs (oxazole and thiazole) are effective in down regulating nuclear β-cat, and reducing cell proliferation. Our findings further indicated a significant decrease in the level of vasculoendothelial growth factor (VEGF), in cells treated with iCRTs. Although our attempts to test the in vivo efficacy of iCRTs in preclinical models are in progress, we provide the first in vitro data evaluation of iCRTs as potential Wnt/β-cat/VEGF pathway antagonists in MM that could effectively block or decrease the disease progression at clinically relevant doses.
Materials and Methods
Compounds
The iCRT compounds (oxazole) iCRT-3 and thiazole (iCRT-5) were procured from “ChemDiv”; http://us.chemdiv.com. The concentrations used for this study were made in DMSO.
Patient samples
Human serum, BMMC (Bone marrow mononuclear cells) and BMSC ((Bone marrow stromal cells) samples (n=16) were obtained from patients with early and active late stage multiple myeloma. Informed consent for the human samples was approved by New York University School of Medicine, Institutional Review Board to Dr. Mazumder (PI, Director of Myeloma Program) for research purpose.
Cell lines and cell culture
MM.1 and U266 cells were kindly provided by Dr. Hearn Cho (Cancer Institute at the Mount Sinai Medical Center, New York). The cells were cultured at 37°C, 5% CO2 in RPMI-1640 (Mediatech-Cellgro) containing 10% heat inactivated fetal bovine serum and 1M HEPES buffer with 20 µg/ml gentamycin (Invitrogen) as described earlier (17). Primary myeloma cells (BMMC) from patient samples were prepared and cultured as described earlier (18). The primary BMSCs used in this study were cultured in Iscove’s modified Dulbecco’s medium containing 20% FBS, 2 mM L-glutamine and 100 g/ml penicillin/streptomycin. Cell culture medium and adherent BMSCs grown in 6 well plates were used for co-culture studies with MM cells and for assays including VEGF analysis and cell migration.
STF16 luciferase reporter assay
To perform the Wnt-β-cat responsive STF16 luciferase reporter assays, MM1 and U266 cells were transfected with 50 ng each of the Wnt responsive STF16 luciferase reporter and pCMV-RL normalization reporter using Lipofectamine LTX (Invitrogen) in 96-well plates. Description of the Wnt response STF16 reporter constructs are presented in earlier publications (16). Transfected cells were then maintained in RPMI with 10% FBS at 37°C for 24 h and subsequently treated with indicated concentrations of iCRT-3 and iCRT-5 (5–50 µM). Luciferase reporter activity was then measured by Dual-Glo system using L-MaxII384 (Molecular Devices) as directed by the manufacturer (Promega). Normalized relative luciferase activity in response to treatments was compared with that obtained from cells treated with DMSO (control). For experiments involving specific Wnt activation MM cells were pretreated with 20 µM LiCl for a period of 24 h.
Transfection with siRNAs
The β-cat gene-specific siRNA duplexes along with HiPerFect transfection reagents were used to transfect MM cells (siRNA-1 and siRNA-2). siRNA sequences of the human β-cat were resuspended in the suspension buffer provided by the manufacturer (Qiagen). Next, 30 nmol siRNA was gently introduced into MM cells by mixing with HiPerFect transfection reagent as described by us in our earlier publications (19). To achieve >85% knockdown, the transfection was continued up to 72 h. Effective reduction in β-cat protein expression was determined by immunofluorescence detection and Western blot analysis.
Immunofluorescence detection of β-catenin
Control, treated or transfected MM cells were seeded into the 2 well chamber slides (LAB-TEK-Nalge Nunc) and grown for 48h. To view the nuclear β-cat localization, cells collected after cytospin preprations were then fixed with 10% neutral buffered formalin (NBF) at RT for 30 min, and washed gently with 1× PBS, premeabilized in 1% triton-X and followed by incubated in 5% FBS at RT for 30 min. After gently removing the blocking solution, the cells were incubated with mouse anti-β-cat antibody (Invitrogen, lot # 940535A) for 1 h followed by staining with phylloidin dye Alexaflour 488 goat antirabbit vs. isotype control. Nuclear staining with 4’,6-diamidino-2-phenylindole (DAPI) was performed before the cells were imaged for localization of β-cat. Green fluorescence signal for β-cat over DAPI was viewed at 40× using an Olympus AX-70 epi-fluorescence microscope (Olympus America, Melville, NY USA) equipped with a computer-controlled digital camera (Spot) for imaging. The positively stained cells were quantified with Image Pro plus software (Media Cybernetics, Silver Spring, MD) as described earlier (19). Phase contrast images of unstained cells were captured using Leitz-LABOVERT microscope.
Cell proliferation analysis
Actively growing cells were plated in triplicate in 96-well plates at a density of 5×105 per well with 100 µl medium containing 5–50 µM iCRT-3 or iCRT-5, and cells treated with 1% DMSO served as the control. After 48h of treatment, cell proliferation analysis was performed using MTS kit (Promega). Absorbance was read after at 490nm using a 96 well plate reader (Spectra Max-M2-Molecular devices). Cells pre-treated with LiCl (20 µM) a GSK3β inhibitor and served as the control for Wnt activation. Cell growth was calculated from the mean relative decrease or increase in the optical density at 490 nm compared to the DMSO treated cells; Inhibition of cell proliferation was calculated based on the mean values of three repeated assays.
Transmigration migration assay
Rate of migration of MM cells was assessed using a 24-well BD FluoroBlok Transwell Inserts (BD Biosciences, Bedford, MA, USA) with 8 µM pore size. Briefly, MM cells (50,000) pretreated with the iCRTs-3 and iCRTs-5 (15 µM) were seeded (in 200 µl) into the inserts with RPMI medium containing 0.25% serum. The bottom well contained RPMI with 10% FBS. After 48 h of incubation the bottom well was filled with 500 µl of Calcein fluorescent dye prepared according to the directions of the manufacturer (BD Biosciences). Calcein AM is the most suitable indicator for staining viable cells due to its low cytotoxicity property. The fluorescence intensity emitted by the migrated cells was measured at 540 nm using a plate reader Max-M2- (Molecular Devices, Sunnyvale, CA). The experiments were repeated three times.
Western blot analysis
Total protein lysate (30 µg/lane) of MM cells treated without or with iCRTs (15 µM) for 48h was prepared in RIPA buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 1% sodium deoxycholate and 0.1% SDS) containing protease inhibitor cocktail (cØmplete™, Boehringer Mannheim, Germany) as described earlier (19). Immunobloting was done by standard SDS-PAGE (12%) using antibodies against β-catenin (Invitrogen, lot # 138400) and DKK1 (Cell signaling, lot 1, 4687S). Secondary antibodies conjugated to horseradish peroxide (Thrermoscientific, # 32430) and the loading control α-tubulin was obtained from Santa Cruz Biotechnology. In addition, protein samples from HEK 293 cells were used to confirm β-cat in nonmyeloma cells. Reactive protein bands for β-cat were developed using an enhanced ECL chemiluminescence detection kit (Amersham Biosciences). All the blots were stripped and re-probed with α-tubulin (Santa Cruz Biotechnology, Santa Cruz, CA) to normalize protein loading. Each experiment was repeated three times using same sets of samples. Quantification of reactive protein bands were performed by densitometric analysis and the fold change was calculated by normalizing with α-tubulin.
VEGF analysis by Enzyme-linked Immunosorbent Assay (ELISA)
MM cells (U266) were grown as described in the earlier section. The primary BMSCs used in this study were obtained from patients and cultured in Iscove’s modified Dulbecco’s medium containing 20% FBS, 2 mM L-glutamine and 5mg/ml penicillin/streptomycin. Cell culture medium collected from U266 cells and co-cultured with adherent BMSCs (grown in 6 well plates) and treated with iCRTs were used for VEGF analysis. ELISA assays were performed using Human VEGF Quantikine ELISA Kits (R&D systems, Minneapolis, MN) by following the manufacturer’s protocol. These assays employ the quantitative sandwich enzyme immunoassay technique. The resultant color was read at 450 nm using an ELISA plate reader Max-M2- (Molecular Devices, Sunnyvale, CA). The concentrations of VEGF in the samples were determined by interpolation from a standard curve made out of the standard provided by the manufacturer. The experiments were performed in triplicate, repeated at least twice.
RNA isolation and quantitative real-time PCR
Total RNA was extracted from the MM cells treated with iCRTs (15 µM) using Trizol reagent (Life Technologies/GibcoBRL) as described earlier (20, ). A two-step RT-PCR was carried out with total RNA (5 µg) extracted from U266 and BMSC treated with 50 µM iCRT-3 was used for initial denaturing for 2 minutes at 95°C and continued the amplification with an extension at 72°C, 7 minutes for 33 cycles using VEGF gene-specific primer sequences upper 5’atttacaacgtctgc gcatctt 3’ lower, 5’ctcgccttgctgctctacctc3’ along with the amplification of glyceraldehyde-3-phosphate dehydrogenase (GAPDH), upper 5’ ggatgaccttgcccacagcct 3’ lower, 5’catctctg ccccctctgctga 3’ as the internal control (IDT). Real-time quantitative PCR was performed in triplicate with a Smart Cycler (Cepheid, Sunnyvale, CA) using SYBR-Green mix (Applied Biosystems, Branchburg, NJ) as described earlier by us (19, ). Results were normalized to amplification of GAPDH and to determine the fold change based on 2ΔΔCt.
Statistical analysis
Data from all the experiments are presented as the mean±SD, from at least three independent experiments. Measures of statistical difference were determined using two-way ANOVA followed by Tukey’s multiple comparison procedure (30). Differences between the treatment and control groups were analyzed using Student’s t-test. Statistical analyses were performed using GraphPad Prism 4 software (San Diego, CA, USA).
Results
β-catenin expression in MM cells
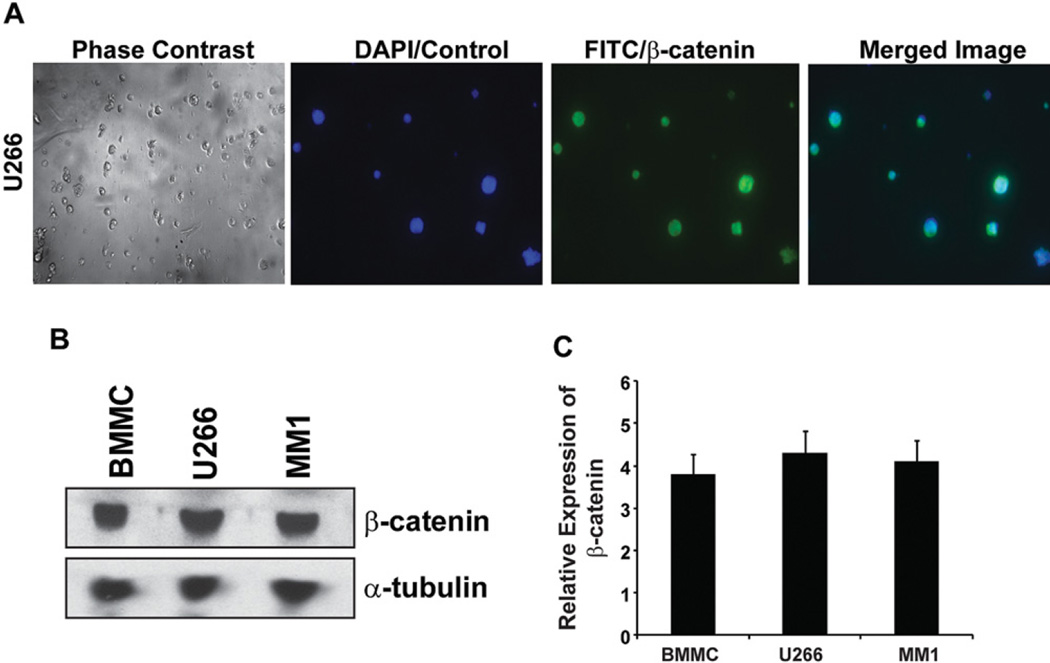
Although, earlier studies have documented Wnt signaling in human MM cells exhibiting nuclear β-cat and its effect on the downstream target genes of the Wnt pathway (2, 21, 22), in order to test the chemosensitivity of iCRTs in myeloma cells, we first confirmed the expression of β-cat in human MM cell types U266 and MM.1 in addition to using patient derived BMMCs. As shown in Figure 1A, immunofluorescence detection of β-cat in U266 cells showed nuclear localization (FITC-merged) in >70 to 80 % of the cells, a similar trend was observed in MM.1 and BMMC. This observation is consistent with the Western blot analysis of β-cat in the total protein lysate, thus indicating a consistently similar level of expression among the cell lines examined (Figure 1B–C). The above data on the expression of β-cat in MM cell lines and cells from patient sample provides the rationale for using these cells to test the efficacy of iCRTs that are specifically designed to target nuclear β-cat signaling (16).
Figure 1.
β-catenin expression in MM. (A) Immunofluorescence detection of nuclear β-cat in the cytospin preparation of U266 cells. Approximately 5000 cells/slide were fixed in 10% NBF, followed by staining for β-cat and counter staining with DAPI to label the nuclei as described in the Methods. (B) Immunoblotting of total β-cat expression in MM and BMMC cell lysates. Western blot analysis using the respective protein lysate (30 µg/lane) was performed as described in the methods. Antibody for α-tubulin was used as loading control. (C) Bar graph represents the relative mean pixel density of the reactive protein bands of β-cat with reference to the loading control a-tubulin from three independent blots. The data presented in each bar represents mean±SD.
Inhibition of Wnt/β-catenin response by iCRTs in myeloma cells
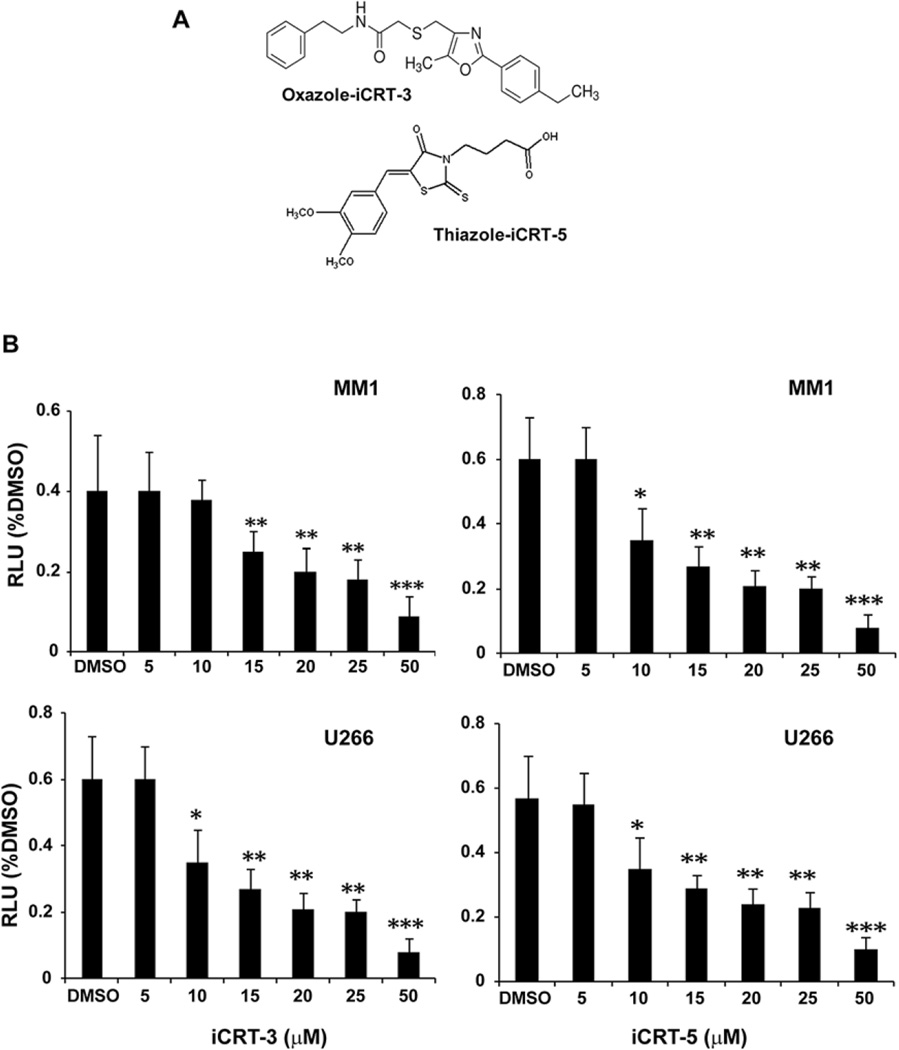
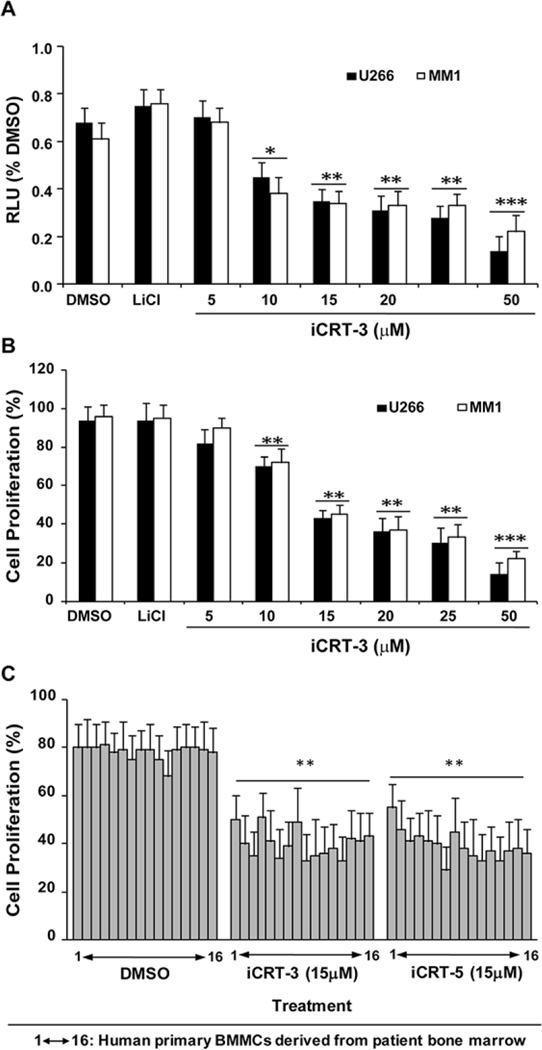
To determine the efficacy of iCRT-3 and iCRT-5 (Figure 2A) in antagonizing Wnt signaling activity in MM cells, we transfected U266 and MM.1 cells with Wnt/β-cat responsive reporter plasmids (STF16) as described earlier (16). Our data confirmed that both U266 and MM.1 cells showed >85% transfection efficiency (determined by immunoflourescence detection) after 24 h. Treatment of STF16-transfected MM cells with iCRT-3 and iCRT-5 showed a dose dependent decrease (2–3 fold) in the reporter activity respectively, with an optimal inhibitory effect at 15µM followed by significant decline at a dose of 50 µM (Figure 2B). To further confirm the specific effect of iCRTs, in separate assays, MM cells were pre-treated for 24 h with LiCl (20 µM), (a GSK3β inhibitor known to activate Wnt signaling by stabilizing β-cat), and then subsequently treated with different doses of iCRTs. As shown in Figure 3A and B, iCRT-3 significantly inhibited both the LiCl-induced reporter activity, and cell proliferation, as determined by MTS assays in U266 and MM1 cell lines. Although effect of LiCl on the Wnt reporter activity compared to DMSO was not much enhanced, the inhibitory effect induced by iCRT-3 is significant (p<0.001). A similar effect was observed in cells treated with iCRT-5 in three repeated assays. iCRTs induced cytotoxic effect induced at 15µM concentration compared to the DMSO control was significant in all the patient derived BMMC samples (n=16) (Figure 3C) (**p<0.01).
Figure 2.
Inhibition of Wnt/β-cat response STF16 luciferase reporter activity by iCRTs in myeloma cells. (A) Chemical structure of iCRTs used in this study. (B) Luciferase reporter activity. The assay was conducted in MM cells transfected with 50 ng each of the Wnt responsive STF16 luciferase reporter and pCMV-RL normalization reporter using Lipofectamine LTX (Invitrogen) as the transfection agent in 96-well plates. After 24 h of transfection, cells were subsequently treated with iCRT-3 or iCRT-5 (5–50 µM). Luciferase reporter activity was then measured using the Dual-Glo system (Promega) as described in the Methods. Normalized relative luciferase activity (% RLU) in response to the treatments for 24 h was compared to cells treated with DMSO (control). The data presented in each bar represents mean±SD. Statistical differences at *p<0.05, **p<0.01, and ***p<0.001.
Figure 3.
Specific Effect of iCRT-3 in MM cells on the STF16 luciferase reporter activity. (A) MM cells were pretreated with 20 µM lithium chloride (LiCl) for 24 h before transfection with the STF16 reporter. Luciferase reporter activity was measured after treatment with iCRTs using the Dual-Glo system (Promega) as described in the Methods. Normalized relative luciferase activity (% RLU) in response to the treatments after 24 h was compared to cells treated with DMSO (control). (B) Cell growth inhibition. MM cells pre-treated with 20 µM LiCl were analyzed for cell growth inhibition in response to iCRTs treatment using MTS assay as described in the Methods. The data presented is mean from three independent assays with error bars reporting standard deviation. Statistical differences at *p<0.05, **p<0.01, and ***p<0.001. (C) Human primary BMMCs derived from bone marrow aspirants were used to test the efficacy of iCRT-3 and iCRT-5 at a dose of 15 µM for 48h; 1–16 represents the number of patient samples. The data presented in each bar represents mean±SD. **Statistical difference at p<0.01.
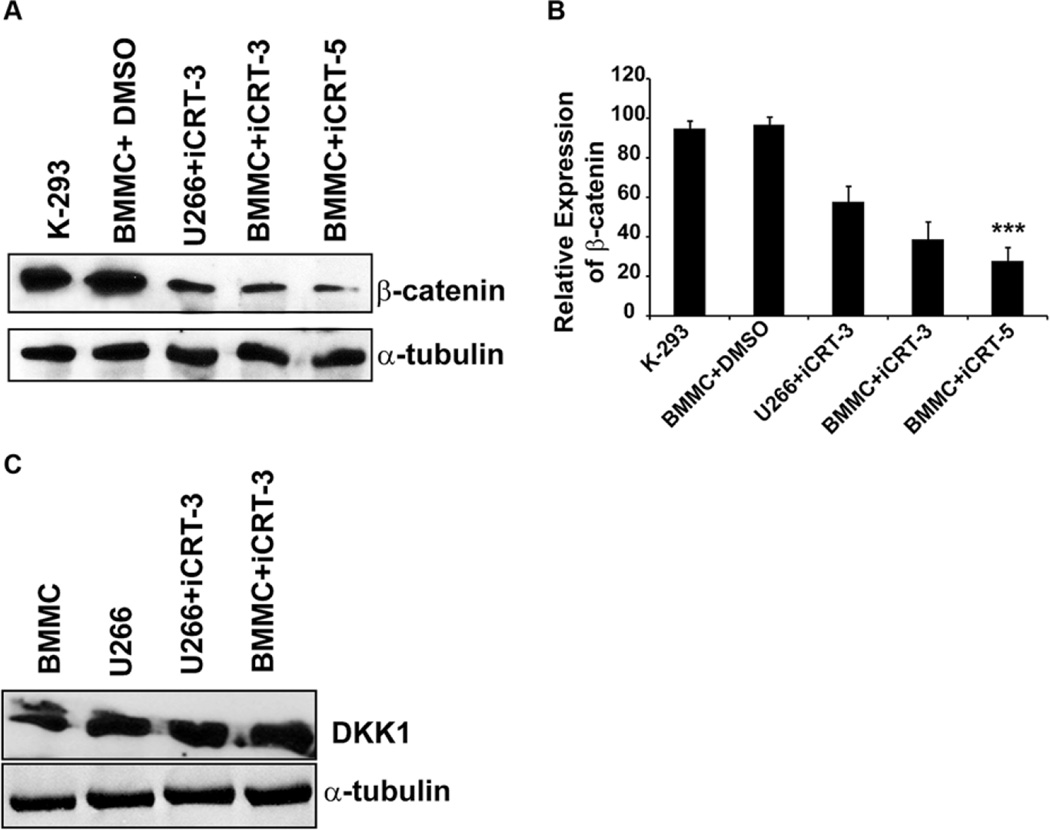
Effect of iCRTs on total β-cat protein vs. DKK1
In this study, iCRT treated cells revealed an inhibitory effect on total β-cat protein (Figure 4A–B), this was typically not observed in few other cancer cell types examined, including colon cancers (HT29, HCT116, SW480) or human breast adenocarcinoma cells (MCF7) (16). As a positive control, protein samples from HEK 293 cells were used to confirm β-cat expression. Given the role of Wnt and the upstream antagonist DKK1 in normal bone formation and MM development, we examined whether inhibition of Wnt-β-cat response by iCRTs indirectly affects the expression of DKK1. Our findings from Western blot analysis of MM cells treated with iCRTs at the optimal or higher doses (15 µM or 50 µM) showed no significant change in the expression of DKK1 protein (Figure 4C). Taken together these data reveal a possibility that in MM cells iCRTs may indirectly influence the levels of β-cat protein.
Figure 4.
Effect of iCRTs on β-catenin and DKK1 expression in MM cells. (A) Standard Western blot analysis was performed as described in the Methods using 30 µg/lane of total protein lysate from BMMC and U266 cells treated with 15 µM of indicated iCRT for 48 h. Protein lysate from untreated HEK-293 cells and MM cells treated with DMSO served as the positive and experimental controls. (B) Quantification of the reactive protein bands in terms of pixel density for total β-cat expression normalized to α-tubulin in BMMC and U266 cells is presented in the bar graph represents the mean±SD. (C) Immunoblotting was performed using DKK1 specific antibody as described in Methods. Total protein lyste was obtained from BMMC and U266 cells treated only with iCRT-3 (15 µM). The antibody for α-tublin served as the loading control. As there was no change in the level of DKK1 in the untreated or treated cells, data on a quantification of the reactive protein bands are not presented.
Effect of iCRTs on vascular endothelial growth factor (VEGF)
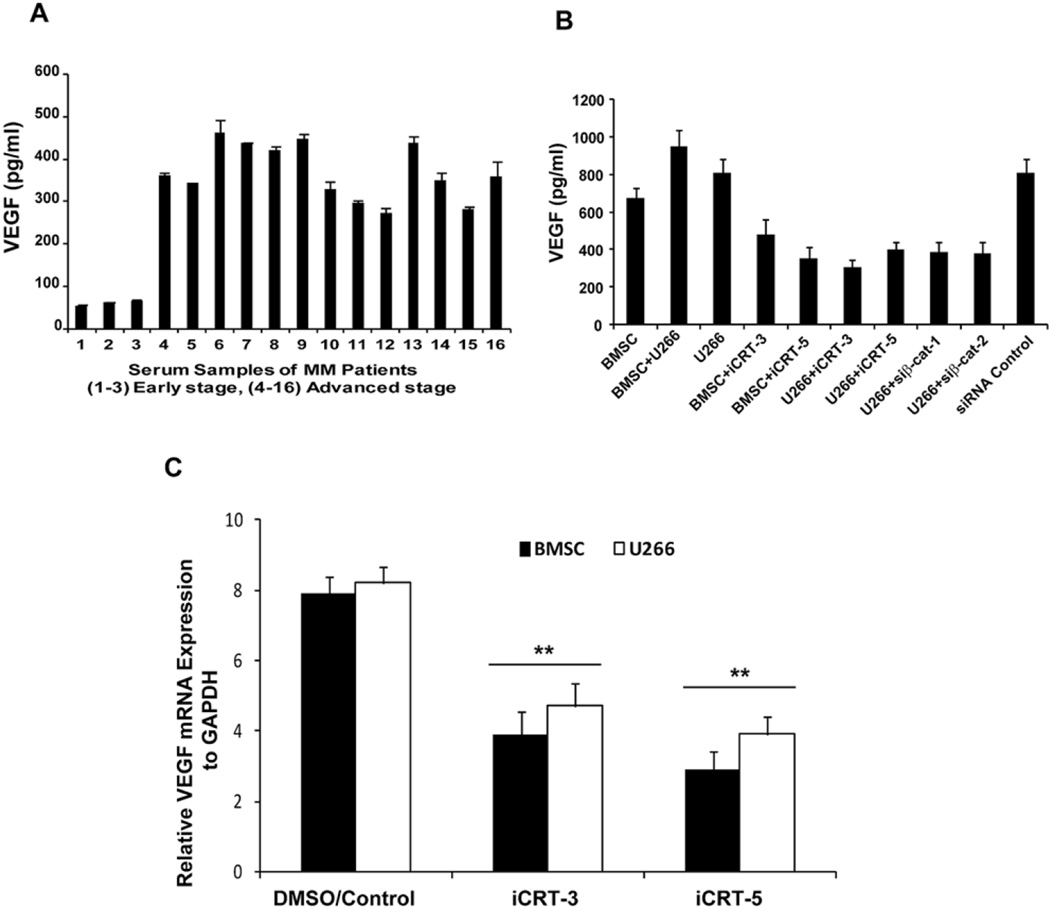
While bone marrow angiogenesis is a hallmark indicating the progression of multiple myeloma, it is also correlated with the severity of the disease. We therefore tested the potential effect of iCRTs on Wnt/β-cat mediated angiogenic processess. With this focus, first, we measured the VEGF level, a transcriptional target of Wnt/β-cat signaling, in the serum samples of MM patients presented with early and late stage disease. As shown in Figure 5A, we observed elevated levels (ranging from 300 to 500 pg/ml) of VEGF in patients with late stage MM.
Figure 5.
Inhibitory effect of iCRTs on VEGF. (A) Serum analysis for VEGF levels in MM patients (n=16) was performed using an ELISA based assay as described in the Methods. The data presented is mean from three independent assays with error bars reporting standard deviation. (B) Human primary BMSCs derived from bone marrow were used in co-culture with U266 cells. Secretion of VEGF into the medium by adherent BMSC co-cultured with U266 in the presence or absence of iCRTs (15 µM) and/or transfected with β-cat siRNA was measured after 24 h. C, RT-PCR was performed as described in the methods using total RNA (5 µg) isolated from BMSC and U266 cells with or without exposure to iCRTS. Relative expression of VEGF-mRNA to GAPDH is presented in the bar graph with the mean±SD. ***Statistical difference at p<0.01.
To test the efficacy of iCRTs on VEGF level in MM cells, the cell culture medium obtained from the co-culture of bone marrow stromal cells (BMSC) with human U266 cells were used. This is based on the findings from earlier studies that binding of MM cells to BMSCs triggers the production of VEGF (23). Treatment of both BMSC and U266 cells with iCRT-3 or iCRT-5 resulted in a marked reduction in VEGF (ranging from 200–400 pg/ml) expression compared to untreated cells (650–900 pg/ml). To determine whether the effect of iCRTs on the VEGF activity is specific to its ability to antagonize β-cat activity, we used culture medium collected from cells transiently transfected with siRNA for β-cat. As shown in Figure 5B, siRNA mediated down regulation of β-cat showed a significant decrease in the VEGF levels (200–400 pg/ml compared to siRNA control (800 pg/ml), thereby confirming that the effect of iCRTs is indeed mediated by their inhibitory activity on β-cat. In addition, qPCR analysis for the VEGF revealed that the iCRT-mediated inhibition (>2 fold, p<0.05) is at the transcription level (Figure 5C), and these findings are consistent with the notion of VEGF being a Wnt/β-cat target gene.
Effect of iCRTs on MM cell migration
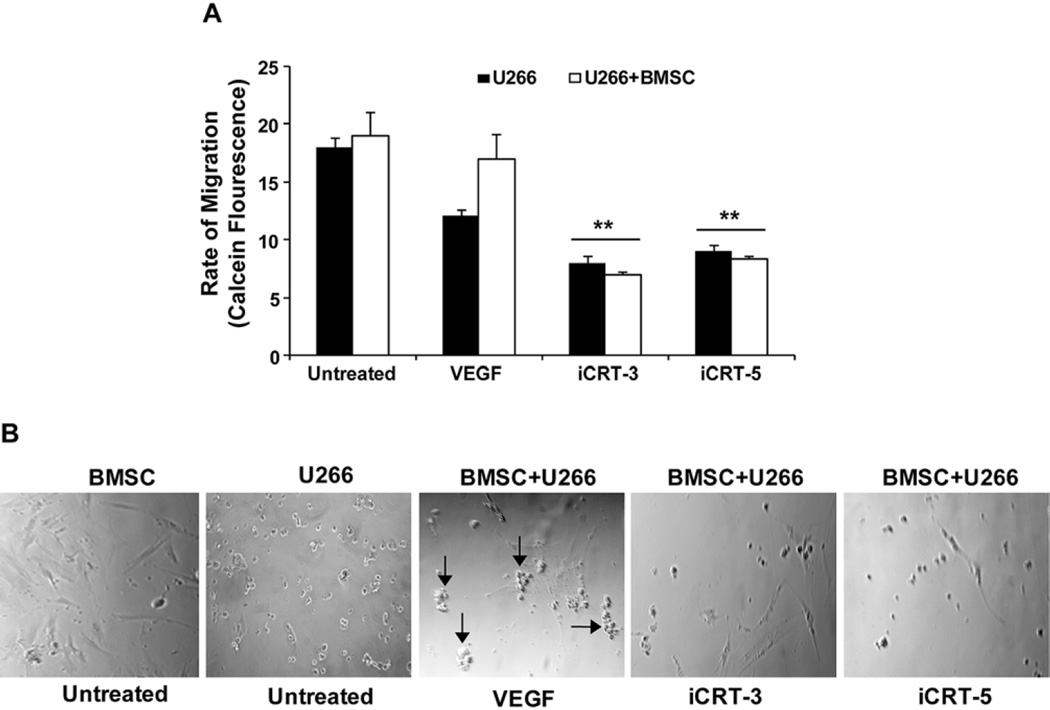
Since our findings revealed a negative effect of iCRTs on VEGF levels, we next determined the effect on Wnt/β-cat/VEGF mediated cell migration. U266 cells transfected with reporter plasmid (STF16) followed by treatment with iCRTs (15 µM) and/or with an exogenous source of VEGF (30 ng) as positive control were assessed for the rate of migration. Our findings after 24h treatments showed a significant decrease in cell migration compared to the untreated control (by 2–3 fold decrease, p<0.05), determined based on the Calcein fluorescence readings of the migrated cells (Figure 6A). Parallel experiments conducted to determine any binding of U266 with BMSC revealed that the cells pretreated with VEGF showed an inhibitory effect by iCRTs on the binding or clustering of cells as shown in the images (arrow) by iCRTs (Figure 6B) compared to the untreated cells or cells supplemented with VEGF alone.
Figure 6.
iCRTs inhibit MM cell migration and adhesion of BMSC with U266 cells. (A) U266 cells in the presence of absence of BMSCs were grown in 24 well-transwell migration plates as described in the Methods. Migratory potential of cells in the presence of iCRTs (15 µM each) was measured after 24 h using Calcein fluorescent intensity of the migrated cells. Cells treated with VEGF showing clustering of cells (arrow) in untreated and stimulated with VEGF serve as controls. Arbitrary relative fluorescent units were used to represent the rate of migration based on the fluorescence intensity. The data presented in each bar represents mean±SD. **Statistical difference at p<0.01. (B) Phase contrast images of untreated or iCRT treated U266 and BMSC cells were taken after 24 h of treatment.
Discussion
Wnt signaling plays a key role the in the regulation of bone mass and in the development of multiple myeloma (24). Interestingly, overexpression of β-cat in osteoblasts has been demonstrated to induce a high bone mass phenotype (25). However the precise functions of specific antagonists of Wnt/βcat signaling and its importance against growth and survival of myeloma cells are unclear. Findings from this study indicate that β-cat is predominantly present in the nucleus in the MM cells. This is consistent with the earlier observations in myeloma cells (9). We further demonstrate that the iCRTs as antagonists of Wnt signaling block Wnt/β-cat reporter activity, down regulate β-cat expression and inhibit cell proliferation in different myeloma cell types in addition to human primary BMMC cells from 16 patients with either advanced or early stage of the disease. We observed a dose dependent effect with an optimum effect at 15µM, however; the clinical relevance of this dose is yet being confirmed in in vivo studies. In this context, it is also important to recall earlier in vivo studies using yet another small interfering RNA (siRNA) targeting β-cat has been shown to suppress the progression of MM in mouse models (26). Interestingly, iCRT treated cells revealed an inhibitory effect on total β-cat protein as shown in Figure 4A–B, this was typically not observed in few other cancer cell types examined, including colon cancers (HT29, HCT116, SW480) or human breast adenocarcinoma cells (MCF7) (16) and thus suggesting a possibility of inducing specific effects in MM cells. The canonical Wnt pathway is regulated by large number antagonists, including the DKK family of proteins, among which DKK1 and DKK2 have been well characterized (12, 27, 28). Given the role of Wnt and DKK1 in normal bone formation and MM development, we examined whether inhibition of Wnt-β-catenin response by iCRTs affects DKK1 protein expression. Our data from Western blot analysis of MM cells treated with iCRTs showed no change in the expression of DKK1 protein even at a higher dose of 50µm for 48h, suggesting that the iCRTs mediated negative regulation of the Wnt pathway is likely to act at the level of regulating downstream components of the Wnt pathway.
Another important aspect of our findings is the possible inhibition of VEGF by iCRTs. VEGF is a crucial cytokine that directs and promotes tumorogenesis and potentiation in the bone marrow. Our findings on demonstrating elevated serum levels of VEGF in patients with advanced MM disease is consistent with earlier reports (29) and is correlated with the risk for angiogenesis (4, 30–33). Although few studies have shown that binding of MM cells to BMSC upregulates VEGF secretion and also triggers IL-6 production (34), our findings on the impact of iCRTs on VEGF mediated migration is significant. Migration is one of the fundamental processes involved in myeloma cell invasion and dissemination, and extravasation of myeloma cells from blood vessels into the BM is likely to be controlled by several chemoattractants including VEGF(35). Our data on the elevated level of VEGF in advanced MM patient samples indicate that VEGF could be potent effectors of myeloma cell transmigration through vascular endothelium and BMSC stromal cells. Consistently, our findings on the co-culture of BMSC cells with U666 cell showed an increase in migration and clustering of cells that was significantly inhibited by iCRTs. Although our findings from cytotoxic assays reflect a similar inhibitory effect on cell proliferation in most of the cell types including human primary cells examined, the clinical relevance of the optimal dose of 15 µM or less has yet to be confirmed in murine models for MM. Taken together, these observations suggest that VEGF produced by bone marrow stromal cells may form a gradient leading to attraction of myeloma cells into the BM cavity where higher concentrations of VEGF may promote cell survival and proliferation. Overall, our findings from this study indicate that Wnt/β-cat/VEGF pathway dependent increase in migration and proliferation of MM cells can be antagonized by specific inhibitors of nuclear β-cat activity, and thereby underscoring the importance of developing iCRTs as a novel class of Wnt-directed therapeutics in human MM.
Acknowledegements
We thank the RNAi core of the NYUSOM Cancer Institute for providing the reagents and Dr. Randall T. Moon for the STF16 reporter plasmids to conduct this study.
References
- 1.Chauhan D, Uchiyama H, Akbarali Y, Urashima M, Yamamoto K, Libermann TA, Anderson KC. Multiple myeloma cell adhesion-induced interleukin-6 expression in bone marrow stromal cells involves activation of NF-kappa B. Blood. 1996;87:1104–1112. [PubMed] [Google Scholar]
- 2.Derksen PW, Tjin E, Meijer HP, Klok MD, MacGillavry HD, van Oers MH, Lokhorst HM, Bloem AC, Clevers H, Nusse R, van der Neut R, Spaargaren M, Pals ST. Illegitimate WNT signaling promotes proliferation of multiple myeloma cells. Proc Natl Acad Sci USA. 2004;101:6122–6127. doi: 10.1073/pnas.0305855101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McWhirter JR, Neuteboom ST, Wancewicz EV, Monia BP, Downing JR, Murre C. Oncogenic homeodomain transcription factor E2A-Pbx1 activates a novel WNT gene in pre-B acute lymphoblastoid leukemia. Proc Natl Acad Sci USA. 1999;96:11464–11469. doi: 10.1073/pnas.96.20.11464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vacca A, Ria R, Ribatti D, Semeraro F, Djonov V, Di Raimondo F, Dammacco F. A paracrine loop in the vascular endothelial growth factor pathway triggers tumor angiogenesis and growth in multiple myeloma. Haematologica. 2003;88:176–185. [PubMed] [Google Scholar]
- 5.Van Den Berg DJ, Sharma AK, Bruno E, Hoffman R. Role of members of the Wnt gene family in human hematopoiesis. Blood. 1998;92:3189–3202. [PubMed] [Google Scholar]
- 6.Kim Y, Schmidt M, Endo T, Lu D, Carson D, Schmidt-Wolf IG. Targeting the Wnt/beta-catenin pathway with the antifungal agent ciclopirox olamine in a murine myeloma model. In Vivo. 2011;25:887–893. [PubMed] [Google Scholar]
- 7.Miller JR, Hocking AM, Brown JD, Moon RT. Mechanism and function of signal transduction by the Wnt/beta-catenin and Wnt/Ca2+ pathways. Oncogene. 1999;18:7860–7872. doi: 10.1038/sj.onc.1203245. [DOI] [PubMed] [Google Scholar]
- 8.You Z, Saims D, Chen S, Zhang Z, Guttridge DC, Guan KL, MacDougald OA, Brown AM, Evan G, Kitajewski J, Wang CY. Wnt signaling promotes oncogenic transformation by inhibiting c-Myc-induced apoptosis. J Cell Biol. 2002;157:429–440. doi: 10.1083/jcb.200201110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sukhdeo K, Mani M, Zhang Y, Dutta J, Yasui H, Rooney MD, Carrasco DE, Zheng M, He H, Tai YT, Mitsiades C, Anderson KC, Carrasco DR. Targeting the beta-catenin/TCF transcriptional complex in the treatment of multiple myeloma. Proc Natl Acad Sci USA. 2007;104:7516–7521. doi: 10.1073/pnas.0610299104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edwards CM, Edwards JR, Lwin ST, Esparza J, Oyajobi BO, McCluskey B, Munoz S, Grubbs B, Mundy GR. Increasing Wnt signaling in the bone marrow microenvironment inhibits the development of myeloma bone disease and reduces tumor burden in bone in vivo. Blood. 2008;111:2833–2842. doi: 10.1182/blood-2007-03-077685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qiang YW, Hu B, Chen Y, Zhong Y, Shi B, Barlogie B, Shaughnessy JD., Jr Bortezomib induces osteoblast differentiation via Wnt-independent activation of beta-catenin/TCF signaling. Blood. 2009;113:4319–4330. doi: 10.1182/blood-2008-08-174300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci. 2003;116:2627–2634. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- 13.Furlong MT, Morin PJ. Rare activation of the TCF/beta-catenin pathway in ovarian cancer. Gynecol Oncol. 2000;77:97–104. doi: 10.1006/gyno.1999.5718. [DOI] [PubMed] [Google Scholar]
- 14.Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta. 2003;1653:1–24. doi: 10.1016/s0304-419x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 15.Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- 16.Gonsalves FC, Klein K, Carson BB, Katz S, Ekas LA, Evans S, Nagourney R, Cardozo T, Brown AM, DasGupta R. An RNAi-based chemical genetic screen identifies three small-molecule inhibitors of the Wnt/wingless signaling pathway. Proc Natl Acad Sci USA. 2011;108:5954–5963. doi: 10.1073/pnas.1017496108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nardiello T, Jungbluth AA, Mei A, Diliberto M, Huang X, Dabrowski A, Andrade VC, Wasserstrum R, Ely S, Niesvizky R, Pearse R, Coleman M, Jayabalan DS, Bhardwaj N, Old LJ, Chen-Kiang S, Cho HJ. MAGE-A inhibits apoptosis in proliferating myeloma cells through repression of Bax and maintenance of survivin. Clin Cancer Res. 2011;17:4309–4319. doi: 10.1158/1078-0432.CCR-10-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhan F, Hardin J, Kordsmeier B, Bumm K, Zheng M, Tian E, Sanderson R, Yang Y, Wilson C, Zangari M, Anaissie E, Morris C, Muwalla F, van Rhee F, Fassas A, Crowley J, Tricot G, Barlogie B, Shaughnessy J., Jr Global gene expression profiling of multiple myeloma, monoclonal gammopathy of undetermined significance, and normal bone marrow plasma cells. Blood. 2002;99:1745–1757. doi: 10.1182/blood.v99.5.1745. [DOI] [PubMed] [Google Scholar]
- 19.Narayanan BA, Narayanan NK, Davis L, Nargi D. RNA interference-mediated cyclooxygenase-2 inhibition prevents prostate cancer cell growth and induces differentiation: modulation of neuronal protein synaptophysin, cyclin D1, and androgen receptor. Mol Cancer Ther. 2006;5:1117–1125. doi: 10.1158/1535-7163.MCT-05-0520. [DOI] [PubMed] [Google Scholar]
- 20.Kim HN, Narayanan NK, Lasano S, Narayanan B. Modulation of PGE2-induced EP4 expression on snail signaling and the impact on epithelial-mesenchymal transition: significance of EP4 antagonism. Anticancer Res. 2011;31:4347–4357. [PubMed] [Google Scholar]
- 21.Carrasco DR, Tonon G, Huang Y, Zhang Y, Sinha R, Feng B, Stewart JP, Zhan F, Khatry D, Protopopova M, Protopopov A, Sukhdeo K, Hanamura I, Stephens O, Barlogie B, Anderson KC, Chin L, Shaughnessy JD, Jr, Brennan C, Depinho RA. High-resolution genomic profiles define distinct clinico-pathogenetic subgroups of multiple myeloma patients. Cancer Cell. 2006;9:313–325. doi: 10.1016/j.ccr.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 22.Qiang YW, Endo Y, Rubin JS, Rudikoff S. Wnt signaling in B-cell neoplasia. Oncogene. 2003;22:1536–1545. doi: 10.1038/sj.onc.1206239. [DOI] [PubMed] [Google Scholar]
- 23.Gupta D, Treon SP, Shima Y, Hideshima T, Podar K, Tai YT, Lin B, Lentzsch S, Davies FE, Chauhan D, Schlossman RL, Richardson P, Ralph P, Wu L, Payvandi F, Muller G, Stirling DI, Anderson KC. Adherence of multiple myeloma cells to bone marrow stromal cells upregulates vascular endothelial growth factor secretion: therapeutic applications. Leukemia. 2001;15:1950–1961. doi: 10.1038/sj.leu.2402295. [DOI] [PubMed] [Google Scholar]
- 24.Westendorf JJ, Kahler RA, Schroeder TM. Wnt signaling in osteoblasts and bone diseases. Gene. 2004;341:19–39. doi: 10.1016/j.gene.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 25.Glass DA, 2nd, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, Taketo MM, Long F, McMahon AP, Lang RA, Karsenty G. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell. 2005;8:751–764. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 26.Ashihara E, Kawata E, Nakagawa Y, Shimazaski C, Kuroda J, Taniguchi K, Uchiyama H, Tanaka R, Yokota A, Takeuchi M, Kamitsuji Y, Inaba T, Taniwaki M, Kimura S, Maekawa T. beta-catenin small interfering RNA successfully suppressed progression of multiple myeloma in a mouse model. Clin Cancer Res. 2009;15:2731–2738. doi: 10.1158/1078-0432.CCR-08-1350. [DOI] [PubMed] [Google Scholar]
- 27.Li J, Sarosi I, Cattley RC, Pretorius J, Asuncion F, Grisanti M, Morony S, Adamu S, Geng Z, Qiu W, Kostenuik P, Lacey DL, Simonet WS, Bolon B, Qian X, Shalhoub V, Ominsky MS, Zhu Ke H, Li X, Richards WG. Dkk1-mediated inhibition of Wnt signaling in bone results in osteopenia. Bone. 2006;39:754–766. doi: 10.1016/j.bone.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 28.Mao B, Wu W, Davidson G, Marhold J, Li M, Mechler BM, Delius H, Hoppe D, Stannek P, Walter C, Glinka A, Niehrs C. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature. 2002;417:664–667. doi: 10.1038/nature756. [DOI] [PubMed] [Google Scholar]
- 29.Di Raimondo F, Azzaro MP, Palumbo GA, Bagnato S, Stagno F, Giustolisi GM, Cacciola E, Sortino G, Guglielmo P, Giustolisi R. Elevated vascular endothelial growth factor (VEGF) serum levels in idiopathic myelofibrosis. Leukemia. 2001;15:976–980. doi: 10.1038/sj.leu.2402124. [DOI] [PubMed] [Google Scholar]
- 30.Lin B, Podar K, Gupta D, Tai YT, Li S, Weller E, Hideshima T, Lentzsch S, Davies F, Li C, Weisberg E, Schlossman RL, Richardson PG, Griffin JD, Wood J, Munshi NC, Anderson KC. The vascular endothelial growth factor receptor tyrosine kinase inhibitor PTK787/ZK222584 inhibits growth and migration of multiple myeloma cells in the bone marrow microenvironment. Cancer Res. 2002;62:5019–5026. [PubMed] [Google Scholar]
- 31.Rajkumar SV. Thalidomide in multiple myeloma. Oncology (Williston Park) 2000;14:11–16. [PubMed] [Google Scholar]
- 32.Rajkumar SV, Fonseca R, Dispenzieri A, Lacy MQ, Lust JA, Witzig TE, Kyle RA, Gertz MA, Greipp PR. Thalidomide in the treatment of relapsed multiple myeloma. Mayo Clin Proc. 2000;75:897–901. doi: 10.4065/75.9.897. [DOI] [PubMed] [Google Scholar]
- 33.Ribatti D, Vacca A, Nico B, Quondamatteo F, Ria R, Minischetti M, Marzullo A, Herken R, Roncali L, Dammacco F. Bone marrow angiogenesis and mast cell density increase simultaneously with progression of human multiple myeloma. Br J Cancer. 1999;79:451–455. doi: 10.1038/sj.bjc.6690070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dankbar B, Padro T, Leo R, Feldmann B, Kropff M, Mesters RM, Serve H, Berdel WE, Kienast J. Vascular endothelial growth factor and interleukin-6 in paracrine tumor-stromal cell interactions in multiple myeloma. Blood. 2000;95:2630–2636. [PubMed] [Google Scholar]
- 35.Vincent L, Jin DK, Karajannis MA, Shido K, Hooper AT, Rashbaum WK, Pytowski B, Wu Y, Hicklin DJ, Zhu Z, Bohlen P, Niesvizky R, Rafii S. Fetal stromal-dependent paracrine and intracrine vascular endothelial growth factor-a/vascular endothelial growth factor receptor-1 signaling promotes proliferation and motility of human primary myeloma cells. Cancer Res. 2005;65:3185–3192. doi: 10.1158/0008-5472.CAN-04-3598. [DOI] [PubMed] [Google Scholar]