Abstract
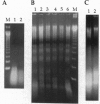
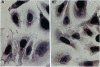
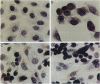
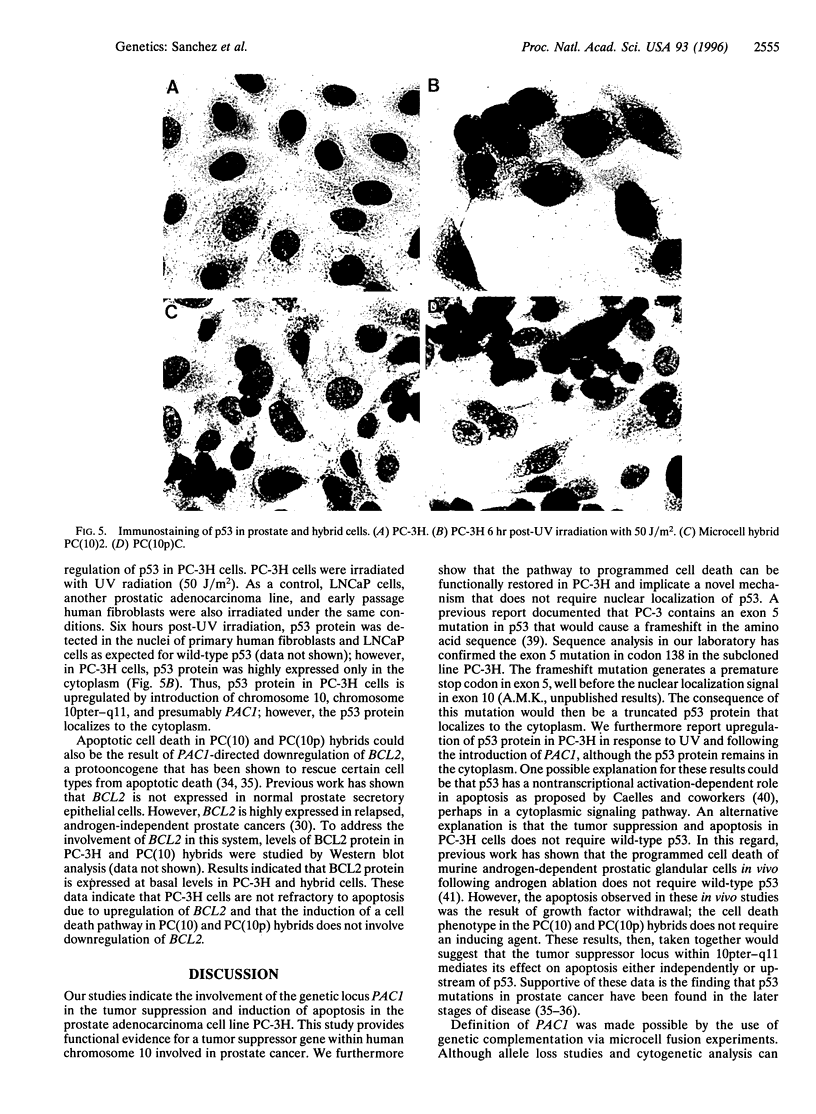
Prostate cancer is the second leading cause of male cancer deaths in the United States. Yet, despite a large international effort, little is known about the molecular mechanisms that underlie this devastating disease. Prostate secretory epithelial cells and androgen-dependent prostate carcinomas undergo apoptosis in response to androgen deprivation and, furthermore, most prostate carcinomas become androgen independent and refractory to further therapeutic manipulations during disease progression. Definition of the genetic events that trigger apoptosis in the prostate could provide important insights into critical pathways in normal development as well as elucidate the perturbations of those key pathways in neoplastic transformation. We report the functional definition of a novel genetic locus within human chromosome 10pter-q11 that mediates both in vivo tumor suppression and in vitro apoptosis of prostatic adenocarcinoma cells. A defined fragment of human chromosome 10 was transferred via microcell fusion into a prostate adenocarcinoma cell line. Microcell hybrids containing only the region 10pter-q11 were suppressed for tumorigenicity following injection of microcell hybrids into nude mice. Furthermore, the complemented hybrids undergo programmed cell death in vitro via a mechanism that does not require nuclear localization of p53. These data functionally define a novel genetic locus, designated PAC1, for prostate adenocarcinoma 1, involved in tumor suppression of human prostate carcinoma and furthermore strongly suggest that the cell death pathway can be functionally restored in prostatic adenocarcinoma.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergerheim U. S., Kunimi K., Collins V. P., Ekman P. Deletion mapping of chromosomes 8, 10, and 16 in human prostatic carcinoma. Genes Chromosomes Cancer. 1991 May;3(3):215–220. doi: 10.1002/gcc.2870030308. [DOI] [PubMed] [Google Scholar]
- Berges R. R., Furuya Y., Remington L., English H. F., Jacks T., Isaacs J. T. Cell proliferation, DNA repair, and p53 function are not required for programmed death of prostatic glandular cells induced by androgen ablation. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):8910–8914. doi: 10.1073/pnas.90.19.8910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookstein R., Allred D. C. Recessive oncogenes. Cancer. 1993 Feb 1;71(3 Suppl):1179–1186. doi: 10.1002/1097-0142(19930201)71:3+<1179::aid-cncr2820711442>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Bookstein R., MacGrogan D., Hilsenbeck S. G., Sharkey F., Allred D. C. p53 is mutated in a subset of advanced-stage prostate cancers. Cancer Res. 1993 Jul 15;53(14):3369–3373. [PubMed] [Google Scholar]
- Boring C. C., Squires T. S., Tong T. Cancer statistics, 1991. CA Cancer J Clin. 1991 Jan-Feb;41(1):19–36. doi: 10.3322/canjclin.41.1.19. [DOI] [PubMed] [Google Scholar]
- Bova G. S., Carter B. S., Bussemakers M. J., Emi M., Fujiwara Y., Kyprianou N., Jacobs S. C., Robinson J. C., Epstein J. I., Walsh P. C. Homozygous deletion and frequent allelic loss of chromosome 8p22 loci in human prostate cancer. Cancer Res. 1993 Sep 1;53(17):3869–3873. [PubMed] [Google Scholar]
- Brothman A. R., Peehl D. M., Patel A. M., MacDonald G. R., McNeal J. E., Ladaga L. E., Schellhammer P. F. Cytogenetic evaluation of 20 cultured primary prostatic tumors. Cancer Genet Cytogenet. 1991 Aug;55(1):79–84. doi: 10.1016/0165-4608(91)90238-p. [DOI] [PubMed] [Google Scholar]
- Brothman A. R., Peehl D. M., Patel A. M., McNeal J. E. Frequency and pattern of karyotypic abnormalities in human prostate cancer. Cancer Res. 1990 Jun 15;50(12):3795–3803. [PubMed] [Google Scholar]
- Caelles C., Helmberg A., Karin M. p53-dependent apoptosis in the absence of transcriptional activation of p53-target genes. Nature. 1994 Jul 21;370(6486):220–223. doi: 10.1038/370220a0. [DOI] [PubMed] [Google Scholar]
- Carter B. S., Ewing C. M., Ward W. S., Treiger B. F., Aalders T. W., Schalken J. A., Epstein J. I., Isaacs W. B. Allelic loss of chromosomes 16q and 10q in human prostate cancer. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8751–8755. doi: 10.1073/pnas.87.22.8751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle L. R., Yin X., Brothman A. R., Williams B. J., Atkin N. B., Prochownik E. V. Mutation of the MXI1 gene in prostate cancer. Nat Genet. 1995 Mar;9(3):249–255. doi: 10.1038/ng0395-249. [DOI] [PubMed] [Google Scholar]
- Effert P. J., Neubauer A., Walther P. J., Liu E. T. Alterations of the P53 gene are associated with the progression of a human prostate carcinoma. J Urol. 1992 Mar;147(3 Pt 2):789–793. doi: 10.1016/s0022-5347(17)37387-1. [DOI] [PubMed] [Google Scholar]
- Ellis R. E., Yuan J. Y., Horvitz H. R. Mechanisms and functions of cell death. Annu Rev Cell Biol. 1991;7:663–698. doi: 10.1146/annurev.cb.07.110191.003311. [DOI] [PubMed] [Google Scholar]
- Fernandez A., Marin M. C., McDonnell T., Ananthaswamy H. N. Differential sensitivity of normal and Ha-ras-transformed C3H mouse embryo fibroblasts to tumor necrosis factor: induction of bcl-2, c-myc, and manganese superoxide dismutase in resistant cells. Oncogene. 1994 Jul;9(7):2009–2017. [PubMed] [Google Scholar]
- Fournier R. E., Ruddle F. H. Microcell-mediated transfer of murine chromosomes into mouse, Chinese hamster, and human somatic cells. Proc Natl Acad Sci U S A. 1977 Jan;74(1):319–323. doi: 10.1073/pnas.74.1.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helminen H. J., Ericsson J. L. Ultrastructural studies on prostatic involution in the rat. Evidence for focal irreversible damage to epithelium, and heterophagic digestion in macrophages. J Ultrastruct Res. 1972 Jun;39(5):443–455. doi: 10.1016/s0022-5320(72)90112-8. [DOI] [PubMed] [Google Scholar]
- Hockenbery D., Nuñez G., Milliman C., Schreiber R. D., Korsmeyer S. J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990 Nov 22;348(6299):334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- Isaacs W. B., Carter B. S., Ewing C. M. Wild-type p53 suppresses growth of human prostate cancer cells containing mutant p53 alleles. Cancer Res. 1991 Sep 1;51(17):4716–4720. [PubMed] [Google Scholar]
- Kaighn M. E., Narayan K. S., Ohnuki Y., Lechner J. F., Jones L. W. Establishment and characterization of a human prostatic carcinoma cell line (PC-3). Invest Urol. 1979 Jul;17(1):16–23. [PubMed] [Google Scholar]
- Kerr J. F., Searle J. Deletion of cells by apoptosis during castration-induced involution of the rat prostate. Virchows Arch B Cell Pathol. 1973 Jun 25;13(2):87–102. doi: 10.1007/BF02889300. [DOI] [PubMed] [Google Scholar]
- Killary A. M., Fournier R. E. Microcell fusion. Methods Enzymol. 1995;254:133–152. doi: 10.1016/0076-6879(95)54011-3. [DOI] [PubMed] [Google Scholar]
- Killary A. M., Wolf M. E., Giambernardi T. A., Naylor S. L. Definition of a tumor suppressor locus within human chromosome 3p21-p22. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10877–10881. doi: 10.1073/pnas.89.22.10877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunimi K., Bergerheim U. S., Larsson I. L., Ekman P., Collins V. P. Allelotyping of human prostatic adenocarcinoma. Genomics. 1991 Nov;11(3):530–536. doi: 10.1016/0888-7543(91)90059-n. [DOI] [PubMed] [Google Scholar]
- Liu P., Siciliano J., Seong D., Craig J., Zhao Y., de Jong P. J., Siciliano M. J. Dual Alu polymerase chain reaction primers and conditions for isolation of human chromosome painting probes from hybrid cells. Cancer Genet Cytogenet. 1993 Feb;65(2):93–99. doi: 10.1016/0165-4608(93)90213-6. [DOI] [PubMed] [Google Scholar]
- Lu X., Lane D. P. Differential induction of transcriptionally active p53 following UV or ionizing radiation: defects in chromosome instability syndromes? Cell. 1993 Nov 19;75(4):765–778. doi: 10.1016/0092-8674(93)90496-d. [DOI] [PubMed] [Google Scholar]
- Lundgren R., Mandahl N., Heim S., Limon J., Henrikson H., Mitelman F. Cytogenetic analysis of 57 primary prostatic adenocarcinomas. Genes Chromosomes Cancer. 1992 Jan;4(1):16–24. doi: 10.1002/gcc.2870040103. [DOI] [PubMed] [Google Scholar]
- McDonnell T. J., Troncoso P., Brisbay S. M., Logothetis C., Chung L. W., Hsieh J. T., Tu S. M., Campbell M. L. Expression of the protooncogene bcl-2 in the prostate and its association with emergence of androgen-independent prostate cancer. Cancer Res. 1992 Dec 15;52(24):6940–6944. [PubMed] [Google Scholar]
- McNeill C. A., Brown R. L. Genetic manipulation by means of microcell-mediated transfer of normal human chromosomes into recipient mouse cells. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5394–5398. doi: 10.1073/pnas.77.9.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micale M. A., Mohamed A., Sakr W., Powell I. J., Wolman S. R. Cytogenetics of primary prostatic adenocarcinoma. Clonality and chromosome instability. Cancer Genet Cytogenet. 1992 Jul 15;61(2):165–173. doi: 10.1016/0165-4608(92)90082-j. [DOI] [PubMed] [Google Scholar]
- Navone N. M., Troncoso P., Pisters L. L., Goodrow T. L., Palmer J. L., Nichols W. W., von Eschenbach A. C., Conti C. J. p53 protein accumulation and gene mutation in the progression of human prostate carcinoma. J Natl Cancer Inst. 1993 Oct 20;85(20):1657–1669. doi: 10.1093/jnci/85.20.1657. [DOI] [PubMed] [Google Scholar]
- Oshimura M., Kugoh H., Koi M., Shimizu M., Yamada H., Satoh H., Barrett J. C. Transfer of a normal human chromosome 11 suppresses tumorigenicity of some but not all tumor cell lines. J Cell Biochem. 1990 Mar;42(3):135–142. doi: 10.1002/jcb.240420304. [DOI] [PubMed] [Google Scholar]
- Sanchez Y., el-Naggar A., Pathak S., Killary A. M. A tumor suppressor locus within 3p14-p12 mediates rapid cell death of renal cell carcinoma in vivo. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3383–3387. doi: 10.1073/pnas.91.8.3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxon P. J., Srivatsan E. S., Stanbridge E. J. Introduction of human chromosome 11 via microcell transfer controls tumorigenic expression of HeLa cells. EMBO J. 1986 Dec 20;5(13):3461–3466. doi: 10.1002/j.1460-2075.1986.tb04670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu M., Yokota J., Mori N., Shuin T., Shinoda M., Terada M., Oshimura M. Introduction of normal chromosome 3p modulates the tumorigenicity of a human renal cell carcinoma cell line YCR. Oncogene. 1990 Feb;5(2):185–194. [PubMed] [Google Scholar]
- Tanaka K., Oshimura M., Kikuchi R., Seki M., Hayashi T., Miyaki M. Suppression of tumorigenicity in human colon carcinoma cells by introduction of normal chromosome 5 or 18. Nature. 1991 Jan 24;349(6307):340–342. doi: 10.1038/349340a0. [DOI] [PubMed] [Google Scholar]
- Trent J. M., Stanbridge E. J., McBride H. L., Meese E. U., Casey G., Araujo D. E., Witkowski C. M., Nagle R. B. Tumorigenicity in human melanoma cell lines controlled by introduction of human chromosome 6. Science. 1990 Feb 2;247(4942):568–571. doi: 10.1126/science.2300817. [DOI] [PubMed] [Google Scholar]
- Weissman B. E., Saxon P. J., Pasquale S. R., Jones G. R., Geiser A. G., Stanbridge E. J. Introduction of a normal human chromosome 11 into a Wilms' tumor cell line controls its tumorigenic expression. Science. 1987 Apr 10;236(4798):175–180. doi: 10.1126/science.3031816. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Yonish-Rouach E., Resnitzky D., Lotem J., Sachs L., Kimchi A., Oren M. Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature. 1991 Jul 25;352(6333):345–347. doi: 10.1038/352345a0. [DOI] [PubMed] [Google Scholar]