Abstract
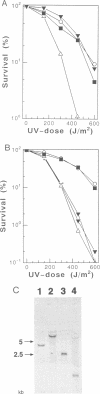
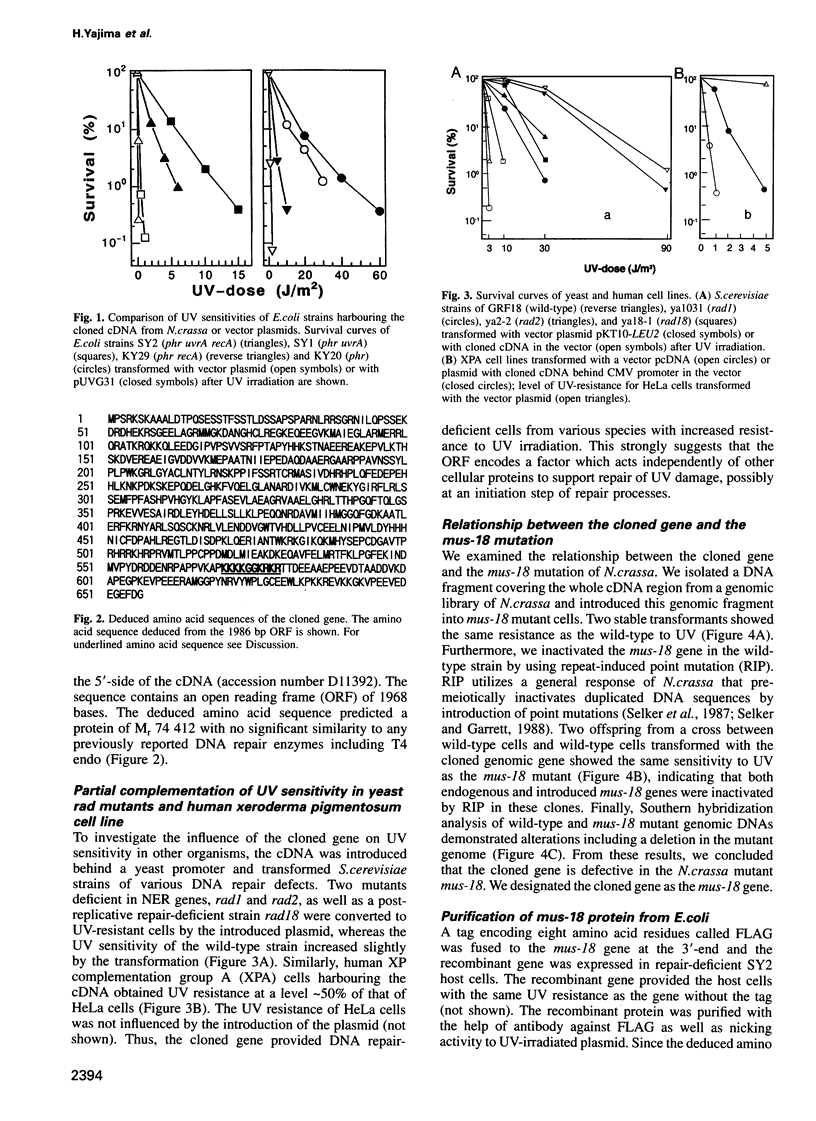
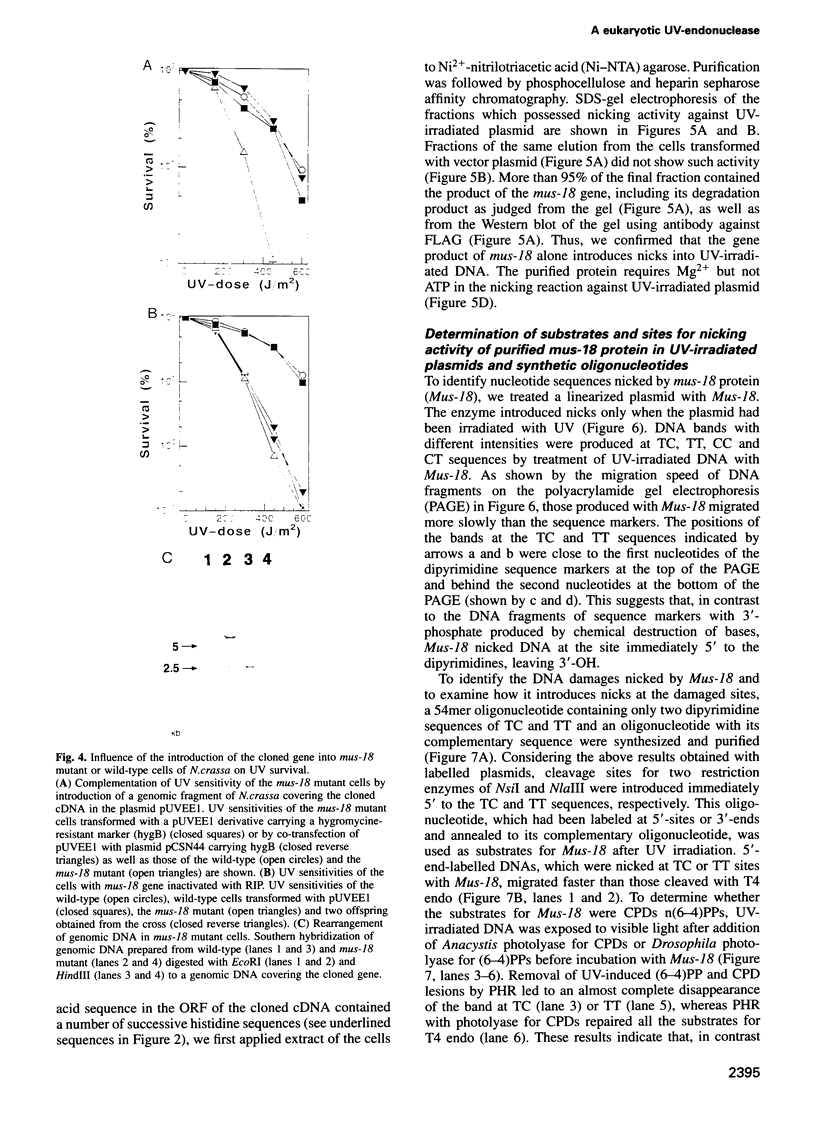
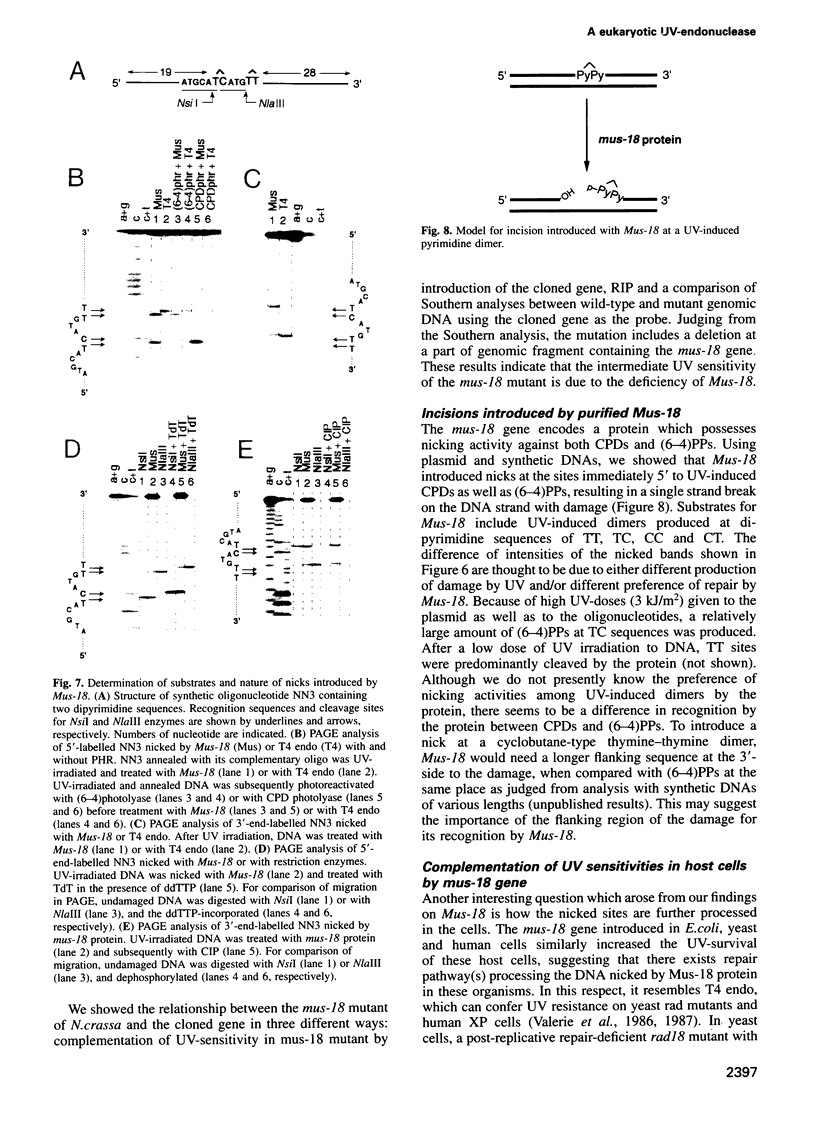
Many eukaryotic organisms, including humans, remove ultraviolet (UV) damage from their genomes by the nucleotide excision repair pathway, which requires more than 10 separate protein factors. However, no nucleotide excision repair pathway has been found in the filamentous fungus Neurospora crassa. We have isolated a new eukaryotic DNA repair gene from N.crassa by its ability to complement UV-sensitive Escherichia coli cells. The gene is altered in a N.crassa mus-18 mutant and responsible for the exclusive sensitivity to UV of the mutant. Introduction of the wild-type mus-18 gene complements not only the mus-18 DNA repair defect of N.crassa, but also confers UV-resistance on various DNA repair-deficient mutants of Saccharomyces cerevisiae and a human xeroderma pigmentosum cell line. The cDNA encodes a protein of 74 kDa with no sequence similarity to other known repair enzymes. Recombinant mus-18 protein was purified from E.coli and found to be an endonuclease for UV-irradiated DNA. Both cyclobutane pyrimidine dimers and (6-4)photoproducts are cleaved at the sites immediately 5' to the damaged dipyrimidines in a magnesium-dependent, ATP-independent reaction. This mechanism, requiring a single polypeptide designated UV-induced dimer endonuclease for incision, is a substitute for the role of nucleotide excision repair of UV damage in N.crassa.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando T., Watanabe S. A new method for fractionation of protamines and the amino acid sequences of salmine and three components of iridine. Int J Protein Res. 1969;1(3):221–224. doi: 10.1111/j.1399-3011.1969.tb01646.x. [DOI] [PubMed] [Google Scholar]
- Bardwell A. J., Bardwell L., Tomkinson A. E., Friedberg E. C. Specific cleavage of model recombination and repair intermediates by the yeast Rad1-Rad10 DNA endonuclease. Science. 1994 Sep 30;265(5181):2082–2085. doi: 10.1126/science.8091230. [DOI] [PubMed] [Google Scholar]
- Bowman K. K., Sidik K., Smith C. A., Taylor J. S., Doetsch P. W., Freyer G. A. A new ATP-independent DNA endonuclease from Schizosaccharomyces pombe that recognizes cyclobutane pyrimidine dimers and 6-4 photoproducts. Nucleic Acids Res. 1994 Aug 11;22(15):3026–3032. doi: 10.1093/nar/22.15.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eker A. P., Kooiman P., Hessels J. K., Yasui A. DNA photoreactivating enzyme from the cyanobacterium Anacystis nidulans. J Biol Chem. 1990 May 15;265(14):8009–8015. [PubMed] [Google Scholar]
- Eker A. P., Yajima H., Yasui A. DNA photolyase from the fungus Neurospora crassa. Purification, characterization and comparison with other photolyases. Photochem Photobiol. 1994 Aug;60(2):125–133. doi: 10.1111/j.1751-1097.1994.tb05078.x. [DOI] [PubMed] [Google Scholar]
- Grafstrom R. H., Park L., Grossman L. Enzymatic repair of pyrimidine dimer-containing DNA. A 5' dimer DNA glycosylase: 3'-apyrimidinic endonuclease mechanism from Micrococcus luteus. J Biol Chem. 1982 Nov 25;257(22):13465–13474. [PubMed] [Google Scholar]
- Harrington J. J., Lieber M. R. Functional domains within FEN-1 and RAD2 define a family of structure-specific endonucleases: implications for nucleotide excision repair. Genes Dev. 1994 Jun 1;8(11):1344–1355. doi: 10.1101/gad.8.11.1344. [DOI] [PubMed] [Google Scholar]
- Hinnen A., Hicks J. B., Fink G. R. Transformation of yeast. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1929–1933. doi: 10.1073/pnas.75.4.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii C., Nakamura K., Inoue H. A novel phenotype of an excision-repair mutant in Neurospora crassa: mutagen sensitivity of the mus-18 mutant is specific to UV. Mol Gen Genet. 1991 Aug;228(1-2):33–39. doi: 10.1007/BF00282444. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McCready S., Carr A. M., Lehmann A. R. Repair of cyclobutane pyrimidine dimers and 6-4 photoproducts in the fission yeast Schizosaccharomyces pombe. Mol Microbiol. 1993 Nov;10(4):885–890. doi: 10.1111/j.1365-2958.1993.tb00959.x. [DOI] [PubMed] [Google Scholar]
- Mitchell D. L., Haipek C. A., Clarkson J. M. (6-4)Photoproducts are removed from the DNA of UV-irradiated mammalian cells more efficiently than cyclobutane pyrimidine dimers. Mutat Res. 1985 Jul;143(3):109–112. doi: 10.1016/s0165-7992(85)80018-x. [DOI] [PubMed] [Google Scholar]
- Mitchell D. L., Nairn R. S. The biology of the (6-4) photoproduct. Photochem Photobiol. 1989 Jun;49(6):805–819. doi: 10.1111/j.1751-1097.1989.tb05578.x. [DOI] [PubMed] [Google Scholar]
- Orbach M. J., Sachs M. S., Yanofsky C. The Neurospora crassa arg-2 locus. Structure and expression of the gene encoding the small subunit of arginine-specific carbamoyl phosphate synthetase. J Biol Chem. 1990 Jul 5;265(19):10981–10987. [PubMed] [Google Scholar]
- Sancar G. B. DNA photolyases: physical properties, action mechanism, and roles in dark repair. Mutat Res. 1990 Sep-Nov;236(2-3):147–160. doi: 10.1016/0921-8777(90)90002-m. [DOI] [PubMed] [Google Scholar]
- Selker E. U., Cambareri E. B., Jensen B. C., Haack K. R. Rearrangement of duplicated DNA in specialized cells of Neurospora. Cell. 1987 Dec 4;51(5):741–752. doi: 10.1016/0092-8674(87)90097-3. [DOI] [PubMed] [Google Scholar]
- Selker E. U., Garrett P. W. DNA sequence duplications trigger gene inactivation in Neurospora crassa. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6870–6874. doi: 10.1073/pnas.85.18.6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda D. L., Smith C. A., Taylor J. S., Sancar A. Effect of sequence, adduct type, and opposing lesions on the binding and repair of ultraviolet photodamage by DNA photolyase and (A)BC excinuclease. J Biol Chem. 1993 May 15;268(14):10694–10700. [PubMed] [Google Scholar]
- Szymkowski D. E., Lawrence C. W., Wood R. D. Repair by human cell extracts of single (6-4) and cyclobutane thymine-thymine photoproducts in DNA. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):9823–9827. doi: 10.1073/pnas.90.21.9823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todo T., Takemori H., Ryo H., Ihara M., Matsunaga T., Nikaido O., Sato K., Nomura T. A new photoreactivating enzyme that specifically repairs ultraviolet light-induced (6-4)photoproducts. Nature. 1993 Jan 28;361(6410):371–374. doi: 10.1038/361371a0. [DOI] [PubMed] [Google Scholar]
- Valerie K., Fronko G., Henderson E. E., de Riel J. K. Expression of the denV gene of coliphage T4 in UV-sensitive rad mutants of Saccharomyces cerevisiae. Mol Cell Biol. 1986 Oct;6(10):3559–3562. doi: 10.1128/mcb.6.10.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valerie K., Green A. P., de Riel J. K., Henderson E. E. Transient and stable complementation of ultraviolet repair in xeroderma pigmentosum cells by the denV gene of bacteriophage T4. Cancer Res. 1987 Jun 1;47(11):2967–2971. [PubMed] [Google Scholar]
- Yajima H., Inoue H., Oikawa A., Yasui A. Cloning and functional characterization of a eucaryotic DNA photolyase gene from Neurospora crassa. Nucleic Acids Res. 1991 Oct 11;19(19):5359–5362. doi: 10.1093/nar/19.19.5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuhira S., Yasui A. Visible light-inducible photolyase gene from the goldfish Carassius auratus. J Biol Chem. 1992 Dec 25;267(36):25644–25647. [PubMed] [Google Scholar]
- Yasui A., Eker A. P., Koken M. Existence and expression of photoreactivation repair genes in various yeast species. Mutat Res. 1989 Jan;217(1):3–10. doi: 10.1016/0921-8777(89)90029-3. [DOI] [PubMed] [Google Scholar]
- Yasui A., Eker A. P., Yasuhira S., Yajima H., Kobayashi T., Takao M., Oikawa A. A new class of DNA photolyases present in various organisms including aplacental mammals. EMBO J. 1994 Dec 15;13(24):6143–6151. doi: 10.1002/j.1460-2075.1994.tb06961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]